Abstract
Cross-sectional positron emission tomography (PET) studies find that cognitively normal carriers of the apolipoprotein E (APOE) ɛ4 allele, a common Alzheimer's susceptibility gene, have abnormally low measurements of the cerebral metabolic rate for glucose (CMRgl) in the same regions as patients with Alzheimer's dementia. In this article, we characterize longitudinal CMRgl declines in cognitively normal ɛ4 heterozygotes, estimate the power of PET to test the efficacy of treatments to attenuate these declines in 2 years, and consider how this paradigm could be used to efficiently test the potential of candidate therapies for the prevention of Alzheimer's disease. We studied 10 cognitively normal ɛ4 heterozygotes and 15 ɛ4 noncarriers 50–63 years of age with a reported family history of Alzheimer's dementia before and after an interval of approximately 2 years. The ɛ4 heterozygotes had significant CMRgl declines in the vicinity of temporal, posterior cingulate, and prefrontal cortex, basal forebrain, parahippocampal gyrus, and thalamus, and these declines were significantly greater than those in the ɛ4 noncarriers. In testing candidate primary prevention therapies, we estimate that between 50 and 115 cognitively normal ɛ4 heterozygotes are needed per active and placebo treatment group to detect a 25% attenuation in these CMRgl declines with 80% power and P = 0.005 in 2 years. Assuming these CMRgl declines are related to the predisposition to Alzheimer's dementia, this study provides a paradigm for testing the potential of treatments to prevent the disorder without having to study thousands of research subjects or wait many years to determine whether or when treated individuals develop symptoms.
Alzheimer's dementia is the most common form of cognitive impairment in older persons, afflicting 10% of those over the age of 65 and almost half of those over 85 (1). As the population ages, this disorder is expected to take a growing toll on afflicted persons, their families, and the communities in which they live. Indeed, the prevalence of this disorder is projected to quadruple in the next 50 years, creating a catastrophic public health problem (2). Scientific progress has raised the hope of identifying treatments to halt the progression and prevent the onset of this catastrophic disorder: the discovery of genetic mutations and at least one susceptibility gene that account for many cases of Alzheimer's disease (3, 4); the characterization of molecular events that could be involved in the development and potential treatment of the disorder (3–5); the production of transgenic mice that could be used to further clarify disease mechanisms and screen candidate treatments (3, 4); evidence suggesting that commonly used medications and dietary supplements (e.g., estrogen-replacement therapy, anti-inflammatory medications, and vitamin E) might be associated with a lower risk of Alzheimer's disease and a later onset of dementia (3, 4); and the development of innovative treatments, such as amyloid β-peptide immunization (6), which have the potential to treat and even prevent the disorder. Even if a prevention therapy is only modestly helpful, it could provide an extraordinary public health benefit. For instance, a therapy that delayed the mean onset of Alzheimer's dementia by only 5 years might reduce the risk of the disorder by half (7). By using cited statistics (1, 2) and projection formulas (2, 8) (available at http://www.jhsph.edu/biostats/softrb.html), it is estimated that in almost 50 years, a treatment with this seemingly modest effect might reduce the expected prevalence of Alzheimer's dementia from 16 to 9 million cases and reduce the cost of this disorder from 750 to 425 billion dollars per year (with no adjustment for inflation).
Unfortunately, it would require thousands of research subjects, many years, and great expense to determine whether or when cognitively normal persons treated with a candidate primary prevention therapy develop Alzheimer's dementia. For instance, the Women's Health Initiative Memory Study has enrolled more than 7,500 postmenopausal women 65–79 years of age in a 10-year randomized, double-blind, placebo-controlled study to test the ability of estrogen replacement therapy to decrease the risk of “all-cause” dementia (9). While the investigators recognize the possibility that women might require treatment soon after menopause for estrogen replacement therapy to decrease the risk of Alzheimer's dementia, it would require too many research subjects, too many years, and an extraordinary amount of money to determine whether or when cognitively normal women treated in their 50s and early 60s develop Alzheimer's dementia. One way to reduce the sample sizes and study duration required to assess the efficacy of an Alzheimer's dementia prevention therapy is to conduct what might be considered a secondary prevention study in patients with mild cognitive impairment (MCI), who in one study developed Alzheimer's dementia at the rate of about 12% per year (10). While this strategy is extremely important, it remains possible that subjects would require treatment at an earlier age or preclinical stage of Alzheimer's disease for a candidate prevention therapy to exert its beneficial effects. We have been developing a strategy to test the potential of treatments to prevent Alzheimer's symptoms without having to study thousands of research subjects or wait many years to determine whether or when treated individuals develop cognitive impairment or dementia.
