Abstract
Cell-surface retention sequence (CRS) binding protein (CRSBP-1) is a membrane glycoprotein identified by its ability to bind PDGF-BB and VEGF-A via their CRS motifs (clusters of basic amino acid residues). CRSBP-1 is identical to LYVE-1 and exhibits dual ligand (CRS-containing proteins and hyaluronic acid) binding activity, suggesting the importance of CRSBP-1 ligands in lymphatic function. Here, we show that CRSBP-1 ligands induce disruption of VE-cadherin-mediated intercellular adhesion and opening of intercellular junctions in lymphatic endothelial cell (LEC) monolayers as determined by immunofluorescence microscopy and Transwell permeability assay. This occurs by interaction with CRSBP-1 in the CRSBP-1–PDGFβR–β-catenin complex, resulting in tyrosine phosphorylation of the complex, dissociation of β-catenin and p120-catenin from VE-cadherin, and internalization of VE-cadherin. Pretreatment of LECs with a PDGFβR kinase inhibitor abolishes ligand-stimulated tyrosine phosphorylation of VE-cadherin, halts the ligand-induced disruption of VE-cadherin intercellular adhesion and blocks the ligand-induced opening of intercellular junctions. These CRSBP-1 ligands also induce opening of lymphatic intercellular junctions that respond to PDGFβR kinase inhibitor in wild-type mice (but not in Crsbp1-null mice) as evidenced by increased transit of injected FITC–dextran and induced edema fluid from the interstitial space into lymphatic vessels. These results disclose a novel mechanism involved in the opening of lymphatic intercellular junctions.
Key words: VE-cadherin intercellular junctions, PDGF β-type receptor, Tyrosine phosphorylation, Endothelial cell permeability, Interstitial–lymphatic transit
Introduction
Cell-surface retention sequence (CRS) binding protein-1 (CRSBP-1) is a membrane glycoprotein first identified by its ability to mediate cell-surface retention (after synthesis and secretion) of the simian sarcoma virus (SSV) oncogene v-sis gene product (PDGF-BB) in SSV-transformed fibroblasts (Boensch et al., 1995; Boensch et al., 1999; Lokeshwar et al., 1990). CRSBP-1 forms complexes with the v-sis gene product (by binding its CRS motif near the C-terminus of the v-sis gene product) during trafficking from the endoplasmic reticulum (ER) to the plasma membrane, thus retaining it at the cell surface (Boensch et al., 1999). All members of the PDGF superfamily, including PDGF-AA, PDGF-BB, placental growth factor (PlGF) and vascular endothelial cell growth factor-A, -C and -D (VEGF-A, VEGF-C and VEGF-D), possess CRS motifs and exhibit cell-surface retention during secretion (Carmeliet et al., 1999; Houck et al., 1991; Joukov et al., 1996; Joukov et al., 1997; LaRochelle et al., 1991; Ostman et al., 1991). These CRS motifs contain clustered basic amino acid residues (Arg, Lys and His) and are evolutionarily conserved (Carmeliet et al., 1999; Joukov et al., 1997; LaRochelle et al., 1991; Ostman et al., 1991). Through cross-linking studies (Boensch et al., 1995; Boensch et al., 1999), CRSBP-1 was identified as a receptor that binds the synthetic oligopeptides containing the CRS motifs of PDGF-BB and VEGF-A. cDNA cloning, sequencing and expression of bovine CRSBP-1 (Huang et al., 2003) revealed that CRSBP-1 is a 120 kDa disulfide-linked homodimeric type I membrane glycoprotein with distinct dual ligand [CRS-containing growth factors and cytokines, and hyaluronic acid (HA)] binding activity. The deduced amino acid sequences of human and murine CRSBP-1 exhibit 61% and 56% identity, respectively, to that of bovine CRSBP-1 and are identical to those of human and murine lymphatic vessel endothelial HA receptor-1 (LYVE-1) (Banerji et al., 1999; Prevo et al., 2001; Huang et al., 2003). LYVE1 was cloned using CD44 sequence homology cloning (Banerji et al., 1999). Both CD44 and LYVE-1 are members of the Link protein superfamily that bind the large extracellular matrix glycosaminoglycan HA (Banerji et al., 1999; Teriete et al., 2004). LYVE-1 is primarily expressed in lymphatic endothelium and localized to both luminal and abluminal faces of lymphatic capillary vessels (Teriete et al., 2004). The primary localization of CRSBP-1/LYVE-1 to lymphatic endothelium has made it a suitable marker for studying lymphangiogenesis (Alitalo and Carmeliet, 2002; Jackson, 2003; Jussila and Alitalo, 2002). This also implies the likely importance of the dual ligand binding activity of CRSBP-1 in regulating the function of lymphatic vessels.
To define the in vivo role of CRSBP-1, we recently generated Crsbp1-null (Crsbp1−/−) mice (Huang et al., 2006). These mice are macroscopically normal. However, their lymphatic vessel lumens are constitutively distended compared with the irregularly shaped, collapsed lumens of lymphatic vessels often found in wild-type mice (Huang et al., 2006). The alteration in the shape of lymphatic vessel lumens is most prominent in the liver and intestine (Huang et al., 2006). High molecular weight FITC–dextran (average MW ~2,000,000) injected subcutaneously in the tail of Crsbp1−/− mice is cleared from the tail more rapidly than in wild-type mice (Huang et al., 2006). Since high molecular weight FITC–dextran can only be cleared via lymphatic drainage, we reasoned that Crsbp1−/− mice might have a constitutive increase in the transit of large molecules, fluids and cells from the interstitial space into the lumen of lymphatic capillaries, a process we call interstitial–lymphatic transit (Huang et al., 2006). To further define the role of CRSBP-1 in the regulation of interstitial–lymphatic transit, FITC–dextran was co-injected with and without PDGF-BB, a putative physiological CRSBP-1 ligand, into tails of wild-type mice. PDGF-BB enhanced the clearance of FITC–dextran near the injection site (Huang et al., 2006). Co-injection of HA, a known CRSBP-1 ligand, also enhanced the clearance of FITC–dextran near the injection site. PDGF-BB was more effective than HA in wild-type mice; however, in Crsbp1−/− mice, neither PDGF-BB nor HA enhanced the clearance of the FITC–dextran (Huang et al., 2006). These results support our hypothesis that the Crsbp1-null mutation and binding of CRSBP-1 by its cognate ligands cause opening of lymphatic intercellular junctions, resulting in increased interstitial–lymphatic transit (Huang et al., 2006).
To define the molecular mechanism by which CRSBP-1 ligands regulate the function of lymphatic intercellular junctions, we determined the effects of several CRSBP-1 ligands on the structure (morphology) and function (permeability) of intercellular junctions in the monolayers of LECs, including SVEC4-10 cells and primary human dermal LECs (HDLECs). These CRSBP-1 ligands included putative physiological ligands (PDGF-BB, VEGF-A165 and hyaluronic acid) and two specific ligands (PDGF peptide and VEGF peptide, which are synthetic oligopeptides containing the CRS motifs of PDGF-BB and VEGF-A165, respectively and do not interact with PDGFβR and VEGFR2) (supplementary material Fig. S1). SVEC4-10 cells are SV40-transformed LEC-like cells (supplementary material Fig. S2). We also determined the effects of these CRSBP-1 ligands on the function of lymphatic intercellular junctions by measuring the transit of intradermally injected high molecular weight FITC–dextran and λ-carrageenan-induced edema fluid from the interstitial space into lymphatic vessels in wild-type and Crsbp1-null mice. In this communication, we show that these CRSBP-1 ligands induce disruption of VE-cadherin-mediated intercellular adhesion and opening of intercellular junctions in a manner dependent on PDGFβR activity in LEC monolayers (as evidenced by increased permeability) and in lymphatic vessels in wild-type mice (as evidenced by rapid clearance of injected FITC–dextran near the injection site and by attenuated λ-carrageenan-induced edema).
Results
CRSBP-1 ligands induce disruption of VE-cadherin-mediated intercellular adhesion
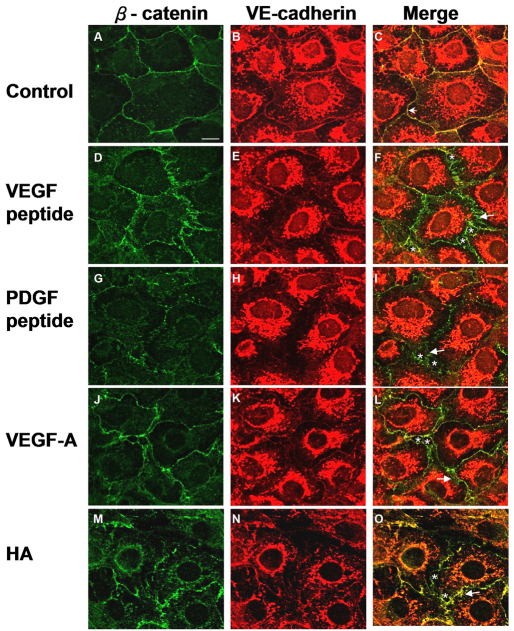
We hypothesized that CRSBP-1 is involved in the formation of overlapping intercellular junctions of lymphatic endothelial cells in lymphatic vessels and that CRSBP-1 ligands induce opening of the lymphatic intercellular junction (Huang et al., 2006). Baluk and colleagues (Baluk et al., 2007) demonstrated that VE-cadherin is required for maintenance of lymphatic junctional integrity. To test the hypothesis that CRSBP-1 ligands cause opening of lymphatic intercellular junctions by compromising VE-cadherin- and β-catenin-mediated intercellular adhesion, we analyzed the subcellular localization of VE-cadherin and its associated protein, β-catenin, in monolayers of SVEC4-10 cells treated with CRSBP-1 ligands using immunofluorescence confocal microscopy. Without stimulation, VE-cadherin colocalized with β-catenin at the plasma membrane (Fig. 1C). Upon stimulation with 10 μM VEGF peptide and 10 μM PDGF peptide for 1 hour, VE-cadherin levels diminished at the plasma membrane, presumably because of increased VE-cadherin endocytosis or internalization (Fig. 1E,H vs 1B). Additionally, β-catenin staining became disorganized into a zigzag pattern (Fig. 1D,G) and there was no colocalization of VE-cadherin and β-catenin at the plasma membrane (Fig. 1F,I). It has been shown that the change in β-catenin membrane distribution from a linear pattern to zigzag pattern is associated with disassembly of the VE-cadherin–β-catenin complex and disruption of VE-cadherin-mediated cell–cell adhesion (Boccellino et al., 2005; Esser et al., 1998). Furthermore, when compared with cells treated with vehicle only, gaps and slits appeared between endothelial cells treated with CRSBP-1 ligand, which we believe is due to loss of cell–cell adhesion (Fig. 1F,I). Stimulation with physiological CRSBP-1 ligands (5 nM VEGF-A165 and 100 μg/ml HA) also induced decreased plasma membrane distribution of VE-cadherin and disorganization of distribution of β-catenin in the membrane from a linear pattern to a zigzag pattern (Fig. 1L,O). VEGF-A165 is a putative physiological CRSBP-1 ligand for LECs that express very little endogenous VEGFR2, which is a specific receptor for VEGF-A165 in blood vascular endothelial cells (Farrara, 2004). Taken together, these results suggest that CRSBP-1 ligands inhibit VE-cadherin trans interaction (Boccellino et al., 2005; Esser et al., 1998; Vincent et al., 2004) and impair cell–cell adhesion by inducing disassembly of the VE-cadherin–β-catenin complex at intercellular junctions in SVEC4-10 cells.
Fig. 1.
