Abstract
Cell migration during vascular remodelling is regulated by crosstalk between growth factor receptors and integrin receptors, which together coordinate cytoskeletal and motogenic changes. Here, we report extracellular matrix (ECM)-directed crosstalk between platelet-derived growth factor receptor (PDGFR)-β and α5β1-integrin, which controls the migration of mesenchymal stem (stromal) cells (MSCs). Cell adhesion to fibronectin induced α5β1-integrin-dependent phosphorylation of PDGFR-β in the absence of growth factor stimulation. Phosphorylated PDGFR-β co-immunoprecipitated with α5-integrin and colocalised with α5β1-integrin in the transient tidemarks of focal adhesions. Adhesion to fibronectin also strongly potentiated PDGF-BB-induced PDGFR-β phosphorylation and focal adhesion kinase (FAK) activity, in an α5β1-integrin-dependent manner. PDGFR-β-induced phosphoinositide 3-kinase (PI3K) and Akt activity, actin reorganisation and cell migration were all regulated by fibronectin and α5β1-integrin. This synergistic relationship between α5β1-integrin and PDGFR-β is a fundamental determinant of cell migration. Thus, fibronectin-rich matrices can prime PDGFR-β to recruit mesenchymal cells at sites of vascular remodelling.
Key words: Platelet-derived growth factor receptor-β, α5β1-Integrin, Fibronectin, Mesenchymal stem cell, Signalling, Migration
Introduction
Signalling through the receptor tyrosine kinase (RTK) platelet-derived growth factor receptor (PDGFR)-β is essential for the migration and differentiation of cells during vascular development (Yancopoulos et al., 2000; Betsholtz et al., 2001; Kinner et al., 2002; Lindblom et al., 2003; Ball et al., 2007; Andrae et al., 2008). Knockout of the genes encoding PDGFR-β or PDGF-B in mice causes death during late embryonic stages from widespread microvascular bleedings caused by deficient mural cell recruitment (Lindahl et al., 1997; Hellström et al., 1999; French et al., 2008). PDGF-BB, the main growth factor ligand of PDGFR-β, is a potent stimulant of smooth muscle cell (SMC) recruitment during neointimal hyperplasia following vascular injury (Andrae et al., 2008). PDGFR signalling has also emerged as a predominant pathway in recruitment of adult perivascular mesenchymal stem cells (MSCs), which play crucial roles in angiogenesis, wound repair and tissue regeneration (Ferrari et al., 1998; Abedin et al., 2004; Fiedler et al., 2004; Tepper et al., 2005; Ball et al., 2007).
Dimerisation of PDGFR-β, induced by ligating growth factor dimers, stimulates autophosphorylation of specific tyrosine residues within its cytoplasmic domain (Kelly et al., 1991). PDGFR-β is mainly activated by PDGF-BB, but also by PDGF-DD and vascular endothelial growth factor (VEGF)-A (Fredriksson et al., 2004; Ball et al., 2007). Autophosphorylation provides docking sites for downstream signal transduction molecules (Kazlauskas and Cooper, 1989), especially phosphoinositide 3-kinase (PI3K), which mediates actin reorganisation and migration, phospholipase Cγ (PLCγ), which stimulates cell growth and motility, and Src family tyrosine kinases, which influence cell proliferation (Heldin et al., 1998; Jiménez et al., 2000; Tallquist and Kazlauskas, 2004; Andrae et al., 2008). Signalling by RTKs such as PDGFR-β is not only regulated by growth factors but also by functional collaboration with integrins (Eliceiri, 2001; Yamada and Even-Ram, 2002; Giancotti and Tarone, 2003; Streuli and Akhtar, 2009). Integrins are αβ heterodimeric cell-surface receptors that act as a transmembrane link between extracellular matrix (ECM) ligands and the actin cytoskeleton. They direct inside-out and outside-in signalling that regulates numerous cellular responses, including survival, growth, migration and differentiation (Hynes, 1992; Danen and Sonnenberg, 2003; Askari et al., 2009). β1- and αVβ3-integrins can influence PDGFR-β activity (Sundberg and Rubin, 1996; Schneller et al., 1997; Woodard et al., 1998; Borges et al., 2000; Minami et al., 2007; Amano et al., 2008; Zemskov et al., 2009), and integrin-linked kinase (ILK) can control SMC migration in response to PDGF (Esfandiarei et al., 2010). However, the molecular mechanisms underlying crosstalk between PDGFR-β and integrins, and how they coordinate cell recruitment, remain obscure.
We have discovered a fundamental ECM-specific receptor crosstalk mechanism that controls MSC migration. Adhesion to fibronectin, through α5β1-integrin, specifically induced MSC migration by activating PDGFR-β signalling in the absence of growth factor stimulation. Fibronectin also strongly potentiated growth-factor-mediated receptor activation in an α5β1-integrin-dependent manner. Phosphorylated PDGFR-β appeared in ruffles at the leading edge of migratory cells and transiently colocalised with α5β1-integrin in the tidemarks of focal adhesions. Corresponding focal adhesion kinase (FAK)-dependent PI3K activity, actin reorganisation, membrane ruffling and MSC migration all exhibited fibronectin- and α5β1-integrin-dependence. This synergistic relationship between α5β1-integrin and PDGFR-β is thus a fundamental determinant of mesenchymal cell migration. Fibronectin-rich matrices might therefore act to prime PDGFR-β to recruit mesenchymal cells to sites of vascular remodelling.
Results
Adhesion to ECM induces PDGFR tyrosine phosphorylation
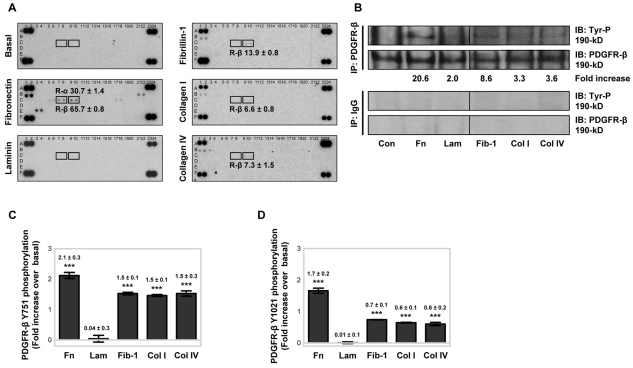
To evaluate how adhesion to ECM regulates PDGFR activation in MSCs, tyrosine phosphorylation of PDGFR-α and PDGFR-β was examined in serum-free conditions, after plating onto fibronectin, laminin, fibrillin-1 PF8 [an Arg-Gly-Asp (RGD)-containing fragment that engages the α5β1-integrin] (Bax et al., 2007; Cain et al., 2005), collagen type I or collagen type IV (all at 10 μg/ml) for 90 minutes. A human array for phosphorylated RTKs (Fig. 1A), containing 42 different immobilised anti-RTK antibodies, was utilised; this allowed the simultaneous relative quantification of tyrosine phosphorylation levels for both PDGFR-α (array coordinates C7 and C8) and PDGFR-β (array coordinates C9 and C10) in the same cell lysate. MSCs plated onto a BSA control substrate (basal) for 90 minutes produced no detectable immunoreactivity for any RTKs, but control reactivity against phosphorylated tyrosine was positive. When MSCs were plated onto fibronectin for 90 minutes, PDGFR-α and PDGFR-β tyrosine phosphorylation was detected. Fibronectin stimulation also induced tyrosine phosphorylation of other RTKs, notably epidermal growth factor receptor (EGFR), EphA7, Axl and c-Ret. When MSCs were plated onto fibrillin-1 PF8, collagen type I or collagen type IV for 90 minutes, PDGFR-β phosphorylation was induced at lower levels compared with that on fibronectin, but no PDGFR-α phosphorylation was detected. Fibrillin-1 PF8, and collagen types I and IV, also induced tyrosine phosphorylation of EGFR (array coordinates B1 and B2). By contrast, MSCs plated onto laminin for 90 minutes showed no detectable RTK phosphorylation, similar to that with MSCs plated onto BSA. The percentage of MSCs adhering to the different ECM substrates after 90 minutes was similar (supplementary material Fig. S1A), indicating that the ECM-induced increases in RTK phosphorylation was not due to differences in cell attachment.
Fig. 1.
ECM-induced tyrosine phosphorylation of PDGFR-β. (A) Human phosphorylated RTK arrays were used to examine ECM-induced RTK phosphorylation levels in MSC lysates. PDGFR-α (R-α; coordinates C7 and C8) and PDGFR-β (R-β; coordinates C9 and C10), respectively, were examined in MSC lysates, taken at 90 minutes, from BSA-coated wells or wells coated with 10 μg/ml fibronectin, laminin, fibrillin-1 (PF8), collagen type I or collagen type IV. Each array was identically exposed to detection reagents and film. Quantitative analysis was evaluated by densitometry with phosphorylated PDGFR duplicate RTK spots, normalised against phosphotyrosine-positive control spots [coordinates (A1, A2), (A23, A24), (F1, F2), (F23, F24)], and is represented as the fold increase above that with BSA control substrate (±s.d.) for duplicate spots. A representative example of two independent experiments is shown for each array analysis. (B) Immunoprecipitation (IP) analysis of the levels of PDGFR-β tyrosine phosphorylation was carried out using lysates from cells plated onto BSA control (Con) substrate or 10 μg/ml immobilised fibronectin (Fn), laminin (Lam), fibrillin-1 (PF8) (Fib-1), collagen type I (Col I) or collagen type IV (Col IV) in serum-free conditions for 90 minutes at 37°C. PDGFR-β was isolated from equivalent MSC lysates by IP analysis using anti-PDGFR-β with anti-rabbit IgG antibodies as a control, then tyrosine phosphorylation was detected by immunoblotting (IB) using an antibody against phosphorylated tyrosine (Tyr-P) (supplementary material Table S1). Membranes were re-probed with anti-PDGFR-β antibody, as a loading control. Quantitative analysis was evaluated by densitometry with Tyr-P normalised to total PDGFR-β and is represented as the fold increase above that with BSA control substrate. A representative example of two independent experiments is shown. ECM-induced PDGFR-β (C) Y751 and (D) Y1021 PDGFR-β phosphorylation was determined using ELISAs for phosphorylated PDGFR-β, with all conditions normalised to the total amount of PDGFR-β. MSCs were plated onto 10 μg/ml immobilised ECM proteins in serum-free conditions for 90 minutes at 37°C. PDGFR-β phosphorylation is represented as the fold increase above the control BSA-induced PDGFR-β tyrosine phosphorylation, against which ECM proteins were compared (***P<0.001 by one-way ANOVA). Experiments were performed in triplicate, on the same microtitre plate, and at least three times. Data are means±s.d. for at least three independent experiments.
