Abstract
Marine microorganisms are presented with unique challenges to obtain essential metal ions required to survive and thrive in the ocean. The production of organic ligands to complex transition metal ions is one strategy to both facilitate uptake of specific metals, such as iron, and to mitigate the potential toxic effects of other metal ions, such as copper. A number of important trace metal ions are complexed by organic ligands in seawater, including iron, cobalt, nickel, copper, zinc, and cadmium, thus defining the speciation of these metal ions in the ocean. In the case of iron, siderophores have been identified and structurally characterized. Siderophores are low molecular weight iron-binding ligands produced by marine bacteria. Although progress has been made toward the identity of in situ iron-binding ligands, few compounds have been identified that coordinate the other trace metals. Deciphering the chemical structures and production stimuli of naturally produced organic ligands and the organisms they come from is fundamental to understanding metal speciation and bioavailability. The current evidence for marine ligands, with an emphasis on siderophores, and discussion of the importance and implications of metal-binding ligands in controlling metal speciation and cycling within the world’s oceans are presented.
Keywords: iron, cobalt, nickel, copper, zinc, cadmium
INTRODUCTION TO TRANSITION METAL IONS IN THE OCEAN
Metals are required in a vast array of enzymatic reactions carried out in marine organisms, including bacterioplankton, phytoplankton, fungi, and macroalgae. The chemistry and speciation of metals in the oceans may largely control metal bioavailability. The speciation of many metals seems to be controlled by biogenic ligands. The sources of these ligands and the purposes for which they are produced appear to be metal ion specific (Bruland et al. 1991). Whereas some organisms are able to substitute metal ions when there is a paucity of a certain metal ion, or even substitute an organic cofactor for a metal ion (Doucette et al. 1996, La Roche et al. 1996), others have absolute growth requirements for a specific metal ion.
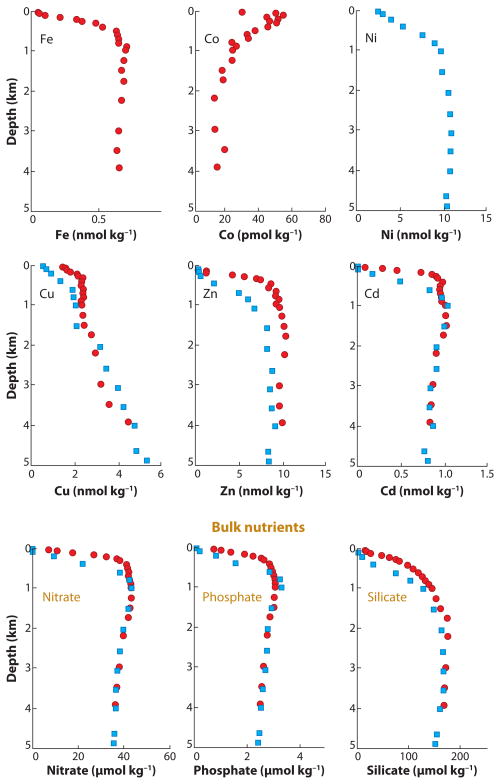
A current goal is to determine the link between the conditional stability constants of metals determined from native seawater and the biota that may be producing the metal-complexing ligands. Conditional stability constants are a measure of how tightly a ligand coordinates a metal ion. In the absence of structural characterization of the ligands, conditional stability constants provide the only means to distinguish between different metal-ligand complexes in the ocean. Transition metal ions that are complexed by organic ligands in seawater include iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), zinc (Zn), and cadmium (Cd). Most transition metal ions in the ocean display nutrient-like distributions, as shown in Figure 1, and are used by microorganisms living in surface waters.
Figure 1.
Depth profiles of metal ions discussed in this review as well as depth profiles of representative bulk nutrients for comparison. Data collected in the North Pacific Ocean and the Gulf of Alaska. Data points in blue squares are from Bruland (1980); data points in red circles are from Martin et al. (1989). Please note the different scales for concentration. The speciation of the metal ions is not included.
Most metals, including iron, are present at picomolar to nanomolar concentrations, which is approximately one-millionth of the cellular concentrations in the plankton (Morel & Price 2003). In oceanic waters, iron, as is the case for many trace metals, is increasingly depleted as water depth decreases (Martin et al. 1989, Morel & Price 2003) (Figure 1). This nonconservative (nutrient-like) distribution in the oceans, with very low concentrations in surface waters and increasing at depth, is a product of primary production and physical processes. Owing to a scarcity of iron and other trace metals in the photic zone, the growth of planktonic microorganisms, including phytoplankton, cyanobacteria, and heterotrophic bacteria, is limited. The unique challenges of a marine environment and a constant battle to attain trace metals for growth and fend off their toxic effects must have evolutionarily equipped marine organisms with methods of survival. The vast majority of Fe(III) in surface seawater is complexed to organic ligands, which might be a possible method of survival. Although phytoplankton are larger than heterotrophic bacteria, some studies find that marine bacteria have a larger overall biomass and higher intracellular iron concentration (Tortell et al. 1999). For heterotrophic bacteria to successfully compete with other pelagic microorganisms that have a greater surface area, bacteria must have an effective method for sequestering iron for growth and reproduction.
We present herein the current evidence for marine ligands, with an emphasis on siderophores (see section on Siderophores), and discuss the importance and implications of metal-binding ligands in controlling metal speciation and cycling within the world’s oceans.
THE CASE FOR Fe(III)-SPECIFIC MARINE LIGANDS
Fe(III)-Ligand
Importance of ligands in the ocean
Iron is one of the most abundant elements in terrestrial environments. Iron-containing metalloenzymes are essential for many life processes, including photosynthesis, respiration, and nitrogen fixation. Iron serves as a redox catalyst in photosynthetic and respiratory electron transport chains. The respiratory chain alone contains approximately 94% of the cellular iron associated with NADH-Q reductase, succinate-Q reductase, cytochrome b1, and cytochrome oxidase (Tortell et al. 1999). Iron has robust effects on the nitrogen cycle because it is involved in the catalysis of all nitrogen redox transformations (Butler 1998, Morel & Price 2003). For example, iron is important for nitrate reductase, found in both phytoplankton and heterotrophic bacteria, and chlorophyll biosynthesis (Rawls 1996, Tortell et al. 1999).
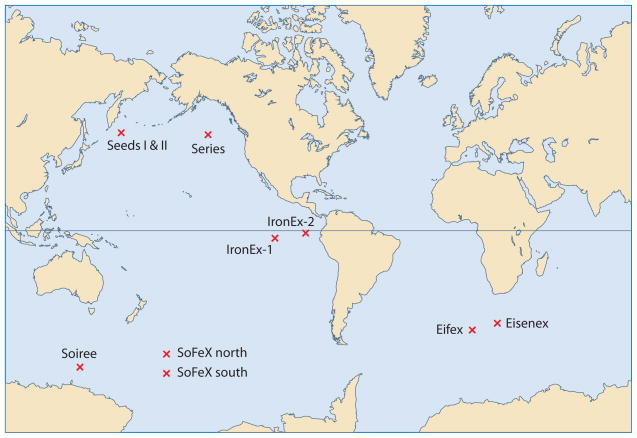
Iron(III) levels in oceanic surface waters are extremely low (i.e., 0.02 to 1 nM), with more than 99% bound to organic ligands. This low concentration of Fe(III) is believed to chronically limit primary productivity by phytoplankton and cyanobacteria in a significant portion of the world’s oceans (Behrenfeld et al. 1996, de Baar et al. 1995, Gledhill & van den Berg 1994, Rue & Bruland 1995, van den Berg 1995, Wu & Luther 1995). Iron is a limiting growth nutrient in seawater, in part as a result of the low solubility of Fe(III) in aqueous aerobic conditions, as well as low levels of input. Both mesoscale iron-enrichment experiments and mesocosm bottle experiments have confirmed the iron hypothesis, that the naturally low concentration of iron limits primary productivity, even in areas replete in bulk nutrients (e.g., nitrate, phosphate, and silicate) (Coale et al. 1996, Martin et al. 1994). The subarctic Pacific, the equatorial Pacific, and the Southern Ocean are replete in macronutrients year round but have low phytoplankton growth and corresponding low levels of chlorophyll. These high-nutrient, low-chlorophyll (HNLC) regions make up more than 20% of the total area of the oceans. Ten in situ iron addition experiments have been carried out in the three major HNLC regions to test the iron hypothesis (Figure 2).
