Abstract
Specialized sensory-transducing hair cells regenerate in response to injury in non-mammalian vertebrates such as birds and fish but not in mammals. Previous work has shown that overexpression of microRNA181a (miR181a) in cultured chicken basilar papillae, the avian counterpart of the cochlea, is sufficient to stimulate proliferation with production of new hair cells. The present study investigates the role of miR181a in hair cell regeneration after injury in explants of chicken auditory epithelia. Basilar papillae were explanted from 0-day-old chickens and transfected with either anti-miR181a, which knocks down endogenous miR181a, or a non-targeting miRNA and cultured with streptomycin to eliminate all hair cells from the epithelium. Labeling with BrdU was used to quantify proliferation. Explants exposed to streptomycin and transfected with anti-miR181a had significantly fewer BrdU positive cells than basilar papillae treated with streptomycin and transfected with a non-targeting miRNA. Activated caspase-3 and myosin VI labeling were used to show that the pattern of hair cell death and loss, respectively, were not affected by anti-miR181a transfection. MiR181a downregulation therefore seems to dimish the proliferative component of hair cell regeneration rather than prevent hair cell death following ototoxic injury.
Keywords: hair cell, regeneration, microRNA181a, microRNA, inner ear, hearing
INTRODUCTION
Auditory hair cells are highly specialized mechanosensitive sensory cells in the inner ear that respond to acoustic stimulation. These cells can be damaged or lost as a result of infection, chemical insult, aging, acoustic trauma, or genetic causes. These cells cannot regenerate in the mammalian organ of Corti, where hearing impairment resulting from their loss is irreversible. In contrast, non-mammalian vertebrates such as birds are able to regenerate hair cells after injury (Stone and Cotanche, 2007) by both mitotic (Corwin and Cotanche, 1988; Ryals and Rubel, 1988; Stone and Cotanche, 1994) and non-mitotic mechanisms (Adler and Raphael, 1996; Baird et al., 1996; Roberson et al., 1996; Baird et al., 2000; Roberson et al., 2004; Taylor and Forge, 2005; Duncan et al., 2006). As yet, little is known about the molecular signals that underlie this phenomenon. A better understanding of the cellular signals for regeneration in the avian ear might point to rational targets for reversing sensorineural hearing loss.
Previous work has shown that many genes are differentially expressed between proliferating and quiescent cultured chicken auditory epithelia (Frucht et al., 2010). Given this observation, a computational approach called gene set enrichment analysis was used to identify regulatory molecules and events that might be responsible for the complex changes in transcriptional profile that occur with proliferation and new hair cell production. This analysis identified myriad microRNAs (miRNA) that were predicted to play a role in this process. One miRNA, miR181a, was selected for functional studies and was found to stimulate proliferation and produce new hair cells when overexpressed in undamaged chicken auditory epithelia in-vitro.
The present study addresses the question of whether endogenous miR181a plays a role in auditory hair cell regeneration after injury in the chicken inner ear. Cochlear ducts containing the basilar papilla (BP), the avian counterpart of the cochlea, were explanted from 0-day-old chickens and immediately transfected with either a nontargeting miRNA or anti-miR181a that knocks down endogenous miR181a. The explants were then cultured with streptomycin to eradicate all hair cells and the thymidine analog, BrdU, to assay for proliferation. The tissue was then fixed and labeled for BrdU. BPs transfected with anti-miR181a had significantly fewer BrdU positive cells than tissue transfected with a nontargeting miRNA, indicating that endogenous miR181a is necessary for the proliferative component of hair cell regeneration after injury in the chicken inner ear. Additionally, the pattern of hair cell loss is unaffected by anti-miR181 transfection, so the decreased rate of BrdU labeling seems to represent direct inhibition of proliferation, rather than cytoprotection. Quantitative PCR (qPCR) was used to measure expression of miR181a, which did not increase after exposure to streptomycin, suggesting that the functional effect of miR181a in hair cell regeneration is not primarily mediated by expression level, but rather by regulation of its activity.