CRSBP-1 ligands induce disruption of VE-cadherin–β-catenin-mediated intercellular adhesion in SVEC4-10 cells. SVEC4-10 cells were seeded on coverslips and treated with vehicle only (control) (A–C), 10 μM VEGF peptide (D–F), 10 μM PDGF peptide (G–I), 100 ng/ml VEGF-A165 (J–L) or 100 μg/ml HA (M–O) for 1 hour. After incubation, cells were fixed with methanol at −20°C for 10 minutes. Cells were stained with anti-β-catenin antibody (green) (A,D,G,J,M) and anti-VE-cadherin antibody (red) (B,E,H,K,N) and visualized with a confocal microscope. Cells treated with vehicle showed colocalization of VE-cadherin and β-catenin on the plasma membrane (as indicated by an arrowhead in C). CRSBP-1 ligand treatment induced disruption of β-catenin distribution from a linear pattern to a zigzag pattern in the cell borders as indicated by arrows (F,I,L,O), diminished plasma membrane distribution of VE-cadherin (E,H,K,N) and decreased colocalization of VE-cadherin and β-catenin (F,I,L,O). CRSBP-1 ligand stimulation also enlarged gaps between cells (as indicated by asterisk in F,I,L,O). Scale bar: 10 μm.
The effects of CRSBP-1 ligands on VE-cadherin–β-catenin intercellular junctions were also determined in HDLECs. HDLECs were stimulated with CRSBP-1 ligands, as described above. Similarly to SVEC4-10 cells, indirect immunofluorescence staining of unstimulated HDLECs using antibodies against VE-cadherin and β-catenin revealed colocalization of VE-cadherin and β-catenin at the plasma membrane along the cell borders. HDLECs stimulated with CRSBP-1 ligands (10 μM PDGF peptide, 10 μM VEGF peptide, 5 nM VEGF-A165 and 100 μg/ml HA) exhibited disorganization of the membrane distribution of VE-cadherin–β-catenin at the intercellular junctions from a linear pattern to a zigzag pattern, with a diminished plasma membrane distribution of VE-cadherin and decreased colocalization of VE-cadherin and β-catenin (data not shown). PDGF and VEGF peptides induced more disorganization of VE-cadherin and β-catenin than was observed with PDGF-BB and HA (data not shown). Additionally, gaps between cells in HDLEC monolayers stimulated with PDGF and VEGF peptides, and VEGF-A165 were increased (data not shown).
Furthermore, the linear and zigzag staining patterns of VE-cadherin–β-catenin were quantified using the method described previously (Vincent et al., 2005). The VE-cadherin–β-catenin immunofluorescence staining of cell borders in HDLECs treated with vehicle only exhibited mainly linear patterns. Treatment with PDGF peptide (10 μM), VEGF peptide (10 μM), VEGF-A165 (5 nM) and HA (100 μg/ml) resulted in increased zigzag patterns in the plasma membrane distribution of VE-cadherin–β-catenin (n=10) (20±3%, 27±3%, 17±4% and 11±3%, respectively). PDGF-BB (2 nM) had moderate effects on the staining patterns of VE-cadherin–β-catenin (6±2% zigzag patterns) compared with those in vehicle-treated cells. This is consistent with the concept that PDGF-BB weakly increases opening of VE-cadherin–β-catenin intercellular junctions in SVEC4-10 cells. This is probably due to the fact that the PDGF-BB (Abcam ab73229) used in these experiments is not the full-length protein and lacks the CRS motif near the C-terminal end of the protein. PDGF-BB is believed to slightly induce opening of intercellular junctions, mainly via interaction with PDGFβR.
CRSBP-1 ligands increase permeability in LEC monolayers in a CRSBP-1-dependent manner
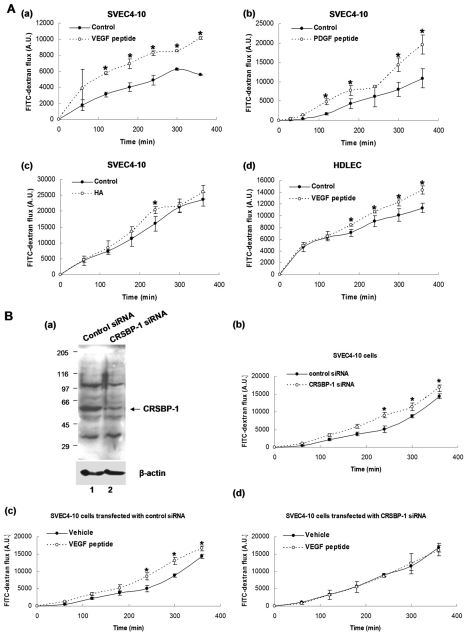
As described above, CRSBP-1 ligands induce opening of VE-cadherin–β-catenin intercellular junctions. This finding raises the possibility that CRSBP-1 ligands are capable of stimulating the permeability of LEC monolayers by inducing opening of intercellular junctions. Using a Transwell system, we determined the permeability of SVEC4-10 cell and HDLEC monolayers stimulated with CRSBP-1 ligands by measuring the passage of FITC–dextran (MW ~40,000) from the upper chamber to the lower chamber. Stimulation of SVEC4-10 cells with 10 μM VEGF (Fig. 2Aa) and 10 μM PDGF peptide (Fig. 2Ab) significantly increased transit of FITC–dextran across the cell monolayer when compared with results obtained with the vehicle alone. Treating SVEC4-10 cell monolayers with 100 μg/ml HA slightly enhanced the transit of FITC–dextran to the lower chamber (Fig. 2Ac). The same experiment was performed using HDLECs. VEGF peptide also increased permeability in HDLEC monolayers as determined using the Transwell permeability assay (Fig. 2Ad). These results indicate that specific CRSBP-1 ligands (VEGF and PDGF peptides) are capable of increasing permeability in SVEC4-10 cell and HDLEC monolayers, presumably by inducing opening of VE-cadherin–β-catenin intercellular junctions.
Fig. 2.
CRSBP-1 ligands stimulate permeability in SVEC4-10 cell monolayers and HDLEC monolayers in a CRSBP-1-dependent manner. (A) CRSBP-1 ligands stimulate permeability in SVEC4-10 cell (a–c) and HDLEC (d) monolayers. Cells (1.5×105) were plated onto 6.5 mm Transwell (collagen-coated, 3 μm pore PTFE) membrane inserts and grown overnight. Cell monolayers were treated with vehicle only (control) (a–d), 10 μM VEGF peptide (a,d), 10 μM PDGF peptide (b) and 100 μg/ml HA (c). FITC–dextran (MW 40 kDa; 1 mg/ml) was then added to the upper chamber. At each time period (0, 60, 120, 180, 240, 300 and 360 minutes), an aliquot was taken from the lower chamber to measure its fluorescence intensity (A.U.). *P<0.05 compared with vehicle-only control. (B) Downregulation of CRSBP-1 expression by transfection with Crsbp1 siRNA abolishes CRSBP-1-ligand-stimulated permeability in SVEC4-10 cells. Cells were transfected with control or Crsbp1 siRNA for 48 hours. Following transfection, the amounts of CRSBP-1 protein and β-actin (as a loading control) were determined by western blot (a). SVEC4-10 cells (1.5×105) transfected with control or Crsbp1 siRNA were plated onto 6.5 mm Transwell (collagen-coated, 3 μm pore PTFE) membrane inserts and grown overnight. Cell monolayers were treated with vehicle only or 10 μM VEGF peptide. FITC–dextran (MW 40 kDa; 1 mg/ml) was then added to the upper chamber. At each time point, an aliquot was taken from the lower chamber to measure its fluorescence intensity (A.U.). Monolayers of cells transfected with Crsbp1 siRNA exhibited greater permeability than monolayers of cells transfected with control siRNA (b). Monolayers of cells transfected with control siRNA showed VEGF-peptide-increased permeability of the cell monolayer (c), whereas in monolayers of cells transfected with Crsbp1 siRNA, VEGF-peptide-increased permeability of the cell monolayer was abolished (d). *P<0.05, compared with control siRNA or vehicle-only control.
To test the hypothesis that CRSBP-1 ligands act specifically with CRSBP-1 to exert its biological activity, we performed gene-specific downregulation of CRSBP-1 by transfection of SVEC4-10 cells with Crsbp1 siRNA. At 48 hours after siRNA transfection, about 80% of CRSBP-1 protein was downregulated compared with levels in cells transfected with control siRNA (Fig. 2Ba). SVEC4-10 cells transfected with control siRNA or Crsbp1 siRNA for 48 hours were used for the Transwell permeability assay. Cells transfected with Crsbp1 siRNA exhibited increased transit of FITC–dextran from the upper chamber to the lower chamber compared with cells transfected with control siRNA (Fig. 2Bb), indicating increased permeability in Crsbp1 siRNA-transfected cells. However, cells transfected with control siRNA exhibited increased permeability upon VEGF peptide stimulation (Fig. 2Bc). Interestingly, the increase in permeability in ligand-stimulated control cells was similar to that observed in cells transfected with Crsbp1 siRNA. In addition, downregulation of CRSBP-1 expression by transfection of cells with Crsbp1 siRNA abolished VEGF-peptide-stimulated permeability of transfected cell monolayers (Fig. 2Bd). These results suggest that CRSBP-1 is required for mediation of CRSBP-1-ligand-stimulated permeability in SVEC4-10 cell monolayers. These results are consistent with the fact that Crsbp1-null mice exhibit constitutively increased interstitial–lymphatic transit (Huang et al., 2006). We hypothesize that this results from constitutive opening of intercellular junctions in lymphatic capillary vessels.
CRSBP-1 ligands induce internalization of VE-cadherin and dissociation of β-catenin and p120-catenin from VE-cadherin
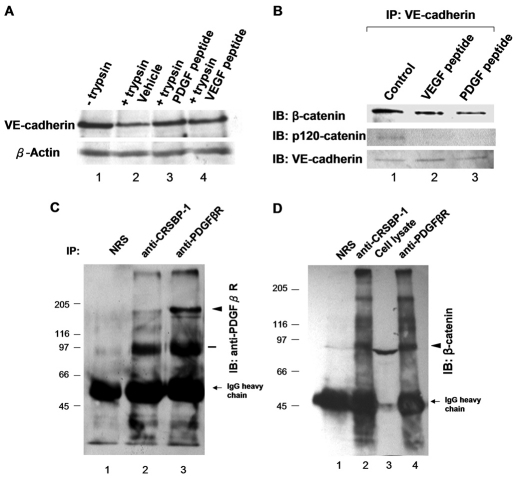
As described above, confocal microscopy of intercellular junction proteins (VE-cadherin and β-catenin) reveals that CRSBP-1 ligand stimulation results in decreased cell-surface staining of VE-cadherin, decreased colocalization of VE-cadherin and β-catenin at the plasma membrane and opening of VE-cadherin intercellular junction in LEC monolayers. These results suggest that CRSBP-1 ligands induce internalization of cell-surface VE-cadherin in LECs. To test this possibility, we performed trypsin digestion experiments to determine whether CRSBP-1 ligands can induce internalization of cell-surface VE-cadherin, because internalized cell-surface proteins are resistant to trypsin digestion. Following treatment of SVEC4-10 cells with vehicle only, 10 μM PDGF peptide and 10 μM VEGF peptide, cells were untreated (for analysis of the total VE-cadherin) or treated with trypsin to remove cell-surface proteins. The lysates of cells untreated and treated with trypsin were subjected to western blot analysis using anti-VE-cadherin antibodies. Lysates of SVEC4-10 cells, which were not subjected to further incubation with trypsin, contained 130 kDa VE-cadherin (Fig. 3A, lane 1). This represented the total VE-cadherin in the cells. In these cells, a fraction (~30%) of VE-cadherin was estimated to be trypsin resistant, representing intracellular or internalized VE-cadherin (Fig. 3A, lane 2). This finding suggests that VE-cadherin undergoes internalization and recycling without stimulation by exogenous CRSBP-1 ligands. However, treatment with PDGF and VEGF peptides increased the amount of trypsin-resistant VE-cadherin to ~80% of the total VE-cadherin (Fig. 3A, lanes 3 and 4 vs lane 1), suggesting that these peptides stimulate internalization of cell-surface VE-cadherin ~2.7-fold. Similar results were also obtained from the experiments measuring trypsin-resistant cell surface (biotinylated) VE-cadherin following treatment of SVEC4-10 cells with VEGF peptide (data not shown).