Figure 2.
World map showing the locations of the ten major iron addition experiments completed thus far. References for studies shown are as follows: IronEx-1 (Martin et al. 1994), IronEx-2 (Coale et al. 1996), SOIREE (Boyd et al. 2000), Eisenex (Gervais et al. 2002), SEEDS I (Tsuda et al. 2003), SEEDS II (Roy et al. 2008), SoFeX North (Coale et al. 2004), SoFeX South (Coale et al. 2004), SERIES (Boyd et al. 2004), and Eifex (Hoffmann et al. 2006).
Although variability exists in the magnitude of the response to iron addition, chlorophyll a concentration, a proxy for specific growth rate, increased in all experiments. Iron availability cycles with the time of year, increasing after times of upwelling during winter months. The nadir coincides with the diatom spring bloom; nevertheless, in most oceanic regions the surface waters in the photic zone remain persistently iron depleted.
Evidence for Fe(III) ligands in the oceans
At the slightly basic pH of oxic seawater, iron has very low solubility. A recent study subdivided iron into five fractions or phases of iron: soluble (<0.03 μm), dissolved (<0.22μm), total dissolved (acidified dissolved, <0.22μm), labile (unfiltered), and total (acidified, unfiltered) (Wong et al. 2006). The majority of iron in seawater resides in the particulate phase, whereas greater than 99% of dissolved Fe(III) is bound by organic ligands (Hutchins et al. 1999a, Rue & Bruland 1995, Wu & Luther 1995). Fe(III)-binding ligands have a range of stability constants (Table 1) and can either be addressed as one pool of ligands, L, (Hutchins et al. 1999a, Rue & Bruland 1995, Wu & Luther 1995) or as two separate classes, L1 and L2 (Hutchins et al. 1999a, Rue & Bruland 1995, Wu & Luther 1995). L1 (~0.44 nM), found predominantly in the surface waters, has a higher conditional stability constant, whereas L2 (~1.5 nM), found throughout the water column, is present at higher concentrations but with a lower conditional stability constant (Table 1).
Table 1.
Representative conditional stability constants and ligand concentrations for iron (III) binding ligands
| Location | Values measured | Method, competing liganda | Reference | |||
|---|---|---|---|---|---|---|
| Dissolved Fe concentration | Ligand concentration (nM) | log K′Fe-(L)b | log KFe3+-Lb | |||
| Central North Pacific (surface waters) | 0.2 nM | L1: 0.44 L2: 1.5 |
L1: 13.08 L2: 11.48 |
CSV, SA | (Rue & Bruland 1995) | |
| Atlantic Ocean | 0.8–1.8 nMc | 3.0–4.9 | 18.8–19.7 | CSV, NN | (Gledhill & van den Berg 1994) | |
| Southern Ocean | 0.1–0.6 nM | 0.72 ± 0.23 | 22.1 ± 0.5 | CSV, NN | (Boye et al. 2001) | |
| Northwest Atlantic Ocean | <0.4 μM | 0.45–0.60 ± 0.20 | >23.22 | CSV, NN | (Wu & Luther 1995) | |
Abbreviations: CSV, cathodic stripping voltammetry; SA, salicylaldoxime; NN, 1-nitroso-2-napthol.
An ~1010 conversion factor relates log K′FeL to log KFe3+-L.
Values vary by depth and station: see Gledhill & van den Berg (1994).
Various iron enrichment studies have examined the presence and concentrations of organic ligands. The second equatorial Pacific iron enrichment experiment, IronEx-2, showed a rapid, fourfold increase in the concentration of ligand (Rue & Bruland 1997). During the Subarctic Ecosystem Response to Iron Enrichment Study (SERIES) iron fertilization experiment, a phase shift occurred in the first week from the colloidal portion of the dissolved iron to labile particulate iron (Wong et al. 2006). Immediately afterward, a physical event resulted in a reduction in the nonlabile particulate iron due to sinking out of the patch. After the second iron injection, the labile particulate pool decreased substantially with a coincident decrease in silicate, which would indicate utilization of this size fraction of iron for diatom growth (Wong et al. 2006). The general decrease in colloidal iron was thought to be a product of aggregation of oxyhydroxides, utilization by mixotrophic phytoplankton, and adsorption to plankton cell surfaces (Wong et al. 2006), or it could be due to sequestration by organic ligands, such as those identified during IronEx-2. The dominance of diatoms could also be due to their ability to extracellularly reduce Fe(III)-ligand complexes during iron uptake (Morel & Price 2003).
Siderophores
Marine bacterial siderophores
Virtually all bacteria, including marine autotrophic (cyanobacteria) and heterotrophic bacteria, require iron for growth. Although it is generally thought that bacteria in common terrestrial environments need approximately micromolar iron concentrations for growth (Lankford 1973), the iron requirements for oceanic bacteria are not well defined. In response to low iron aerobic conditions, bacteria often secrete siderophores to solubilize and sequester Fe(III) as one of several possible strategies to facilitate iron uptake. Siderophores are low molecular weight organic ligands that complex Fe(III) with high affinity. An important part of the siderophore-mediated iron uptake process is the concomitant production of outer membrane receptor proteins that recognize a specific ferric siderophore complex with a remarkable degree of selectivity. The transport of iron across both the outer membrane and inner membrane is an energy-dependent process coupled to ATP hydrolysis (Armstrong et al. 2004, Martinez & Butler 2007, Reid & Butler 1991). The biosyntheses of both siderophores and their outer membrane receptor proteins are repressed when the intracellular iron demand is satisfied. As Fe(III) chelators, the marine siderophores could form a portion of the iron binding ligands found in seawater, and as such play critical roles in iron cycling and speciation and an indirect yet vital part in the global carbon cycle.
Structures
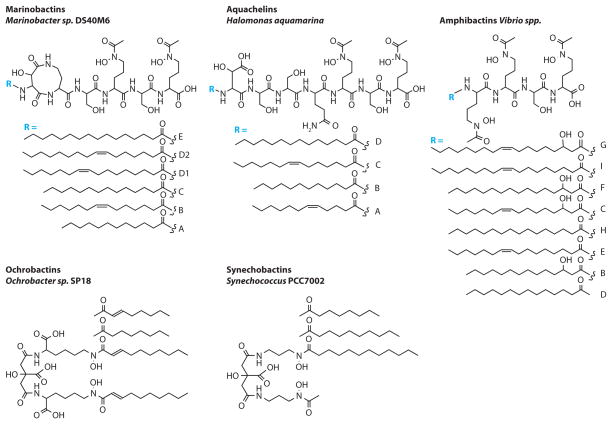
The structures of the marine siderophores known today are summarized in Figures 3 and 4. The majority of these siderophores comes from gamma and alpha proteobacteria, which may be a result of current culturing techniques. Two structural features dominate the marine siderophores: (a) siderophores that are produced as suites of amphiphiles with variation in the chain length of a fatty acid appendage (Figure 3), and (b) siderophores that are produced with an α-hydroxy carboxylic acid moiety, which when coordinated to Fe(III) is photoreactive (Figure 4a).