MATERIALS AND METHODS
Culture and transfection of explants
Animals were treated in accordance with policies set forth by the Yale Institutional Animal Care and Use Committee (protocol number 2007-10439). Cochlear ducts containing the BPs were explanted from 0-day-old chicks and then transfected with either anti-miR181a or a non-targeting miRNA (Ambion, Austin, TX) as described below. Explants were then cultured for 48 hours with or without streptomycin (78 µM, a concentration sufficient to eradicate all hair cells) and BrdU (0.01%, to allow for quantification of proliferation) in DMEM with 10% FBS at 37 °C with 5% CO2. BPs were then transfected again with either pre-miR181a or a non-targeting miRNA (pre-miR Negative Control #1, Ambion), and then cultured for an additional 48 hours with BrdU but no streptomycin to allow recovery. The anti- and non-targeting miRNAs were transfected at a final concentration of 100 nM using the lipid-based X-tremeGENE SiRNA Transfection Reagent (Roche, Indianapolis, IN). This, and all subsequent reagent kits were used per the manufacturer’s instructions. The efficacy of this transfection reagent in the chicken auditory epithelium has been previously established (Frucht et al., 2010).
BrdU labeling
BPs were fixed in 4% PFA in PBS for 30 minutes. Between each step three 5-minute PBS washes were performed. All BrdU labeling steps were performed at room temperature. The tissue was blocked and permeabilized using a solution of PBS with FBS (10%) and Triton-X (0.1%) for one hour. Each BP was then incubated with mouse anti-BrdU antibodies (1:40, BD, Franklin Lakes, NJ) in PBS for one hour. Alkaline phosphatase-conjugated goat anti-mouse IgG (1:400, Santa Cruz, Santa Cruz, CA) in PBS with Triton-X (0.1%) was applied for one hour. Alkaline phosphatase substrate was produced using the NBT/BCIP Reagent Kit (Invitrogen, Carlsbad, CA) and then added to the tissue for approximately five minutes.
Statistics
BPs immunohistochemically labeled for BrdU were viewed using brightfield microscopy and were analyzed following digitization. The borders of the sensory epithelium were easily appreciated by direct microscopy. Only those nuclei that were clearly within the epithelium were counted. Unpaired Student’s t-tests were performed to determine the statistical significance of the comparisons discussed in the text using a cut-off of p < 0.05.
Cell death assay
Explanted BPs cultured with or without streptomycin (78 µM) for 48 hours. These conditions cause a complete loss of hair cells in the proximal (i.e., high frequency) segment of the BP (Shang et al., 2010). The CaspaTag kit (Millipore, Billerica, MA) was then used to label for activated caspase-3. The BPs were then fixed, permeabilized and incubated with rabbit anti-myosin VI (1:350, Proteus, Ramona, CA) in blocking solution for one hour and then incubated in Alexa Fluor 546 conjugated anti-rabbit secondary antibodies (1:1,000, Invitrogen, Carlsbad, CA) with Alexa Fluor 633-conjugated phalloidin (1:100, Invitrogen, Carlsbad, CA) for one hour. Sections were mounted on glass slides in Vectashield fluorescent mounting medium (Vector Laboratories, Burlingame, CA). Images were captured using a Zeiss LSM 510 confocal microscope.
RNA isolation
Some BPs were microdissected after 48 or 72 hours in culture with or without 78 µM streptomycin. Total plus small RNA isolation was performed using the MiRNEasy kit (Qiagen, Duesseldorf, Germany). Reverse transcription and qPCR were performed using the TaqMan MicroRNA Reverse Trancription Kit and TaqMan MicroRNA Assay for miR181a (Applied Biosystems, Foster City, CA).
qPCR
Amplification was performed using the SYBR Green Supermix kit (Bio-Rad, Hercules, CA) on an iCycler system (Bio-Rad, Hercules, CA). Expression levels were normalized to the total RNA concentration. Three replicates were performed and the data averaged for each cDNA sample and primer pair combination. Those fold-changes with a 95% confidence interval excluding a fold-change of one (which would represent no change) were considered significant.
RESULTS
To determine miR181a’s role in hair cell regeneration in the post-hatch chicken inner ear, BPs were cultured for 48 hours with streptomycin to eliminate all hair cells in the epithelium, and then an additional 48 hours without streptomycin (Shang et al., 2010). The explanted tissue was also transfected with either anti-miR181a, to knock down endogenous miR181a, or a nontargeting miRNA at 0 and 48 hours of exposure to streptomycin. Also present in the culture medium was BrdU, a thymidine analog that is incorporated into the nuclei of cells entering s-phase. At the end of the entire 4-day culture duration, BPs were fixed and labeled for BrdU to quantify proliferation.