ECM-induced PDGFR-β tyrosine phosphorylation in serum-free conditions was also examined by immunoprecipitation and immunoblotting, normalised to the total amount of PDGFR-β (Fig. 1B). Fibronectin induced the greatest PDGFR-β tyrosine phosphorylation activity, but cells cultured on fibrillin-1 PF8 also exhibited an increase in the level of phosphorylated PDGFR-β. By contrast, MSC adhesion to laminin or collagen types I or IV had little effect on PDGFR-β activity compared with that in cells on the BSA control substrate.
To confirm that adhesion to ECM in serum-free conditions differentially activates PDGFR-β, the phosphorylation status of specific PDGFR-β tyrosine residues was examined using immunoassays. Analysis of PDGFR-β tyrosine phosphorylation revealed that plating MSCs onto fibronectin, fibrillin-1 PF8 and collagen types I or IV for 90 minutes all triggered phosphorylation of Y751 (Fig. 1C) and Y1021 (Fig. 1D) above that in the basal BSA control. MSCs plated onto fibronectin had the greatest level of PDGFR-β Y751 and Y1021 phosphorylation. By contrast, laminin had no more of an effect on PDGFR-β Y751 and Y1021 phosphorylation than exposure to BSA. Because PDGFR-β phosphorylation was ECM-dependent, we checked whether the abundance of PDGFR-β, at protein and mRNA levels, was altered following the adhesion of MSCs to ECM ligands for 90 minutes in serum-free conditions. Immunoblotting, reverse-transcription-PCR (RT-PCR) and quantitative real-time PCR (qPCR) revealed that PDGFR-β expression was not increased by adhesion to the ECM over this timeframe (supplementary material Fig. S1B). RT-PCR and qPCR assays also revealed only trace levels of endogenous PDGF-BB, PDGF-DD and VEGF-A transcripts, which were not upregulated by plating MSCs onto fibronectin for 90 minutes (supplementary material Fig. S1C). Enzyme-linked immunosorbent assays (ELISAs) showed that adhesion of MSCs to fibronectin or laminin did not induce secretion of PDGF-BB or VEGF-A above that on the BSA control substrate (supplementary material Fig. S1D). In addition, treatment of MSCs with a PDGFR-β-neutralising antibody (AF385), which prevents PDGF-BB from binding and activating the receptor, failed to block fibronectin-induced Y751 or Y1021 PDGFR-β activation (see Fig. 5A).
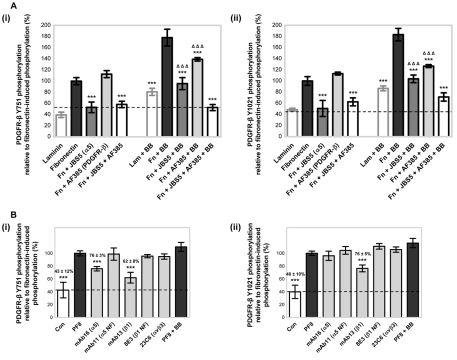
Fig. 5.
Fibronectin enhances PDGF-BB-induced PDGFR-β tyrosine phosphorylation. (A) The effects of fibronectin (Fn) or laminin (Lam) on PDGF-BB (BB)-stimulated PDGFR-β (i) Y751 and (ii) Y1021 phosphorylation were examined using an ELISA for phosphorylated PDGFR-β with all conditions normalised to the total amount of PDGFR-β. MSCs in serum-free conditions were plated onto 10 μg/ml immobilised fibronectin or laminin in the absence or presence of 50 ng/ml PDGF-BB for 90 minutes at 37°C. The broken line indicates basal tyrosine phosphorylation of PDGFR-β induced by BSA. The involvement of integrin subunit α5 in fibronectin-regulated PDGF-BB stimulation of PDGFR-β (i) Y751 or (ii) Y1021 phosphorylation levels was also determined. MSCs in serum-free conditions, treated with either 10 μg/ml anti-integrin-α5 inhibitory antibody (JBS5) or anti-PDGFR-β neutralisation antibody (AF385), or both, were plated onto 10 μg/ml immobilised fibronectin in the absence or presence of 50 ng/ml PDGF-BB for 90 minutes at 37°C. Antibody specificity is shown in brackets next to the antibody name. Conditions are expressed relative to fibronectin-induced PDGFR-β (i) Y751 or (i) Y1021 phosphorylation (100%) in the absence of PDGF-BB, and are compared against the respective fibronectin-induced phosphorylation of PDGFR-β in the absence or presence of PDGF-BB (***P<0.001 by one-way ANOVA), or in the presence of both JBS5 and AF385 (ΔΔΔP<0.001 by one-way ANOVA). Laminin-induced phosphorylation of PDGFR-β in the presence of PDGF-BB is compared with that of laminin in the absence of PDGF-BB (***P<0.001 by one-way ANOVA). Experiments were performed in triplicate, on the same microtitre plate, and at least three times. Data are means±s.d. for at least three independent experiments. (B) The effects of inhibitory anti-integrin antibodies and PDGF-BB on fibrillin-1 PF8-induced PDGFR-β (i) Y751 and (ii) Y1021 phosphorylation levels were determined using an ELISA for phosphorylated PDGFR-β with all conditions normalised to the total amount of PDGFR-β. MSCs in serum-free conditions were plated onto 10 μg/ml immobilised fibrillin-1 (PF8) in the presence of 10 μg/ml anti-integrin inhibitory mAbs or 50 ng/ml PDGF-BB for 90 minutes at 37°C. Antibodies were specific for the integrin α5 subunit (mAb16, mAb11), integrin β1 subunit (mAb13, 8E3) and αvβ3-integrin (23C6). Antibody specificity is indicated in brackets next to the antibody name; NF denotes a non-functional antibody, as a control. All conditions are expressed relative to PF8-induced PDGFR-β (i) Y751 and (ii) Y1021 phosphorylation (100%) in the absence of antibody (***P<0.001 by one-way ANOVA). Control (Con; broken line) indicates the basal tyrosine phosphorylation of PDGFR-β induced by BSA. All experiments were performed in triplicate on the same microtitre plate at least three times. Data are means±s.d of at least three independent experiments.
α5β1-integrin mediates fibronectin-induced PDGFR-β tyrosine phosphorylation
Integrins are the principal receptors for ECM ligands, serving as transmembrane links between extracellular adhesion molecules and the intracellular actin cytoskeleton (Askari et al., 2009). As the integrin expression profile of a cell will dictate its interactions with the ECM, the expression of integrins by cultured MSCs was analysed by flow cytometry (supplementary material Fig. S2A). Integrin subunits αv, α3, α5 and β1, and the αvβ5-integrin, were all prominently expressed by MSCs, whereas the αvβ3-integrin, and integrin subunits α1, α2, α4, α6 and β4, had a lower level of expression. In comparison with the isotype-matched IgG controls, α9β1-integrin and αvβ6-integrin were not detectable.
Having identified the integrins expressed by MSCs, we investigated which of these receptors mediated adhesion to two contrasting ECM proteins: fibronectin, which induced the greatest level of PDGFR-β phosphorylation in MSCs, and laminin, which induced no substantial PDGFR-β phosphorylation (see Fig. 1). Adhesion assays were performed for 90 minutes in the presence of function-blocking monoclonal antibodies (mAbs) against integrin subunits and dimeric integrin receptors. MSC adhesion to fibronectin was greatly reduced by the function-blocking anti-β1-integrin antibody (mAb13) and was also substantially inhibited by each of three different function-blocking antibodies against the integrin subunit α5 (mAb16, JBS5 and P1D6) (supplementary material Fig. S2B). By contrast, non-inhibitory antibodies against the integrin subunits β1 (8E3) and α5 (mAb11) had no effect on attachment. MSC adhesion to fibronectin was partially reduced by integrin function-blocking antibodies against the integrin subunits α4 (HP2/1) or αv (17E6), and the αvβ3-integrin (LM609 and 23C6). As expected, MSC adhesion to laminin was unaffected by function-blocking antibodies against the integrin subunits α5 or αv, and the αvβ3-integrin (data not shown); however, MSC attachment to laminin was almost completely blocked by the inhibitory anti-β1-integrin antibody (mAb13) (supplementary material Fig. S2C). MSC adhesion to laminin was also substantially reduced by the inhibitory anti-α1-integrin antibody (FB12) and partially reduced by the inhibitory anti-α6-integrin antibody (GoH3), but the non-inhibitory anti-β1-integrin antibody (8E3) had little effect.