Figure 3.
Amphiphilic marine siderophores, including marinobactins (Martinez et al. 2000), aquachelins (Martinez et al. 2000), amphibactins (Martinez et al. 2003), ochrobactins (Martin et al. 2006), and synechobactins (Ito & Butler 2005).
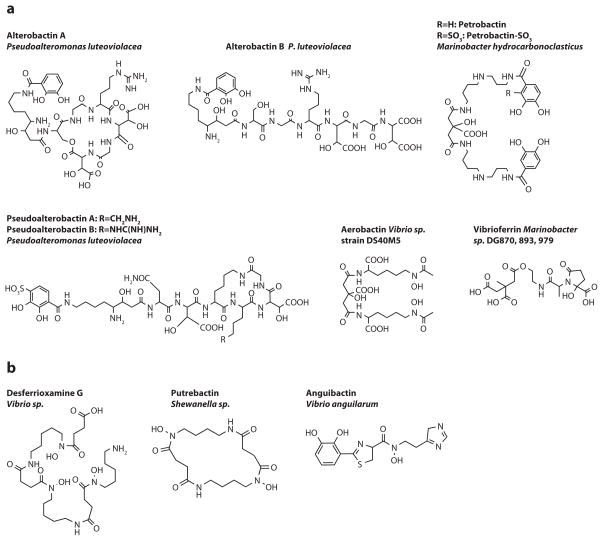
Figure 4.
Other marine siderophores, including (a) alterobactins (Reid et al. 1993), pseudoalterobactins (Kanoh et al. 2003), aerobactin (Haygood et al. 1993), petrobactin (Barbeau et al. 2002), petrobactin-SO3 (Hickford et al. 2004), and vibrioferrin (Amin et al. 2007) and (b) desferrioxamine G (Martinez et al. 2001), putrebactin (Ledyard & Butler 1997), and anguibactin (Lorenzo et al. 2004).
The aquachelins, marinobactins, ochrobactins, and synechobactins in Figure 3 coordinate Fe(III) by both oxygen atoms of each hydroxamate group and both oxygen atoms of the α-hydroxy carboxylate group (i.e., either β-hydroxy aspartic acid as in the aquachelins and marinobactins or citric acid as in the ochrobactins and synechobactins). The amphibactins coordinate Fe(III) with the three hydroxamate groups.
The siderophores in Figure 4a all contain one or two α-hydroxy carboxylate groups (i.e., either β-hydroxy aspartic acid as in the alterobactins and pseudoalterobactins or citric acid as in aerobactin and the petrobactins). The alterobactins and pseudoalterobactins coordinate Fe(III) via the two β-hydroxy aspartate moieties and one catecholate group, whereas petrobactin and petrobactin sulfonate coordinate Fe(III) with the two catecholates and the α-hydroxy acid portion of the citrate backbone.
Photoreactive Fe(III)-siderophore complexes
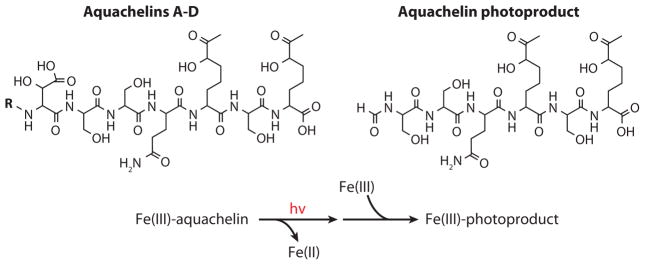
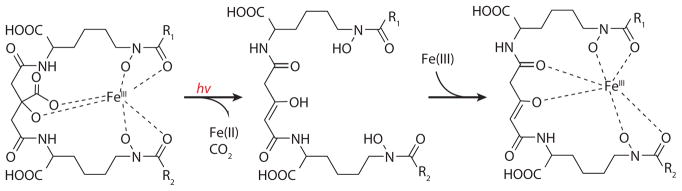
One of the distinguishing characteristics of the marine siderophores that contain an α-hydroxy carboxylic acid moiety (e.g., citric acid or β-hydroxy aspartic acid) is the UV photoreactivity of the Fe(III) complexes, which leads to oxidation of the ligand and reduction of Fe(III) to Fe(II). Photoreactivity was first demonstrated with the Fe(III)-aquachelins (Figure 5) (Barbeau et al. 2001). Upon UV photolysis (~300 nm) of Fe(III)-aquachelins, Fe(III) is reduced to Fe(II) and the ligand is oxidized, producing a photoproduct (Figure 5) that can still coordinate Fe(III) via the remaining hydroxamate moieties (Barbeau et al. 2001).
Figure 5.
Photooxidation of the Fe(III)-aquachelin ligand and production of Fe(II) (Barbeau et al. 2001). See Figure 3 for R groups in aquachelins A–D.
The ferric conditional stability constant of the Fe(III)-photoproduct (log K = 11.5) is somewhat lower than that of the native aquachelin siderophore (log K = 12.2) as a result of the loss of the α-hydroxy carboxylate moiety (Barbeau et al. 2001). When the photolysis reaction is carried out under aerobic conditions, but with a catalytic amount of Fe(III) relative to the aquachelin concentration, all the aquachelin is still oxidized; thus the Fe(II) that is produced is reoxidized by dioxygen, forming Fe(III) that can coordinate to another equivalent of the native aquachelin siderophore. The other β-hydroxyaspartate-containing siderophores in Figure 4a, e.g., the alterobactins and pseudoalterobactins, are also photoreactive when coordinated to Fe(III) (Barbeau et al. 2002), although the photoproduct structures have not been fully identified yet.
Aerobactin, the ochrobactins, the synechobactins, petrobactin, petrobactin-SO3, and other citrate-based siderophores contain the α-hydroxy carboxylate group that is stable as the uncomplexed acid, but when this group is coordinated to Fe(III), it also undergoes photooxidation of the citrate backbone of the siderophore and reduction of Fe(III) to Fe(II) (Figure 6).
Figure 6.
Photoreaction of Fe(III)-aerobactin (R = CH3) (Kupper et al. 2006).
Although a predominance of marine siderophores contains an α-hydroxy carboxylic acid moiety, which is photoreactive when coordinated to Fe(III), the biological significance of this photoreactivity, if any, remains unknown. The fate of the photolytically derived Fe(II) is uncertain and is open to further study. The oxidized ligand photoproduct of both aerobactin and aquachelin still coordinates Fe(III) and still transports Fe(III) into the bacterial cell. Much work remains to elucidate the extent and importance of the photoreactivity in the ocean.
