Abstract
Overview:
The purpose of this study was to evaluate the role of Tumor Necrosis Factor-α (TNF-α) in insulin resistance (IR) during normal pregnancy.
Approach:
This cross sectional study was carried out on 86 healthy pregnant women including 26, 23 and 37 individuals in the 1st, 2nd and 3rd trimesters, respectively, and in 21 healthy non pregnant women. Serum TNF-α concentration was measured by Enzyme Linked Immunosorbent Assay (ELISA) method.
Results:
There were significant differences between serum TNF-α and IL-6 levels in pregnant women as compared with maternal healthy controls. There was significant correlation between gestational age and Body Mass Index (BMI) (r = 0.28, P = 0.01). There was no significant correlation between gestational age and insulin resistance (IR). We also did not find correlations between IR and TNF-α and IR and IL-6 in pregnant women.
Conclusion:
In conclusion, our findings suggest that TNF-α and IL-6 are not greatly contributed to pregnancy induced insulin resistance in normal pregnancy.
Keywords: TNF-α, IL-6, normal pregnancy, gestational age, body mass index, insulin resistance
Introduction
Pregnancy is related to glucose metabolism disorders and insulin resistance.1–3 Insulin resistance may facilitate supply of appropriate nutrients particularly of glucose to the fetus for growth and metabolism. The mechanism responsible for insulin resistance has not been clearly established. Recent research shows that adipokinins including leptin4 resistin5 interleukin-6 (IL-6)6 and TNF-α7–10 play an important role in insulin resistance. TNF-α is one of the most widely studied cytokinins produced by adipose tissue. This cytokinin is also secreted by placenta.11,12 TNF-α has an important role in obesity-induced insulin resistance and diabetes.7,10,13,14 A few studies suggest that TNF-α may play a role in insulin resistance in normal and diabetogenic pregnant women8,13,15 Interleukine-6 is another cytokinine produced by immune and non immune cells.16–18
There are reports regarding its role in the pathogenesis of insulin resistance.7,19–21 Insulin sensitivity changes from an enhanced state during early pregnancy to an insulin resistant state in late pregnancy.3,8,22 Therefore, it is suspected, subsequent to increase in IR during pregnancy, its related factors change too. However, at the time of our study we did not find any research had been done about changes in serum IL-6 and TNF-α levels during different trimesters of normal pregnancy and their relationships to insulin resistance. Therefore, the aim of this study is to determine the association of insulin resistance with serum interleukin-6 and TNF-α levels during normal pregnancy.
Methods
This study was conducted at the Department of Obstetrics and Gynecology of Honary Clinic, Jahrom, Iran. Subjects were 86 pregnant women with different gestational ages (first trimester: 26, second trimester: 23, third trimester: 37, and 21 non pregnant women similar in age and body mass index (BMI)). All subjects met the following criteria: no history of pregestational diabetes; no history of liver, respiratory, thyroid or other illness or any current infectious condition. They were not on any drug therapy.
Body mass index (BMI, Kg/m2) was calculated according to the maternal height and prepregnacy weight. Serum samples were analyzed for concentrations of IL-6, TNF-α, insulin and glucose. Blood sugar was measured by glucose oxidase/peroxidase (GOD-POD) method. Serum insulin was determined by ELISA (Diaplus; based on the direct sandwich technique in which two monoclonal antibodies are directed against separate antigenic determinants on the insulin molecule. During incubation insulin in the sample reacts with enzyme (HRP) conjugated anti-insulin antibody and anti-insulin antibody bound to micro-titration well. A sample washing step removed unbound enzyme labeled antibody. In the insulin ELISA, the bound HRP complex is detected by reaction with TMB substrate. The reaction is stopped by adding acid to give a colorimetric endpoint that is read using ELISA reader). Serum TNF-α was assayed by ELISA (Bendermed, Austria Ref NO: BMF 223; It is based on the direct sandwich technique with biotin-Streptavidin, in which two monoclonal antibodies are directed against human TNF-α. Human TNF- present in the sample or standard binds to antibodies adsorbed to the microwells. A biotin-conjugated anti-human TNF- antibody is added and binds to human TNF- captured by the first antibody. Following incubation unbound biotin-conjugated anti-human TNF- antibody is removed during a wash step. Streptavidin-HRP is added and binds to the biotin-conjugated anti-human TNF- antibody. Following incubation unbound Streptavidin-HRP is removed during a wash step, and substrate solution reactive with HRP is added to the wells. A coloured product is formed in proportion to the amount of human TNF- present in the sample or standard. The reaction is terminated by addition of acid and absorbance is measured at 450 nm. A standard curve is prepared from 7 human TNF- standard dilutions and human TNF- sample concentration determined). Serum IL-6 was measured by ELISA (Bendermed, Austria Ref NO: BMS213HS;). Insulin resistance value were calculated using the homeostasis model assessment, HOMA-IR, as (fasting insulin IU/L) × (fasting glucose mmol/L)/22.5 as previously reported by Matthows.23
The study was approved by the ethical committee of Jahrom University of Medical Sciences. All participants in the study filled and signed informed consent letter.