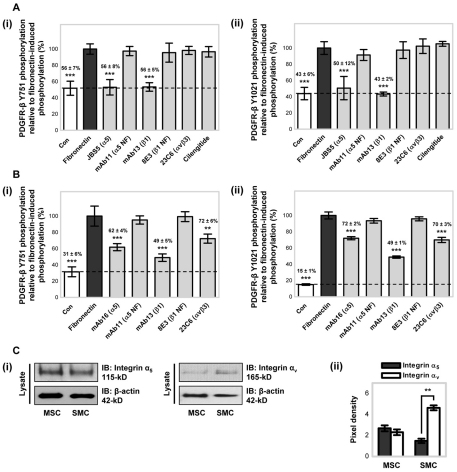
To investigate whether α5β1- and αvβ3-integrin, which supported MSC adhesion to fibronectin, modulated fibronectin-induced PDGFR-β activity, PDGFR-β Y751 and Y1021 phosphorylation levels were assayed in the presence of function-blocking antibodies against the integrin subunits α5 and β1, and the αvβ3-integrin, or with the cyclic RGD peptide cilengitide that inhibits αv integrins (Fig. 2A). MSCs plated onto fibronectin for 90 minutes in the presence of function-blocking antibodies against the α5 (JBS5) or β1 (mAb13) integrin subunits exhibited a substantial reduction in the level of PDGFR-β Y751 and Y1021 phosphorylation compared with the level of fibronectin-induced phosphorylation (set at 100%). By contrast, the presence of inhibitory antibodies against αvβ3-integrin (23C6), cilengitide, non-functional anti-α5-integrin antibody (mAb11), or non-inhibitory anti-β1-integrin antibody (8E3), resulted in no substantial change in the level of PDGFR-β phosphorylation. As the same concentration of an anti-αvβ3-integrin antibody (23C6) was shown to reduce MSC adhesion to fibronectin (see supplementary material Fig. S2B), failure to inhibit PDGFR-β phosphorylation was not due to the concentration of antibody used. MSCs plated onto laminin for 90 minutes had no detectable change in the level of PDGFR-β Y751 or Y1021 phosphorylation compared with that of cells on the control BSA substrate, in either the absence or presence of blocking antibodies against the integrin α5 or β1 subunits or the αvβ3-integrin (data not shown). Thus, fibronectin-induced phosphorylation of PDGFR-β in MSCs is dependent upon the α5 and β1 integrin subunits, confirming the fibronectin receptor α5β1-integrin, but not αvβ3-integrin, as the mediator of fibronectin-induced ligand-independent PDGFR-β phosphorylation.
Fig. 2.
Fibronectin-induced integrin-mediated PDGFR-β tyrosine phosphorylation. The effects of inhibitory anti-integrin antibodies on fibronectin-induced PDGFR-β (i) Y751 and (ii) Y1021 phosphorylation were determined in (A) MSCs or (B) SMCs, using ELISAs for phosphorylated PDGFR-β, with all conditions normalised to the total amount of PDGFR-β. Cells in serum-free conditions were plated onto 10 μg/ml immobilised fibronectin for 90 minutes in the presence of 10 μg/ml anti-integrin inhibitory mAbs or 10 μM integrin-αv-inhibiting peptide cilengitide. Antibodies were specific for the integrin α5 subunit (JBS5, mAb11), the integrin β1 subunit (mAb13, 8E3) and αvβ3-integrin (23C6). Antibody specificity is indicated in brackets next to the antibody name; NF denotes a non-functional antibody, as a control. All conditions are expressed relative to fibronectin-induced PDGFR-β (i) Y751 and (ii) Y1021 phosphorylation (100%) in the absence of antibody (**P<0.01, ***P<0.001 by one-way ANOVA). Control (Con; broken line) indicates the basal tyrosine phosphorylation of PDGFR-β induced by BSA. All experiments were performed in triplicate, on the same microtitre plate, and at least three times. Data are means±s.d. for at least three independent experiments. (C) Expression of the integrin α5 and integrin αv subunits was examined in MSCs and SMCs plated onto 10 μg/ml immobilised fibronectin, in serum-free conditions, for 90 minutes at 37°C. (i) Protein expression was detected by immunoblotting (IB) equal amounts (10 μg) of cell lysates using anti-integrin-α5 or anti-integrin-αv antibodies. Membranes were reprobed with anti-β-actin antibody, as a loading control. A representative example of two independent experiments is shown. (ii) Quantitative analysis was evaluated by densitometry with data normalised to the level of β-actin. Data are represented as the mean pixel density (±s.d.) for two independent experiments (**P<0.01 by one-way ANOVA).
To determine whether α5β1-integrin-dependent fibronectin-induced phosphorylation of PDGFR-β is a generalised mechanism, the effect of integrins in modulating fibronectin-induced PDGFR-β activity was examined in human aortic SMCs (Fig. 2B). In a manner similar to MSCs, SMCs plated onto fibronectin for 90 minutes had a substantial increase in PDGFR-β Y751 and Y1021 phosphorylation compared with cells on BSA control substrate. Furthermore, SMCs on fibronectin in the presence of function-blocking antibodies against the α5 (mAb16) or β1 (mAb13) integrin subunits exhibited a substantial reduction in the level of PDGFR-β Y751 and Y1021 phosphorylation compared with the fibronectin-induced phosphorylation (set at 100%). However, in contrast with MSCs, fibronectin-induced PDGFR-β activity in SMCs was also reduced in the presence of an inhibitory antibody against the αvβ3-integrin (23C6) integrin. Thus, fibronectin-induced phosphorylation of PDGFR-β in SMCs is mediated by α5β1- and αvβ3-integrin. As fibronectin-induced PDGFR-β phosphorylation was differentially mediated by integrins in different cell types, the abundance of the integrin subunits α5 and αv at the protein level was determined following the adhesion of MSCs or SMCs to fibronectin for 90 minutes in serum-free conditions (Fig. 2C). Immunoblotting revealed that integrin α5 protein expression was similar in MSCs and SMCs, whereas integrin αv was more abundantly expressed in SMCs compared with its expression in MSCs.
Fibronectin induces the association of PDGFR-β and α5β1-integrin in serum-free conditions
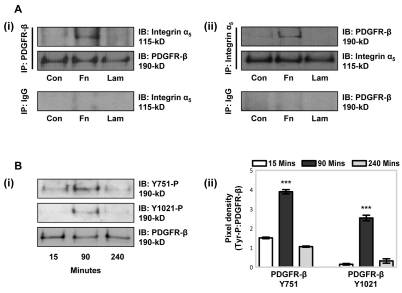
Crosstalk mechanisms between PDGFR-β and α5β1-integrin in MSCs might involve physical interactions between the integrin and PDGFR-β receptors and/or intracellular signalling events leading to PDGFR-β activation. Co-immunoprecipitation experiments were conducted in serum-free conditions to examine receptor associations in MSCs plated onto fibronectin or laminin for 90 minutes (Fig. 3A). Co-immunoprecipitation of PDGFR-β and integrin subunit α5 occurred in lysates derived from MSCs plated onto fibronectin. By contrast, no association of PDGFR-β and integrin subunit α5 was detected in lysates derived from MSCs plated onto laminin.
Fig. 3.
Fibronectin-induced association of PDGFR-β and α5β1-integrin. (A) The association of PDGFR-β with integrin subunit α5 was examined by immunoprecipitation (IP). MSCs in serum-free conditions were incubated on tissue culture plastic, as a control (Con), or on 10 μg/ml immobilised fibronectin (Fn) or laminin (Lam), for 90 minutes at 37°C. (i) PDGFR-β was isolated from equivalent MSC lysates by IP using anti-PDGFR-β antibody, with anti-rabbit IgG antibody as a control, then integrin subunit α5 association was detected by immunoblotting (IB) using an antibody against integrin α5. Membranes were re-probed with anti-PDGFR-β as a loading control. (ii) Integrin subunit α5 was isolated by IP using antibodies against integrin α5 or IgG, as a control, then PDGFR-β association was detected by IB analysis using anti-PDGFR-β antibody. Membranes were reprobed with an antibody against integrin α5 as a loading control. A representative example of three independent experiments is shown. (B) Immunoblotting of PDGFR-β Y751 or Y1021 phosphorylation was carried out using MSC lysates, taken at 15, 90 or 240 minutes, from wells coated with 10 μg/ml fibronectin. (i) Levels of PDGFR-β phosphorylation were detected in equal (10 μg) amounts of cell lysates using antibodies against phosphorylated PDGFR-β Y751 or Y1021, or anti-PDGFR-β antibody, as a loading control. A representative example of two independent experiments is shown. (ii) Quantitative analysis, evaluated by densitometry, with data normalised to the total amount of PDGFR-β. Data are means±s.d. for two independent experiments (***P<0.001 compared with other time points).
To determine whether the stimulatory effect of fibronectin on PDGFR-β activation was attributable to a physical interaction between the extracellular regions of PDGFR-β and α5β1-integrin, surface plasmon resonance (BIAcore) analysis was performed (supplementary material Fig. S3A,B). No direct interaction was detected between the extracellular domains of either PDGFR-β and α5β1-integrin using either immobilised PDGFR-β or immobilised α5β1-integrin, although positive controls PDGF-BB and fibronectin (50 kDa adhesion fragment) bound PDGFR-β and α5β1-integrin, respectively. However, both the extracellular domains of α5β1-integrin and PDGFR-β (Kd=19 nM) strongly bound immobilised heparin (supplementary material Fig. S3C), and fibronectin-induced co-immunoprecipitation of PDGFR-β and integrin subunit α5 was prevented by excess heparin and treatment with K5 heparan lyase (supplementary material Fig. S3D). Thus, cell surface heparan sulphate proteoglycans mediate the association between PDGFR-β and α5β1-integrin.
Fibronectin induces temporal phosphorylation of PDGFR-β and colocalisation with α5β1-integrin in serum-free conditions
Maximal fibronectin-induced Y751 and Y1021 PDGFR-β phosphorylation was detected at 90 minutes by immunoblotting, compared with that upon exposure for 15 or 240 minutes (Fig. 3B). Temporal phosphorylation of PDGFR-β was confirmed by phosphorylated RTK array analysis (supplementary material Fig. S4A). Fibronectin also induced tyrosine phosphorylation of other RTKs throughout the timecourse of 15, 90 and 240 minutes, notably PDGFR-α and EGFR, which were induced in a fibronectin-dependent manner (see Fig. 1A), but the phosphorylation remained constant over the time period examined (supplementary material Fig. S4A). Although the level of PDGFR-α phosphorylation was of lower magnitude compared with that of PDGFR-β phosphorylation under the same conditions, an ELISA for phosphorylated PDGFR-α showed that MSCs plated onto fibronectin for 90 minutes in the presence of function-blocking antibodies against integrin subunits α5 (JBS5), β1 (mAb13) or αv (17E6), or cilengitide, exhibited a substantial reduction in the level of PDGFR-α Y742 phosphorylation compared with the fibronectin-induced phosphorylation (set at 100%) (supplementary material Fig. S4B). Thus, whereas fibronectin–α5β1-integrin engagement mediates PDGFR-β activity in MSCs, fibronectin-induced phosphorylation of PDGFR-α is mediated by α5β1-integrin and αv integrins.