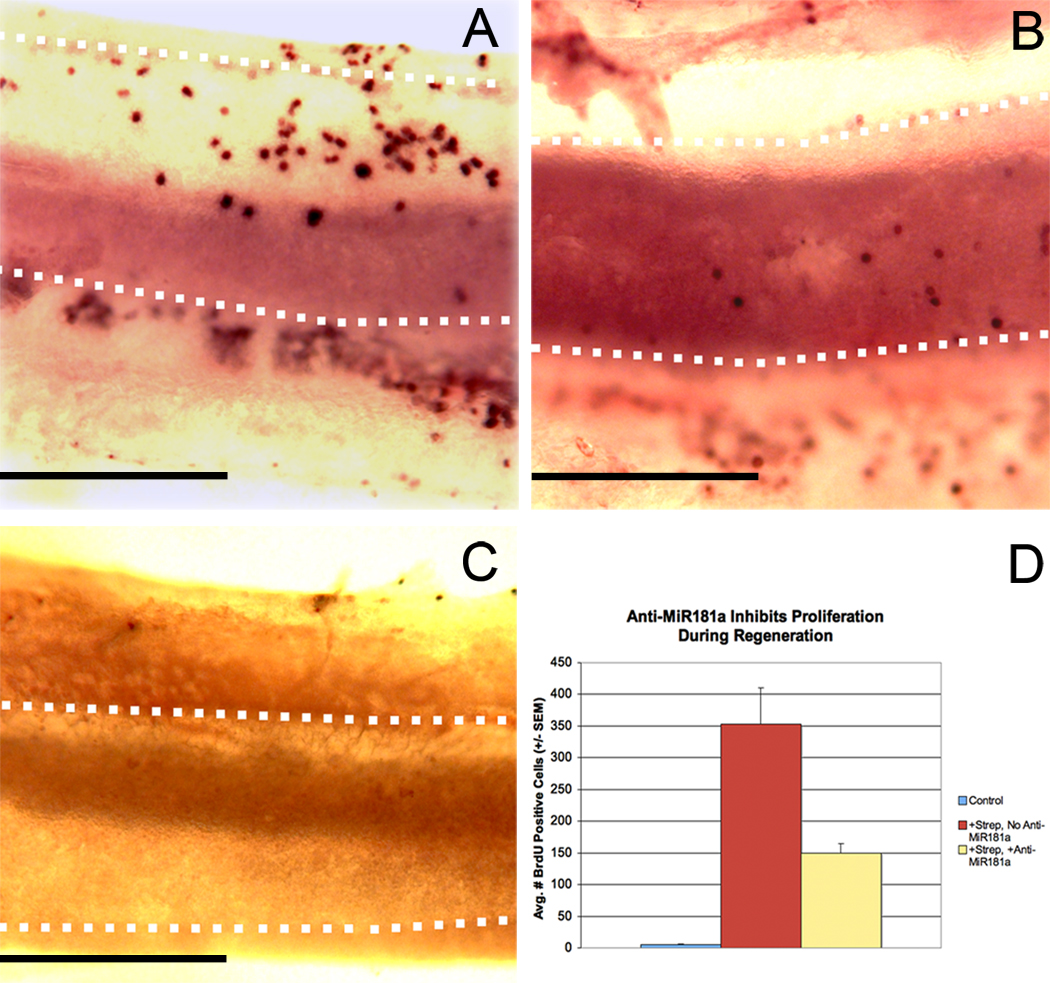
BPs injured with streptomycin and transfected with the non-targeting miRNA had an average of 353.1 (+/− SEM = 56.5) BrdU positive cells (Figure 1). In contrast, anti-miR181a transfection and subsequent culture alone apparently does not cause much injury to the epithelium, as these BPs had on average 5.3 (+/− SEM = 2.6) BrdU positive cells. Similarly, BPs that were neither transfected nor exposed to streptomycin all had fewer than 10 BrdU positive cells per epithelium (data not shown). Explants that were cultured with streptomycin and transfected with anti-miR181a had on average 149.9 (+/− SEM = 14.5) BrdU positive cells per epithelium, significantly fewer than epithelia exposed to streptomycin and transfected with the non-targeting miRNA (p < 0.01).