Amphiphilicity
The fatty acid–containing siderophores (Figure 3) vary significantly in their degree of amphiphilicity. The amphibactins and ochrobactins are isolated by extraction of the bacterial pellet after centrifugation of the bacterial culture medium in the siderophore isolation process (Martin et al. 2006, Martinez et al. 2003), which indicates they are quite hydrophobic, whereas the aquachelins are isolated from the supernatant, and thus are much more hydrophilic. The synechobactins are isolated from both the bacterial pellet as well as the supernatant (Ito & Butler 2005). The marinobactins A–E are isolated from the supernatant (Martinez et al. 2000), but recently small quantities of a new marinobactin, marinobactin F, which contains a C18:1 fatty acid, were extracted from the bacterial pellet (Martinez & Butler 2007). Because the location of the double bond has not been determined yet in marinobactin F, the structure is not included in Figure 3.
The hydrophobic amphibactins have the shortest head group of the peptidic siderophores, with only four amino acids versus six or seven amino acids in the marinobactins and aquachelins, respectively, and the fatty acids tend to be somewhat longer than the fatty acids of the marinobactins and aquachelins (Figure 4); thus the combination of a smaller hydrophilic head group with longer fatty acids makes the amphibactins much more hydrophobic than the aquachelins and marinobactins (Martinez et al. 2003). The ochrobactins are also quite hydrophobic as a result of having two fatty acid appendages, as well as a small head group, whereas the synechobactins, with a similarly small head group, are less hydrophobic than the ochrobactins as a result of having only one fatty acid.
The close association of amphiphilic siderophores with the bacterial membrane could be a defense against siderophore diffusion in the oceanic environment, although studies to investigate partitioning of the amphiphilic siderophores are at a very early stage. Partitioning studies using bacteria are complicated by uptake of the siderophore in many cases (Martinez & Butler 2007). However, to get an idea of the relative extent of partitioning, studies using vesicles of phosphatidyl-choline as a model for bacterial cells have revealed some interesting trends (Martin et al. 2006, Martinez et al. 2003, Xu et al. 2002). In general, within a family of siderophores, the siderophores with longer fatty acids partition more than those with shorter fatty acids, as observed for the marinobactins, amphibactins, and ochrobactins, and the siderophores with cis double bonds partition less than those with saturated fatty acids, as observed for marinobactins. Curiously, however, the Fe(III) siderophore complexes partition less than the corresponding apo siderophore [i.e., without Fe(III) coordinated] for marinobactin E (ME) and ochrobactins B and C (Martin et al. 2006, Xu et al. 2002).
Progress on the Identity of L
Although siderophores can be isolated and characterized from marine bacteria in culture, uncertainties exist regarding the source(s) and chemical structures of dissolved Fe(III)-binding ligands in the ocean. Gledhill and coworkers (2004) isolated siderophores from natural bacterial assemblages from a nutrient-enriched seawater sample taken from the English Channel. Members of the amphibactin family as well as a number of ferrioxamines were tentatively identified on the basis of mass spectral data (m/z and fragmentation patterns) by comparison with the previously characterized ferrioxamine and amphibactin siderophores (Gledhill et al. 2004, Martinez et al. 2003, Matzanke 1991). Thus, coupling of high-performance liquid chromatography (HPLC) with electrospray ionization-mass spectrometry (ESI-MS) proved to be an effective strategy for separation and identification of siderophores and siderophore-like compounds in complex samples (Gledhill et al. 2004, McCormack et al. 2003).
The conditional stability constants of organic ligands in seawater and model siderophores were determined with stripping voltammetric techniques (see Luther et al. 2001 for an overview of voltammetric methods; Reid et al. 1993, Rue & Bruland 1995, Witter et al. 2000). Macrellis and coworkers (2001) attempted to identify siderophores directly from seawater by analyzing volumes greater than 200 liters. Partially isolated compounds had conditional stability constants (with respect to inorganic iron, Fe′; log KFeL,Fe′) of 11.5–11.9 (Macrellis et al. 2001), which is similar to the siderophores produced and purified from culture and the in situ Fe(III)-binding ligands in seawater (log KFe′L1 = 12, from Rue & Bruland 1997) (Barbeau et al. 2001, Hutchins et al. 1999b, Lewis et al. 1995, Rue & Bruland 1995, Rue & Bruland 1997, Witter et al. 2000). Macrellis and coworkers (2001) also identified hydroxamates and catecholates, i.e., two common Fe(III)-binding functional groups, whenever iron binding was detected in a sample (Macrellis et al. 2001).
The similarities between the conditional stability constants and structural groups of the natural organic Fe(III) ligands and siderophores provide evidence that siderophores or siderophore-like compounds account for a portion of the organic ligands in seawater (Gledhill et al. 2004, Lewis et al. 1995, Macrellis et al. 2001, Martinez et al. 2003, Rue & Bruland 1995). Evidence also points to porphyrins as a potential source of L2 class ligands on the basis of their conditional stability constants (Rue & Bruland 1995, Rue & Bruland 1997, Witter et al. 2000). We will, however, not know the biological significance of siderophore production and organic Fe(III)-binding ligands until appreciable quantities of organic ligands with a range of binding affinities can be isolated directly from seawater. Direct isolation would allow the determination of their in situ concentrations, the environmental stimuli that initiate production, and their exact chemical structures.
Porphyrins
Intracellular iron-binding molecules, most notably protoporphyrin IX, are widely produced by terrestrial and marine organisms. Porphyrins may be released into seawater by cell lysis, passive excretion, or during zooplankton feeding, and have been detected at nanomolar concentrations (Vong et al. 2007). Iron-porphyrin-like complexes may play an important role in the oceans (Rue & Bruland 1995). Using protoporphyrin IX as a model ligand, Luther and colleagues (2001) reported a conditional stability constant of 11.9 ± 0.5 (log KFe′L, kinetic), which is within the range of the L2 class ligands (e.g., log KFe′L2 = 11, from Rue & Bruland 1997), indicating that porphyrins could contribute to the pool of dissolved Fe(III)-binding ligands (Rue & Bruland 1995, 1997; Witter et al. 2000).
THE CASE FOR OTHER METAL-SPECIFIC MARINE LIGANDS
Introduction
Complexation by organic ligands dominates the chemical speciation of a variety of trace metals other than Fe, including Cu, Zn, and Co (Coale & Bruland 1990, Laglera & van den Berg 2003). Speciation is important because it affects the biological availability of metals, as well as the possible toxicity of these metal ions. Organic ligands in seawater have been characterized mainly by their binding affinity. Precise measurements of conditional stability constants emerged with titration methods that use cathodic stripping voltammetry competitive ligand experiments (CSV-CLE) (van den Berg 1984). The general classes of organic ligands identified to date in seawater have conditional stability constants that range from 108.6 to 1018.7 (Table 2). Deciphering the chemical structures and production stimuli of naturally produced organic ligands and the organisms they come from is fundamental to understanding metal speciation and bioavailability.
Table 2.