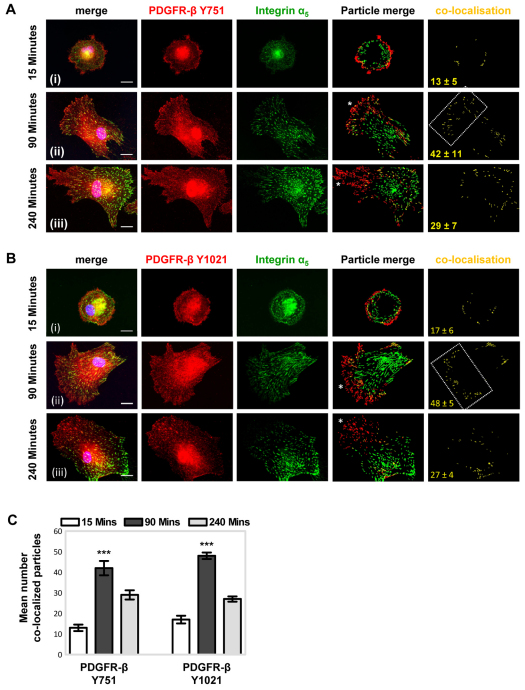
To investigate further the relationship between PDGFR-β and α5β1-integrin in MSCs following adhesion to fibronectin, the cellular distribution of phosphorylated PDGFR-β and integrin subunit α5 was examined by immunofluorescence microscopy. PDGFR-β phosphorylated on Y751 and Y1021 was detected at the cell periphery following a 15-minute incubation on fibronectin (Fig. 4A,Bi), whereas MSCs had not adhered to laminin at this timepoint (data not shown). After MSCs had been plated for 90 minutes on fibronectin (Fig. 4A,Bii), but not laminin (supplementary material Fig. S5A), PDGFR-β Y751 and Y1021 phosphorylation was detected at the leading edge and in a tidemark of integrin-α5-containing focal adhesions behind the leading edge. Fibronectin-induced colocalisation of phosphorylated PDGFR-β and integrin subunit α5 at 90 minutes was supported by colocalisation analysis (Fig. 4C). After 4 hours of incubation on fibronectin (Fig. 4A,Biii), PDGFR-β phosphorylated on Y751 and Y1021 was detected predominantly at the leading edge of MSCs, whereas integrin subunit α5 was present towards their trailing edge. Thus, in serum-free conditions, fibronectin first induces PDGFR-β phosphorylation at the leading edge, followed by transient colocalisation of phosphorylated PDGFR-β with α5β1-integrin in focal adhesions, then dissociation and translocation of α5β1-integrin towards the rear of the cells.
Fig. 4.
Fibronectin-induced temporal colocalisation of phosphorylated PDGFR-β with α5β1-integrin. Following adhesion to fibronectin, the cellular distribution of phosphorylated PDGFR-β and integrin subunit α5 was examined over time using immunofluorescence microscopy. MSCs in serum-free conditions were plated onto 10 μg/ml immobilised fibronectin for (i) 15 minutes, (ii) 90 minutes or (iii) 240 minutes, at 37°C, before fixation with paraformaldehyde. Representative examples of MSCs double-labelled for (A) Y751- or (B) Y1021-phosphorylated PDGFR-β (red) and integrin subunit α5 (green), and merged images, are shown. Nuclei appear blue owing to DAPI staining. To quantify receptor colocalisation, images were analysed using the ImageJ colocalisation plugin (see Materials and Methods). Merged particle analysis images are shown, with red and green channels having similar threshold values and the same particle size range (1–500 pixels), together with colocalisation events represented in yellow. The white asterisks highlight the leading edge and the boxed area highlights the transient tidemark of receptor colocalisation. The mean number of colocalised particles (±s.d.) derived from five different single-cell images is denoted in yellow and is represented graphically (C) (***P<0.001 compared with other timepoints). Images are representative of at least three independent experiments. Scale bar: 20 μm.
Fibronectin and PDGF-BB additively contribute to PDGFR-β tyrosine phosphorylation
Given that MSC adhesion to fibronectin in serum-free conditions was shown to induce ligand-independent phosphorylation of PDGFR-β and association with α5β1-integrin, we investigated the effect of fibronectin on PDGF-BB-stimulated PDGFR-β phosphorylation. PDGF-BB, the major ligand of PDGFR-β, is an important mitogen and chemoattractant for mesenchymal cells (Betsholtz et al., 2001). ELISA analysis demonstrated that, compared with ligand-independent fibronectin-induced PDGFR-β activity, exposure to 50 ng/ml PDGF-BB increased the levels of Y751 (Fig. 5Ai) and Y1021 (Fig. 5Aii) phosphorylation by 78% and 83%, respectively. The addition of PDGF-AA, which binds only the PDGFR-α homodimer, was used as a negative control and did not induce PDGFR-β activity above that induced by fibronectin (data not shown). MSCs cultured on laminin did not induce receptor phosphorylation above that with BSA, although PDGF-BB did induce a substantial increase in PDGFR-β activity (Fig. 5A). These results show that fibronectin and PDGF-BB both contribute to PDGFR-β phosphorylation, whereas on laminin PDGFR-β phosphorylation is only induced in the presence of PDGF-BB.
To confirm that the fibronectin-enhanced PDGF-BB stimulation of PDGFR-β Y751 and Y1021 phosphorylation in MSCs was α5β1-integrin-dependent, the effects of the function-blocking antibody against integrin subunit α5 (JBS5) and/or a neutralising anti-PDGFR-β (AF385) antibody, which inhibits receptor phosphorylation, were examined (Fig. 5A). In the absence of the PDGF-BB ligand, only JBS5 substantially reduced the level of PDGFR-β phosphorylation, to near the basal levels induced by BSA, whereas AF385 had no detectable effect. However, when MSCs plated onto fibronectin were stimulated with PDGF-BB, addition of either JBS5 or AF385 resulted in a substantial reduction in PDGFR-β Y751 and Y1021 phosphorylation levels, compared with the levels in MSCs in the absence of inhibitory antibodies. Phosphorylation of Y751 and Y1021 was further reduced when MSCs were cultured on fibronectin and stimulated with PDGF-BB in the presence of both JBS5 and AF385. Taken together, these antibody blocking experiments show that fibronectin α5β1-integrin-dependent regulation of PDGFR-β enhances PDGF-BB-stimulated PDGFR-β Y751 and Y1021 phosphorylation. We also found that PDGF-BB directly binds α5β1-integrin (supplementary material Fig. S3B), suggesting that receptor co-clusters of α5β1-integrin with PDGFR-β might enhance the interaction of PDGF-BB with its receptor. However, although PDGFR-β was also shown to be activated by the α5β1-integrin-binding fragment (PF8) of fibrillin-1 (see Fig. 1) in an α5β1-integrin-dependent (but not integrin αvβ3-dependent) manner (Fig. 5B), in contrast with fibronectin-enhanced PDGF-BB stimulation of PDGFR-β phosphorylation, PDGF-BB had no further effect on PDGFR-β Y751 and Y1021 phosphorylation when MSCs were cultured on PF8 (Fig. 5B).
PDGFR-β and α5β1-integrin crosstalk mediates MSC migration
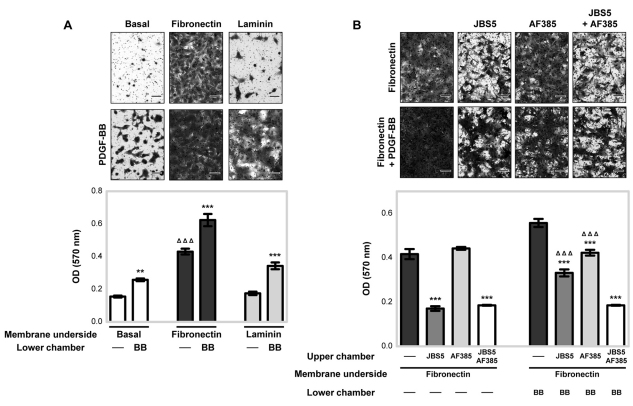
We next investigated the functional importance of the crosstalk between α5β1-integrin and PDGFR-β for MSC migration. In serum-free conditions, MSC migration in fibronectin-coated Boyden chambers was increased by ~2.8-fold above that on BSA-coated inserts, whereas laminin had no effect above that with the basal BSA control (Fig. 6A). PDGF-BB substantially increased MSC migration towards fibronectin, laminin and BSA above that induced by serum-free conditions (Fig. 6A). To confirm that the fibronectin-enhanced PDGF-BB stimulation of MSC migration was α5β1-integrin-dependent, the effects of JBS5 and/or AF385 antibody on MSC migration were examined (Fig. 6B). In the absence of PDGF-BB ligand, only JBS5 inhibited MSC migration, whereas the anti-PDGFR-β antibody had no detectable effect. MSCs exposed to PDGF-BB in the presence of JBS5 or AF385 resulted in a substantial reduction in MSC migration towards fibronectin, compared with the amount of migration in the absence of inhibitory antibodies. MSC migration towards fibronectin was further reduced upon exposure to PDGF-BB in the presence of both JBS5 and AF385. By contrast, incubation of MSCs with a function-blocking antibody against αvβ3-integrin (LM609) had no detectable effect on fibronectin-induced MSC migration in the absence or presence of PDGF-BB (supplementary material Fig. S5B). Thus, α5β1-integrin-dependent regulation of PDGFR-β enhances PDGF-BB-stimulated MSC migration towards fibronectin.