Figure 1. Suppression of endogenous miR181a expression with anti-miR181a blunts proliferation during hair cell regeneration after streptomycin exposure.
Basilar papillae from 0-day-old post-hatch chickens were transfected with either anti-miR181a or a non-targeting miRNA and then cultured with BrdU and streptomycin (78 µM) or in drug-free medium (n = 7) as described in the text. There were a total of 7 BPs in each condition. After culture, explants were immunolabeled for BrdU to assay for proliferation. Panels A-C show basilar papillae labeled for BrdU, with the sensory epithelia outlined with dotted white lines. The neural edges of the epithelia are toward the bottom, and the proximal ends are to the left. Epithelia exposed to streptomycin exhibit far more proliferation when transfected with a non-targeting miRNA (A) than anti-miR181a (B). Epithelia transfected with a non-targeting miRNA and cultured only in the absence of streptomycin typically have very few BrdU positive cells (C). Shown in D is the average number of BrdU positive cells per epithelium in each condition. All pair wise comparisons are statistically significant (t-test, p < 0.05). Scale bars in A-C = 0.2 mm.
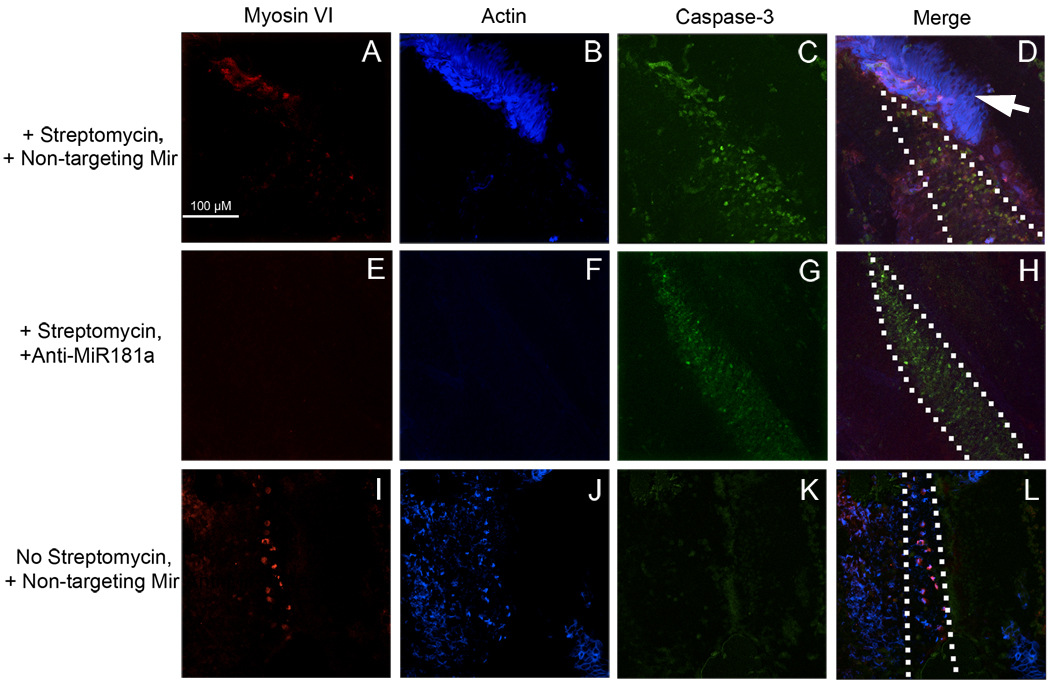
The BrdU labeling data suggest that endogenous miR181a plays a crucial role in the post-injury proliferative response in the chicken auditory epithelium but does not exclude the possibility that anti-miR181a transfection simply prevents cell death. To address this issue it was necessary to compare the patterns of hair cell loss after streptomycin exposure with and without prior anti-miR181a transfection. BPs were therefore transfected with either anti-miR181a or a non-targeting miRNA and then cultured for 48 hours with 78 µM streptomycin, before fixation and labeling for the apoptosis marker activated caspase-3 (Kaiser et al., 2008), the hair cell marker myosin VI, and the major hair bundle component actin (Figure 2). Streptomycin treatment causes complete hair cell loss at the high frequency segment of the BP (Figure 2). Accordingly there are many activated caspase-3 positive cells in BPs exposed to streptomycin, but not in those cultured without streptomycin. This pattern of cell death and hair cell loss is consistent regardless of anti-miR181a transfection, where there is still hair cell loss and caspase-3 activation at the high frequency end of the BP. At the low frequency end, there is less of a loss of hair cells and accordingly less caspase-3 activation (data not shown). This pattern also is independent of anti-miR181a transfection, mirroring the data from the high frequency BP segment. It therefore appears as though the reduction in BrdU labeling with anti-miR181a transfection of streptomycin treated BPs is due to direct inhibition of the regenerative response, rather than cytoprotection with a resulting decrease in the number of cells damaged by streptomycin.