Compilation of selected conditional stability constants and ligand concentrations of natural organic ligands for cobalt, nickel, copper, and zinc
| Location | Values measured | Method, competing liganda,b | Reference | ||
|---|---|---|---|---|---|
| Metal concentration pM | Ligand concentration pM | log K′ (Metal)-L | |||
| Cobalt | |||||
| Antarctic polar front | 10–120 | 15–50 | – | CSV, nioxime | (Ellwood et al. 2005) |
| Costa Rica upwelling dome | 57–12c 45–93d |
50 | ≥16.8 | CSV, DMG | (Saito et al. 2005) |
| East equatorial Pacific | 27–315e | – | – | CSV, DMG | (Saito et al. 2004) |
| Atlantic Ocean, Sargasso Sea | 17–73d 20 ± 10e,f 19–133c |
– | – | CSV, DMG | (Saito & Moffett 2002) |
| Sargasso Sea | 19–73d | 9–83 | 16.3 ± 0.9 | CSV, DMG | (Saito & Moffett 2001b) |
| Northeast Atlantic Ocean | 25–103c | 22–60 | 15.6–16.1 | CSV, nioxime | (Ellwood & van den Berg 2001) |
| Nickel | nM | nM | |||
| Costa Rica upwelling dome | 3.0 ± 0.3f | – | – | CSV, DMG | (Saito et al. 2005) |
| Coastal Britain | – | 2–4 | 17.3–18.7 | CSV, DMG | (van den Berg & Nimmo 1987) |
| Copper | nM | nM | |||
| Subarctic northwest Pacific | 3–4 | 3.7–5 (1500–2500 m) | 12.7–14.1 | CSV, salicylaldoxime, and benzoylacetone | (Moffett & Dupont 2007) |
| Estuarine waters | 9–23g | L1 = 10–33 L2 = 14–300 |
L1 = 14.8 – 15.8 L2 = 13–13.5 |
CSV, salicylaldoxime | (Laglera & van den Berg 2003) |
| North Pacific | 0.58–1.88d | L1 = 1.5–3 L2 = 5–10 |
L1 = 11.6 L2 = 8.6 |
DPASV | (Coale & Bruland 1990) |
| Zinc | nM | nM | |||
| Northeastern Atlantic Ocean | 0.3–2.0 | 0.4–2.5 | 10.0–10.5 | CSV, PDC | (Ellwood & van den Berg 2000) |
| Central North Pacific | 0.1–3.0d | 1.2 | 11.0 | DPASV | (Bruland 1989) |
Abbreviations: CSV, cathodic stripping voltammetry; DMG, dimethylglyoxime; DPASV, differential pulse anodic stripping voltammetry; PDC, pyrrolidinedithiocarbamate.
Where applicable.
Values vary by location, see reference for details (i.e., open ocean to near shore).
Values vary by depth: shallow to deep, see reference for details.
Range of values for a specific depth, see reference for details.
Mean concentration.
Values go from high to low salinity.
The trace metals all occur in picomolar to nanomolar quantities in the world’s oceans (Bruland 1980, Martin et al. 1989). Table 2 shows the metal concentrations, ligand concentrations, and conditional stability constants for a number of different metal-ligand complexes in various regions of the oceans. For the most part, these metals are absolutely required for very specific enzymatic reactions, but too much of a good thing is also bad, especially in the cases of copper and cadmium. Toxic effects must be thwarted and organic ligands may play a role in attaining the benefits and hindering the negative effects of the presence of trace metals. Although enzymes often require specific metal ions or metal cofactors, cobalt, cadmium, and zinc are known to functionally substitute for each other in marine diatoms to maintain optimal growth (Lane et al. 2005). Interestingly, this same feature is not always present in cyanobacteria, which appear to have an absolute cobalt requirement (Saito & Moffett 2001b, Sunda & Huntsman 1995).
Cobalt Ligands
Cobalt displays a nutrient-like profile and occurs in very low concentrations due to scavenging by particulate matter and high reactivity in seawater (Martin et al. 1989) (Figure 1). Whereas iron is present in the most diverse array of metalloproteins, very few cobalt-containing proteins are known. Despite the relative paucity of cobalt-containing metalloenzymes, cyanobacteria produce cobalt-complexing ligands in laboratory culture (Saito & Moffett 2001a, Saito et al. 2002), and some strains have an absolute cobalt requirement for growth (Saito & Moffett 2001b, Sunda & Huntsman 1995). Synechococcus bacillaris has an obligate cobalt requirement for which Zn cannot substitute (Sunda & Huntsman 1995), and another cyanobacterium, Prochlorococcus, also has an absolute cobalt requirement (Saito et al. 2002).
Cobalt complexation is dominated by natural organic ligands with high conditional stability constants: logK′Co-L = 15.6–16.8 (Ellwood & van den Berg 2001). The concentrations of these strong ligands correlate inversely with cobalt concentration, suggesting biological control via production of natural organic cobalt-complexing ligands (Ellwood et al. 2005). In support of biological control of the ligand pool, Saito & Moffett (2001b) found an excess of ligand in the chlorophyll maximum in the ocean, where Prochlorococcus and Synechococcus dominated the picoplankton. In addition, cobalt speciation was dominated by strong-binding ligands at concentrations that paralleled the total cobalt concentration (Saito & Moffett 2001b) (Table 2).
Although a number of studies indicate the presence of high-affinity cobalt ligands in the open ocean, the identity of the organisms producing them and their chemical structures remain unknown (Ellwood & van den Berg 2001, Saito & Moffett 2001b, Saito et al. 2004). Saito and coworkers (2002) used previously conditioned media to stimulate Prochlorococcus cultures to assimilate Co(II) faster than in fresh media. The stimulant, a byproduct of previous Prochlorococcus growth (present in the conditioned media), could be an excreted ligand, because the study ruled out bacterial causes. Additional culture experiments have pointed toward both Synechococcus and Prochlorococcus as potential sources of cobalt ligands (Saito & Moffett 2001a; Saito et al. 2002, 2005). Further investigation is required to determine whether cobalt is complexed in the Co(II) or Co(III) oxidation state (Saito et al. 2005).
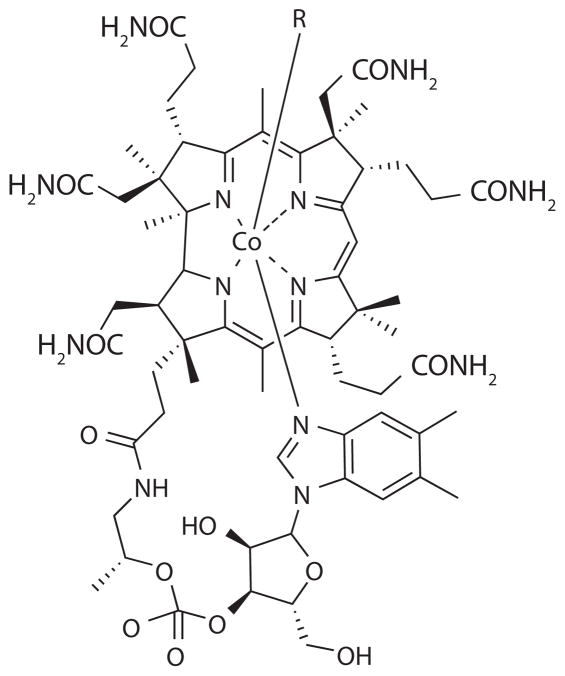
In an attempt to elucidate the chemical identity of the organic ligands, vitamin B12 (Figure 7) and coenzyme B12 were added to seawater and the conditional stability constants were determined (Ellwood & van den Berg 2001). The conditional stability constants for these complexes, log K′Co-B12 = 16.4 and 15.5, respectively, fit in the same range as those measured directly for seawater (Ellwood & van den Berg 2001) (Tables 2 and 3). The detection of ligands in deep waters indicates the presence of recalcitrant compounds with long residence times (Saito et al. 2002), for example apo-cobalamin (vitamin B12) or its breakdown products (Saito & Moffett 2001b).
Figure 7.