All results are displayed as mean ± SD (standard devation of mean). Insulin resistance (IR), BMI and body weight data were analyzed with One Way analysis of Variance (ANOVA). Serum IL-6, TNF-α and insulin concentration data were analyzed with non-parametric kruskal-wallis test followed by Mann Whitney U-test. Correlations were calculated using liner correlation (Pearson). Statistical analysis was performed using SPSS 11 for Windows. P < 0.05 was considered statistically significant for all analysis.
Results
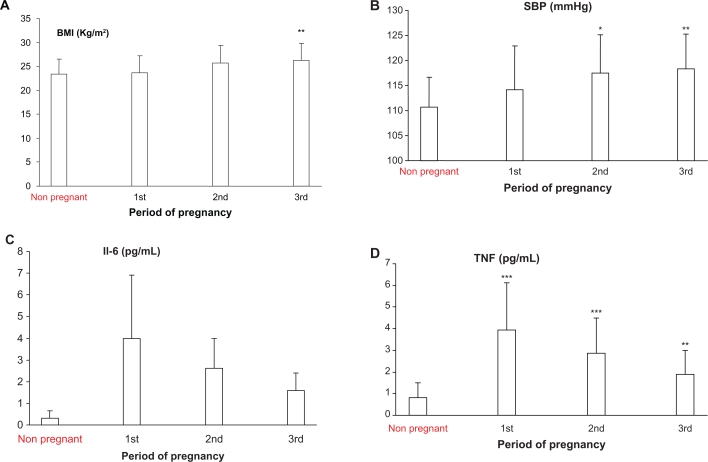
A total of 86 pregnant women and 21 non pregnant subjects participated in the study. Clinical and laboratory characteristics of subjects are summarized in Table 1. Body mass index was significantly different in the third trimester as compared with non pregnant and women with first trimester of pregnancy (Fig. 1A, Table 2). In terms of systolic pressure, subjects in the second and third trimester of pregnancy were significantly different compared to non pregnant women (Fig. 1B). There was statistical difference in serum IL-6 concentration between the non pregnant women and subjects in first, second and third trimester of pregnancy (Table 2, Fig. 1C). TNF-α level was also significantly higher in patients in all gestational age as compared to non pregnant women (Fig. 1D). However, during pregnancy TNF-α level decreased by increasing in the gestational age (Table 2). Pregnant women exhibited higher score of HOMA IR compared to non pregnant subjects group, however, there was no difference in this score between pregnant subjects in different gestational age (Tables 1 and 2). There was significant correlation between gestational age and BMI (r = 0.28, P = 0.01), diastolic pressure (r = 0.28, P = 0.01) IL-6 (r = −0.52, P = 0.000) and TNF-α level (r = −0.44, P < 0.0001). There was no significant correlation between gestational age and IR. IL-6 level in pregnancy and no correlation with IR, fasting insulin, BMI and body weight. TNF-α level also did not show correlation with IR, fasting insulin, BMI and body weight.
Table 1.
Clinical and laboratory characteristic of subjects.
| Non pregnant women | Pregnant women | |
|---|---|---|
| Number of case | 21 | 86 |
| Age (year) | 27.2 ± 5.6 | 26.4 ± 4.1 |
| Gestational age (week) | 23.9 ± 9.8 | |
| HT (m) | 1.58 ± 0.07 | 1.6 ± 0.06 |
| WT (Kg) | 58.6 ± 6.4 | 64.99 ± 11.5** |
| BMI (Kg/m2) | 23.4 ± 3 | 25.4 ± 3.7* |
| SBP (mmHg) | 110.7 ± 5.9 | 117 ± 7.8** |
| DBP (mmHg) | 70.9 ± 11.4 | 72.8 ± 6.9 |
| BGL (mg/100) | 80.2 ± 8.7 | 81.5 ± 8.7 |
| Insulin (μLU/mL) | 8.7 ± 1.9 | 10.9 ± 6 |
| IL-6 (pg/mL) | 0.3 ± 0.35 | 2.6 ± 2.1*** |
| TNF (pg/mL) | 0.8 ± 0.7 | 2.8 ± 1.9*** |
| IR | 1.7 ± 0.4 | 2.1 ± 0.7* |
Notes:
P < 0.05 (control);
P < 0.01 (control);
P < 0.0001 (control).