Fig. 6.
PDGFR-β and α5β1-integrin crosstalk mediates MSC migration towards fibronectin. (A) MSC migration towards fibronectin with or without PDGF-BB (BB) stimulation was evaluated using a Boyden chamber migration assay. BSA (basal) and laminin were used as control substrates. MSCs in serum-free conditions in the upper chamber were cultured for 4 hours at 37°C in Boyden chambers pre-coated on the underside with BSA as a basal control, or 10 μg/ml fibronectin or laminin, with or without 50 ng/ml PDGF-BB in the lower chamber. Conditions are compared against the respective MSC migration in the absence of PDGF-BB (**P<0.01; ***P<0.001 by one-way ANOVA), or against basal in the absence of PDGF-BB (ΔΔΔP<0.001 by one-way ANOVA). (B) The involvement of integrin subunit α5 in PDGF-BB-induced MSC migration towards fibronectin was also determined. MSCs in serum-free conditions in the upper chamber treated with either 10 μg/ml anti-integrin-α5 inhibitory antibody (JBS5), anti-PDGFR-β neutralisation antibody (AF385), or both, were cultured for 4 hours at 37°C in Boyden chambers pre-coated on the underside with 10 μg/ml fibronectin, with or without 50 ng/ml PDGF-BB in the lower chamber. Conditions are compared against the respective MSC migration in the absence or presence of PDGF-BB (***P<0.001 by one-way ANOVA), or in the presence of both JBS5 and AF385 (ΔΔΔP<0.001 by one-way ANOVA). All data are the mean (±s.d.) optical density (OD 570 nm) of stained migratory cells from two readings in an individual experiment, repeated three times. Images above each bar graph are representative of the migratory cells per field on the membrane underside of three independent experiments. Scale bar: 100 μm.
Fibronectin–α5β1-integrin engagement and FAK activation mediates PDGFR-β-induced PI3K activity and actin arrangement
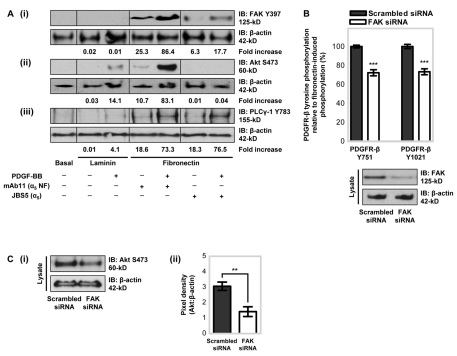
Having established that fibronectin- and α5β1-integrin-dependent regulation of PDGFR-β enhances PDGF-BB-stimulated PDGFR-β phosphorylation and MSC migration, we examined the involvement of specific intracellular signalling pathways. FAK is an important receptor-proximal link between PDGFR and integrin signalling pathways (Sieg et al., 2000). Therefore to determine whether FAK integrates the signalling pathways activated by both fibronectin–α5β1-integrin engagement and PDGFR-β, FAK activation at the major autophosphorylation Y397 site was examined by immunoblotting (Fig. 7Ai). In a manner similar to ECM-induced PDGFR-β phosphorylation, MSCs plated onto fibronectin for 90 minutes induced an increase in FAK Y397 phosphorylation, whereas MSCs plated onto laminin or BSA had no detectable FAK Y397 phosphorylation. MSCs stimulated with PDGF-BB showed markedly increased fibronectin-induced phosphorylation of FAK. By contrast, however, MSCs exposed to PDGF-BB when plated on laminin did not display an increase in FAK Y397 phosphorylation, suggesting that MSC adhesion to fibronectin, and engagement of α5β1-integrin, is necessary for PDGF-BB-induced FAK phosphorylation. To confirm that fibronectin-induced PDGF-BB-stimulated phosphorylation of FAK was α5β1-integrin-dependent, the effect of JBS5 was examined (Fig. 7Ai). In the absence or presence of PDGF-BB ligand, FAK phosphorylation was markedly reduced by adding JBS5. Furthermore, partial knockdown of FAK by small interfering RNA (siRNA) was sufficient to substantially reduce fibronectin-induced PDGFR-β Y751 and Y1021 phosphorylation (Fig. 7B). Thus, FAK is a mediator of fibronectin- and α5β1-integrin-dependent regulation of PDGFR-β.
Fig. 7.
α5β1-Integrin and FAK activation mediates PDGFR-β-induced PI3K activation. (A) MSCs in serum-free conditions treated with 10 μg/ml anti-integrin-α5 inhibitory antibody (JBS5) or non-functional (NF) anti-integrin-α5 antibody (mAb11), were plated onto tissue culture plastic, as a control (basal), or 10 μg/ml immobilised fibronectin or laminin in the absence or presence of 50 ng/ml PDGF-BB for 90 minutes at 37°C. Phosphorylation levels of (i) FAK, (ii) Akt and (iii) PLCγ-1 were detected in equal (10 μg) amounts of cell lysates by immunoblotting (IB) using antibodies against (i) phosphorylated FAK (Y397), (ii) phosphorylated Akt (S473) or (iii) phosphorylated PLCγ-1 (Y783). Membranes were reprobed with anti-β-actin antibody as a loading control. Quantitative analysis was performed by densitometry with (i) phosphorylated FAK, (ii) phosphorylated Akt or (iii) phosphorylated PLCγ-1 normalised to the level of β-actin. Data are the fold increase above that with basal control substrate. A representative example of two independent experiments is shown. (B) The effect of FAK siRNA knockdown on fibronectin-induced PDGFR-β Y751 and Y1021 phosphorylation levels was determined using an ELISA for phosphorylated PDGFR-β, with all conditions normalised to that with total PDGFR-β. MSCs transfected with 3 μg of siRNA FAK or scrambled siRNA, as a control, were plated onto 10 μg/ml immobilised fibronectin for 90 minutes at 37°C. Conditions are expressed relative to fibronectin-induced PDGFR-β Y751 or Y1021 phosphorylation (100%) in the presence of scrambled siRNA (***P<0.001 by one-way ANOVA). Experiments were performed in triplicate, on the same microtitre plate, and at least three times. Data are the means±s.d. for at least three independent experiments. The knockdown efficiency of the FAK siRNA as assessed by immunoblotting is shown below the graph. (C) The effect of FAK siRNA knockdown on fibronectin-induced Akt S473 phosphorylation levels was determined by immunoblotting. MSCs transfected with 3 μg of siRNA FAK or scrambled siRNA, as a control, were plated onto 10 μg/ml immobilised fibronectin for 90 minutes at 37°C. (i) Phosphorylation levels of Akt were detected in equal (10 μg) amounts of cell lysates using an antibody against phosphorylated Akt S473 (S473). The membrane was re-probed with anti-β-actin antibody as a loading control. A representative example of two independent experiments is shown. (ii) Quantitative analysis, as determined by densitometry, with the levels of phosphorylated Akt normalised to those of β-actin. Data are the means±s.d. for two independent experiments (**P<0.01 by Student's t-test). The knockdown efficiency of the FAK siRNA is shown in B.
As FAK was shown to mediate fibronectin-induced PDGFR-β Y751 and Y1021 phosphorylation, we examined the activity of PI3K and PLCγ, which are known to associate with these specific tyrosine residues within the PDGFR-β cytoplasmic domain, respectively. PI3K and PLCγ are important regulators of motogenic signal transduction (Kundra et al., 1994; Wennström et al., 1994), and cells expressing a PDGFR-β mutant lacking the PI3K-binding site are unable to migrate directionally towards PDGF (Ruusala et al., 1998). Phosphorylation of Akt at S473, as an indicator of PI3K activity, and phosphorylation of PLCγ-1 at Y783, was examined by immunoblotting. MSCs plated onto fibronectin for 90 minutes induced S473 phosphorylation of Akt (Fig. 7Aii) and Y783 phosphorylation of PLCγ-1 (Fig. 7Aiii). By contrast, MSCs plated onto laminin or BSA, displayed neither Akt nor PLCγ-1 phosphorylation. MSC stimulation with PDGF-BB markedly increased fibronectin-induced phosphorylation of both Akt and PLCγ-1. Fibronectin-induced S473 Akt phosphorylation in the absence or presence of PDGF-BB, was abolished by adding JBS5 (Fig. 7Aii), indicating that α5β1-integrin regulates PDGFR-β-ligand-induced PI3K activity in MSCs. By contrast, incubation of MSCs with JBS5 did not alter PLCγ-1 phosphorylation (Fig. 7Aiii). Furthermore, partial knockdown of FAK by siRNA was sufficient to substantially reduce fibronectin-induced Akt S473 phosphorylation levels (Fig. 7C). Thus, FAK is a mediator of fibronectin- and α5β1-integrin-dependent regulation of Akt-PI3K activity.
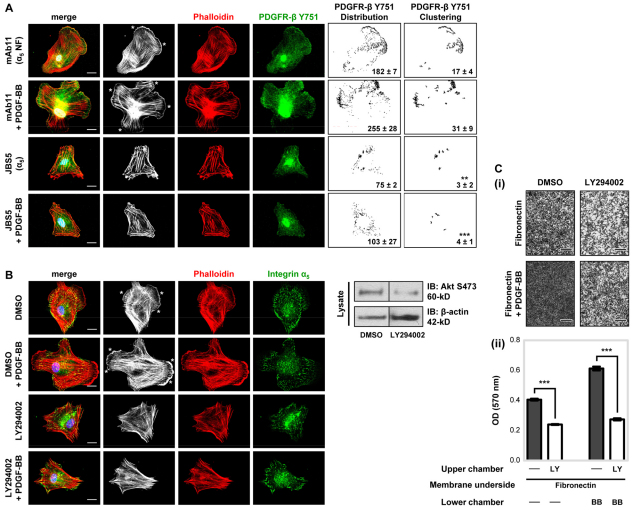
PDGF-BB induces cell migration in conjunction with marked reorganisation of actin filaments and the appearance of F-actin clusters at membrane protrusions (Meima et al., 2009), whereas mutant PDGF-stimulated cells expressing the non-phosphorylatable Tyr-to-Phe (Y751F) PDGFR-β lack ruffled edges (Wennström et al., 1994). Our finding that α5β1-integrin engagement of fibronectin regulates PDGFR-β-mediated Akt-PI3K activity in MSCs led us to examine the effect of α5β1-integrin inhibition on F-actin organisation and the localisation of Y751 phosphorylated PDGFR-β (Fig. 8A). Plating MSCs on fibronectin in serum-free conditions induced Y751 phosphorylated PDGFR-β clustering and membrane ruffling; these cellular changes were more pronounced in the presence of PDGF-BB, with intensely stained patches of actin at the rim of the cells. Inhibition of α5β1-integrin using the JBS5 function-blocking antibody substantially reduced the clustering of fibronectin-induced Y751-phosphorylated PDGFR-β and F-actin in membrane ruffles, both in the absence and presence of PDGF-BB (Fig. 8A). By contrast, when MSCs were plated onto laminin, Y751-phosphorylated PDGFR-β and F-actin clustering were only induced in the presence of PDGF-BB (supplementary material Fig. S5C).