Figure 2. Anti-miR181a transfection does not prevent hair cell death after streptomycin exposure.
Basilar papillae were cultured with streptomycin for 48 hours following transfection with either a non-targeting miRNA (A–D) or anti-miR181a (E–H). Whole-mounts were then labeled for the hair cell marker myosin VI, sterocilia bundle component actin, and the early cell death marker caspase-3. Hair cells were completely eliminated from the high frequency segment of basilar papillae exposed to streptomycin, regardless of transfection with anti-miR181a (A, B, E, F). Further, streptomycin exposure triggers extensive cell death with or without anti-miR181a transfection (C, G). In contrast, epithelia transfected with a non-targeting miRNA and unexposed to streptomycin retain hair cells (I, J) and do not show extensive apoptosis (K). The neural edge is to the right of each panel, and the proximal segment is to the top. The arrow in panel D shows what are likely homogene cells remaining after microdissection. The scale bar in A also applies to B-L.
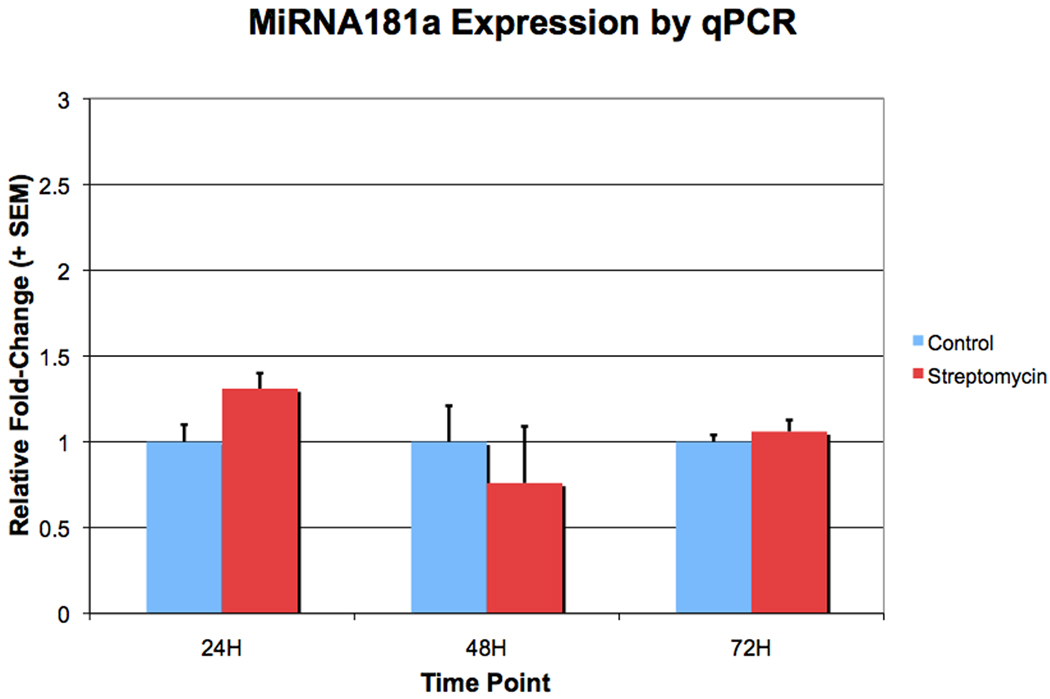
QPCR was used to ascertain whether expression of mature miR181a increases following exposure to streptomycin. BPs were cultured in streptomycin for 24, 48 or 72 hours, at which point the sensory epithelium was isolated by microdissection. After RNA isolation, first-strand-synthesis and qPCR was performed using primers for miR181a. At none of these time points were there significant miR181a expression level differences (Figure 3). Therefore, while knocking down endogenous miR181a interferes with post-injury hair cell regeneration, it appears as though an increase of activity, rather than expression levels alone, mediates this miRNA’s role in regeneration.