Abbreviations: BMI, body max index; HT, height of women; WT, weight of body; SBP, systolic blood pressure; DBP, diastolic blood pressure; BGL, blood glucose level; IR, insulin resistance.
Figure 1.
A) BMI in patients in different trimesters of pregnancy and non pregnant subjects. BMI were significantly higher in 3rd trimesters compared with the control (**P < 0.01). B) Systolic blood pressure in different trimesters of pregnancy and non pregnant subjects. B) Systolic blood pressure was significantly higher in the 2nd and 3rd trimesters as compared with non pregnant subjects (*P < 0.05; **P < 0.01). C) Serum IL-6 level in different trimesters of pregnancy and non pregnant subjects. Serum IL-6 level was significantly higher in the 1st, 2nd and 3rd trimester as compared with non pregnant subjects (*P < 0.05). D) Serum TNF level in different trimesters of pregnancy and non pregnant subjects. TNF level was significantly higher in the 1st, 2nd and 3rd trimesters of pregnancy as compared non pregnant subjects (***P < 0.0001; **P < 0.01).
Table 2.
Clinical and laboratory characteristics of pregnant women with different gestational age.
| 3rd trimester mean ± SD | 2nd trimester mean ± SD | 1st trimester mean ± SD | |
|---|---|---|---|
| Number of cases | 37 | 23 | 26 |
| Age (year) | 27.6 ± 4.7 | 24.9 ± 2.3 | 25.5 ± 4.1 |
| GA (week) | 32.9 ± 4.3 | 22.2 ± 2.7 | 11.2 ± 1.6 |
| WT (Kg) | 68.2 ± 10.6** | 65.7 ± 12.6 | 59.2 ± 10.2 |
| HT (m) | 1.6 ± 0.07 | 1.58 ± 0.06 | 1.58 ± 0.06 |
| SBP (mmHg) | 118.3 ± 7* | 117.5 ± 7.7 | 114.2 ± 8.7 |
| DBP (mmHg) | 74.7 ± 6.5 | 71.7 ± 7.1 | 70.5 ± 6.6 |
| BMI (Kg/m2) | 26.2 ± 3.6** | 25.7 ± 3.7 | 23.6 ± 3.6 |
| BGL (mg/100) | 84.1 ± 21.2 | 79.4 ± 6.7 | 78.6 ± 6.7 |
| Insulin (μL/mL) | 10.05 ± 3.9 | 10.6 ± 3.8 | 10.5 ± 3.4 |
| IL-6 (pg/mL) | 1.6 ± 0.8** | 2.6 ± 1.4 | 4 ± 2.9 |
| TNF (pg/mL) | 1.9 ± 1.1*** | 2.88 ± 1.6* | 3.93 ± 2.2 |
| IR | 2.1 ± 0.8 | 2.1 ± 0.6 | 2 ± 0.6 |
Notes:
P < 0.05;
P < 0.01;
P < 0.001 (significantly different from pregnant women in 1st trimester).
Abbreviations: BMI, body mass index; GA, gestational age; WT, wight of body; HT, height; SBP, systolic blood pressure; DBP, diastolic blood pressure; BGL, blood glucose level; IR, insulin resistance.
Discussion
Glucose metabolism disorder is a common complication during pregnancy and its pathology is associated with IR and deficiency of insulin secretion.2 This study showed that insulin resistance was significantly different in pregnant group in general compared to non pregnant subjects. In spite of a previous report we did not found correlation between gestational age and insulin resistance. Our finding was in contrast to the results of Kirwan et al,8 Melczer et al9 and Consultant et al3 in which they showed insulin resistance was significantly increased in the late pregnancy compared with either non pregnant or early pregnancy. This difference may be due to differences in dietary composition, life style between western and eastern societies,24 variability between insulin assays in different experimental researches,25 differences in the population study and sampling time during pregnancy.