Fig. 3.
CRSBP-1 ligands stimulate VE-cadherin internalization and dissociation of β-catenin and p120-catenin from VE-cadherin, but not CRSBP-1 complex formation. (A) SVEC4-10 cells were stimulated with vehicle only (lanes 1 and 2), 10 μM PDGF peptide (lane 3) or 10 μM VEGF peptide (lane 4) for 30 minutes. After stimulation, SVEC4-10 cells were untreated (lane 1) or treated with trypsin (lanes 2, 3 and 4) and trypsin-untreated and trypsin-treated cell lysates were then analyzed by western blot analysis using anti-VE-cadherin antibody. PDGF peptide and VEGF peptide enhanced internalization of VE-cadherin ~2.7 fold (~80% of the total VE-cadherin; lanes 3 and 4) when compared with that observed in cells simulated with vehicle only (~30% of the total VE-cadherin (lane 2). (B) SVEC4-10 cells were treated with vehicle only (control) (lane 1), 10 μM VEGF peptide (lane 2) or 10 μM PDGF peptide (lane 3) for 30 minutes. Cell lysates were immunoprecipitated using anti-VE-cadherin antibody. Immunoprecipitated proteins were subjected to SDS-PAGE followed by quantitative western blot analysis using anti-β-catenin and anti-p120-catenin antibodies. VEGF peptide and PDGF peptide induced dissociation of β-catenin and p120-catenin from VE-cadherin (lanes 2 and 3, respectively). (C,D) CRSBP-1 forms complexes with PDGFβR and β-catenin in the absence of exogenous CRSBP-1 ligands. Cell lysates of SVEC4-10 cells were immunoprecipitated using non-immunized rabbit serum (NRS) (C,D, lane 1), anti-CRSBP-1 serum (C,D, lane 2) or anti-PDGFβR serum (C, D, lanes 3 and 4, respectively). Immunoprecipitated proteins were resolved by SDS-PAGE, followed by western blot analysis using anti-PDGFβR serum (C) or anti-β-catenin antibody (D). Anti-CRSBP-1 serum immunoprecipitated PDGFβR (C, lane 2), which was ~10% of the total PDGFβR (C, lane 2 vs lane 3). β-catenin was present in the immunoprecipitates using anti-CRSBP-1 serum and anti-PDGFβR serum (D, lanes 2 and 4, respectively), and in the cell lysates (D, lane 3). Arrowheads indicate the protein band that corresponds to PDGFβR (180 kDa) or β-catenin (90 kDa). The bar indicates the location of a protein that might be a proteolytic product of PDGFβR (C, lanes 2 and 3). The three high molecular weight species might be post-translationally modified products of β-catenin, which were immunoprecipitated and concentrated by anti-CRSBP-1 antibody (D, lanes 2 and 4).
In blood vascular endothelial cells, internalization of VE-cadherin might result from the dissociation of β-catenin or p120-catenin from VE-cadherin. To determine the interaction between VE-cadherin and β-catenin or p120-catenin in LECs, SVEC4-10 cells were stimulated with vehicle only, 10 μM VEGF peptide or 10 μM PDGF peptide for 30 minutes. Stimulated cell lysates were subjected to immunoprecipitation using anti-VE-cadherin antibody followed by SDS-PAGE and immunoblot analysis using anti-β-catenin antibody or anti-p120-catenin antibody. In vehicle-treated cells, VE-cadherin and β-catenin or p120-catenin were found to be co-immunoprecipitated (Fig. 3B, lane 1). Upon stimulation by VEGF and PDGF peptides, there was a decrease in the amount of β-catenin (~50%) and p120-catenin (~90%) co-immunoprecipitated with VE-cadherin (Fig. 3B, lanes 2 and 3 vs lane 1). These results indicate that specific CRSBP-1 ligands are capable of increasing dissociation of β-catenin and p120-catenin from VE-cadherin.
CRSBP-1 forms complexes with PDGFβR and β-catenin
CRSBP-1 is known to form complexes with PDGFβR in SSV-transformed cells (Huang et al., 2006). We determined whether this complex formation also occurs in SVEC4-10 cells by immunoprecipitation with rabbit anti-CRSBP-1 serum or non-immune serum followed by immunoblot analysis with rabbit anti-PDGFβR serum. PDGFβR was present in the immunoprecipitates using anti-CRSBP-1 serum (Fig. 3C, lane 2) but not non-immune serum (Fig. 3C, lane 1). Interestingly, ~10% of the total PDGFβR was found to form complexes with CRSBP-1 (Fig. 3C, lane 2 vs lane 3). Because CRSBP-1 ligands stimulate dissociation of β-catenin from VE-cadherin, we hypothesized that the CRSBP-1–PDGFβR complex interacts with VE-cadherin or β-catenin in the absence of CRSBP-1 ligands. To test this hypothesis, SVEC4-10 cell lysates were immunoprecipitated using pre-immune serum, anti-CRSBP-1 serum or anti-PDGFβR serum, followed by immunoblot analysis with anti-β-catenin antibody or anti-VE-cadherin antibody. The immunoprecipitates using anti-CRSBP-1 serum (Fig. 3D, lane 2) or anti-PDGFβR serum (Fig. 3D, lane 4) contained β-catenin but not VE-cadherin (data not shown). These results suggest that CRSBP-1 forms complexes with PDGFβR and β-catenin and that the CRSBP-1–PDGFβR complex might associate with β-catenin to form ternary complexes. These results also suggest that PDGFβR is involved in mediating CRSBP-1-ligand-induced disassembly and opening of VE-cadherin–β-catenin intercellular junctions.
CRSBP-1 ligands stimulate tyrosine phosphorylation of PDGFβR
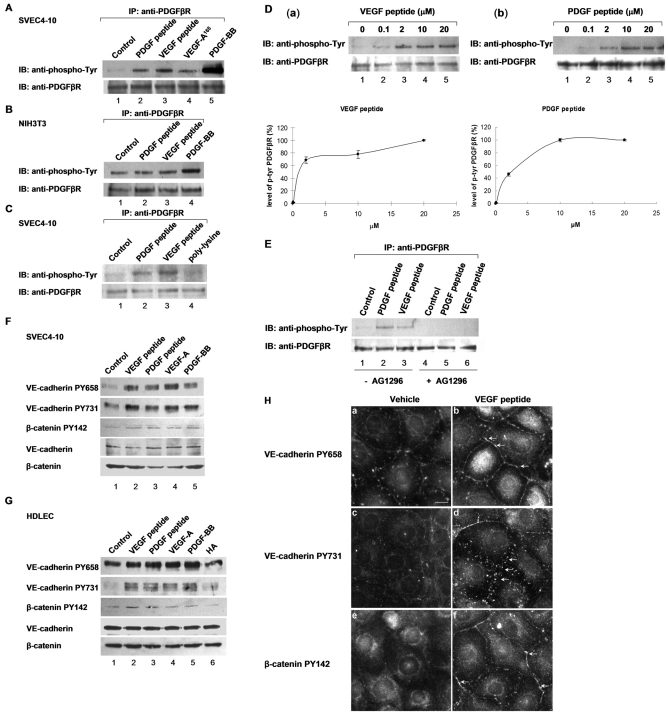
As described above, CRSBP-1 forms complexes with PDGFβR and β-catenin in SVEC4-10 cells. It is possible that CRSBP-1 ligands induce disruption of VE-cadherin- and β-catenin-mediated intercellular adhesion by stimulating the protein tyrosine kinase activity of PDGFβR in the CRSBP-1–PDGFβR complex and subsequent tyrosine phosphorylation of VE-cadherin and β-catenin (probably by stimulated PDGFβR). It has recently been reported that VEGF-A stimulates serine phosphorylation of VE-cadherin, resulting in disruption of intercellular adhesion and opening of intercellular junctions in blood vascular endothelial cells (Gavard and Gutkind, 2006). To test a similar possibility, we determined the levels of tyrosine phosphorylation of several protein species in SVEC4-10 cells stimulated with 10 μM VEGF peptide or vehicle only using western blot analysis with anti-phosphotyrosine antibody. Upon VEGF peptide stimulation, there was a remarkable increase in tyrosine phosphorylation of a protein at ~180 kDa, which coincides with the molecular mass of PDGFβR (Hellberg et al., 2010). Based on this preliminary result and the finding of the complex formation of CRSBP-1 and PDGFβR in SVEC4-10 cells and other cell types expressing both CRSBP-1 and PDGFβR (Boensch et al., 1999), we hypothesized that CRSBP-1 ligands can stimulate tyrosine phosphorylation of PDGFβR by activation of PDGFβR in the CRSBP-1–PDGFβR complex. To test this hypothesis, we determined the level of tyrosine phosphorylation of PDGFβR by immunoprecipitation with anti-PDGFβR serum, followed by immunoblotting for phosphotyrosine in SVEC4-10 cells stimulated by PDGF peptide, VEGF peptide, VEGF-A165 or PDGF-BB. CRSBP-1 ligands, including 10 μM PDGF peptide, 10 μM VEGF peptide, 5 nM VEGF-A165 and 5 nM PDGF-BB all stimulated tyrosine phosphorylation of PDGFβR after 30 minutes of stimulation in SVEC4-10 cells (Fig. 4A, lanes 2–5, respectively). By contrast, in NIH3T3 cells, which are fibroblasts that express a very low level of endogenous CRSBP-1 but a high level of endogenous PDGFβR, 10 μM concentrations of these CRSBP-1 ligands did not significantly stimulate tyrosine phosphorylation of PDGFβR (Fig. 4B, lanes 2 and 3). However, PDGF-BB is a potent stimulator of tyrosine phosphorylation of PDGFβR in both cell types (Fig. 4A, lane 5 and Fig. 4B, lane 4). PDGF-BB-stimulated tyrosine phosphorylation of PDGFβR was ~tenfold greater than that stimulated by PDGF peptide or VEGF peptide (Fig. 4A, lane 5 vs lanes 2 and 3). These results are consistent with the observation that only ~10% of the total PDGFβR forms complexes with CRSBP-1. CRSBP-1 ligands appear to stimulate PDGFβR in the CRSBP-1–PDGFβR complex.
Fig. 4.