Structure of cobalamin (Vitamin B12) (Bertini et al. 2007).
Table 3.
Compilation of representative conditional stability constants and concentrations of potential metal-binding ligands
| Location | Additional measurements | Methoda | Reference |
|---|---|---|---|
| Iron | |||
| Central California coastal upwelling system | log KFeL,Fe′ = 11.5–11.9 | CSV, SA; performed on partially isolated ligand(s) | Macrellis et al. 2001 |
| Cobalt | |||
| Northeast Atlantic Ocean | log K′Co-Vit. B12 = 16.4 log K′Co-coenz. B12 = 15.5 |
CSV, nioxime | Ellwood & van den Berg 2001 |
| Sargasso Sea | log KCoHDMG2 = 11.5 ± 0.3 at pH 8 | CSV, DMG | Saito & Moffett 2001b |
| Coastal waters | concentration of Vit. B12 = 47–446 pM | C18 solid phase extraction and quantification by reverse-phase HPLC with UV-Vis detector | Okbamichael & Sanudo-Wilhelmy 2004 |
| Zinc | |||
| Northeastern Atlantic Ocean | free Zn ion conc. = 6–20 pM | CSV, pyrrolidinedithiocarbamate | Ellwood & van den Berg 2000 |
| Copper | |||
| Subarctic northwest Pacific | log K′Cudeep = 13.8–14.1 | CSV, SA, and benzoylacetone | Moffett & Dupont 2007 |
| Estuarine waters | log K′CuThiol = 12.3–14.1 | CSV, SA | Laglera & van den Berg 2003 |
Abbreviations: CSV, cathodic stripping voltammetry; SA, salicylaldoxime; DMG, dimethylglyoxime; HPLC, high-performance liquid chromatography.
Copper Ligands
Copper occurs at nanomolar concentrations in the open ocean with a nutrient-like distribution profile (Figure 1). Copper is strongly and uniformly complexed throughout the water column; 99% of the copper is complexed by organic ligands to depths as great as 3000 m in the subarctic Northwest Pacific (Moffett & Dupont 2007). Although copper is vital for growth, it can also be toxic. The free metal is more toxic than the complexed metal, because the former is considered more available (Brand et al. 1986, Moffett & Brand 1996). Thus, the high degree of complexation may act to decrease toxicity, but it could also lead to copper limitation if species are not able to take up the complexed form. A careful balance exists between the positive and negative effects of copper, which may be mediated by organic ligands.
Copper ligands are grouped into two distinct classes on the basis of their conditional stability constants (Coale & Bruland 1988, 1990; van den Berg et al. 1987) (Table 2). Class 1 is generally a small pool of strong ligands with log K′Cu-L1 = 12–14; a larger pool of weaker ligands belongs to class 2 with log K′Cu-L2 = 9–12. Although algae and bacteria are known to release extracellular copper-complexing ligands (Gledhill et al. 1999, Moffett & Brand 1996), the sources and structures of these copper ligands are under current investigation.
A variety of species of planktonic microorganisms produce organic substances with conditional stability constants that would characterize them as class 2 ligands (Croot et al. 2000). The strength of these chelators is correlated to the degree of copper sensitivity that the host species displays (Croot et al. 2000). For example, numerous species of the marine cyanobacterium Synechococcus are sensitive to their chemical environment (Brand et al. 1986) and produce a chelator with a strong conditional stability constant, in the reported range of class 1 ligands, when grown in culture under elevated copper levels (copper toxicity) (Moffett et al. 1990). In fact, regardless of the source of the copper ligands, copper-sensitive species such as Synechococcus benefit greatly. Synechococcus’ growth rate would be significantly decreased at copper concentrations higher than those reported in regions where class 1 ligands have also been identified (Croot et al. 2000).
Using CSV-CLE, Gledhill et al. (1999) showed that the brown algae Fucus vesiculosus produces copper-complexing ligands with conditional stability constants of log K′Cu-L = 11.2 in response to increasing copper levels in culture. The concentration of the ligand exceeded that of copper, possibly in an attempt to quench the toxic effects of elevated copper levels. In response to increasing copper concentrations, ligand concentration in culture also increases, which indicates a clear link between ligand production and copper concentration (Croot et al. 2000, Gledhill et al. 1999).
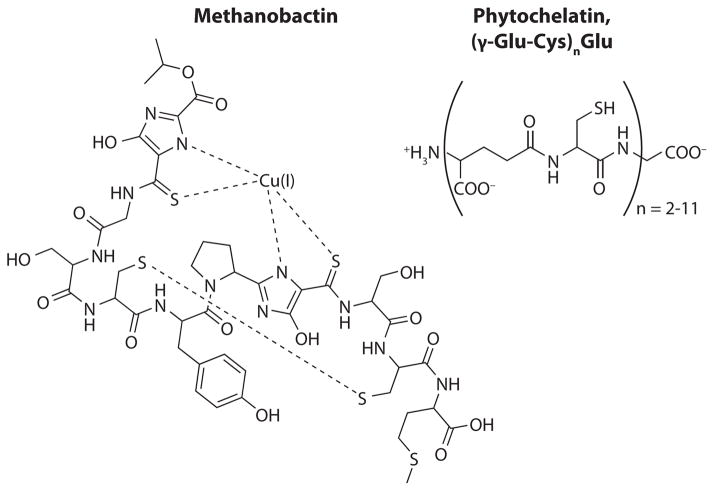
Although the chemical structures of marine copper-binding ligands or classes of ligands are still unknown, a number of different candidates for the chemical nature of these ligands are emerging. Methanobactin (Figure 8) has been isolated from Methylosinus trichosporium OB3b, where it facilitates copper uptake (Kim et al. 2004). Although methanobactin is not produced by a marine microorganism, phytochelatins (Figure 8) and glutathione have been isolated from eukaryotic phytoplankton (Kawakami et al. 2006). Gordon and coworkers (1996) isolated copper ligands from estuarine waters and found that a portion of the ligands are humic substances, primarily low in molecular weight. Their study indicates that the ligands are produced by picoplankton, which is corroborated by the finding that Synechococcus sp. WH7803 produces high-affinity copper-complexing ligands in culture (Gordon et al. 1996, Moffett & Brand 1996). In addition to humic and fulvic acids, thiols complex copper in estuarine waters (Laglera & van den Berg 2003).
Figure 8.
Structures of methanobactin (Kim et al. 2004) and phytochelatin (Kawakami et al. 2006).
The free thiol concentration decreased in response to increasing copper concentrations, and the conditional stability constant of the thiol complexes was determined to be log K′CuThiol = 12.3–14.1 (Laglera & van den Berg 2003) (Table 3). Thiol release in response to copper addition was induced in Emiliania huxleyi, a coccolithophorid (Croot et al. 2000, Dupont et al. 2004, Moffett & Brand 1996). With increased copper levels in a culture experiment, the production of two novel copper-binding thiols, arginine-cysteine (Arg-Cys) and glutamine-cysteine (Gln-Cys), as well as cysteine, was determined (Dupont et al. 2004). Thus, sulfur-containing compounds of low molecular weight are an important part of the ligand pool that complexes nearly all copper in surface seawater.
A current debate that requires further investigation is whether the ligands (e.g., thiols) bind copper intracellularly and then are released as a detoxification method or if the ligands are first released and then bind copper extracellularly. In either scenario, complexation to copper acts to decrease the amount of biologically available copper in the immediate surrounding of the cell (Lee et al. 1996, Moffett & Brand 1996). The chemical speciation is dominated by complexed copper, which may aid in copper uptake in regions of low copper and hinder toxicity in regions with high copper concentration (Moffett & Dupont 2007).