A number of studies have reported concentrations of IL-6 in pregnant subjects.21,26–28 Our data are inconsistent with several pervious observations that found an increase in IL-6 level in pregnant compared to non-pregnant subjects.26 IL-6 is a pro inflammatory cytokine that has important role in on-time parturition and success of pregnancy.27,29 There are limited and partially contradictory reports concerning the pattern of IL-6 secretion during normal pregnancy.26–28 In the present study, maternal serum IL-6 has decreased with further increasing in pregnancy period. In this regard, our results were in contrast with Doria et al and Curry et al.29,30 We speculate, that this discrepancy was probably caused by different biochemical assays for determination of serum IL-6,28 and differences in survey population.30 In addition, several other variables may influence cytokine levels during pregnancy including maternal age, prepregnancy body mass index and prior preterm delivary status.31 In the present study, we did not find any correlation between maternal IL-6 and insulin resistance.
Our results are in agreement with several previous observations that found an increase in TNF-α level in pregnant as compared to non pregnant subjects9,15,32 but in contradiction with these studies, we found a negative correlation between gestational age and TNF-α level. Explanations accounting for this finding may be related to lifestyle of our subjects. Clapp et al reported that regular weight bearing exercise during pregnancy suppressed the usual pregnancy- associated changes in the circulating level of TNF-α.33 In our study most of pregnant subjects lived in a rural area with a high level of physical activity. In addition, pregnancy is associated with changes of several hormones such as estrogen, progesterone, cortisol and 1,25 dihydroxyvitamin D3.34–36 Some of these hormones, for instance cortisol and catecholamines and 1,25 dihydroxy D3, are potent inhibitors of TNF -α production by monocyte/macrophage.37–40 Plasma concentration of cortisol was more elevated during late pregnancy than early pregnancy.8 Therefore, it is possible to increase production of these hormones during pregnancy to be responsible for reducing maternal TNF-α production.
In conclusion, our findings suggest that TNF-α and IL-6 do not greatly contribute to pregnancy induced insulin resistance in normal pregnancy.
Acknowledgments
This work was supported by the research project of the Jahrom University of Medical Sciences. Authors thank the staffs of Honary Clinic, Motahary Hospital particularly, Miss Neda Abaszade and Mr Shadman. Authors are grateful to subjects who accepted to enter this study.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Hadden DR, McLaughlin C. Normal and abnormal maternal metabolism during pregnancy. Seminars in Fetal and Neonatal Medicine. 2009;14:66–71. doi: 10.1016/j.siny.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CA. Glucose homeostasis during canine pregnancy: Insulin resistance, ketosis, and hypoglycemia. Theriogenology. 2008;70:1418–23. doi: 10.1016/j.theriogenology.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Stanley K, Fraser R, Bruce C. Physiological changes in insulin resistance in human pregnancy: longitudinal study with the hyperinsulinaemic euglycaemic clamp technique. BJOG: An International Journal of Obstetrics and Gynaecology. 1998;105(7):756–9. doi: 10.1111/j.1471-0528.1998.tb10207.x. [DOI] [PubMed] [Google Scholar]
- 4.McLachlan KA, O’Neal D, Jenkins A, Alford FP. Do adiponectin, TNFalpha, leptin and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabetes Metab Res Rev. 2006;22(2):131–8. doi: 10.1002/dmrr.591. [DOI] [PubMed] [Google Scholar]
- 5.Lu HL, Wang HW, Wen Y, Zhang MX, Lin HH. Roles of adipocyte derived hormone adiponectin and resistin in insulin resistance of type 2 diabetes. World J Gastroenterol. 2006;12(11):1747–51. doi: 10.3748/wjg.v12.i11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51(12):3391–9. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- 7.Zinman B, Hanley AJG, Harris SB, Kwan J, Fantus G. Circulating tumor necrosis factor-α concentration in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. 1999;84(1):272–8. doi: 10.1210/jcem.84.1.5405. [DOI] [PubMed] [Google Scholar]