Figure 3. MiR181a expression does not increase after exposure to streptomycin for 24, 48 or 72 hours.
Basilar papillae were cultured with 78 µM streptomycin for 24, 48, or 72 hours, and sensory epithelia were isolated by microdissection. Shown are relative fold-changes of miR181a expression with exposure to streptomycin, as detected by qPCR. The fold-changes shown are relative to expression in untreated controls. There was no significant difference in miR181a expression between the basilar papillae that were and were not treated with streptomycin at any of the three time points (t-test, p < 0.05).
DISCUSSION
Presented here is the first direct functional biological evidence for the role of miRNA in auditory hair cell regeneration after injury. Previously, there was limited, indirect evidence that the let-7 family of miRNA play a role in hair cell regeneration in the newt inner ear (Tsonis et al., 2007). The present observation that transfection with anti-miR181a results in a markedly blunted regenerative response suggests that miR181a plays a key role in hair cell regeneration in the chicken inner ear. This finding agrees with a previous study showing that miR181a overexpression in the uninjured chicken BP can stimulate proliferation with the production of new hair cells (Frucht et al., 2010). Additionally, miR181a has a pro-proliferative role in cultured human myeloid leukemia cells that seems to be mediated at least partly by repressing expression of the cell cycle inhibitor p27 (Wang et al., 2009). This is worth pointing out given that p27 is thought to represent a significant barrier to regeneration in the mammalian inner ear (Lowenheim et al., 1999; Lee et al., 2006). Further experiments will be necessary to determine whether miR181a’s ability to stimulate proliferation in the chicken inner ear involves targeted downregulation of p27 expression.
The observation that miR181a expression does not increase during regeneration despite its clear role in this process suggests regulation of its activity mediates this effect. It is possible that upregulation of miR181a occurs earlier than 24 hours after onset of exposure, or transiently between 24, 48 or 72 hours, but these possibilities seem unlikely given the sustained nature of the regenerative response. Our understanding of the mechanisms of control of miRNA biogenesis is more detailed than that of the ways in which miRNA biological activity is controlled (Cai et al., 2009). MiRNA activity can be modulated by different mechanisms including association with RNA-binding factors that influence miRNA-target interactions (Breving and Esquela-Kerscher, 2010). Unfortunately, there are presently no methods for directly assaying miRNA activity. One indirect method would be to look at changes in expression of predicted miR181a targets with exposure to streptomycin once such targets have been validated in chickens. Future studies on changes in expression of predicted miR181a targets in regeneration may be helpful in elucidating the precise mechanism of this miRNA’s role in hair cell regeneration.
It also bears mentioning that miR181a is not the only miR181 family miRNA expressed in chickens. MiR181b is also expressed in chickens, and its sequence differs from that of miR181a by 4 bases. (www.mirbase.org). It is theoretically possible that the anti-miR181a may also cause downregulation of miR181b, but this seems highly unlikely given evidence that there is a significant decrease in anti-miRNA activity with just one mismatch and a lack of activity with as few as three mismatches (Esau, 2008). Once there are known, biologically validated targets of both miR181a and miR181b in chickens, experiments should aim to confirm the predicted specificity of anti-miR181a.