The apolipoprotein E (APOE) ɛ4 allele is an Alzheimer's disease susceptibility gene that accounts for many cases of the disorder. Persons homozygous for the ɛ4 allele comprise 2–3% of the general population (11) and have an especially high risk for Alzheimer's disease (12, 13). Persons heterozygous for the ɛ4 allele comprise about 24% of the general population (11) and also have an increased risk for Alzheimer's disease (12, 13). Positron emission tomography (PET) studies find that patients with Alzheimer's dementia have progressive reductions in posterior cingulate, parietal, temporal, and prefrontal measurements of the cerebral metabolic rate for glucose (CMRgl) (14). As compared with cognitively normal noncarriers of the APOE ɛ4 allele who were matched for gender, age, and educational level, we previously found that cognitively normal ɛ4 homozygotes and ɛ4 heterozygotes 50–63 years of age had abnormally low CMRgl at the time of their baseline scans in the same brain regions as patients with Alzheimer's dementia (15, 16). [The homozygotes also have abnormally low CMRgl in additional prefrontal regions, which appear to be preferentially affected by normal aging (15).] If these CMRgl abnormalities are progressive, and if they reflect preclinical Alzheimer's disease or aging processes necessary for the development of this disorder, PET studies of ɛ4 carriers could provide a relatively rapid way to test treatments to prevent the disorder.
In this study, we characterized longitudinal CMRgl declines in late-middle-aged cognitively normal ɛ4 heterozygotes, determined whether these declines were significantly greater than those in ɛ4 noncarriers, and estimated the number of ɛ4 heterozygotes in this age group needed per active and placebo treatment group to detect attenuation of these CMRgl declines in just 1 or 2 years. Assuming these CMRgl declines are related to the predisposition to Alzheimer's disease, we consider how this paradigm could be used to efficiently test the potential of candidate Alzheimer's prevention therapies.
Methods
Subjects.
Newspaper ads were used to recruit volunteers 50–65 years of age who reported a family history of probable Alzheimer's disease in at least one first-degree relative (15, 16). [A family history of dementia was elicited by using a questionnaire and confirmed in the volunteer's interview with the examining neurologist (R.J.C.).] The participants agreed they would not be given information about their APOE genotype, provided informed consent, and were studied under guidelines approved by human-subjects committees at Good Samaritan Regional Medical Center (Phoenix, AZ) and the Mayo Clinic (Rochester, MN). Venous samples were drawn, leukocytes were isolated, and APOE genotypes were characterized with analysis involving restriction-fragment-length polymorphism (17). Investigators unaware of the subjects' APOE genotypes obtained baseline data from medical and family histories, a neurologic examination, a structured psychiatric interview, the Folstein modified Mini-Mental State Examination (MMSE), a battery of neuropsychological tests, a T1-weighted volumetric magnetic resonance brain image, and a PET CMRgl image in ɛ4 heterozygotes and ɛ4 noncarriers initially matched for gender, age, and educational level. The investigators acquired the same data approximately 2 years later in 10 ɛ4 heterozygotes, and 15 noncarriers 50–63 years of age. (Because follow-up data from clinical ratings and neuropsychological tests were not obtained due to a scheduling error in one ɛ4 heterozygote, who was 54 years of age with an MMSE score of 30 at the time of her baseline PET scan, none of her clinical ratings or test scores were included in the data analysis.)
The 10 ɛ4 heterozygotes included 7 women and 3 men, all with the ɛ3/ɛ4 genotype, were 55.9 ± 3.4 years of age (mean ± SD), and had 15.4 ± 2.9 years of education. The ɛ4 noncarriers included 10 women and 5 men, 10 with the ɛ3/ɛ3 genotype and 5 with the ɛ2/ɛ3 genotype, were 57.1 ± 4.4 years of age, and had 16.1 ± 1.9 years of education. The subjects denied an impairment in memory or other cognitive skills, did not satisfy criteria for a current psychiatric disorder, had no known cardiovascular or cerebrovascular disease, denied use of centrally acting medications for at least 2 weeks before their PET sessions, and had a normal neurologic examination. One subject in each group reported a brief loss of consciousness caused by a closed head injury in the remote past; 4 ɛ4 heterozygotes and 8 ɛ4 noncarriers, all females, reported use of estrogen replacement therapy; 2 subjects in each group reported use of a nonsteroidal anti-inflammatory medication; and 1 subject in each group reported use of vitamin E. The interval between baseline and follow-up PET scans was 2.2 ± 0.2 years in the ɛ4 heterozygotes and 2.5 ± 0.8 years in the ɛ4 noncarriers.
PET Imaging and Image Analysis.