CRSBP-1 ligands stimulate tyrosine phosphorylation of PDGFβR, VE-cadherin and β-catenin in a range of cell lines. (A–C) CRSBP-1 ligands stimulate tyrosine phosphorylation of PDGFβR in SVEC4-10 cells but not in NIH 3T3 cells. SVEC4-10 (A,C) and NIH 3T3 (B) cells were stimulated with vehicle only (control) (A–C, lane 1), 10 μM PDGF peptide (A–C, lane 2), 10 μM VEGF peptide (A–C, lane 3), 5 nM VEGF-A165 (A, lane 4), 20 μM poly-L-lysine (C, lane 4) and 2 nM PDGF-BB (A, lane 5 and B, lane 4) for 30 minutes. PDGF-BB was used as a positive control. Stimulated cell lysates were subjected to immunoprecipitation using anti-PDGFβR serum followed by immunoblot analysis using anti-phosphotyrosine antibody. CRS-containing peptides (PDGF peptide and VEGF peptide) and proteins (e.g. VEGF-A165) were found to stimulate tyrosine phosphorylation of PDGFβR in SVEC4-10 cells (A,C). The tyrosine phosphorylation of PDGFβR stimulated by PDGF peptide, VEGF peptide and VEGF-A165 was ~10% of that stimulated by PDGF-BB (A, lanes 2, 3 and 4 vs lane 5). Poly-L-lysine induced little increase of tyrosine phosphorylation of PDGFβR (C, lane 4). In NIH3T3 cells, PDGF peptide (B, lane 2) and VEGF peptide (B, lane 3) induced only slight and insignificant enhancement of tyrosine phosphorylation of PDGFβR. By contrast, PDGF-BB effectively stimulated tyrosine phosphorylation of PDGFβR in these fibroblasts (B, lane 4). NIH3T3 cells are known to express PDGFβR, which mediates cell proliferation in response to PDGF-BB stimulation. These cells express very low levels of CRSBP-1. (D) CRSBP-1 ligands stimulate tyrosine phosphorylation of PDGFβR in a concentration-dependent manner in SVEC4-10 cells. Cells were stimulated with vehicle only (a and b, lane 1), VEGF peptide (a) or PDGF peptide (b) at 0.1, 2, 10 and 20 μM for 30 minutes. Cell lysates were subjected to immunoprecipitation using anti-PDGFβR serum followed by SDS-PAGE and immunoblot analysis using anti-phosphotyrosine antibody (a and b, top). The intensities of the protein bands were quantified by densitometry (a and b, bottom). Both VEGF peptide and PDGF peptide stimulated tyrosine phosphorylation of PDGFβR with IC50 values of ~2 μM. (E) Tyrphostin abolishes CRSBP-1-stimulated tyrosine phosphorylation of PDGFβR in SVEC4-10 cells. Cells were pretreated with either vehicle only (–AG1296, lanes 1 to 3) or 1 μM of Tyrphostin (+AG1296, lanes 4 to 6) for 1 hour. Cells were then stimulated with vehicle only (control) (lanes 1 and 4), 10 μM PDGF peptide (lanes 2 and 5) or 10 μM VEGF peptide (lanes 3 and 6). Cell lysates were immunoprecipitated using anti-PDGFβR serum followed by immunoblot analysis using anti-phosphotyrosine antibody. PDGF peptide and VEGF peptide stimulated tyrosine phosphorylation of PDGFβR (lanes 2 and 3, respectively). Pretreatment with Tyrphostin abolished the peptide-stimulated tyrosine phosphorylation of PDGFβR (lanes 5 and 6). (F,G) CRSBP-1 ligands stimulate tyrosine phosphorylation of VE-cadherin and β-catenin in SVEC4-10 cells and HDLECs. SVEC4-10 cells (F) and HDLECs (G) were treated with vehicle only (control) (lane 1), 10 μM VEGF peptide (lane 2), 10 μM PDGF peptide (lane 3), 5 nM VEGF-A165 (lane 4), 2 nM PDGF-BB (lane 5), and 150 μg/ml HA (F, lane 6) for 30 minutes. Cell lysates were subjected to SDS-PAGE, followed by western blot analysis using anti-VE-cadherin PY658, anti-VE-cadherin PY731, and anti-β-catenin PY142 antibodies. The membranes were also blotted using anti-VE-cadherin and anti-β-catenin antibodies as loading controls. CRS-containing peptides (VEGF peptide and PDGF peptide) and proteins (VEGF-A165 and PDGF-BB) stimulated tyrosine phosphorylation of VE-cadherin at Tyr658 and Tyr731, and of β-catenin at Tyr142 in both cell types (F,G, lanes 2–5). HA stimulation in HDLECs slightly increased the tyrosine phosphorylation of VE-cadherin at Tyr731 but had no effect on tyrosine phosphorylation of VE-cadherin at Tyr658 and of β-catenin at Tyr142 (G, lane 6). (H) VEGF peptide stimulates tyrosine phosphorylation of VE-cadherin and β-catenin at intercellular junctions in SVEC4-10 cells. Cells were stimulated with vehicle only or 10 μM VEGF peptide for 30 minutes. Stimulated cells were fixed and stained with anti-VE-cadherin PY658, anti-VE-cadherin PY731, and anti-β-catenin PY142 antibodies, followed by FITC-conjugated goat anti-rabbit IgG and visualized with a fluorescence microscope. VEGF peptide stimulation increased tyrosine phosphorylation of VE-cadherin at Tyr-658 and Tyr731 (b and d) compared with vehicle alone (a and c). VEGF peptide also stimulated tyrosine phosphorylation of β-catenin at Tyr142 (f) when compared with vehicle alone (e). The arrow indicates the discontinuous punctuate distribution of tyrosine-phosphorylated VE-cadherin and β-catenin at the plasma membrane (b, d and f). Scale bar: 10 μm.
Because VEGF and PDGF peptides are rich in basic amino acid residues, we determined whether poly-L-lysine is also capable of activating PDGFβR. SVEC4-10 cells were treated with 10 μM VEGF peptide, 10 μM PDGF peptide or 20 μM poly-L-lysine (average molecular mass ~40 kDa) for 30 minutes and the level of tyrosine phosphorylation in PDGFβR was determined as described above. Poly-L-lysine was only capable of slightly increasing tyrosine phosphorylation of PDGFβR when compared with vehicle-only treatment in SVEC4-10 cells (Fig. 4C, lanes 1 and 4). CRS-containing peptides (PDGF peptide and VEGF peptide; Fig. 4C, lanes 2 and 3, respectively), which contain a cluster of basic amino acid residues (Boensch et al., 1995), elicited a stronger effect on PDGFβR tyrosine phosphorylation than poly-L-lysine did (Fig. 4C, lane 4). This suggests that certain amino acid sequences in the CRS motifs, which are rich in basic amino acid residues, are required for the optimal activity of CRSBP-1 ligands. The dose-response relationship of CRSBP-1-ligand-stimulated tyrosine phosphorylation of PDGFβR was further dissected by treating SVEC4-10 cells with VEGF peptide and PDGF peptide at 0, 0.1, 2, 10, 20 μM (Fig. 4D). VEGF peptide (Fig. 4Da) and PDGF peptide (Fig. 4Db) stimulated tyrosine phosphorylation in a dose-dependent manner with EC50 values of ~2 μM.
To determine whether CRSBP-1 ligands stimulate and activate PDGFβR, SVEC4-10 cells were pretreated with the specific PDGFβR kinase inhibitor Tyrphostin AG 1296 (Kovalenko et al., 1997) before they were stimulated with CRSBP-1 ligands. Pretreatment of cells with 1 μM Tyrphostin abolished PDGF and VEGF peptide-stimulated tyrosine phosphorylation of PDGFβR (Fig. 4E, lanes 5 and 6 vs lanes 2 and 3). These results indicate that this specific PDGFβR kinase inhibitor abolishes CRSBP-1-ligand-stimulated tyrosine phosphorylation and activation of PDGFβR. These results also suggest that CRSBP-1 ligands probably stimulate activation and tyrosine phosphorylation (most likely autophosphorylation) of PDGFβR through interaction with CRSBP-1 in the CRSBP-1–PDGFβR complex. This explanation is supported by other observations: (1) CRSBP-1 ligands fail to stimulate tyrosine phosphorylation of PDGFβR in cells expressing very low levels of CRSBP-1 (e.g. NIH3T3 cells), and (2) specific CRSBP-1 ligands stimulate tyrosine phosphorylation of PDGFβR ~10% as efficiently as PDGF-BB in SVEC4-10 cells. In these cells, ~10% of the total PDGFβR form complexes with CRSBP-1 as determined by immunoprecipitation and immunoblot analysis.
CRSBP-1 ligands stimulate tyrosine phosphorylation of VE-cadherin and β-catenin
The adhesive state of the VE-cadherin–β-catenin intercellular junction can be regulated via tyrosine phosphorylation of VE-cadherin and β-catenin (Piedra et al., 2003; Potter et al., 2005). In SVEC4-10 cells and HDLECs stimulated with or without CRSBP-1 ligands, we determined the levels of tyrosine phosphorylation of VE-cadherin and β-catenin by western blot analysis and indirect immunofluorescence staining using tyrosine-phosphorylation-site-specific anti-VE-cadherin and anti-β-catenin antibodies. In SVEC4-10 cells, specific CRSBP-1 ligands (VEGF peptide and PDGF peptide) and putative physiological CRSBP-1 ligands (VEGF-A165 and PDGF-BB) stimulated tyrosine phosphorylation of VE-cadherin at Tyr658 and Tyr731 ~two- to threefold (Fig. 4F, lanes 2–5) more efficiently than the control vehicle treatment (Fig. 4F, lane 1). These CRSBP-1 ligands stimulated tyrosine phosphorylation of β-catenin at Tyr142 ~1.6-fold. In HDLECs, the same CRSBP-1 ligands also stimulated tyrosine phosphorylation of VE-cadherin at Tyr658 and Tyr731 ~two- to threefold (Fig. 4G, lanes 2–5) more efficiently than the control vehicle treatment (Fig. 4G, lane 1). The other CRSBP-1 ligand HA at 100 μg/ml only slightly stimulated tyrosine phosphorylation of VE-cadherin at Tyr731, but had no effect on tyrosine phosphorylation of VE-cadherin at Tyr658 (Fig. 4G, lane 6). CRSBP-1 ligands (VEGF peptide, PDGF peptide, VEGF-A165 and PDGF-BB) stimulated tyrosine phosphorylation of β-catenin at Tyr142 by ~1.4-fold (Fig. 4G, lanes 2–5), whereas HA stimulation had no effect on β-catenin tyrosine phosphorylation (Fig. 4G, lane 6). The effects of CRSBP-1 ligands on tyrosine phosphorylation of VE-cadherin and β-catenin were also determined with indirect immunofluorescence staining using tyrosine-phosphorylation-site-specific antibodies to VE-cadherin and β-catenin. In SVEC4-10 cells, VEGF peptide stimulation increased the localization of tyrosine-phosphorylated VE-cadherin and β-catenin at the plasma membrane in a discontinuous punctate distribution (Fig. 4Hb,d,f vs 4Ha,c,e). These results indicate that CRSBP-1 ligands (PDGF peptide, VEGF peptide, PDGF-BB and VEGF-A165) stimulate tyrosine phosphorylation of VE-cadherin and β-catenin in SVEC4-10 cells, whereas HA, which is also a CRSBP-1 ligand, only stimulates limited tyrosine phosphorylation of VE-cadherin and does not affect β-catenin tyrosine phosphorylation in these cells. Similar results were also obtained when HDLECs were used for the same set of experiments (data not shown). Because phosphorylation of intercellular junction proteins VE-cadherin and β-catenin has a pivotal role in regulation of permeability in endothelial cell monolayers (Boccellino et al., 2005; Esser et al., 1998; Gavard and Gutkind, 2006; Piedra et al., 2003; Potter et al., 2005; Vincent et al., 2004), these results might explain why HA is weaker than other CRSBP-1 ligands in stimulating permeability in LEC monolayers and interstitial–lymphatic transit in mice (Huang et al., 2006).