Other Metals: Zn(II), Cd(II), and Ni(II) Ligands
Zinc displays a nutrient-like distribution in the water column, with lower concentrations at the surface and increasing concentrations at depth (Bruland 1980) (Figure 1). Zinc is used extensively in many different metalloenzymes, including carbonic anhydrase, an enzyme of paramount importance to photosynthetic organisms. Low zinc levels could thus limit growth in species that are not able to substitute cobalt or cadmium, or in waters where all trace metals are found in very low abundance (Badger & Price 1994). Measurements of zinc complexation have been made using CSV-CLE and indicate that greater than 98% of zinc (in the North Pacific surface water) is strongly complexed by unidentified organic ligands with conditional stability constants of log K′cond,Zn2+ = 11.0 (Bruland 1989, 1992). Open-ocean zinc speciation is dominated by complexation to natural organic ligands with conditional stability constants between log K′ZnL = 10.0–10.5 (Ellwood & Van den Berg 2000) (Table 2).
In addition to zinc, cadmium also displays a nutrient-like distribution profile in the ocean and is complexed by organic ligands (Abe 2002; Bruland 1980, 1989; Martin et al. 1989) (Figure 1). Few enzymes are known in which cadmium is required; however, Lane et al. (2005) recently discovered a cadmium-containing carbonic anhydrase from Thalassiosira weissflogii, a marine diatom. Lee et al. (1996) showed that cadmium is likely exported from Thalassiosira weissflogii as a phytochelatin complex.
Both zinc and cadmium are more highly complexed in surface waters than at depth, indicating that the origin of the ligands is from surface waters (Bruland 1989, 1992; Sakamotoarnold et al. 1987). A portion of the pool of cadmium and zinc complexing ligands may be low-molecular-weight thiols, similar to those that complex copper, as previously mentioned (Dupont & Ahner 2005).
Ni(II) is found in low nanomolar concentrations (3–4 nM) in the open ocean and exhibits a nutrient-like depth profile (Bruland 1980) (Figure 1). 10–60% of the nickel in coastal and open-ocean waters is bound by organic ligands (i.e., it is partially complexed) (Donat et al. 1994, Saito et al. 2004). Nickel is primarily labile in the Peru Upwelling Zone (Saito et al. 2004). Nickel is required in some organisms, such as cyanobacteria, which contain Ni-superoxide dismutase (Dupont et al. 2008). Hence, although it appears that less of the total nickel concentration is complexed by organic ligands, the ligands still play an important role in determining the speciation and the availability to bacteria and phytoplankton.
A LOOK TO THE FUTURE
Although one can say with certainty that trace metals in the ocean are in part complexed by organic ligands, these ligands have yet to be fully identified and characterized in terms of their chemical structures and metal-binding selectivities. Because speciation of the metal ions is affected by complexation by biologically produced organic ligands, metal ion speciation and planktonic microorganism community structure likely have as yet unidentified feedback effects on one another.
As we attempt to determine the identity of metal-complexing substances via laboratory growth experiments, we must keep one question in mind: Does the exudation of ligands produced in laboratory settings occur at environmentally relevant metal concentrations and by organisms present in the marine environment? Studies by Gledhill and others, who are investigating iron-binding ligands, suggest that indeed they are (Gledhill et al. 2004, Macrellis et al. 2001). Although the metal concentrations in experiments reported by Gledhill et al. mirror those in the natural environment, the experiments employ nutrient addition to attain large enough quantities of the ligands to identify, which may bias the growth of microorganisms, and thus skew what would be the in situ proportion of different iron-binding ligands.
Studies of marine bacterial siderophores have afforded us insight into the possible structures, binding groups, and affinities of certain metal-binding ligands. In addition to these studies, a more in-depth look at siderophore production by other marine organisms is warranted. We are far from exhausting the work to be done on bacteria. The siderophores thus characterized are produced mainly by gamma-proteobacteria, which may be due to a bias in our ability to culture bacterial cells in laboratory settings. With the advent of new culturing techniques for marine microbes (Giovannoni & Stingle 2007), the future holds the promise of discoveries of new bacteria that may produce new chelating agents that are relevant to the speciation and cycling of metal ions in the ocean.
Acknowledgments
Support from NIH GM38130 (A.B.) and The Center for Environmental BioInorganic Chemistry (CEBIC), an NSF Environmental Molecular Science Institute (CHE-0221978), is gratefully acknowledged. J.M.V. is supported by a National Science Foundation Graduate Research Fellowship. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
Contributor Information
Julia M. Vraspir, Email: jvraspir@chem.ucsb.edu.
Alison Butler, Email: butler@chem.ucsb.edu.
LITERATURE CITED
- Abe K. Preformed Cd and PO4 and the relationship between the two elements in the northwestern Pacific and the Okhotsk Sea. Mar Chem. 2002;79:27–36. [Google Scholar]
- Amin SA, Kupper FC, Green DH, Harris WR, Carrano CJ. Boron binding by a siderophore isolated from marine bacteria associated with the toxic dinoflagellate Gymnodinium catenatum. J Am Chem Soc. 2007;129:478–79. doi: 10.1021/ja067369u. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Granger J, Mann EL, Price NM. Outer-membrane siderophore receptors of heterotrophic oceanic bacteria. Limnol Oceanogr. 2004;49:579–87. [Google Scholar]
- Badger MR, Price GD. The role of carbonic anhydrase in photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:369–92. [Google Scholar]
- Barbeau K, Rue EL, Bruland KW, Butler A. Photochemical cycling of iron in the surface ocean mediated by microbial iron(III)-binding ligands. Nature. 2001;413:409–13. doi: 10.1038/35096545. [DOI] [PubMed] [Google Scholar]
- Barbeau K, Zhang G, Live DH, Butler A. Petrobactin, a photoreactive siderophore produced by the oil-degrading marine bacterium Marinobacter hydrocarbonoclasticus. J Am Chem Soc. 2002;124:378–79. doi: 10.1021/ja0119088. [DOI] [PubMed] [Google Scholar]
- Behrenfeld MJ, Bale AJ, Kolber ZS, Aiken J, Falkowski PG. Confirmation of iron limitation of phytoplankton photosynthesis in the equatorial Pacific Ocean. Nature. 1996;383:508–11. [Google Scholar]
- Bertini I, Gray HB, Stiefel EI, Valentine JS. Biological Inorganic Chemistry: Structure & Reactivity. Sausalito, CA: Univ. Sci. Books; 2007. [Google Scholar]