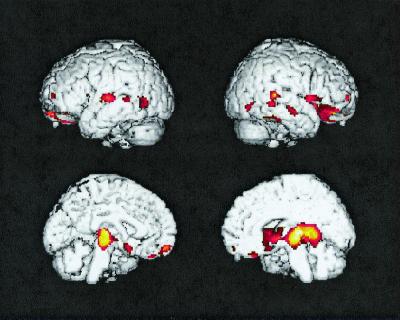
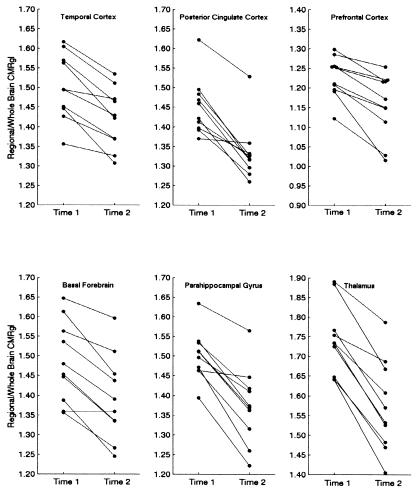
PET was performed as previously described with a 951/31 ECAT scanner (Siemens, Knoxville, TN), a 20-min transmission scan, the i.v. injection of 10 mCi of [18F]fluorodeoxyglucose, and a 60-min dynamic sequence of emission scans as the subjects, who fasted for at least 4 h, lay quietly in a darkened room with their eyes closed and directed forward; PET images were reconstructed from the final 30-min scan by using a back-projection method, a Hanning filter of 0.40 cycle per sec, and a procedure to correct for radiation attenuation (15). Automated algorithms (SPM96, Wellcome Department of Cognitive Neurology, Functional Imaging Laboratory, London) (18) were used to align the sequential PET images from each subject, deform the images into the coordinates of a standard brain atlas (19), normalize PET data for the variation in absolute measurements by proportionate scaling, and generate statistical parametric maps of significant CMRgl declines in the APOE ɛ4 heterozygotes, significant CMRgl declines in the ɛ4 noncarriers, and significantly greater CMRgl declines in the ɛ4 heterozygotes than in the noncarriers (Table 2, P < 0.001, one-tailed and uncorrected for multiple comparisons; Fig. 1). Normalized data from each scan were extracted from the temporal, posterior cingulate, parahippocampal/lingual, prefrontal, basal forebrain, and thalamic locations specified in Table 2; these locations were associated with significant CMRgl declines in the ɛ4 heterozygotes, as well as significantly greater declines in the ɛ4 heterozygotes than in the noncarriers (Fig. 2).
Table 2.
Location and magnitude of maximal declines in regional glucose metabolism
| Region | Atlas
coordinates*
|
Z score† | ||
|---|---|---|---|---|
| x | y | z | ||
| ɛ4 heterozygotes | ||||
| Temporal cortex | 66 | −38 | 8‡ | 4.36 |
| −34 | −24 | 16 | 4.53 | |
| −52 | −40 | 2 | 3.74 | |
| Posterior cingulate cortex | 12 | −46 | 8‡ | 4.32 |
| Prefrontal cortex | 62 | 12 | 6‡ | 4.25 |
| 54 | 30 | −10 | 4.49 | |
| 30 | 62 | 4 | 4.17 | |
| −64 | 14 | 12 | 3.58 | |
| −36 | 56 | 8 | 4.04 | |
| −4 | 60 | −14 | 4.01 | |
| Anterior cingulate cortex | 0 | 22 | 28 | 3.77 |
| 0 | 48 | −2 | 3.22 | |
| Basal forebrain | −8 | 8 | −14‡ | 5.03 |
| Parahippocampal/lingual gyri | 12 | −46 | 4‡ | 4.79 |
| Thalamus | 8 | −22 | 2‡ | 4.97 |
| −6 | −20 | 4 | 4.07 | |
| Lentiform nucleus | 30 | −6 | 4 | 4.70 |
| Caudate nucleus | −8 | 10 | −4 | 3.40 |
| Midbrain | −2 | −24 | −12 | 3.59 |
| ɛ4 noncarriers | ||||
| Posterior cingulate cortex | 8 | −22 | 34§ | 3.92 |
| Parietal cortex | 70 | −34 | 34 | 3.46 |
| 56 | −58 | 32§ | 3.35 | |
| −60 | −30 | 34 | 3.70 | |
| Anterior cingulate cortex | −6 | 38 | 12§ | 3.49 |
| Caudate nucleus | 14 | 2 | 14§ | 3.50 |
| ɛ4 heterozygotes > ɛ4 noncarriers | ||||
| Temporal cortex | 66 | −38 | 12 | 3.69 |
| −50 | −14 | 6 | 4.25 | |
| −52 | −38 | 4 | 3.41 | |
| −36 | −24 | 16 | 3.16 | |
| Occipitotemporal cortex | 42 | −70 | −8 | 3.52 |
| −36 | −62 | 8 | 3.75 | |
| Posterior cingulate | 12 | −46 | 8 | 4.33 |
| Prefrontal cortex | 62 | 12 | 6 | 4.20 |
| 38 | 62 | 6 | 3.78 | |
| 32 | 40 | −14 | 3.89 | |
| −64 | 12 | 12 | 3.46 | |
| −40 | 58 | 8 | 3.44 | |
| −12 | 60 | −14 | 3.60 | |
| Basal forebrain | −8 | 8 | −14 | 4.47 |
| Hippocampal formation | 28 | −36 | −2 | 4.19 |
| Parahippocampal/lingual gyri | 12 | −46 | 4 | 4.88 |
| Fusiform gyrus | −32 | −50 | −10 | 3.20 |
| Thalamus | 6 | −26 | 2 | 5.01 |
| −4 | −22 | 0 | 4.20 | |
| Lentiform nucleus | 30 | −6 | 4 | 3.79 |
| Midbrain | 0 | −24 | −10 | 4.14 |
| Cerebellum | 18 | −48 | −8 | 3.64 |
Coordinates from the atlas of Talairach and Tournoux (19). x is the distance in mm to the right (+) or left (−) of midline; y is the distance anterior (+) or posterior (−) to the anterior commissure, and z is the distance superior (+) or inferior (−) to a horizontal plane through the anterior and posterior commissures.