Fig. 8.
PI3K mediates PDGFR-β-induced actin arrangement and migration. (A) The cellular distribution of F-actin and phosphorylated PDGFR-β Y751 was examined by immunofluorescence microscopy. MSCs in serum-free conditions treated with 10 μg/ml anti-integrin α5 inhibitory antibody (JBS5) or non-functional (NF) anti-α5-integrin antibody (mAb11), as a control, were plated onto 10 μg/ml immobilised fibronectin in the absence or presence of 50 ng/ml PDGF-BB for 90 minutes at 37°C, before fixation with paraformaldehyde. Representative examples of MSCs double-labelled for phalloidin (white and red) and Y751 phosphorylated PDGFR-β (green), and merged images are shown. Nuclei appear blue owing to DAPI staining. F-actin membrane ruffling is highlighted by a white asterisk. To quantify receptor distribution and clustering (Chen et al., 2010), images were analysed using the ImageJ ‘analyse particles’ function (as described in the Materials and Methods). Corresponding particle analysis images of PDGFR-β distribution (1–500 pixels) or PDGFR-β clustering (10–500 pixels) are shown next to each original image. The mean number of particles (±s.d.) derived from five different single-cell images is denoted in black (**P<0.01; ***P<0.001 compared with the fibronectin-induced PDGFR-β clustering in the absence of JBS5). Images are representative of at least three independent experiments. Scale bar: 20 μm. (B) The cellular distribution of F-actin and integrin subunit α5 was examined by immunofluorescence microscopy. MSCs in serum-free conditions treated with 20 μM LY294002, or DMSO as a control, were plated onto 10 μg/ml immobilised fibronectin in the absence or presence of 50 ng/ml PDGF-BB for 90 minutes at 37°C, before fixation with paraformaldehyde. Representative examples of MSCs double-labelled for phalloidin (white and red) and integrin α5 (green), and merged images are shown. Nuclei appear blue after DAPI staining. F-actin membrane ruffling is highlighted by white asterisks. Images are representative of at least three independent experiments. Scale bar: 20 μm. Inhibition efficiency of LY294002 on Akt phosphorylation by immunoblotting is shown to the right of the image panel. (C) The involvement of PI3K in MSC migration towards fibronectin was evaluated using a Boyden chamber migration assay. MSCs in serum-free conditions in the upper chamber treated with 20 μM LY294002 (LY) or DMSO (−), as a control, were cultured for 4 hours at 37°C in Boyden chambers pre-coated on the underside with 10 μg/ml fibronectin, with or without 50 ng/ml PDGF-BB in the lower chamber. (i) Images are representative of the migratory cells per field on the membrane underside of three independent experiments. Scale bar: 400 μm. (ii) Data are represented as mean (±s.d.) optical density (OD 570 nm) of stained migratory cells of two readings of an individual experiment, repeated three times. Conditions are compared against their respective MSC migration in the absence or presence of PDGF-BB (***P<0.001 by one-way ANOVA).
As α5β1-integrin engagement of fibronectin regulated PDGFR-β-mediated Akt-PI3K activity (Fig. 7Aii) and F-actin organisation (Fig. 8A), we investigated the effect of PI3K inhibition using the chemical inhibitor LY294002 on MSC F-actin organisation and migration. Inhibition of PI3K activity ablated both fibronectin- and PDGF-BB-induced F-actin membrane ruffles (Fig. 8B), and substantially attenuated MSC migration towards fibronectin in the absence or presence of PDGF-BB (Fig. 8C).
In summary, our results show that the adhesion of MSCs to fibronectin through α5β1-integrin both directly stimulates PDGFR-β and potentiates PDGF-BB-stimulated PDGFR-β signalling. In turn, PDGFR-β-mediated Akt-PI3K activation drives the cytoskeletal changes that regulate cell migration.
Discussion
The crucial importance of PDGFR-β in directing vascular cell behaviour is well documented, yet ECM-dependent mechanisms that regulate its signalling are not well understood. Using human MSCs, we have shown that adhesion to fibronectin specifically induces PDGFR-β signalling in an α5β1-integrin-dependent manner (Fig. 9). Fibronectin regulates PDGFR-β-dependent actin reorganisation and cell migration in the absence of growth factor ligand and also potentiates PDGF-BB-induced PDGFR-β signalling. Thus, fibronectin-rich matrices are crucial regulators of PDGFR-β-mediated mesenchymal cell migration during vascular remodelling.
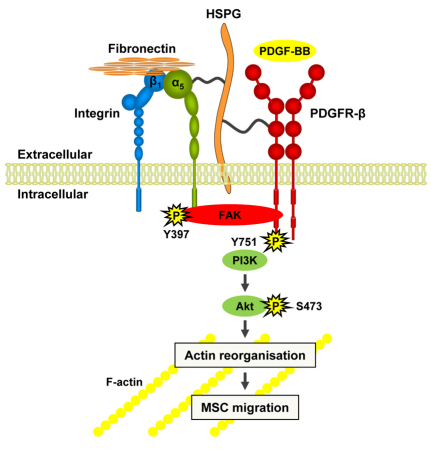
Fig. 9.
MSC migration is regulated by fibronectin through α5β1-integrin-mediated activation of PDGFR-β. Schematic model depicting the synergistic connection between α5β1-integrin-mediated adhesion to fibronectin and PDGFR-β, which controls Akt-PI3K activity and MSC migration. Adhesion to fibronectin promotes receptor clustering and association, which is mediated by heparan sulphate proteoglycans (HSPG), induces α5β1-integrin- and FAK-dependent PDGFR-β phosphorylation in the absence of growth factor and strongly potentiates PDGF-BB-stimulated PDGFR-β signalling. The potentiation of PI3K-Akt activity by crosstalk between α5β1-integrin and PDGFR-β is an essential event in the cascade that induces actin reorganisation and cell motility.
Nascent blood vessels contain many ECM molecules, including fibronectin, laminin, collagens and fibrillins. Our study shows that PDGFR-β signalling is differentially affected by cell adhesion to specific ECM ligands. In particular, adhesion to fibronectin and fibrillin-1, but not laminin, induces tyrosine phosphorylation of PDGFR-β in a manner independent of growth factor ligand. The stimulatory effect of fibronectin (a key regulator of cell adhesion, migration and survival during tissue formation, and a regulator of wound repair) is integrin-dependent as blocking α5β1-integrin but not αvβ3-integrin prevented PDGFR-β activation. Thus ECM, through specific integrins, directly stimulates PDGFR-β.
PDGFR-β activation induces dynamic changes in the cytoskeleton and membrane ruffles during cell migration (Mellström et al., 1988; Ruusala et al., 2008). We found that, in MSCs on fibronectin, crosstalk with α5β1-integrin regulates PDGFR-β-mediated cell adhesion, spreading and migration. Adhesion to fibronectin induces a rapid movement of phosphorylated PDGFR-β to the leading edge of MSCs, and a tidemark of phosphorylated PDGFR-β transiently colocalises with α5β1-integrin-containing focal adhesions behind the leading edge. Thus, PDGFR-β activity is a crucial element of α5β1-integrin-mediated adhesion and migration in these cells. Although co-immunoprecipitations showed that PDGFR-β and integrin subunit α5 associate in complexes, even in the absence of PDGF, we did not detect a direct interaction between the extracellular domains of PDGFR-β and α5β1-integrin. However, we did find that both receptor ectodomains strongly bound heparin, and fibronectin-induced co-immunoprecipitation of PDGFR-β and integrin subunit α5 was prevented by excess heparin and treatment with K5 heparan lyase. Thus, cell surface heparan sulphate proteoglycans mediate the association between PDGFR-β and α5β1-integrin. The receptor crosstalk could also involve molecules such as pericellular tissue transglutaminase, which binds to PDGFR-β (Zemskov et al., 2009), or tetraspanins, which form membrane complexes with integrin receptors and are implicated in integrin-mediated cell migration (Berditchevski and Odintsova, 1999).
Previous studies have indicated that the β1 integrin subunit and αVβ3-integrin can influence PDGFR-β activity (Sundberg and Rubin, 1996; Schneller et al., 1997; Woodard et al., 1998; Borges et al., 2000; Minami et al., 2007; Amano et al., 2008; Zemskov et al., 2009). Our study is the first to identify the major fibronectin α5β1-integrin as a key partner in PDGFR-β-mediated mesenchymal cell migration, both in the absence of growth factors and in potentiating PDGF-BB-mediated MSC migration. Integrin-mediated activation of PDGFR-β could occur as a consequence of integrin clustering, which is known to alter the spatial distribution of PDGFR-β within focal adhesions (Burridge et al., 1988; Miyamoto et al., 1996; Boudreau and Jones, 1999) enhancing PDGFR-β dimerisation and activation. This hypothesis is supported by our finding that α5β1-integrin engagement of fibronectin and PDGF-BB can induce both clusters of phosphorylated PDGFR-β and membrane ruffles, and that PDGF-BB can bind α5β1-integrin, which might further enhance the activity of PDGFR-β. Interactions of phosphorylated PDGFR-β with cytoskeletal proteins have been reported (Schneller, 2001), but it is not known whether PDGFR-β can directly bind actin. However, EGFR binds actin (den Hartigh et al., 1992; Tang and Gross, 2003) and localises in signalling complexes at membrane ruffles (Diakonova et al., 1995), thereby enhancing signalling efficiency (Gronowski and Bertics, 1993).