PDGFβR mediates CRSBP-1-ligand-stimulated tyrosine phosphorylation of VE-cadherin
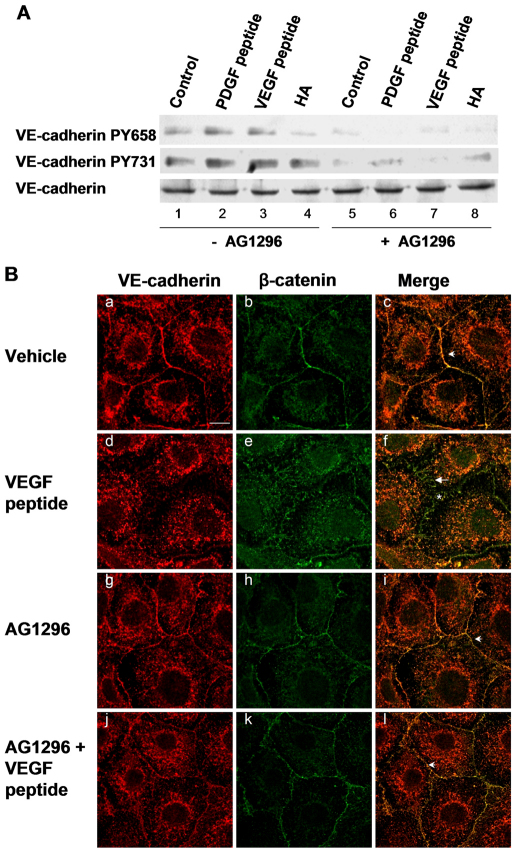
To determine whether PDGFβR is involved in CRSBP-1-ligand-stimulated tyrosine phosphorylation of VE-cadherin, we determined the effects of CRSBP-1 ligands on tyrosine phosphorylation of VE-cadherin in SVEC4-10 cells pretreated with and without Tyrphostin using SDS-PAGE and western blot analysis. SVEC4-10 cells were pretreated with or without 1 μM Tyrphostin for 30 minutes and then treated with vehicle only, 10 μM VEGF peptide, 10 μM PDGF peptide or 100 μg/ml HA. PDGF peptide (Fig. 5A, lane 2) and VEGF peptide (Fig. 5A, lane 3) stimulated phosphorylation of VE-cadherin at Tyr658 and Tyr731 ~1.6-fold and ~2.5-fold, respectively, when compared with vehicle-only treatment, whereas HA stimulated phosphorylation of VE-cadherin at only Tyr731 (Fig. 5A, lane 4). Pretreatment of SVEC4-10 cells with Tyrphostin abolished CRSBP-1-ligand-stimulated tyrosine phosphorylation of VE-cadherin (Fig. 5A, lanes 6 and 7 vs lanes 2 and 3). Tyrphostin also attenuated ligand-unstimulated tyrosine phosphorylation of VE-cadherin in these cells (Fig. 5A, lane 5 vs lane 1).
Fig. 5.
Tyrphostin inhibits CRSBP-1-ligand-stimulated tyrosine phosphorylation of VE-cadherin, VEGF-peptide-induced disassembly and opening of VE-cadherin–β-catenin intercellular junctions in SVEC4-10 cells. (A) Cells were either pretreated with vehicle only (–AG1296, lanes 1–4) or with 1 μM Tyrphostin for 1 hour (+AG1296, lanes 5–8). Cells were then treated with vehicle only (control) (lanes 1 and 5), 10 μM PDGF peptide (lanes 2 and 6), 10 μM VEGF peptide (lanes 3 and 7) or 100 μg/ml HA (lanes 4 and 8) for 30 minutes. Cell lysates were subjected to SDS-PAGE analysis followed by immunoblot analysis using anti-VE-cadherin PY658 and anti-VE-cadherin PY731 antibodies. The membranes were also blotted using anti-VE-cadherin as a loading control. CRS-containing peptides (PDGF and VEGF peptides) stimulated tyrosine phosphorylation of VE-cadherin at Tyr658 and Tyr731 (lanes 2 and 3, respectively). HA stimulated phosphorylation of VE-cadherin at Tyr731 but had no effect on phosphorylation of VE-cadherin at Tyr658. Pretreatment with Tyrphostin abolished CRSBP-1-ligand-stimulated tyrosine phosphorylation of VE-cadherin (lanes 6–8). (B) Confluent cells were pretreated with vehicle only (a–c) or 1 μM Tyrphostin (g–l) for 1 hour. Cells were then stimulated with vehicle only (a–c) or 10 μM VEGF peptide (d–f) for 1 hour. Cells were fixed and stained with anti-VE-cadherin and anti-β-catenin antibodies followed by confocal immunofluorescence microscopy. VEGF peptide induced disorganization of β-catenin and internalization of VE-cadherin (d–f,j–l). Pretreatment of cells with this PDGFβR kinase inhibitor abolished disassembly of VE-cadherin junctions induced by VEGF peptide and microscopically visible gaps between cells (l). Arrowheads (c,i,l) and arrows (f) indicate the localization of the linear and zigzag patterns of β-catenin at the plasma membrane, respectively. Asterisk indicates the localization of the gaps between cells (f). Scale bar: 10 μm.
PDGFβR kinase activity is crucial in CRSBP-1 ligand-induced opening of VE-cadherin–β-catenin intercellular junctions
As described above, the binding of specific CRSBP-1 ligands to CRSBP-1 triggers the molecular disassembly of VE-cadherin–β-catenin intercellular junctions and formation of microscopically visible intercellular gaps, leading to increased permeability of LEC monolayers. Because CRSBP-1 ligands stimulate tyrosine phosphorylation and activation of PDGFβR, we wished to determine whether PDGFβR is the crucial signaling protein that mediates CRSBP-1-ligand-stimulated effects in LECs. SVEC4-10 cell monolayers were pretreated with or without 1 μM Tyrphostin, followed by stimulation with vehicle only, 10 μM VEGF peptide (Fig. 5B) or 10 μM PDGF peptide (data not shown). In vehicle-stimulated cells without Tyrphostin pretreatment, VE-cadherin and β-catenin colocalized at the plasma membrane and displayed linear patterns in the plasma membrane distribution (Fig. 5Bc). Stimulation with VEGF peptide (Fig. 5Bd,e,f) and PDGF peptide (data not shown) resulted in decreased plasma membrane distribution of VE-cadherin and disorganization of β-catenin from a linear pattern to a zigzag pattern (Fig. 5Bf vs 5Bc). However, pretreatment of SVEC4-10 cells with 1 μM Tyrphostin abolished VEGF-peptide-induced (Fig. 5Bl vs 5Bf) and PDGF peptide-induced (data not shown) disorganization (formation of zigzag patterns) of VE-cadherin–β-catenin intercellular junctions. In the control experiments, pretreatment of SVEC4-10 cells with 1 μM Tyrphostin only did not alter the distribution of either VE-cadherin or β-catenin when compared with cells without inhibitor pretreatment (Fig. 5Bi vs 5Bc). These results suggest that Tyrphostin effectively abolishes VEGF-peptide- and PDGF-peptide-induced opening of VE-cadherin–β-catenin intercellular junctions.
Specific CRSBP-1 ligands stimulate interstitial–lymphatic transit in mice in a CRSBP-1-dependent manner
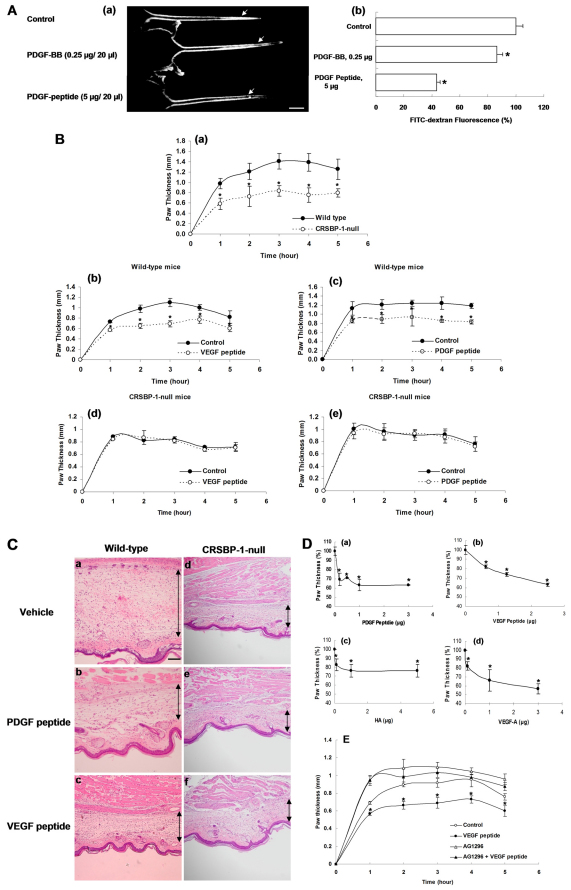
We previously demonstrated that PDGF-BB and HA increase entry of large molecules from the interstitial space into lymphatic vessels (interstitial–lymphatic transit) in wild-type mice but not Crsbp1−/− mice (Huang et al., 2006), as determined by intradermal injection of high molecular weight FITC–dextran in mouse tails and by monitoring the decay in fluorescence intensity near the injection site. The results from these studies indicate that CRSBP-1 mediates PDGF-BB- and HA-induced increase in interstitial–lymphatic transit. However, it is possible that, in addition to CRSBP-1, PDGFβR is also involved in PDGF-BB-stimulated interstitial–lymphatic transit. To test this possibility, we determined the effect of PDGF peptide, a specific CRSBP-1 ligand which does not bind to PDGFβR, on interstitial–lymphatic transit, as determined by using the FITC–dextran tail injection assay (Huang et al., 2006). PDGF peptide (5 μg/20 μl) was capable of stimulating interstitial–lymphatic transit (Fig. 6Aa). It appeared to accelerate the egress of FITC–dextran more than PDGF-BB at their optimal concentrations (Fig. 6Ab). The reason that PDGF peptide is more potent than PDGF-BB is unknown. This might be due to the fact that PDGF-BB used in the experiments is not a full-length protein and lacks the putative CRS motif. PDGF peptide and PDGF-BB might exert their different effects by binding to CRSBP-1 and PDGFβR, respectively.
Fig. 6.
CRSBP-1 ligands stimulate interstitial–lymphatic transit in wild-type mice but not in Crsbp1−/− mice. (A) Specific CRSBP-1 ligand stimulated egress of high molecular weight FITC–dextran injected intracutaneously into tails of wild-type mice. 20 μl FITC–dextran (8 mg/ml) were intradermally co-injected with vehicle only (control), human PDGF-BB (0.25 μg), or PDGF-peptide (5 μg) into tails of mice (female, 6 weeks old). Five mice were used in each experimental group. After 10 minutes, photographs were taken under UV illumination. A representative sample of five mice is shown (a). The arrow indicates the injection site of FITC–dextran and PDGF-BB or PDGF peptide. FITC–dextran fluorescence near the injection site was measured (b). The fluorescence intensity near the site co-injected with vehicle only was taken as 100%. Bars represent mean ± s.d. (n=5). *P<0.05 compared with control. Scale bar: 10 μm. (B) Specific CRSBP-1 ligand-induced attenuation in λ-carrageenan-induced paw edema in wild-type mice (a–c) but not in Crsbp1−/− mice (a,d,e). Wild-type and Crsbp1−/− (Crsbp1-null) mice (female, 6 weeks old) received subplantar injection of 25 μl of 1% λ-carrageenan and vehicle only (a). Mice received subplantar injection of 25 μl of 1% λ-carrageenan with vehicle only (control) (b–e), 1.5 μg VEGF peptide (b,d) or PDGF peptide (c,e). After injection of λ-carrageenan ± PDGF peptide or VEGF peptide, paw thickness was measured using calipers (Mitatoyo, Japan) at 1, 2, 3, 4, and 5 hours after the injection. The increase in paw thickness was determined by the difference between the paw volume at each time point and the basal volume. Bars represent mean ± s.d. (n=5). *P<0.05 compared with control and wild-type mice. (C) Histological analysis of mouse paw edema induced by λ-carrageenan in the presence or absence of PDGF peptide or VEGF peptide in wild-type mice (a–c) and Crsbp1−/− mice (d–f). Crsbp1+/+ mice and wild-type mice received subplantar injection of 25 μl of 1% λ-carrageenan with vehicle only (a,d), PDGF peptide (b,e) or VEGF peptide (c,f). After 2 hours, mice were killed and paws were fixed in formalin and embedded in paraffin. The tissue slides were stained with hematoxylin and eosin (H&E). Edema was measured as increased thickness between muscularis and epidermal layers. The black line in the figure indicates the thickness of the edema. Scale bar: 2 mm. (D) Dose-dependence of CRSBP-1-ligand-induced attenuation in λ-carrageenan-induced paw edema in wild-type mice. Wild-type mice received subplantar injection of 25 μl of 1% λ-carrageenan alone or with varying amounts of PDGF-peptide (a), VEGF-peptide (b), HA (c) and VEGF-A165 (d). At 2 hours, paw thickness was measured by caliper. CRSPB-1 ligand treatment attenuated paw edema. Bars represent mean ± s.d. (n=5). *P<0.05 compared with control. (E) Tyrphostin abolishes CRSBP-1-ligand-attenuated λ-carrageenan-induced paw edema in wild-type mice. Wild-type C57BL/6J mice (female, 6 weeks old and five mice for each experimental group) were separated into groups. Hind paws of mice received subplantar injection of 25 μl of λ-carrageenan (1% in H2O). Hind paws of mice were also co-injected with vehicle only (control) (○), 2 μg VEGF peptide (●), 10 nmole Tyrphostin (▵), or 10 nmole Tyrphostin with 2 μg VEGF peptide (▴). VEGF peptide treatment attenuated λ-carrageenan-induced paw edema. Tyrphostin treatment alone increased λ-carrageenan-induced paw edema. When co-injected with VEGF peptide, Tyrphostin abolished the VEGF-peptide-attenuated paw edema.