P < 0.001, uncorrected for multiple comparisons.
Location in which data were extracted from each scan, plotted graphically (Fig. 2), and used to estimate the number of ɛ4 heterozygotes needed to test the potential efficacy of Alzheimer's prevention therapies (Table 3).
Location in which data were extracted from each scan and used to estimate the number of ɛ4 noncarriers needed to detect an attenuation in age-related CMRgl decline (Table 3).
Figure 1.
Statistical parametric map of significantly greater 2-year declines in regional CMRgl in cognitively normal APOE ɛ4 heterozygotes than in ɛ4 noncarriers (P < 0.001, uncorrected for multiple comparisons). Significant CMRgl declines (in color) are superimposed onto the left lateral, right lateral, left medial, and right medial surfaces of a spatially standardized volume-rendered MRI.
Figure 2.
Two-year declines in regional CMRgl in cognitively normal APOE ɛ4 heterozygotes. These data were extracted from locations indicated in Table 2 and used to estimate the number of subjects needed to test the potential efficacy of Alzheimer's prevention therapies shown in Table 3.
Power analyses (20) (stplan software, Department of
Biomathematics, Univ. of Texas, Houston) were performed on the
extracted data, shown in Fig. 2, to estimate the number of ɛ4
heterozygotes needed in a 2-year, randomized, double-blind,
placebo-controlled study to test the efficacy of a candidate therapy to
attenuate CMRgl declines (i.e., to evaluate its potential to prevent
Alzheimer's disease-related cognitive decline and dementia). More
specifically, we calculated the number of ɛ4 heterozygotes per active
treatment and placebo control group needed to detect 50%, 33%, and
25% attenuations in CMRgl declines with 80% power using unpaired
t tests and P = 0.005, one-tailed and
uncorrected for multiple comparisons in the preferentially affected
brain regions (Table 3). Calculations were performed iteratively by
using the formula n ≥
2S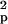 [t(α, ν) +
t(β,
ν)]2/(x̄1
− x̄2)2,
where n is the estimated sample size,
S
[t(α, ν) +
t(β,
ν)]2/(x̄1
− x̄2)2,
where n is the estimated sample size,
S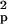 is the pooled variance, α is the
significance level, β is 1 − power, ν is the degrees of
freedom related to the estimated sample size, t(α,
ν) and t(β, ν) are the t values, and
x̄1 and
x̄2 are the mean CMRgl
differences in the active treatment and placebo groups.
is the pooled variance, α is the
significance level, β is 1 − power, ν is the degrees of
freedom related to the estimated sample size, t(α,
ν) and t(β, ν) are the t values, and
x̄1 and
x̄2 are the mean CMRgl
differences in the active treatment and placebo groups.
Table 3.
Number of cognitively normal persons per group needed for PET to detect a treatment effect (i.e., an attenuation in CMRgl decline) in 2 years
| Region | Treatment
effect*
|
||
|---|---|---|---|
| 25% | 33% | 50% | |
| APOE ɛ4 heterozygotes | |||
| Temporal cortex | 100 | 57 | 27 |
| Posterior cingulate cortex | 84 | 48 | 22 |
| Prefrontal cortex | 115 | 66 | 29 |
| Basal forebrain | 107 | 62 | 29 |
| Parahippocampal gyrus | 84 | 48 | 22 |
| Thalamus | 50 | 29 | 14 |
| APOE ɛ4 noncarriers | |||
| Posterior cingulate cortex | 289 | 165 | 73 |
| Parietal cortex | 244 | 139 | 62 |
| Anterior cingulate cortex | 233 | 136 | 60 |
| Caudate nucleus | 207 | 120 | 53 |
The upper part of the table gives the estimated number of cognitively normal 50- to 63-year-old APOE ɛ4 heterozygotes per active treatment and placebo group needed to test the potential of a candidate Alzheimer's prevention therapy (i.e., detect an attenuation in CMRgl decline) in 2 years. The lower part gives the estimated number of APOE ɛ4 noncarriers per group needed to detect an attenuation in age-related CMRgl decline.
80% power and P = 0.005 (one-tailed and uncorrected for the number of resolution elements in the implicated brain regions).
Results
There were no significant differences between the ɛ4 heterozygotes and ɛ4 noncarriers in gender, age, years of education, or duration between scans. MMSE and neuropsychological test scores at the time of the baseline and follow-up scans are shown in Table 1. There were no significant differences between the subject groups in scores on the MMSE or neuropsychological tests at the time of either scan, no significant declines in these scores from time 1 to time 2 in either group, and no significant Group × Time interactions.
Table 1.