We have found that crosstalk between PDGFR-β and α5β1-integrin induce synergistic signalling responses. FAK and Akt-PI3K activity was cooperatively stimulated by fibronectin and PDGF-BB in an α5β1-integrin-dependent manner, whereas FAK knockdown reduced fibronectin-induced PDGFR-β and Akt phosphorylation, thereby linking FAK and Akt-PI3K signals. This is in agreement with a previous study that found FAK to be an important receptor-proximal link between PDGFR and integrin signalling pathways (Sieg et al., 2000). Inhibition of Akt-PI3K inhibited both fibronectin- and PDGF-induced actin reorganisation and migration of MSCs. These findings are consistent with previous reports that show a PDGFR-β mutant that is unable to bind and activate PI3K fails to mediate actin reorganisation and chemotaxis (Wennström et al., 1994). Moreover, in the absence of PDGFR-β-driven PI3K signalling, epicardial cells adopt an irregular actin cytoskeleton, leading to aberrant migration into the myocardium and defective coronary artery formation (Mellgren et al., 2008).
In summary, we have identified a novel synergistic connection between α5β1-integrin-mediated adhesion to fibronectin and PDGFR-β that controls receptor signalling and mesenchymal cell migration. We envisage that the functional interaction between α5β1-integrin and PDGFR-β promotes receptor clustering, which increases PDGFR-β affinity for its growth factor ligands and enhances PDGFR-β activity. The physical proximity of α5β1-integrin and PDGFR-β might facilitate the recruitment of numerous shared signalling and adapter proteins; the potentiation of FAK and Akt-PI3K activity by crosstalk between α5β1-integrin and PDGFR-β is an essential event in the cascade that induces cell motility. Thus, ECM controls mesenchymal cells through crosstalk with the potent vascular receptor PDGFR-β.
Materials and Methods
Cells and reagents
Human MSCs derived from the normal bone marrow of five different individuals (CD44, CD73 and CD105 positive and CD11b, CD14, CD34 and CD45 negative; from 28- and 34-year-old females and 19-, 25- and 33-year-old males; Lonza) were tested for their ability to differentiate into osteogenic, adipogenic and chondrogenic lineages. MSCs were maintained as previously described (Ball et al., 2007). Human aortic SMCs (32-year-old female; Lonza), were maintained in SMC growth medium (Invitrogen). Cells were cultured on human plasma fibronectin (Chemicon), murine laminin (α1β1γ1), bovine collagen type I, murine collagen type IV (BD Biosciences), recombinant fibrillin-1 protein fragment (PF8) (prepared in-house) (Cain et al., 2005) or a recombinant 50-kDa fibronectin fragment comprising type III repeats 6–10 (a gift from Martin Humphries, University of Manchester, Manchester, UK). All growth factors and the anti-PDGFR-β neutralising antibody (AF385) were obtained from R&D Systems. The cyclic RGD-blocking peptide cilengitide (Nisato et al., 2003) was a gift from Simon L. Goodman, Merck, Garching, Germany, and the PI3K inhibitor LY294002 was from Merck. For the antibodies used, see supplementary material Table S1.
Phosphorylated RTK array
A human phosphorylated RTK array kit (R&D Systems) was used to simultaneously detect the relative tyrosine phosphorylation levels of 42 different RTKs, as previously described (Ball et al., 2007).
Immunoblotting
Isolated proteins were resolved using pre-cast Tris-acetate (3–8%) or Bis-Tris (4–12%) gels (Invitrogen), transferred onto nitrocellulose, incubated with primary antibody overnight and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 2 hours. Detection of proteins was performed with enhanced chemiluminescence (ECL) western blotting reagent, as previously described (Ball et al., 2007). The pixel density of bands was determined with the Gene Tools v3 software.
Immunoprecipitation
Cell lysates (500 μg) were pre-cleared using 10% protein-A–Sepharose, immunoprecipitated with primary antibody overnight, and then the immunocomplexes were isolated by incubation with 10% protein-A–Sepharose for 2 hours, as previously described (Ball et al., 2007).
siRNA transfection
MSCs (5×105 cells), together with 3 μg of small interfering RNA (siRNA), were transfected by electroporation using a human Amaxa Nucleofector kit (Lonza), then cultured in growth medium overnight. Validated siRNA, functionally tested to provide ≥70% gene knockdown for FAK, was obtained from Qiagen (SI02622130), and scrambled siRNA was used as a control (Qiagen).
Phosphorylated PDGFR immunoassays
Phosphorylated PDGFR-β immunoassays (R&D Systems) were used to measure the levels of tyrosine residue (Y751 and Y1021) phosphorylation for PDGFR-β, according to the manufacturer's instructions. Assays were modified to measure phosphorylated PDGFR-α (Y742) utilising an antibody against phosphorylated PDGFR-α (Y742) (R&D Systems).
Immunofluorescence analysis
Cells were fixed using 4% paraformaldehyde for 20 minutes, permeabilised with 0.5% Triton X-100 for 4 minutes, blocked with 2% fish-skin gelatin (Sigma–Aldrich) for 1 hour and then incubated with primary antibody overnight at 4°C. Alexa-Fluor-488 or -594-conjugated secondary antibodies (Invitrogen), and Rhodamine-conjugated phalloidin (Invitrogen) to stain filamentous actin, were added for 2 hours, then coverslips were mounted using Vectashield containing DAPI (Vector Laboratories). Images were collected on a wide-field microscope (Leica DM RXA) using a 60× oil objective and captured using a Coolsnap EZ camera (Photometrics) driven by MetaVue Software (Molecular Devices). For colocalisation analysis, images were analysed using ImageJ software and the colocalisation analysis plugin. For receptor distribution and clustering analysis, images were analysed using the ‘analyse particles’ function of ImageJ software (Chen et al., 2010). Similar best-fit lower threshold values were determined for each image to reduce signal background, with the upper threshold always set at 255. Particle sizes for colocalisation were set at a minimum of 1 pixel and maximum of 500 pixels, and colocalisation is represented by a yellow image. Particle sizes for receptor distribution and clustering were set at a minimum of 1 or 10 pixels, respectively, and a maximum of 500 pixels, and are represented by a black and white image.
Cell migration assay
Modified Boyden chamber assays were conducted using filter inserts of 8-μm pore size and 6.5-mm diameter (BD Biosciences), as previously described (Ball et al., 2007). Migratory cells were imaged by phase-contrast microscopy (Leica DM RXA brightfield) and captured using a Coolsnap EZ camera driven by MetaVue software.
Statistical analysis
In all quantification experiments, results are expressed as means±s.d. Statistical differences between two sets of data were determined using an unpaired Student's t-test, and differences between more than two data sets were determined by one-way ANOVA using the Tukey comparison test. P<0.05 was considered statistically significant. All statistical calculations were performed using the GraphPad Prism 5 software.