- 8.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Claude Challier J, Huston-Presley L, Friedman JE, et al. TNF-α is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–13. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 9.Melczer Z. Role of tumour necrosis factor-α in insulin resistance during normal pregnancy. Eur J Obstet Gynecol Reprod Biol. 2002;105(1):7. doi: 10.1016/s0301-2115(02)00108-2. [DOI] [PubMed] [Google Scholar]
- 10.Gwozdziewiczová S, Lichnovská R, Hrebícek J. Cesk Fysiol. Tumor necrosis factor alfa (TNF alpha) and insulin resistance. Cesk Fysiol. 2004;53(4):167–75. [PubMed] [Google Scholar]
- 11.Zavalza-Gómez AB, Anaya-Prado R, Rincón-Sánchez AR, Mora-Martínez JM. Adipokines and insulin resistance during pregnancy. Diabetes Res Clin Pract. 2008;80(1):8–15. doi: 10.1016/j.diabres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Chen HL, Yang YP, Hu XL, Yelavarthi KK, Fishback JL, Hunt JS. Tumor necrosis factor alpha mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol. 1991;139(2):327–35. [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira FO, Frode TS, Medeiros YS. Evaluation of Tumour Necrosis Factor Alpha, Interleukin-2 Soluble Receptor, Nitric Oxide Metabolites, and Lipids as Inflammatory Markers in Type 2 Diabetes Mellitus. Mediators Inflamm. 2006;(1):39062. doi: 10.1155/MI/2006/39062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gwozdziewiczova S, Lichnovska R, Yahia RB, Chlup R, Hřebiček J. TNF-α in the development of insulin resistance and other disorders in metabolic syndrome. HDL Biomed Papers. 2005;149(1):109–17. [PubMed] [Google Scholar]
- 15.Xue-lian G, Hui-Xia Y, Yi Z. Variations of tumor necrosis factor-α, leptin and adiponectin in mid-trimester of gestational diabetes mellitus. Chin Med J. 2008;121(8):701–5. [PubMed] [Google Scholar]
- 16.Bréard E, Benhaïm A, Féral C, Leymarie P. Rabbit ovarian production of nterleukin-6 and its potential effects on gonadotropin-induced progesterone secretion in granulosa and theca cells. J Endocrinol. 1998;159:479–87. doi: 10.1677/joe.0.1590479. [DOI] [PubMed] [Google Scholar]
- 17.Spangelo BL, Macleod RM, Isakson PC. Production of interleukin-6 by anterior pituitary cells in vitro. Endocrinology. 1990b;126:582–6. doi: 10.1210/endo-126-1-582. [DOI] [PubMed] [Google Scholar]
- 18.Van Snick J. In terleukin6: an overreview. Annaual Review of immunology. 1990;8:253–78. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 19.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) Induces Insulin Resistance in 3T3-L1 Adipocytes and Is, Like IL-8 and Tumor Necrosis Factor-α, Overexpressed in Human Fat Cells from Insulin-resistant Subjects. J Biol Chem. 2003 Nov 14;278(46):45777–84. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Bachmann RA, Chen J. Interleukin-6 and insulin resistance. Vitam Horm. 2009;80:613–33. doi: 10.1016/S0083-6729(08)00621-3. [DOI] [PubMed] [Google Scholar]
- 21.Yu F, Xue YM, Li CZ, et al. Association of serum interleukin-6 and high sensitive C-reactive protein levels with insulin resistance in gestational diabetes mellitus. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27(6):799–801. [PubMed] [Google Scholar]
- 22.Leturque A, Burnol AF, Ferre P, Girard J. Pregnancy-induced insulin resistance in the rat: assessment by glucose clamp technique. Am J Physiol Endocrinol Metab. 1984;246:E25–31. doi: 10.1152/ajpendo.1984.246.1.E25. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DJ, Hoskers JP, Rudenski AS, Waylur BA, Trencher DY, Turner RC. Homeostasis model assessment insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Clapp JF. Effects of Diet and Exercise on Insulin Resistance during Pregnancy. Metab Syndr Relat Disord. 2006;4(2):84–90. doi: 10.1089/met.2006.4.84. [DOI] [PubMed] [Google Scholar]