Clinical ratings and neuropsychological test scores
| Test | ɛ4
heterozygotes
|
ɛ4 noncarriers
|
Group P | Group × Time P | ||||
|---|---|---|---|---|---|---|---|---|
| Time 1 | Time 2 | P | Time 1 | Time 2 | P | |||
| MMSE | 29.8 ± 0.7 | 29.8 ± 0.4 | 1.00 | 29.7 ± 0.7 | 29.9 ± 0.3 | 0.33 | 0.72 | 0.56 |
| AVLT | ||||||||
| Total learning | 51.4 ± 6.7 | 55.7 ± 4.9 | 0.11 | 49.5 ± 6.2 | 52.2 ± 5.3 | 0.08 | 0.21 | 0.55 |
| Short-term recall | 10.9 ± 1.3 | 10.2 ± 2.1 | 0.50 | 10.0 ± 1.9 | 10.0 ± 2.5 | 1.0 | 0.46 | 0.47 |
| Long-term recall | 10.7 ± 2.0 | 9.4 ± 2.0 | 0.25 | 9.5 ± 2.6 | 9.9 ± 2.5 | 0.59 | 0.63 | 0.19 |
| Complex Figure Test | ||||||||
| Copy | 33.9 ± 3.1 | 35.0 ± 1.0 | 0.34 | 34.6 ± 2.2 | 33.7 ± 3.5 | 0.37 | 0.75 | 0.20 |
| Recall | 20.2 ± 7.2 | 21.8 ± 6.2 | 0.33 | 17.4 ± 6.9 | 19.9 ± 7.3 | 0.13 | 0.39 | 0.71 |
| Boston Naming Test | 57.3 ± 2.2 | 57.9 ± 1.8 | 0.43 | 56.5 ± 4.0 | 57.1 ± 2.1 | 0.39 | 0.44 | 0.92 |
| WAIS—R | ||||||||
| Information | 12.3 ± 1.8 | 12.6 ± 2.1 | 0.68 | 11.5 ± 2.3 | 12.1 ± 2.5 | 0.014 | 0.51 | 0.44 |
| Digit span | 11.6 ± 2.2 | 10.8 ± 2.0 | 0.19 | 11.6 ± 2.0 | 11.5 ± 3.1 | 0.92 | 0.66 | 0.48 |
| Block design | 11.3 ± 2.7 | 13.0 ± 2.4 | 0.042 | 11.3 ± 2.6 | 12.0 ± 3.5 | 0.16 | 0.65 | 0.27 |
| Mental arithmetic | 11.7 ± 2.7 | 12.3 ± 2.3 | 0.33 | 11.2 ± 3.2 | 11.1 ± 2.8 | 0.89 | 0.47 | 0.36 |
| Similarities | 12.1 ± 1.5 | 11.9 ± 1.9 | 0.75 | 12.9 ± 2.4 | 12.3 ± 2.3 | 0.35 | 0.46 | 0.73 |
| COWA | 44.4 ± 8.9 | 48.9 ± 8.3 | 0.032 | 44.2 ± 11.4 | 42.5 ± 8.8 | 0.46 | 0.34 | 0.07 |
The Folstein MMSE is a dementia rating scale; the Auditory Verbal Learning Memory Test (AVLT) assesses verbal learning and recall; the Complex Figure Test assesses constructional praxis and visuospatial memory; the Boston Naming Test assesses visual naming; the Information, Digit span, Mental arithmetic, Similarities, and Block design subtests of the Wechsler Adult Intelligence Scale—Revised (WAIS—R) assess general intellect, attention, abstraction skills, psychomotor speed, and spatial skills; and the Controlled Oral Word Association Test (COWA) assesses verbal associative fluency and psychomotor speed. Scores at times 1 and 2 are means ± SD. Analyses were performed with a Group × Time repeated-measures analysis of variance. Pairwise comparisons for time in each group were performed with paired t tests and are presented to emphasize the lack of a significant decline in either group.
In the absence of cognitive alterations, the ɛ4 heterozygotes had significant CMRgl declines from time 1 to time 2 in the vicinity of temporal cortex, posterior cingulate cortex, prefrontal cortex, basal forebrain, parahippocampal/lingual gyri, and thalamus, and these declines were significantly greater than those in the ɛ4 noncarriers (Table 2, Fig. 1, and Fig. 2). Although smaller in magnitude, the ɛ4 noncarriers had significant declines in posterior cingulate cortex, parietal cortex, anterior cingulate cortex, and the caudate nucleus (Table 2). (Means and standard deviations of the CMRgl declines are available on request.)
To test a candidate treatment's potential in the primary prevention of Alzheimer's symptoms, we estimate from our power analyses that between 50 and 115 cognitively normal ɛ4 heterozygotes 50–63 years of age per active and placebo treatment group are needed to detect a 25% attenuation in CMRgl decline in temporal cortex, posterior cingulate cortex, basal forebrain, parahippocampal/lingual gyri, and thalamus with 80% power and P = 0.005, one-tailed and uncorrected for multiple comparisons in these six postulated regions, in just 2 years (Table 3). Assuming a linear rate of CMRgl decline and no change in the variability of this decline, the same number of cognitively normal ɛ4 heterozygotes per active and placebo treatment group would be needed to detect a 50% attenuation in CMRgl declines with 80% power and P = 0.005 in a single year. In addition, we estimate that between 207 and 289 cognitively normal ɛ4 noncarriers 50–63 years of age per active and placebo treatment group are needed to detect a 25% attenuation in age-related CMRgl declines in anterior cingulate cortex, parietal cortex, posterior cingulate cortex, and the caudate nucleus with 80% power and P = 0.005 in 2 years.