Supplementary Material
Acknowledgments
This study was funded by the Medical Research Council (UK). We thank Stuart Cain for assistance with the BIAcore experiments, Mike Jackson for assistance with flow cytometry and Peter March from the Faculty Bioimaging Facility for advice on image analysis. Deposited in PMC for release after 6 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/124/8/1288/DC1
References
- Abedin M., Tintut Y., Demer L. (2004). Mesenchymal stem cells and the artery wall. Circ. Res. 95, 671-676 [DOI] [PubMed] [Google Scholar]
- Amano H., Ikeda W., Kawano S., Kajita M., Tamaru Y., Inoue N., Minami Y., Yamada A., Takai Y. (2008). Interaction and localization of Necl-5 and PDGF receptor beta at the leading edges of moving NIH3T3 cells: implications for directional cell movement. Genes Cells 13, 269-284 [DOI] [PubMed] [Google Scholar]
- Andrae J., Gallini R., Betsholtz C. (2008). Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 15, 1276-1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari J. A., Buckley P. A., Mould A. P., Humphries M. J. (2009). Linking integrin conformation to function. J. Cell Sci. 122, 165-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball S. G., Shuttleworth C. A., Kielty C. M. (2007). Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J. Cell Biol. 177, 489-500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax D. V., Mahalingam Y., Cain S. A., Mellody K., Freeman L., Younger K., Shuttleworth C. A., Humphries M. J., Couchman J. R., Kielty C. M. (2007). Cell adhesion to fibrillin-1: identification of an Arg-Gly-Asp-dependent synergy region and a heparin-binding site that regulates focal adhesion formation. J. Cell Sci. 120, 1383-1392 [DOI] [PubMed] [Google Scholar]
- Berditchevski F., Odintsova E. (1999). Characterization of integrin-tetraspanin adhesion complexes: role of tetraspanins in integrin signaling. J. Cell Biol. 146, 477-492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Karisson L., Lindahl P. (2001). Developmental roles of platelet-derived growth factors. Bioessays 23, 494-507 [DOI] [PubMed] [Google Scholar]
- Borges E., Yiwen J., Ruoslahti E. (2000). PDGF-receptor-β and VEGF-receptor-2 bind to the β3 integrin through its extracellular domain. J. Biol. Chem. 275, 39867-39873 [DOI] [PubMed] [Google Scholar]
- Boudreau N. J., Jones P. L. (1999). Extracellular matrix and integrin signaling: the shape of things to come. Biochem. J. 339, 481-488 [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. (1988). Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu. Rev. Cell Biol. 4, 487-525 [DOI] [PubMed] [Google Scholar]
- Cain S. A., Baldock C., Gallagher J., Morgan A., Bax D. V., Weiss A. S., Shuttleworth C. A., Kielty C. M. (2005). Fibrillin-1 interactions with heparin: implications for microfibril and elastic fiber assembly. J. Biol. Chem. 280, 30526-30537 [DOI] [PubMed] [Google Scholar]
- Chen T. T., Luque A., Lee S., Anderson S. M., Segura T., Iruela-Arispe M. L. (2010). Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J. Cell Biol. 188, 595-609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen E. H., Sonnenberg A. (2003). Integrins in regulation of tissue development and function. J. Pathol. 200, 471-480 [DOI] [PubMed] [Google Scholar]
- den Hartigh J. C., van Bergen en Henegouwen P. M., Verkleij A. J., Boonstra J. (1992). The EGF receptor is an actin-binding protein. J. Cell Biol. 119, 349-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakonova M., Payrastre B., van Velzen A. G., Hage W. J., van Bergen en Henegouwen P. M., Boonstra J., Cremers F. F., Humbel B. M. (1995). Epidermal growth factor induces rapid and transient association of phospholipase C-γ1 with EGF-receptor and filamentous actin at membrane ruffles of A431 cells. J. Cell Sci. 108, 2499-2509 [DOI] [PubMed] [Google Scholar]
- Eliceiri B. P. (2001). Integrin and growth factor receptor crosstalk. Circ. Res. 89, 1104-1110 [DOI] [PubMed] [Google Scholar]
- Esfandiarei M., Yazdi S. A., Gray V., Dedhar S., van Breemen C. (2010). Integrin-linked kinase functions as a downstream signal of platelet-derived growth factor to regulate actin polymerization and vascular smooth muscle cell migration. BMC Cell Biol. 11, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G., Cusella-De Angelis G., Coletta M., Paolucci E., Stornaiuolo A., Cossu G., Mavilio F. (1998). Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279, 1528-1530 [DOI] [PubMed] [Google Scholar]
- Fiedler J., Etzel N., Brenner R. E. (2004). To go or not to go: migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. J. Cell. Biochem. 93, 990-998 [DOI] [PubMed] [Google Scholar]
- Fredriksson L., Li H., Eriksson U. (2004). The PDGF family: four gene products form five different isoforms. Cytokine Growth Factor Rev. 15, 197-204 [DOI] [PubMed] [Google Scholar]
- French W. J., Creemers E. E., Tallquist M. D. (2008). Platelet-derived growth factor receptors direct vascular development independent of vascular smooth muscle cell function. Mol. Cell. Biol. 28, 5646-5657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti F. G., Tarone G. (2003). Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 19, 173-206 [DOI] [PubMed] [Google Scholar]
- Gronowski A. M., Bertics P. J. (1993). Evidence for the potentiation of epidermal growth factor receptor tyrosine kinase activity by association with the detergent-insoluble cellular cytoskeleton: analysis of intact and carboxyterminally truncated receptors. Endocrinology 133, 2838-2846 [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Ostman A., Ronnstrand L. (1998). Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta 1378, F79-F113 [DOI] [PubMed] [Google Scholar]
- Hellström M., Kalén M., Lindahl P., Abramsson A., Betsholtz C. (1999). Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047-3055 [DOI] [PubMed] [Google Scholar]
- Humphries M. J. (2001). Cell adhesion assays. Mol. Biotechnol. 18, 57-61 [DOI] [PubMed] [Google Scholar]
- Hynes R. O. (1992). Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69, 11-25 [DOI] [PubMed] [Google Scholar]
- Jiménez C., Portela R. A., Mellado M., Rodríguez-Frade J. M., Collard J., Serrano A., Martínez A. C., Avila J., Carrera A. C. (2000). Role of the PI3K regulatory subunit in the control of actin organization and cell migration. J. Cell Biol. 151, 249-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. (1989). Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell 58, 1121-1133 [DOI] [PubMed] [Google Scholar]
- Kelly J. D., Haldeman B. A., Grant F. J., Murray M. J., Seifert R. A., Bowen-Pope D. F., Cooper J. A., Kazlauskas A. (1991). Platelet-derived growth factor (PDGF) stimulates PDGF receptor subunit dimerization and intersubunit trans phosphorylation. J. Biol. Chem. 266, 8987-8992 [PubMed] [Google Scholar]
- Kinner B., Zaleskas J. M., Spector M. (2002). Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp. Cell Res. 278, 72-83 [DOI] [PubMed] [Google Scholar]
- Kundra V., Escobedo J. A., Kazlauskas A., Kim H. K., Rhee S. G., Williams L. T., Zetter B. R. (1994). Regulation of chemotaxis by the platelet-derived growth factor receptor-beta. Nature 367, 474-476 [DOI] [PubMed] [Google Scholar]
- Lindahl P., Johansson B. R., Levéen P., Betsholtz C. (1997). Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277, 242-245 [DOI] [PubMed] [Google Scholar]
- Lindblom P., Gerhardt H., Liebner S., Abramsson A., Enge M., Hellstrom M., Backstrom G., Fredriksson S., Landegren U., Nystrom H. C., et al. (2003). Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 17, 1835-1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402-408 [DOI] [PubMed] [Google Scholar]
- Meima M. E., Webb C. L., Witkowska H. E., Barber D. L. (2009). The sodium-hydrogen exchanger NHE1 is an Akt substrate necessary for actin filament reorganization by growth factors. J. Biol. Chem. 284, 26666-26675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellgren A. M., Smith C. L., Olsen G. S., Eskiocak B., Zhou B., Kazi M. N., Ruiz F. R., Pu W. T., Tallquist M. D. (2008). Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circ. Res. 103, 1393-1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellström K., Heldin C. H., Westermark B. (1988). Induction of circular membrane ruffling on human fibroblasts by platelet-derived growth factor. Exp. Cell Res. 177, 347-359 [DOI] [PubMed] [Google Scholar]
- Minami Y., Ikeda W., Kajita M., Fujito T., Amano H., Tamaru Y., Kuramitsu K., Sakamoto Y., Monden M., Takai Y. (2007). Necl-5/poliovirus receptor cis-interacts with integrin αvβ3 and regulates its clustering and formation of focal complexes. J. Biol. Chem. 282, 18481-18496 [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Teramoto H., Gutkind J. S., Yamada K. M. (1996). Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 135, 1633-1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisato R. E., Tille J. C., Jonczyk A., Goodman S. L., Pepper M. S. (2003). αvβ3 and αvβ5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis 6, 105-119 [DOI] [PubMed] [Google Scholar]
- Rozen S., Skaletsky H. (2000). Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132, 365-386 [DOI] [PubMed] [Google Scholar]
- Ruusala A., Sundberg C., Arvidsson A. K., Rupp-Thuresson E., Heldin C. H., Claesson-Welsh L. (1998). Platelet-derived growth factor (PDGF)-induced actin rearrangement is deregulated in cells expressing a mutant Y778F PDGF beta-receptor. J. Cell Sci. 111, 111-120 [DOI] [PubMed] [Google Scholar]
- Ruusala A., Pawson Y., Heldin C. H., Aspenström P. (2008). Nck adapters are involved in the formation of dorsal ruffles, cell migration, and Rho signaling downstream of the platelet-derived growth factor beta receptor. J. Biol. Chem. 283, 30034-30044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneller M. (2001). Identification of a candidate integrin-fraction associated with the activated form of the PDGF-receptor. Biochem. Biophys. Res. Commun. 281, 595-602 [DOI] [PubMed] [Google Scholar]
- Schneller M., Vuori K., Ruoslahti E. (1997). αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J. 16, 5600-5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg D. J., Hauck C. R., Ilic D., Klingbeil C. K., Schaefer E., Damsky C. H., Schlaepfer D. D. (2000). FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2, 249-256 [DOI] [PubMed] [Google Scholar]
- Streuli C. H., Akhtar N. (2009). Signal co-operation between integrins and other receptor systems. Biochem. J. 418, 491-506 [DOI] [PubMed] [Google Scholar]
- Sundberg C., Rubin K. (1996). Stimulation of β1 integrins on fibroblasts induces PDGF independent tyrosine phosphorylation of PDGF β-receptors. J. Cell Biol. 132, 741-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist M., Kazlauskas A. (2004). PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 15, 205-213 [DOI] [PubMed] [Google Scholar]
- Tang J., Gross D. J. (2003). Regulated EGF receptor binding to F-actin modulates receptor phosphorylation. Biochem. Biophys. Res. Commun. 312, 930-936 [DOI] [PubMed] [Google Scholar]
- Tepper O. M., Capla J. M., Galiano R. D., Ceradini D. J., Callaghan M. J., Kleinman M. E., Gurtner G. C. (2005). Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 105, 1068-1077 [DOI] [PubMed] [Google Scholar]
- Wennström S., Siegbahn A., Yokote K., Arvidsson A. K., Heldin C. H., Mori S., Claesson-Welsh L. (1994). Membrane ruffling and chemotaxis transduced by the PDGF beta-receptor require the binding site for phosphatidylinositol 3′ kinase. Oncogene 9, 651-660 [PubMed] [Google Scholar]
- Woodard A. S., Garcia-Cardena G., Leong M., Madri J. A., Sessa W. C., Languino L. R. (1998). The synergistic activity of αvβ3 integrin and PDGF receptor increases cell migration. J. Cell Sci. 111, 469-478 [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Even-Ram S. (2002). Integrin regulation of growth factor receptors. Nat. Cell Biol. 4, E75-E76 [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Davis S., Gale N. W., Rudge J. S., Wiegand S. J., Holash J. (2000). Vascular specific growth factors and blood vessel formation. Nature 407, 242-248 [DOI] [PubMed] [Google Scholar]
- Zemskov E. A., Loukinova E., Mikhailenko I., Coleman R. A., Strickland D. K., Belkin A. M. (2009). Regulation of platelet-derived growth factor receptor function by integrin-associated cell surface transglutaminase. J. Biol. Chem. 284, 16693-16703 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.