- 25.Manley SE, Luzio SD, Stratton IM, et al. Preanalytical, analytical and computational factors affect homeostasis model assessment. Diabetes care. 2008;31:1877–83. doi: 10.2337/dc08-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Opsjłn SL, Wathen NC, Tingulstad S, et al. Tumor necrosis factor, interleukin-1, and interleukin-6 in normal human pregnancy. Am J Obstet Gynecol. 1993 Aug;169(2 Pt 1):397–404. doi: 10.1016/0002-9378(93)90096-2. [DOI] [PubMed] [Google Scholar]
- 27.Saarelainen H, Valtonen P, Punnonen K, et al. Flow mediated vasodilation and circulating concentrations of high sensitive C-reactive protein, interleukin-6 and tumor necrosis factor-alpha in normal pregnancy—The Cardiovascular Risk in Young Finns Study. Clin Physiol Funct Imaging. 2009 Sep;29(5):347–52. doi: 10.1111/j.1475-097X.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- 28.Curry AE, Vogel I, Skogstrand K, et al. Maternal plasma cytokines in early and mud gestation of normal human pregnancy and their association with maternal factors. J Reproductive Immunology. 2008;77(2):152–60. doi: 10.1016/j.jri.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 29.Robertson SA, Christiaens I, Dorian CL, et al. Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology. 2010 Aug;151(8):3996–4006. doi: 10.1210/en.2010-0063. [DOI] [PubMed] [Google Scholar]
- 30.Doria A, Ghirardello A, Laccarino L, et al. Pregnancy, cytokines, and disease activity in systemic lupus Erythematosus. Arthritis and Rheumatism. 2004;51(6):989–95. doi: 10.1002/art.20837. [DOI] [PubMed] [Google Scholar]
- 31.Curry AE. Early and mid-gestation maternal plasma cytokines in term pregnancy and in association with spontaneous preterm delivery. ProQuest Dissertions and Theses. 2007;230:3264067. [Google Scholar]
- 32.Daher S, Fonseca F, Ribeiro OG, Musatti CC, Gerbase-DeLima M. Tumor necrosis factor during pregnancy and at the onset of labor and spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 1999;83(1):77–9. doi: 10.1016/s0301-2115(98)00252-8. [DOI] [PubMed] [Google Scholar]
- 33.Clapp JF, III, Kiess W. Effects of pregnancy and exercise on concentrations of the metabolic markers tumor necrosis factor alpha and leptin. Am J Obstet Gynecol. 2000;182(2):300–6. doi: 10.1016/s0002-9378(00)70215-8. [DOI] [PubMed] [Google Scholar]
- 34.Henricks DM, Dickey JF, HILL JR, Johnston WE. Plasma Estrogen and Progesterone Levels After Mating, and During Late Pregnancy and Postpartum in Cows. Endocrinology. 1972;90(5):1336–42. doi: 10.1210/endo-90-5-1336. [DOI] [PubMed] [Google Scholar]
- 35.Smith VG, Edgerton LA, Hafs HD, Convey EM. Bovine Serum Estrogens, Progestins and Glucocorticoids during Late Pregnancy, Parturition and Early Lactation. J Anim Sci. 1973;36:391–6. doi: 10.2527/jas1973.362391x. [DOI] [PubMed] [Google Scholar]
- 36.Ardawi MS, Nasrat HA, BA’Aqueel HS. Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: a longitu dinal study. Eur J Endocrinol. 1997;137(4):402–9. doi: 10.1530/eje.0.1370402. [DOI] [PubMed] [Google Scholar]
- 37.DeRijk R, Michelson D, Karp B, Petrides J, Galliven E, Deuster P, et al. Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 beta (IL-1 beta), IL-6, and tumor necrosis factor-alpha (TNF alpha) production in humans: high sensitivity of TNF alpha and resistance of IL-6. Clin Endocrinol Metab. 1997;82(7):2182–91. doi: 10.1210/jcem.82.7.4041. [DOI] [PubMed] [Google Scholar]
- 38.Guirao X, Kumar A, Katz J, Smith M, Lin E, Keogh C, et al. Catecholamines increase monocyte TNF receptors and inhibit TNF through β2-adrenoreceptor activation. Am J Physiol Endocrinol Metab. 1997;273(6):E1203–8. doi: 10.1152/ajpendo.1997.273.6.E1203. [DOI] [PubMed] [Google Scholar]
- 39.Anand SP, Selvarajand P, Narayanan PR. Effect of 1,25 dihydroxyvitamin D3 on intracellular IFN-γ and TNF-α positive T cell subsets in pulmonary tuberculosis. Cytokine. 2009;45(2):105–10. doi: 10.1016/j.cyto.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Ito A, Buenafe AC, Matejuk A, Zamora A, Silverman M, Dwyer J, et al. Estrogen Inhibits Systemic T Cell Expression of TNF-α and Recruitment of TNF-α+ T Cells and Macrophages into the CNS of Mice Developing Experimental Encephalomyelitis. Clin Immun. 2002;102(3):275–82. doi: 10.1006/clim.2001.5175. [DOI] [PubMed] [Google Scholar]