Discussion
We previously found that cognitively normal late-middle-aged carriers of a common Alzheimer's disease susceptibility gene have abnormally low CMRgl at the time of their baseline PET scans in the same brain regions as patients with Alzheimer's dementia (15, 16). We now find that regional CMRgl continues to decline in these individuals during the 2-year interval between their baseline and follow-up scans. The decline in regional PET measurements is found in APOE ɛ4 heterozygotes (who constitute almost one-fourth of the population), precedes any evidence of cognitive decline, and is significantly greater than that in ɛ4 noncarriers. Using the maximal rate of CMRgl decline in each of the six preferentially affected brain regions, we estimate that between 50 and 115 cognitively normal ɛ4 heterozygotes are needed per active and placebo treatment group to test the efficacy of candidate Alzheimer's prevention therapies to detect a 25% attenuation in these CMRgl declines in just 2 years. Assuming these CMRgl declines are related to the predisposition to Alzheimer's disease, we propose that this paradigm could be used to efficiently test the potential of treatments to prevent cognitive impairment and dementia.
During the 2-year interval between the baseline and follow-up scans, the ɛ4 heterozygotes had significant CMRgl declines in the vicinity of temporal, posterior cingulate, prefrontal, basal forebrain, parahippocampal/lingual gyri, and thalamus, and these declines were significantly greater than those in the ɛ4 noncarriers. Abnormal CMRgl reductions in temporal, posterior cingulate, and prefrontal cortex have been consistently found in PET studies of patients with Alzheimer's dementia and nondemented ɛ4 carriers (14–16). Histopathological changes in basal forebrain and the parahippocampal gyrus are found early in the course of Alzheimer's disease (21). Although reductions in thalamic CMRgl might not have been expected in persons affected by or at risk for Alzheimer's disease, a circuit linking the anterior thalamus, posterior cingulate cortex, and hippocampal formation has been implicated in an animal model of discriminative learning (22); CMRgl was preferentially reduced in several thalamic nuclei (and the posterior cingulate cortex) in a [18F]fluorodeoxyglucose autoradiographic study of aged transgenic mice overexpressing a mutant form of the amyloid precursor protein associated with a form of early-onset Alzheimer's disease (23); and abnormal reductions in thalamic, posterior and anterior cingulate, and hippocampal perfusion in patients with questionable Alzheimer's disease predicted their subsequent conversion to probable Alzheimer's disease (24). The CMRgl declines observed in ɛ4 noncarriers with a reported family history of probable Alzheimer's disease may be related to normal aging, the earliest preclinical stages of Alzheimer's disease, or their combination (15). Studies of ɛ4 noncarriers without a reported family history of Alzheimer's disease could help distinguish those changes associated with normal aging from those associated with an early preclinical stage of the disorder.
In complementary PET studies, Small and his colleagues used PET to study nondemented ɛ4 carriers and ɛ4 noncarriers with memory concerns (25, 26). The ɛ4 carriers in their most recent study were 66 ± 9 years of age, had baseline MMSE scores of 28.4 ± 1.6, included persons with and without a reported family history of Alzheimer's dementia, and had abnormally low CMRgl at the time of their baseline PET scans in the same brain regions as patients with Alzheimer's dementia (26). Some of the baseline PET abnormalities were correlated with (i.e., predictive of) subsequent declines in neuropsychological test scores. Like our ɛ4 carriers, the ɛ4 carriers in their study had progressive declines in regional CMRgl during the 2-year interval between their baseline and follow-up scans. Together, these studies strongly suggest that PET could be used to track the progression of Alzheimer's disease and test the potential of candidate primary prevention therapies in nondemented persons at risk for the disorder.
In our original cross-sectional studies (15, 16) and this longitudinal study, PET detected declines in regional brain function in late-middle-aged APOE ɛ4 carriers in the absence of any detectable cognitive impairment. The dissociation between neurophysiological and cognitive findings in our study could be attributable to differences in the sensitivity of the brain imaging and psychometric methods used, neurophysiological effects that are not sufficient to cause cognitive declines, or compensatory processes that mask any cognitive effects.
We have not yet compared the longitudinal decline in PET measurements of regional CMRgl to longitudinal changes in MRI measurements of regional or whole brain atrophy (27, 28) or other potential markers in terms of their ability to track the progression of Alzheimer's disease before the onset of symptoms or test treatments to prevent the disorder; nor have we determined how some of these measures might be combined to track Alzheimer's disease and test prevention therapies with even greater statistical power. We have not yet determined how declines in different PET and MRI measurements are differentially related to age, preclinical and clinical stages of Alzheimer's disease, or their interaction. Finally, we have not yet determined the extent to which our findings in ɛ4 heterozygotes with a reported family history of probable Alzheimer's disease can be generalized to ɛ4 heterozygotes who do not report this family history; however, similar PET findings were reported in a group of ɛ4 carriers with and without a reported family history (26).