To further define the effects of specific CRSBP-1 ligands on interstitial–lymphatic transit, we used the system of λ-carrageenan-induced paw edema. The system has been well characterized (Otterness and Moore, 1988; Wise et al., 2007). An acute inflammatory response induced by λ-carrageenan is characterized by an increase in vascular permeability and inflammatory cell infiltration, leading to edema formation, as a result of extravasation of fluid and protein accumulation at the inflammatory site. It can provide a quantitative measurement of alterations in the transit of fluid from the interstitial space into the lumen of lymphatic capillaries. For example, the degree of the attenuation of λ-carrageenan-induced paw edema corresponds to the magnitude of the opening of intercellular junctions in lymphatic vessels and increased interstitial-lymphatic transit of fluid. Using this assay, Crsbp1−/− mice exhibited attenuated swelling (or edema) compared with wild-type mice (Fig. 6Ba). Significant attenuation of paw swelling was observed 1 hour after injection of λ-carrageenan. A maximal attenuation of ~40% was observed 3 hour after λ-carrageenan injection (Fig. 6Ba). This suggests that constitutively increased interstitial–lymphatic transit of fluid results in attenuation of edema in Crsbp1−/− mice. This result is consistent with the observation of constitutively increased interstitial–lymphatic transit of fluid in Crsbp1−/− mice as determined using the FITC–dextran tail injection assay (Huang et al., 2006). Furthermore, specific CRSBP-1 ligands (VEGF peptide and PDGF peptide) attenuated paw swelling by ~30% in wild-type mice (Fig. 6Bb,c). By contrast, these peptides did not affect λ-carrageenan-induced swelling in Crsbp1−/− mice (Fig. 6Bd,e). The histological analysis of paw edema (Fig. 6C) revealed that PDGF peptide and VEGF peptide effectively reduced fluid accumulation in paw edema lesions in wild-type mice (Fig. 6Cb,c vs 6Ca), whereas these peptides did not significantly affect λ-carrageenan-induced paw edema in Crsbp1−/− mice (Fig. 6Ce,f vs 6Cd). PDGF peptide, VEGF peptide, HA and VEGF-A165 decreased the paw edema in a dose-dependent manner (Fig. 6Da–d, respectively). It is important to note that HA was not as potent as PDGF peptide, VEGF peptide and VEGF-A165 in decreasing paw edema at their optimal doses. These results indicate that Crsbp1-null mice and wild-type mice treated with CRSBP-1 ligands exhibit attenuated λ-carrageenan-induced swelling in mouse paws compared with wild-type mice and wild-type mice treated with vehicle only, respectively and that this attenuation is due to opening of intercellular junctions in lymphatic vessels and increased interstitial–lymphatic transit of fluid in the paw tissues of Crsbp1-null mice and wild-type mice treated with CRSBP-1 ligands. These results also support the contention that Crsbp1-null mutation and CRSBP-1 ligand stimulation can cause opening of intercellular junctions in lymphatic vessels, resulting in increased interstitial–lymphatic transit of fluid.
PDGFβR kinase activity is required for CRSBP-1-ligand-stimulated interstitial–lymphatic transit
As described above, the PDGFβR kinase activity is pivotal in CRSBP-1-ligand-induced opening of lymphatic intercellular junctions. To define the role of the PDGFβR kinase activity in CRSBP-1-ligand-stimulated interstitial–lymphatic transit in wild-type mice, we determined the effect of the PDGFβR kinase activity inhibition on interstitial-lymphatic transit in mice using the system of λ-carrageenan-induced mouse paw edema. Hind paws of wild-type mice were injected with 1% λ-carrageenan and either Tyrphostin or Tyrphostin + VEGF peptide. In this experiment, two additional groups of mice were injected with 1% λ-carrageenan and 1% λ-carrageenan + VEGF peptide, as negative and positive controls, respectively. VEGF peptide stimulation was capable of attenuating λ-carrageenan-induced paw edema compared with vehicle-treated animals (Fig. 6E). Treatment with Tyrphostin alone enhanced paw edema when compared with the vehicle control; it is likely that this abolished the action of endogenous CRSBP-1 ligands under the experimental conditions. Moreover, treatment with Tyrphostin abolished VEGF-peptide-induced attenuation of paw edema (Fig. 6E). Together with the results from the cell experiments, these results suggest that inhibition of PDGFβR protein kinase activity can effectively abolish CRSBP-1-ligand-induced opening of lymphatic intercellular junctions in lymphatic vessels.
Discussion
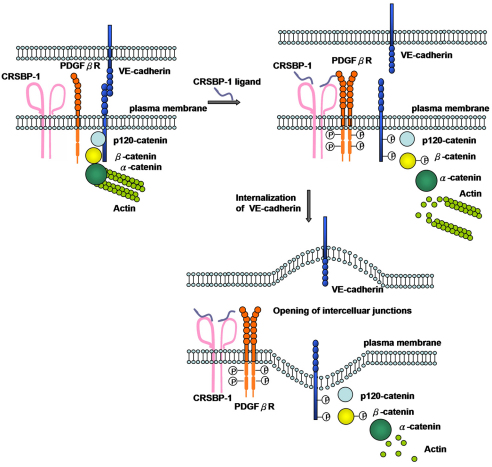
Here, we demonstrate that CRSBP-1 forms complexes with PDGFβR in LECs. We also demonstrate that pretreatment or co-injection with the PDGFβR inhibitor abolishes the CRSBP-1-ligand-induced effects in cultured LECs and in animals. Based on our results and results published by others (Knudsen et al., 1995; Piedra et al., 2003; Potter et al., 2005; Dejana et al., 2008; Hartsock et al., 2008), we propose a model (Fig. 7) to illustrate the mechanism by which CRSBP-1 ligands induce opening of intercellular adherens junctions in LECs. This model stipulates that, in the unstimulated state, VE-cadherin mediates intercellular adhesion by forming trans homodimers with VE-cadherin from adjacent cells. VE-cadherin-mediated adhesion is maintained through the interaction of the VE-cadherin cytoplasmic tail with β-catenin and p120-catenin. These catenin interactions maintain localization of VE-cadherin at the plasma membrane. Furthermore, β-catenin couples VE-cadherin to the actin cytoskeleton by interacting with α-catenin. Our model proposes that CRSBP-1, a 120 kDa disulfide-linked homodimeric membrane protein that forms complexes with the monomeric form of PDGFβR, has an important role in lymphatic vessel biology. Upon ligand binding to CRSBP-1, the PDGFβR in the CRSBP-1 complex is induced to form dimers or oligomers, resulting in activation and tyrosine phosphorylation (autophosphorylation) of the PDGFβR. Activated PDGFβR or PDGFβR downstream effectors (cytoplasmic protein tyrosine kinases) (Adam et al., 2010) are responsible for tyrosine phosphorylation of VE-cadherin at Tyr658 and Tyr731 and of β-catenin at Tyr142. Tyrosine phosphorylation of VE-cadherin at these residues prevents β-catenin and p120-catenin from binding to VE-cadherin. β-catenin and p120-catenin then become dissociated from VE-cadherin, resulting in destabilization and internalization of VE-cadherin. Furthermore, tyrosine phosphorylation of β-catenin hinders the association of β-catenin with α-catenin, causing VE-cadherin to uncouple from the actin cytoskeleton. Consequently, VE-cadherin-mediated intercellular adhesion is disrupted, leading to opening of intercellular junctions with resulting modulation of properties of lymphatic vessels (Fig. 7). This model describes how CRSBP-1 mediates ligand-stimulated interstitial–lymphatic transit through regulation of VE-cadherin intercellular junctions.
Fig. 7.
A model for CRSBP-1 regulation in VE-cadherin-mediated intercellular adhesion in LECs. At the cell surface, disulfide-linked homodimeric CRSBP-1 forms complexes with monomeric PDGFβR and β-catenin. Upon CRSBP-1 ligand binding, CRSBP-1 is induced to form oligomers or have a conformational change, resulting in the formation of dimers or oligomers of PDGFβR. The PDGFβR becomes activated and undergoes tyrosine autophosphorylation. Activation of PDGFβR or activated PDGFβR-mediated signaling cascades lead to increased tyrosine phosphorylation of VE-cadherin at Tyr658 and Tyr731 and β-catenin at Tyr142, resulting in dissociation of β-catenin and p120-catenin from VE-cadherin (which trans dimerizes between cells) and subsequent internalization of VE-cadherin. α-Catenin becomes dissociated from β-catenin, leading to uncoupling of VE-cadherin junctions from actin cytoskeleton, disruption of VE-cadherin-mediated intercellular adhesion and opening of intercellular junctions in LECs and increased interstitial–lymphatic transit in intact mice. Under the conditions of CRSBP-1 downregulation or null mutation, VE-cadherin-mediated intercellular adhesion (VE-cadherin trans-dimerization) is compromised.
The CRSBP-1-mediated regulation of interstitial–lymphatic transit demonstrated here might have an important role in interstitial–lymphatic transit or trafficking of immune and carcinoma cells during immune responses and lymphatic metastasis, respectively. Immune cells such as dendritic cells initiate immune responses after they acquire antigens from the interstitial space and migrate to lymphatic vessels and lymph nodes where they activate naive T-cells. The primary tumor can be spread to other tissues and organs through blood and lymphatic systems (Ji, 2009). Lymphatic vessels appear to be used often by certain carcinomas such as breast cancer to metastasize to other tissues (Eccles et al., 2007). These immune and carcinoma cells in the interstitial space might migrate into the lumen of lymphatic vessels by secreting or using CRSBP-1 ligands to open intercellular junctions in lymphatic vessels, which results in increased interstitial–lymphatic transit. The interstitial–lymphatic transit of immune and carcinoma cells has been shown to require physical contact between these cells and lymphatic vessels. These cells are also known to produce CRSBP-1 ligands, such as members of the PDGF superfamily and CRS-containing cytokines (Cao, 2005; Eccles et al., 2007; Robbiani et al., 2000). The expression levels of PDGF-BB in tumors are correlated with their tendency to produce lymphatic metastasis (Cao, 2005; Cao et al., 2006). VEGF-C produced by tumors promotes tumor-cell invasion by increasing lymphatic metastasis (He et al., 2005). Antibodies against CCL19, which contains a typical CRS motif and is a putative CRSBP-1 ligand, block accumulation of dendritic cells in the lymph nodes (Robbiani et al., 2000).