Though presented here is the first functional evidence for a role for miRNA in hair cell regeneration, previous work has shown that certain miRNAs are not only expressed in the mammalian inner ear (Weston et al., 2006; Wang et al., 2010), but are also important for the proper function and/or development of this structure. For example, conditional Dicer knockout mouse embryos that are depleted of all inner ear miRNA develop grossly malformed inner ears (Soukup et al., 2009). Similarly, mice with conditionally silenced Dicer expression in hair cells only are deaf and have markedly aberrant hair cell morphology (Friedman et al., 2009). Interestingly, mutations in the target-defining seed region of miR96 cause a progressive hearing loss in both mice (Lewis et al., 2009) and humans (Mencía et al., 2009). These results suggest inter-species similarities in ear miRNA function, at least within the class of mammals and therefore raise hopes that insights into the role of miRNA in hair cell regeneration in birds may shed light on why the mammalian auditory epithelium lacks regenerative capacity.
There are as yet very few studies that have directly examined the role of specific miRNAs in development, function, or regeneration of the inner ear. The miR183 family, (miR96, miR182, miR183) have been examined closely following the observation that miR96 mutations are associated with progressive hearing loss. Specifically, miR183 is expressed in mammalian hair cells and spiral and vestibular ganglia (Weston et al., 2006). Interestingly, overexpression of miR96 or miR182 causes production of ectopic hair cells in the zebrafish embryo (Li et al., 2010). In light of existing expression and functional data, it is clear that the miR183 family is important for inner ear development across species. These observations affirm that miRNA may prove to be suitable targets for rational therapies to reverse hearing loss.
In addition to miR181a, other miRNAs have been identified whose roles in hair cell regeneration warrant further investigation (Frucht et al., 2010). Increasing evidence suggests this is a promising avenue of investigation for those interested in producing rational therapy for sensorineural hearing loss. It is attractive to consider the possibility of miRNA based therapeutics, specifically, in light of the transient nature of their overexpression which may result in side effect profiles that are more favorable than those of hypothetical DNA-based gene therapies for hearing loss. Further functional studies are necessary to test the hypothesis that miRNA transfection, perhaps with a carefully chosen combination of miRNA, can result in the production of new hair cells in the mammalian inner ear, and may therefore result in alleviation of sensorineural hearing loss.
ACKNOWLEDGEMENTS
This work is part of a dissertation submitted to fulfill the requirements for the Degree of Doctor of Philosophy at Yale University.
The authors wish to thank Jennifer Stone, PhD, University of Washington, for helpful technical advice and critical feedback on this manuscript.
CSF was supported by NIH MSTP TG 2T32GM07205 and an Ohse Grant for Surgical Research at Yale School of Medicine. CSF, JSS and DSN were supported by NIH/NIDCD grants DC 007894, DC 000273 and DC 008130.
Abbreviations
- BP
basilar papilla
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- Baird RA, Steyger PS, Schuff NR. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Annals of the New York Academy of Sciences. 1996;781:59–70. doi: 10.1111/j.1749-6632.1996.tb15693.x. [DOI] [PubMed] [Google Scholar]
- Baird RA, Burton MD, Lysakowski A, Fashena DS, Naeger RA. Hair cell recovery in mitotically blocked cultures of the bullfrog saccule. Proc Natl Acad Sci USA. 2000;97:11722–11729. doi: 10.1073/pnas.97.22.11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breving K, Esquela-Kerscher A. The complexities of microRNA regulation: mirandering around the rules. Int J Biochem Cell Biol. 2010;42:1316–1329. doi: 10.1016/j.biocel.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Cai Y, Yu X, Hu S, Yu J. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics. 2009;7:147–154. doi: 10.1016/S1672-0229(08)60044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Duncan LJ, Mangiardi DA, Matsui JI, Anderson JK, McLaughlin-Williamson K, Cotanche DA. Differential expression of unconventional myosins in apoptotic and regenerating chick hair cells confirms two regeneration mechanisms. J Comp Neurol. 2006;499:691–701. doi: 10.1002/cne.