We propose that PET (and, when better established, other surrogate markers of Alzheimer's disease progression) could be used in randomized clinical trials of cognitively normal ɛ4 carriers to test the potential of treatments to prevent Alzheimer's disease. Not only does this approach provide a cost-effective way to test candidate prevention therapies, it also provides a way to identify those treatments that might be more beneficial when administered in late middle age to cognitively normal persons than when administered at older ages or in the more advanced preclinical or clinical stages of Alzheimer's disease. Further support for the use of PET to identify the potential of treatments to prevent Alzheimer's disease would come from studies in which short-term longitudinal CMRgl declines in persons at risk for Alzheimer's disease predict the subsequent onset of dementia.
Before PET measurements or other biological markers are used in normal APOE ɛ4 carriers to test the potential of candidate treatments in the primary prevention of Alzheimer's disease-related cognitive impairment and dementia, several potential limitations must be recognized. First, positive or negative findings from a prevention study in ɛ4 carriers may not be generalizable to ɛ4 noncarriers, who account for nearly half of persons with Alzheimer's dementia (29). [Still, new discoveries (30, 31) could increase the spectrum of cognitively normal persons at risk for Alzheimer's disease who are eligible for such prevention studies.] The CMRgl declines do not appear to correspond to aspects of the APOE ɛ4 allele unrelated to the predisposition to Alzheimer's disease: ɛ4 carriers and noncarriers with Alzheimer's dementia have similar declines in regional CMRgl (32); since the CMRgl declines are progressive, they appear to reflect an interaction between the APOE ɛ4 allele and aging rather than a static trait; CMRgl deficits in some of the implicated brain regions predicted the subsequent onset of cognitive decline in older ɛ4 carriers with memory concerns (26); and perfusion deficits in some of the implicated brain regions predicted the subsequent development of Alzheimer's dementia in persons with very mild cognitive impairment, irrespective of their APOE genotype (24).
Second, although it is well established that PET CMRgl measurements provide a neurophysiological marker of disease progression in Alzheimer's dementia (14), it remains to be confirmed that an attenuation in CMRgl decline before the onset of clinical symptoms is actually associated with a decreased risk of Alzheimer's disease symptoms and histopathology. The CMRgl reductions in APOE ɛ4 carriers could provide a preclinical marker of Alzheimer's disease progression (15), in which case declines in regional brain function should predict the subsequent development of cognitive impairment and dementia. This possibility is supported by the CMRgl study of older APOE ɛ4 carriers with memory concerns (26) and the cerebral perfusion study of persons with very mild cognitive impairment (24). Alternatively, the CMRgl reductions in ɛ4 carriers could be related to an acceleration in aging processes which are necessary but not sufficient for Alzheimer's disease (15), in which case CMRgl reductions might be observed in all of our ɛ4 carriers, even though at least half of ɛ4 carriers with a family history of Alzheimer's disease are unlikely to develop dementia. Even if the CMRgl declines are necessary but not sufficient for dementia, an attenuation in CMRgl declines could be used to test the potential of Alzheimer's prevention therapies.
Finally, APOE genotypes and PET measurements are not yet clinically indicated to predict a cognitively normal person's risk of developing Alzheimer's symptoms: this information cannot yet accurately predict whether or when someone might develop symptoms; and prevention therapies have not yet been identified that might outweigh the psychological or social risks involved in making predictions about such a catastrophic illness. Researchers and ethicists need to consider ways to address the risks of providing genetic information to potential research subjects in future Alzheimer's prevention studies.
Despite these limitations, our strategy promises to play an important role in the effort to identify treatments to prevent the cognitive and behavioral symptoms of Alzheimer's disease. We hope that this study, the potential public health benefits of an Alzheimer's dementia prevention therapy, and the impediments related to the performance of large, time-consuming, and expensive clinical trials in cognitively normal persons will encourage researchers, clinicians, pharmaceutical companies, and public policy makers to clarify the role that this strategy should play in testing Alzheimer's disease prevention therapies.
This study provides a paradigm for characterizing the potential of treatments to prevent Alzheimer's disease without having to study thousands of research subjects or wait many years to determine whether or when treated individuals develop symptoms.
Acknowledgments
We thank Stephen Thibodeau for determining the APOE genotypes, David Osborne for neuropsychological testing, Lawrence Mayer for statistical advice, and Sandy Goodwin, Les Mullen, Tricia Giurlani, David Stith, Christine Burns, Jonathon Cong, Lindsay Herseth, Carolyn Barbieri, Anita Palant, and Debbie Intorcia for technical assistance. This study was supported by grants from the National Institutes of Health (MH57899–01A1), the Samaritan and Mayo Clinic Foundations, and the Arizona Center for Alzheimer's Disease Research.