A well-known mechanism that governs interstitial–lymphatic flow is interstitial fluid (hydrostatic) pressure (IFP) dynamics (Aukland and Reed, 1993). In blood vascular capillary beds, plasma fluid and protein are filtrated into the interstitial compartment. Increased fluid in the interstitial compartment results in an increased hydrostatic pressure gradient between the interstitial space and the lymphatic capillary lumen. As interstitial fluid volume and hydrostatic pressure increase, the anchoring filaments that connect LECs to the collagen and elastin fibers are stretched, resulting in opening of intercellular junctions and blind ends of lymphatic capillaries as well as distention of lumens of lymphatic capillaries (Leak, 1976; Skobe and Detmar, 2000). Consequently, increased interstitial fluid enters lymphatic capillaries, thus increasing interstitial–lymphatic transit. This IFP-regulated interstitial–lymphatic transit appears to be involved in fluid homeostasis in tissues, but is not likely to be responsible for driving immune and carcinoma cells into lymphatic vessels during immune responses and lymphatic metastasis. The mechanisms regulating interstitial–lymphatic transit other than that regulated by IFP are not yet clear (Aukland and Reed, 1993). Here, we provide evidence indicating a novel ligand-regulatory mechanism of interstitial–lymphatic transit.
According to the model (Fig. 7), PDGFβR in the CRSBP-1–PDGFβR complex has an important role in mediating CRSBP-1-ligand-stimulated permeability in LECs and interstitial–lymphatic transit in whole animals. This model raises several questions, including: (1) is the signaling pathway mediated by the CRSBP-1–PDGFβR complex different from that mediated by free PDGFβR (which contributes 90% of the total PDGFβR in LECs)? (2) If so, are the cellular responses also different? The signaling and cellular responses mediated by CRSBP-1–PDGFβR and by free PDGFβR appear to be different, as evidenced by the observation that PDGF-BB (without a putative CRS motif) is much weaker than other CRSBP-1 ligands (PDGF peptide, VEGF peptide and VEGF-A165) in stimulating permeability in LEC monolayers and interstitial–lymphatic transit in mice. Apparently, alterations in the morphology (distended lumens) and function (constitutively increased interstitial lymphatic-transit) of lymphatic capillary vessels cannot be compensated for, or rescued by, the presence of PDGFβR and PDGF-BB in Crsbp1−/− mice. Similarly to other CRSBP-1 ligands, PDGF-BB (Abcam ab73229), which a proteolytic product of PDGF-BB lacking a typical CRS motif, is unable to effectively stimulate interstitial–lymphatic transit in Crsbp1−/− mice as determined by high molecular weight FITC–dextran mouse tail assay (Huang et al., 2006). This suggests that PDGF-BB, which lacks a typical CRS motif, stimulates interstitial–lymphatic transit through interaction with PDGFβR in the CRSBP-1–PDGFβR complex in wild-type mice.
Materials and Methods
Materials
Na[125I] (17 Ci/mg) was obtained from ICN Biochemicals (Irvine, CA). PDGF peptide (a 19-mer peptide containing the amino acid sequence of YVRVRRPPKGKHRKFKHTH), VEGF peptide (a 25-mer peptide containing the amino acid sequence of KKSVRGKGKGQKRKRKKSRYKSWSV) and scrambled VEGF peptide (a 25-mer peptide which contained the same amino acid composition of VEGF peptide but with a scrambled amino acid sequence: KSKVKVRSKSKGKWKGRSKYRGKQR) were synthesized by C. S. Bio Co. (Menlo Park, CA). PDGF-BB, which lacks the putative CRS motif, was obtained from Abcam (Cambridge, MA). VEGF-A165 was a gift from Daniel T. Connolly (Mosanto, St Louis, MO). FITC–dextran (molecular weights ~40,000 and ~2,000,000), DMEM, λ-carrageenan, chloramine-T and other biochemical reagents were obtained from Sigma (St Louis, MO). PDGF-BB, anti-phosphotyrosine, anti-VE-cadherin, anti-β-catenin, anti-p120-catenin, anti-VE-cadherin PY658, anti-VE-cadherin PY731 and anti-β-catenin PY142 antibodies were obtained from Abcam. H1299/CRSBP-1 and H1299/vector cells were prepared as described (Huang et al., 2003). SVEC4-10 cells and human dermal LECs (HDLECs) were obtained from American Type Culture Collection (Manassas, VA) and PromoCell (Heidelberg, Germany), respectively. Wild-type mice (C57/BL/6) were obtained from the Jackson Laboratory (Bar Harbor, ME). Crsbp1−/− mice were generated as described previously (Huang et al., 2006). Crsbp1−/− mice have been included in the Induced Mutant Resources at the Jackson Laboratory, Bar Harbor, ME 04609 (Stock #006221,B6. 129Sl-XlkdlÆtmJhuaæ/J). All experiments on live animals were performed in accordance with guidelines and regulations from the IACUC Office at St Louis University.
Immunofluorescence
Cells grown on coverslips for 2 days (~50% confluency) were treated with CRSBP-1 ligands (10 μM PDGF peptide, 10 μM VEGF peptide, 0.2 μM VEGF-A and 120 μg/ml HA) or vehicle only for 30 minutes or 1 hour in serum-free DMEM. After treatment, cells were first washed with PBS twice, then fixed in 100% methanol at −20°C for 10 minutes. Following a rinse with PBS, cells were quenched in 0.1% sodium borohydride in PBS for 5 minutes. Cells were washed with PBS and blocked with 0.2% gelatin in PBS at room temperature for 1 hour. Cells were incubated for 1 hour with a 1:200 or 1:500 dilution of anti-CRSBP-1 antiserum, anti-VE-cadherin antibody, anti-β-catenin antibody, anti-VE-cadherin PY658 antibody, anti-VE-cadherin PY731 antibody, or anti-β-catenin PY142 antibody in a solution of 0.2% gelatin in PBS followed by incubation with an 1:200 dilution of fluorescence-labeled secondary antibodies in a solution of 0.2% gelatin in PBS for 1 hours. Coverslips were then mounted on glass slides. The slides were viewed under an Olympus AHBT3 fluorescence microscope using interference green filters.
For immunofluorescence confocal microscopy of VE-cadherin–β-catenin intercellular junctions, SVEC4-10 cells and HDLECs were treated with CRSBP-1 ligands and fixed as described above. To estimate colocalization of VE-cadherin and β-catenin in SVEC4-10 cells, confluent cells grown on coverslips were treated with CRSBP-1 ligands. Following fixation and blocking, cells were incubated overnight with anti-VE-cadherin antibody and anti-β-catenin antibody at 1:200 dilution. After extensive washing, cells were incubated with Rhodamine-Red-conjugated donkey anti-goat IgG antibody and FITC–conjugated mouse anti-rabbit antibody at 1:200 dilution for 1 hours. Images were acquired using a Leica TCS SP confocal microscope (Leica, Heidelberg, Germany).
siRNA transfection
Transfection of cells with Crsbp1 siRNA and control siRNA was performed according to the manufacturer's protocol (Santa Cruz Biotechnology). Briefly, SVEC4-10 cells were grown on P-60 dishes to 60–70% confluence. Cells were then washed twice with serum-free DMEM and 1.8 ml of transfection medium (sc-36868) was added to the cells. A 0.2 ml aliquot of transfection medium containing 0.15 nmoles Crsbp1 siRNA (sc-42902) or control siRNA (sc-37007) and 15 μl of transfection reagent (sc-29528) was incubated at room temperature for 30 minutes before adding to the cells. The transfected cells were incubated at 37°C overnight. Following incubation, transfected cells were trypsinized and plated for experiments. Cells were used for experiments 48 hours after transfection.
Transwell permeability assay
SVEC4-10 cells transfected with Crsbp1 siRNA or control siRNA at 1.25×105 cells or HDLEC at 1.5×105 were grown on Transwell permeable supports (Corning, pore size 3.0 μm, membrane diameter 6.5 mm, collagen coated) overnight. Inserts were washed once with serum-free DMEM and the lower chamber was filled with 0.6 ml of DMEM. Then 0.1 ml of vehicle, PDGF peptide (10 μM) or VEGF peptide (10 μM) in serum-free DMEM along with FITC–dextran 1 mg/ml (average MW ~40,000) was added to the top chamber. At several time points after FITC–dextran administration, a 50 μl aliquot of DMEM was taken from the lower chamber. The amount of FITC–dextran diffused to the bottom chamber was determined and expressed as arbitrary units (AU).
Internalization of cell-surface VE-cadherin
SVEC4-10 cells grown in six-well plates were treated with vehicle only, 10 μM PDGF peptide or 10 μM VEGF peptide at 37°C for 30 minutes. Following treatment, cells were untreated or treated with trypsin as previously described (Boensch et al., 1999) to remove cell-surface proteins. Trypsin-undigested and trypsin-digested cells were subsequently lysed and subjected to reducing SDS-PAGE and electro-transferred onto PVDF membranes. Internalized or intracellular VE-cadherin, which was resistant to trypsin digestion, on PVDF membranes was visualized using streptavidin-HRP and the ECL system.
Tyrosine phosphorylation measurements
SVEC4-10 cells were grown on six-well plates until confluent. After washing with serum-free DMEM, cells were incubated with CRSBP-1 ligands (10 μM PDGF peptide, 10 μM VEGF peptide and 0.2 μM VEGF-A165) for 30 minutes in serum-free DMEM. Cells were then lysed with 500 μl of 1% Triton X-100 lysis buffer (50 mM Tris-HCl, 1 mM EDTA, 1 mM EGTA, 0.5 mM Na3VO4, 10 mM β-glycerophosphate, 50 mM NaF, 5 mM sodium pyrophosphate). The Triton X-100 extracts were incubated with 5 μl rabbit anti-PDGFβR serum at 4°C overnight. A 50 μl aliquot of agarose-protein-G gel (50% gel suspension) was then rotated with Triton X-100 extracts at 4°C. After 1 hour, the gel was washed three times with lysis buffer and eluted with 35 μl SDS sample buffer. The eluted solution was subjected to reducing 7.5% SDS-PAGE. The separated proteins on SDS gels were electrotransferred onto PVDF membranes followed by western blot analysis using anti-phosphotyrosine antibody (Santa Cruz sc-7020). The membranes were also blotted using anti-VE-cadherin and anti-β-catenin antibodies as loading controls. The antigens on the blots were visualized by using horseradish-peroxidase-conjugated anti-mouse IgG antibody and the ECL system. The relative intensities of antigen bands on X-ray films were quantified by densitometry. For determination of tyrosine phosphorylation of VE-cadherin and β-catenin, cell lysates of cells treated with CRSBP-1 ligands were subjected to SDS-PAGE, followed by quantitative western blot analysis using anti-VE-cadherin PY658, anti-VE-cadherin PY731 and anti-β-catenin PY142 antibodies (Santa Cruz Biotechnology). The PVDF membranes were also blotted using anti-VE-cadherin and anti-β-catenin antibodies as loading controls.
Co-immunoprecipitation
Confluent cells were collected and lysed with 1% Triton X-100 lysis buffer. The Triton X-100 extracts were incubated with 5 μl rabbit anti-CRSBP-1 serum, non-immune serum and anti-PDGFβR serum at 4°C overnight. Fifty μl of agarose-protein-G gel (50% gel suspension) was then rotated with Triton X-100 extracts at 4°C for 1 hour. The agarose-protein-G gel was then washed three times with lysis buffer and eluted with 35 μl SDS sample buffer. The eluted solution was subjected to reducing 7.5% SDS-PAGE. The separated proteins on SDS gels were electrotransferred onto PVDF membranes followed by western blot analysis using rabbit anti-PDGFβR serum, anti-β-catenin antibody and p120-catenin. The antigens on the blots were visualized using horseradish-peroxidase-conjugated anti-rabbit IgG antibody and the ECL system.