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau CC. Inhibition of microRNA with antisense oligonucleotides. Methods. 2008;44:55–60. doi: 10.1016/j.ymeth.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Friedman LM, Dror AA, Mor E, Tenne T, Toren G, Satoh T, Biesemeier DJ, Shomron N, Fekete DM, Hornstein E, Avraham KB. MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proc Natl Acad Sci USA. 2009;106:7915–7920. doi: 10.1073/pnas.0812446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frucht CS, Uduman M, Duke JL, Kleinstein SH, Santos-Sacchi J, Navaratnam DS. Gene Expression Analysis of Forskolin Treated Basilar Papillae Identifies MicroRNA181a as a Mediator of Proliferation. PLoS ONE. 2010;5:e11502. doi: 10.1371/journal.pone.0011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C, Chapman B, Guidi J, Terry C, Mangiardi D, Cotanche D. Comparison of activated caspase detection methods in the gentamicin-treated chick cochlea. Hearing Research. 2008;240:1–11. doi: 10.1016/j.heares.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Liu F, Segil N. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development. 2006;133:2817–2826. doi: 10.1242/dev.02453. [DOI] [PubMed] [Google Scholar]
- Lewis MA, Quint E, Glazier AM, Fuchs H, De Angelis MH, Langford C, Van Dongen S, Abreu-Goodger C, Piipari M, Redshaw N, Dalmay T, Moreno-Pelayo MA, Enright AJ, Steel KP. An ENU-induced mutation of miR-96 associated with progressive hearing loss in mice. Nat Genet. 2009;41:614–618. doi: 10.1038/ng.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Kloosterman W, Fekete DM. MicroRNA-183 Family Members Regulate Sensorineural Fates in the Inner Ear. Journal of Neuroscience. 2010;30:3254–3263. doi: 10.1523/JNEUROSCI.4948-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackney CM, Zenner HP. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci U S A. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencía A, Modamio-Høybjør S, Redshaw N, Morín M, Mayo-Merino F, Olavarrieta L, Aguirre LA, Del Castillo I, Steel KP, Dalmay T, Moreno F, Moreno-Pelayo MA. Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat Genet. 2009;41:609–613. doi: 10.1038/ng.355. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Kreig CS, Rubel EW. Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Aud Neurosci. 1996;2:195–205. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004;78:461–471. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Shang J, Cafaro J, Nehmer R, Stone J. Supporting Cell Division Is Not Required for Regeneration of Auditory Hair Cells After Ototoxic Injury In Vitro. J Assoc Res Otolaryngol. 2010 doi: 10.1007/s10162-009-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup GA, Fritzsch B, Pierce ML, Weston MD, Jahan I, McManus MT, Harfe BD. Residual microRNA expression dictates the extent of inner ear development in conditional Dicer knockout mice. Dev Biol. 2009;328:328–341. doi: 10.1016/j.ydbio.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Cotanche D. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51:633–647. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Identification of the timing of S phase and the patterns of cell proliferation during hair cell regeneration in the chick cochlea. J Comp Neurol. 1994;341:50–67. doi: 10.1002/cne.903410106. [DOI] [PubMed] [Google Scholar]
- Taylor RR, Forge A. Hair cell regeneration in sensory epithelia from the inner ear of a urodele amphibian. J Comp Neurol. 2005;484:105–120. doi: 10.1002/cne.20450. [DOI] [PubMed] [Google Scholar]
- Tsonis P, Call M, Grogg M, Sartor M, Taylor R, Forge A, Fyffe R, Goldenberg R, Cowpersallari R, Tomlinson C. MicroRNAs and regeneration: Let-7 members as potential regulators of dedifferentiation in lens and inner ear hair cell regeneration of the adult newt. Biochemical and Biophysical Research Communications. 2007;362:940–945. doi: 10.1016/j.bbrc.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gocek E, Liu C-G, Studzinski GP. MicroRNAs181 regulate the expression of p27Kip1 in human myeloid leukemia cells induced to differentiate by 1,25-dihydroxyvitamin D3. Cell Cycle. 2009;8:736–741. doi: 10.4161/cc.8.5.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-R, Zhang X-M, Zhen J, Zhang P-X, Xu G, Jiang H. MicroRNA expression in the embryonic mouse inner ear. NeuroReport. 2010;21:611–617. doi: 10.1097/WNR.0b013e328338864b. [DOI] [PubMed] [Google Scholar]
- Weston MD, Pierce ML, Rocha-Sanchez S, Beisel KW, Soukup GA. MicroRNA gene expression in the mouse inner ear. Brain Res. 2006;1111:95–104. doi: 10.1016/j.brainres.2006.07.006. [DOI] [PubMed] [Google Scholar]