Abbreviations
- PET
positron emission tomography
- APOE
apolipoprotein E
- CMRgl
cerebral metabolic rate for glucose
- MMSE
Mini-Mental State Examination
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Evans D A, Funkenstein H H, Albert M S, Scherr P A, Cook N R, Chown M J, Hebert L E, Hennekens C H, Taylor J O. J Am Med Assoc. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 2.Brookmeyer R, Gray S, Kawas C. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiman E M, Caselli R J. Maturitas. 1999;31:185–200. doi: 10.1016/s0378-5122(98)00110-8. [DOI] [PubMed] [Google Scholar]
- 4.Terry R D, Katzman R, Bick K L, Sisodia S S, editors. Alzheimer Disease. Philadelphia: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 5.Vassar R, Bennett B D, Babu-Khan S, Kahn S, Mendiaz E A, Denis P, Teplow D B, Ross S, Amarante P, Loeloff R, et al. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 6.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Nature (London) 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 7.Khachaturian Z S. Neurobiol Aging. 1992;13:197–198. doi: 10.1016/0197-4580(92)90030-2. (editorial). [DOI] [PubMed] [Google Scholar]
- 8.Brookmeyer R, Gray S. Stat Med. 2000;19:1481–1493. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1481::aid-sim440>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Shumaker S A, Reboussin B A, Espeland M A, Rapp S R, McBee W L, Dailey M, Bowen D, Terrell T, Jones B N. Control Clin Trials. 1998;19:604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 10.Petersen R C, Smith G E, Waring S C, Ivnik R J, Tangalos E G, Kokmen E. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 11.Mahley R W. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 12.Corder E H, Saunders A M, Strittmatter W J, Schmechel D E, Gaskell P C, Small G W, Roses A D, Haines J L, Pericak-Vance M A. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 13.Saunders A M, Strittmatter W J, Schmechel D, George-Hyslop P H, Pericak-Vance M A, Joo S H, Rosi B L, Gusella J F, Crapper-MacLachlan D R, Alberts M J. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 14.Minoshima S, Giordani B, Berent S, Frey K A, Foster N L, Kuhl D E. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 15.Reiman E M, Caselli R J, Yun L S, Chen K, Bandy D, Minoshima S, Thibodeau S N, Osborne D. New Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 16.Reiman E M, Caselli R J, Yun L S, Chen K, Bandy D. In: Fourth Annual Flinn Foundation Biomedical Research Symposium, Tucson, AZ. Reiman E M, Read W A, editors. Phoenix, AZ: Flinn Foundation; 1998. p. 19. , abstr. 21. [Google Scholar]
- 17.Hixson J E, Vernier D T. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 18.Frackowiak R S J, Friston K J, Frith C D, Dolan R J, Mazziotta J C, editors. Human Brain Function. San Diego: Academic; 1997. [Google Scholar]
- 19.Talairach J, Tournoux P. Co-Planar Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 20.Mace A E. Sample Size Determination. Huntington, NY: Krieger; 1974. [Google Scholar]
- 21.Braak H, Braak E. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel M, Sparenborg S P, Stolar N. Exp Brain Res. 1987;67:131–152. doi: 10.1007/BF00269462. [DOI] [PubMed] [Google Scholar]
- 23.Reiman E M, Uecker A, Gonzalez-Lima F, Minear D, Chen K, Callaway N L, Berndt J D, Games D. NeuroReport. 2000;11:987–991. doi: 10.1097/00001756-200004070-00018. [DOI] [PubMed] [Google Scholar]
- 24.Johnson K A, Jones K, Holman B L, Becker J A, Spiers P A, Satlin A, Albert M S. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- 25.Small G W, Mazziotta J C, Collins M T, Baxter L R, Phelps M E, Mandelkern M A, Kaplan A, La Rue A, Adamson C F, Chang L, et al. J Am Med Assoc. 1995;273:942–947. [PubMed] [Google Scholar]
- 26.Small G W, Ercoli L M, Silverman D H, Huang S-C, Komo S, Bookheimer S Y, Lavretsky H, Miller K, Siddarth P, Rasgon N L, et al. Proc Natl Acad Sci USA. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. . (First Published May 16, 2000; 10.1073/pnas.090106797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiman E M, Uecker A, Caselli R J, Lewis S, Bandy D, de Leon M J, De Santi S, Convit A, Osborne D, Weaver A, et al. Ann Neurol. 1998;44:288–291. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- 28.Fox N, Freeborough P, Rossor M. Lancet. 1998;348:94–97. doi: 10.1016/s0140-6736(96)05228-2. [DOI] [PubMed] [Google Scholar]
- 29.Rubinsztein D C, Easton D F. Dement Geriatr Cogn Disord. 1999;10:199–209. doi: 10.1159/000017120. [DOI] [PubMed] [Google Scholar]
- 30.Bertram L, Blacker D, Mullin K, Keeney D, Jones J, Basu S, Yhu S, McInnis M G, Go R C, Vekrellis K, et al. Science. 2000;290:2302–2303. doi: 10.1126/science.290.5500.2302. [DOI] [PubMed] [Google Scholar]
- 31.Myers A, Holmans P, Marshall H, Kwon J, Meyer D, Ramic D, Shears S, Booth J, DeVrieze F W, Crook R, et al. Science. 2000;290:2304–2305. doi: 10.1126/science.290.5500.2304. [DOI] [PubMed] [Google Scholar]
- 32.Corder E H, Jelic V, Basun H, Lannfelt L, Valind S, Winblad B, Nordberg A. Arch Neurol. 1997;54:273–277. doi: 10.1001/archneur.1997.00550150035013. [DOI] [PubMed] [Google Scholar]