FITC–dextran tail injection assay
The FITC–dextran tail injection assay was carried out as described (Huang et al., 2006). To determine the interstitial–lymphatic transit in mice (five female mice per experimental group), 20 μl of 8 mg/ml fluorescein isothiocyanate (FITC)-dextran (average MW ~2,000,000; Sigma) with and without PDGF-BB (0.25 μg/20 μl) or PDGF peptide (1 μg/20 μl) in phosphate-buffered saline (PBS) was injected intradermally into the tails of mice. The progressive diffusion (in the absence of injection pressure) of FITC–dextran fluorescence near the injection site was monitored over time with a UV lamp and a camera by fluorescence stereomicroscopy. The fluorescence intensity was determined using the NIH Image J program following photography. The relative fluorescence intensity in mice injected with PBS was taken as 100% (control). The interstitial–lymphatic transit analysis appeared to be quantitative and specific, as previously reported (Huang et al., 2006).
λ-Carrageenan-induced edema in mouse paw
Wild-type and Crsbp1−/− mice around 5–6 weeks old were separated into experimental groups. Each group of animals received subplantar injection without anesthesia of 25 μl of λ-carrageenan (1% w/v in H2O) with and without 1.5 μg VEGF peptide or PDGF peptide into footpads. The use of anesthetics to facilitate the handling of animals has been shown to inhibit the induction of paw swelling (Otterness and Moore, 1988). Paw thickness was measured by using calipers (Mitutoyo, Japan) immediately before the subplantar injection and 1, 2, 3, 4, 5 hours afterward. The increase in paw thickness was determined as the difference between the paw thickness at each time point and the basal paw thickness. After experiments, animals were killed. The paws were fixed in formalin, and paraffin block sections were stained with hematoxylin and eosin (H&E) to examine paw edema and cellularity.
Statistical analysis
The values are presented as means ± s.d. (n=4). Two-tailed unpaired Student's t-test was used to determine the significance of differences between groups. P<0.05 was considered significant. The data shown are representative of three to four independent experiments.
Supplementary Material
Acknowledgments
We thank Thomas Heyduk for providing assistance with confocal fluorescence microscopy. This work was supported by NIH grants HL 087463-01 to S.S.H. and DK 078438/AA 019223 to J.S.H. The authors declare that they have no conflict of interest. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/124/8/1231/DC1
References
- Adam A. P., Sharenko A. L., Pumiglia K., Vincent P. A. (2010). Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers. J. Biol. Chem. 285, 7045-7055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K., Carmeliet P. (2002). Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1, 219-227 [DOI] [PubMed] [Google Scholar]
- Aukland K., Reed R. K. (1993). Interstitil-lymphatic mechanisms in the control of extracellular fluid volume. Am. J. Physiol. 73, 1-75 [DOI] [PubMed] [Google Scholar]
- Baluk P., Fuxe J., Hashizume H., Romano T., Lashnits E., Butz S., Vestweber D., Corada M., Molendini C., Dejana E., et al. (2007). Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349-2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S., Ni J., Wang S. X., Clasper S., Su J., Tammi R., Jones M., Jackson D. G. (1999). LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 144, 789-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccellino M., Camussi G., Giovane A., Ferro L., Calderaro V., Balestrieri C., Quagliuolo L. (2005). Platelet-activating factor regulates cadherin-catenin adhesion system expression and beta-catenin phosphorylation during Kaposi's sarcoma cell motility. Am. J. Pathol. 166, 1515-1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boensch C., Kuo M. D., Connolly D. T., Huang S. S., Huang J. S. (1995). Identification, purification, and characterization of cell-surface retention sequence-binding proteins from human SK-Hep cells and bovine liver plasma membranes. J. Biol. Chem. 270, 1807-1816 [DOI] [PubMed] [Google Scholar]
- Boensch C., Huang S. S., Connolly D. T., Huang J. S. (1999). Cell surface retention sequence binding protein-1 interacts with the v-sis gene product and platelet-derived growth factor beta-type receptor in simian sarcoma virus-transformed cells. J. Biol. Chem. 274, 10582-10589 [DOI] [PubMed] [Google Scholar]
- Cao R., Bjorndahl M. A., Religa P., Clasper S., Garvin S., Galter D., Meister B., Ikomi F., Tritsaris K., Dissing S., et al. (2006). PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 6, 333-345 [DOI] [PubMed] [Google Scholar]
- Cao Y. (2005). Direct role of PDGF-BB in lymphangiogenesis and lymphatic metastasis. Cell Cycle 4, 228-230 [DOI] [PubMed] [Google Scholar]
- Carmeliet P., Ng Y. S., Nuyens D., Theilmeier G., Brusselmans K., Cornelissen I., Ehler E., Kakkar V. V., Stalmans I., Mattot V., et al. (1999). Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat. Med. 5, 495-502 [DOI] [PubMed] [Google Scholar]
- Dejana E., Orsenigo F., Lampugnani M. G. (2008). The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 121, 2115-2122 [DOI] [PubMed] [Google Scholar]
- Eccles S., Paon L., Sleeman J. (2007). Lymphatic metastasis in breast cancer: importance and new insights into cellular and molecular mechanisms. Clin. Exp. Metastasis 24, 619-636 [DOI] [PubMed] [Google Scholar]
- Esser S., Lampugnani M. G., Corada M., Dejana E., Risau W. (1998). Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J. Cell Sci. 111, 1853-1865 [DOI] [PubMed] [Google Scholar]
- Ferrara N. (2004). Vascular endothelial growth factor: basic science and clinical progress. Endocr. Rev. 25, 581-611 [DOI] [PubMed] [Google Scholar]
- Gavard J., Gutkind J. S. (2006). VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 8, 1223-1234 [DOI] [PubMed] [Google Scholar]
- Hartsock A., Nelson W. J. (2008). Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 1778, 660-669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Rajantie I., Pajusola K., Jeltsch M., Holopainen T., Yla-Herttuala S., Harding T., Jooss K., Takahashi T., Alitalo K. (2005). Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 65, 4739-4746 [DOI] [PubMed] [Google Scholar]
- Hellberg C., Ostman A., Heldin C.-H. (2010). PDGF and vessel maturation. Recent Results Cancer Res. 180, 103-114 [DOI] [PubMed] [Google Scholar]
- Houck K. A., Ferrara N., Winer J., Cachianes G., Li B., Leung D. W. (1991). The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol. Endocrinol. 5, 1806-1814 [DOI] [PubMed] [Google Scholar]
- Huang S. S., Tang F. M., Huang Y. H., Liu I.-H., Hsu S. C., Chen S. T., Huang J. S. (2003). Cloning, expression, characterization, and role in autocrine cell growth of cell surface retention sequence binding protein-1. J. Biol. Chem. 278, 43855-43869 [DOI] [PubMed] [Google Scholar]
- Huang S. S., Liu I.-H., Smith T., Shah M. R., Johnson F. E., Huang J. S. (2006). CRSBP-1/LYVE-1-null mice exhibited identifiable morphological and functional alterations of lymphatic capillary vessels. FEBS Lett. 580, 6259-6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. G. (2003). The lymphatics revisited: new perspectives from the hyaluronan receptor LYVE-1. Trends Cardiovasc. Med. 13, 1-7 [DOI] [PubMed] [Google Scholar]
- Ji R. C. (2009). Lymph node lymphangiogenesis: a new concept for modulating tumor metastasis and inflammatory process. Histol. Histopathol. 24, 377-384 [DOI] [PubMed] [Google Scholar]
- Joukov V., Pajusola K., Kaipainen A., Chilov D., Lahtinen I., Kukk E., Saksela O., Kalkkinen N., Alitalo K. (1996). A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 15, 290-298 [PMC free article] [PubMed] [Google Scholar]
- Joukov V., Sorsa T., Kumar V., Jeltsch M., Claesson-Welsh L., Cao Y., Saksel O., Kalkkinen N., Alitalo K. (1997). Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 16, 3898-3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jussila L., Alitalo K. (2002). Vascular growth factors and lymphangiogenesis. Physiol. Rev. 82, 673-700 [DOI] [PubMed] [Google Scholar]
- Knudsen K. A., Soler A. P., Johnson K. R., Wheelock M. J. (1995). Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J. Cell Biol. 130, 67-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko M., Rönnstrand L., Heldin C.-H., Loubtchenkov M., Gazit A., Levitzki A., Böhmer F. D. (1997). Phosphorylation site-specific inhibition of platelet-derived growth factor beta-receptor autophosphorylation by the receptor blocking tyrphostin AG1296. Biochemistry 36, 6260-6269 [DOI] [PubMed] [Google Scholar]
- LaRochelle W. J., May-Siroff M., Robbins K. C., Aaronson S. A. (1991). A novel mechanism regulating growth factor association with the cell surface: identification of a PDGF retention domain. Genes Dev. 5, 1191-1199 [DOI] [PubMed] [Google Scholar]
- Leak L. V. (1976). The structure of lymphatic capillaries in lymph formation. Fed. Proc. 35, 1863-1871 [PubMed] [Google Scholar]
- Lokeshwar V. B., Huang S. S., Huang J. S. (1990). Intracellular turnover, novel secretion, and mitogenically active intracellular forms of v-sis gene product in simian sarcoma virus-transformed cells. Implications for intracellular loop autocrine transformation. J. Biol. Chem. 265, 1665-1675 [PubMed] [Google Scholar]
- Ostman A., Andersson M., Betsholtz C., Westermark B., Heldin C.-H. (1991). Identification of a cell retention signal in the B-chain of platelet-derived growth factor and in the long splice version of the A-chain. Cell Regul. 2, 503-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterness I. G., Moore P. F. (1988). Carregeenan foot edema test. Methods Enzymol. 162, 320-327 [DOI] [PubMed] [Google Scholar]
- Piedra J., Miravet S., Castaño J., Pálmer H. G., Heisterkamp N., García de Herreros A., Duñach M. (2003). p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin interaction. Mol. Cell. Biol. 23, 2287-2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M. D., Barbero S., Cheresh D. A. (2005). Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J. Biol. Chem. 280, 31906-31912 [DOI] [PubMed] [Google Scholar]
- Prevo R., Banerji S., Ferguson D. J., Clasper S., Jackson D. G. (2001). Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J. Biol. Chem. 276, 19420-19430 [DOI] [PubMed] [Google Scholar]
- Robbiani D. F., Finch R. A., Jager D., Muller W. A., Sartorelli A. C., Randolph G. J. (2000). The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell 103, 757-768 [DOI] [PubMed] [Google Scholar]
- Skobe M., Detmar M. (2000). Structure, function, and molecular control of the skin lymphatic system Expression of vascular endothelial growth factor induces an invasive phenotype in human squamous cell carcinomas. J. Investig. Dermatol. Symp. Proc. 5, 14-19 [DOI] [PubMed] [Google Scholar]
- Teriete P., Banerji S., Noble M., Blundell C. D., Wright A. J., Pickford Lowe E., Mahoney D. J., Tammi M. I., Kahmann J. D., Campbell I. D., et al. (2004). Structure of the regulatory hyaluronan binding domain in the inflammatory leukocyte homing receptor CD44. Mol. Cell 13, 483-496 [DOI] [PubMed] [Google Scholar]
- Vincent L., Kermani P., Young L. M., Cheng J., Zhang F., Shido K., Lam G., Bompais-Vincent H., Zhu Z., Hicklin D. J., et al. (2005). Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling. J. Clin. Invest. 115, 2992-3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent P. A., Xiao K., Buckley K. M., Kowalczyk A. P. (2004). VE-cadherin: adhesion at arm's length. Am. J. Physiol. Cell Physiol. 286, C987-C997 [DOI] [PubMed] [Google Scholar]
- Wise L. E., Cannavacciulo R., Cravatt B. F., Martin B. F., Lichtman A. H. (2007). Evaluation of fatty acid amides in the carrageenan-induced paw edema model. Neuropharmacology 54, 181-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.