- Boyd PW, Law CS, Wong CS, Nojiri Y, Tsuda A, et al. The decline and fate of an iron-induced subarctic phytoplankton bloom. Nature. 2004;428:549–53. doi: 10.1038/nature02437. [DOI] [PubMed] [Google Scholar]
- Boyd PW, Watson AJ, Law CS, Abraham ER, Trull T, et al. A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization. Nature. 2000;407:695–702. doi: 10.1038/35037500. [DOI] [PubMed] [Google Scholar]
- Boye M, Van Den Berg CMG, de Jong JTM, Leach H, Croot P, de Baar HJW. Organic complexation of iron in the Southern Ocean. Deep-Sea Res Part I-Oceanogr Res Pap. 2001;48:1477–97. [Google Scholar]
- Brand LE, Sunda WG, Guillard RRL. Reduction of marine phytoplankton reproduction rates by copper and cadmium. J Exp Mar Biol Ecol. 1986;96:225–50. [Google Scholar]
- Bruland KW. Oceanographic distributions of cadmium, zinc, nickel, and copper in the North Pacific. Earth Planet Sci Lett. 1980;47:176–98. [Google Scholar]
- Bruland KW. Complexation of zinc by natural organic-ligands in the central North Pacific. Limnol Oceanogr. 1989;34:269–85. [Google Scholar]
- Bruland KW. Complexation of cadmium by natural organic ligands in the central North Pacific. Limnol Oceanogr. 1992;37:1008–16. [Google Scholar]
- Bruland KW, Donat JR, Hutchins DA. Interactive influences of bioactive trace metals on biological production in oceanic waters. Limnol Oceanogr. 1991;36:1555–77. [Google Scholar]
- Butler A. Acquisition and utilization of transition metal ions by marine organisms. Science. 1998;281:207–10. doi: 10.1126/science.281.5374.207. [DOI] [PubMed] [Google Scholar]
- Coale KH, Bruland KW. Copper complexation in the Northeast Pacific. Limnol Oceanogr. 1988;33:1084–101. [Google Scholar]
- Coale KH, Bruland KW. Spatial and temporal variability in copper complexation in the North Pacific. Deep-Sea Res. 1990;47:317–36. [Google Scholar]
- Coale KH, Johnson KS, Chavez FP, Buesseler KO, Barber RT, et al. Southern ocean iron enrichment experiment: carbon cycling in high- and low-Si waters. Science. 2004;304:408–14. doi: 10.1126/science.1089778. [DOI] [PubMed] [Google Scholar]
- Coale KH, Johnson KS, Fitzwater SE, Gordon RM, Tanner S, et al. A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature. 1996;383:495–501. doi: 10.1038/383495a0. [DOI] [PubMed] [Google Scholar]
- Croot PL, Moffett JW, Brand LE. Production of extracellular Cu complexing ligands by eucaryotic phytoplankton in response to Cu stress. Limnol Oceanogr. 2000;45:619–27. [Google Scholar]
- de Baar HJW, de Jong JTM, Bakker D, Loescher B, Veth C, et al. Importance of iron for plankton blooms and carbon dioxide drawdown in the Southern Ocean. Nature. 1995;373:412–15. [Google Scholar]
- Donat JR, Lao K, Bruland KW. Speciation of dissolved copper and nickel in South San Francisco Bay: a multi-method approach. Anal Chim Acta. 1994;284:547–71. [Google Scholar]
- Doucette GJ, Erdner DL, Peleato ML, Hartman JJ, Anderson DM. Quantitative analysis of iron-stress related proteins in Thalassiosira weissflogii: measurement of flavodoxin and ferredoxin using HPLC. Mar Ecol Prog Ser. 1996;130:269–76. [Google Scholar]
- Dupont CL, Ahner BA. Effects of copper, cadmium, and zinc on the production and exudation of thiols by Emiliania huxleyi. Limnol Oceanogr. 2005;50:508–15. [Google Scholar]
- Dupont CL, Barbeau K, Palenik B. Ni uptake and limitation in marine Synechococcus strains. Appl Environ Microbiol. 2008;74:23–31. doi: 10.1128/AEM.01007-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont CL, Nelson RK, Bashir S, Moffett JW, Ahner BA. Novel copper-binding and nitrogen-rich thiols produced and exuded by Emiliania huxleyi. Limnol Oceanogr. 2004;49:1754–62. [Google Scholar]
- Ellwood MJ, Van Den Berg CMG. Zinc speciation in the Northeastern Atlantic Ocean. Mar Chem. 2000;68:295–306. [Google Scholar]
- Ellwood MJ, Van Den Berg CMG. Determination of organic complexation of cobalt in seawater by cathodic stripping voltammetry. Mar Chem. 2001;75:33–47. [Google Scholar]
- Ellwood MJ, Van Den Berg CMG, Boye M, Veldhuis M, de Jong JTM, et al. Organic complexation of cobalt across the Antarctic Polar Front in the Southern Ocean. Mar Freshw Res. 2005;56:1069–75. [Google Scholar]
- Gervais F, Riebesell U, Gorbunov MY. Changes in primary productivity and chlorophyll a in response to iron fertilization in the Southern Polar Frontal Zone. Limnol Oceanogr. 2002;47:1324–35. [Google Scholar]
- Giovannoni S, Stingle U. The importance of culturing bacterioplankton in the ‘omics’ age. Nat Rev Microbiol. 2007;5:820–26. doi: 10.1038/nrmicro1752. [DOI] [PubMed] [Google Scholar]
- Gledhill M, McCormack P, Ussher S, Achterberg EP, Mantoura RFC, Worsfold PJ. Production of siderophore type chelates by mixed bacterioplankton populations in nutrient enriched seawater incubations. Mar Chem. 2004;88:75–83. [Google Scholar]
- Gledhill M, Nimmo M, Hill SJ. The release of copper-complexing ligands by the brown alga Fucus vesiculosus (Phaeophyceae) in response to increasing total copper levels. J Phycol. 1999;35:501–9. [Google Scholar]
- Gledhill M, Van Den Berg CMG. Determination of complexation of iron(III) with natural organic complexing ligands in seawater using cathodic stripping voltammetry. Mar Chem. 1994;47:41–54. [Google Scholar]
- Gordon AS, Dyer BJ, Kango RA, Donat JR. Copper ligands isolated from estuarine water by immobilized metal affinity chromatography: temporal variability and partial characterization. Mar Chem. 1996;53:163–72. [Google Scholar]
- Haygood MG, Holt PD, Butler A. Aerobactin production by a planktonic marine Vibrio sp. Limnol Oceanogr. 1993;38:1091–97. [Google Scholar]
- Hickford SJH, Kuepper FC, Zhang G, Carrano CJ, Blunt JW, Butler A. Petrobactin sulfonate, a new siderophore produced by the marine bacterium Marinobacter hydrocarbonoclasticus. J Nat Prod. 2004;67:1897–99. doi: 10.1021/np049823i. [DOI] [PubMed] [Google Scholar]
- Hoffmann LJ, Peeken I, Lochte K, Assmy P, Veldhuis M. Different reactions of Southern Ocean phytoplankton size classes to iron fertilization. Limnol Oceanogr. 2006;51:1217–29. [Google Scholar]
- Hutchins DA, Franck VM, Brzezinski MA, Bruland KW. Inducing phytoplankton iron limitation in iron-replete coastal waters with a strong chelating ligand. Limnol Oceanogr. 1999a;44:1009–18. [Google Scholar]
- Hutchins DA, Witter AE, Butler A, Luther GW. Competition among marine phytoplankton for different chelated iron species. Nature. 1999b;400:858–61. [Google Scholar]
- Ito Y, Butler A. Structure of synechobactins, new siderophores of the marine cyanobacterium Synechococcus sp. PCC 7002. Limnol Oceanogr. 2005;50:1918–23. [Google Scholar]
- Kanoh K, Kamino K, Leleo G, Adachi K, Shizuri Y. Pseudoalterobactin A and B, new siderophores excreted by marine bacterium Pseudoalteromonas sp. KP20-4. J Antibiot. 2003;56:871–75. doi: 10.7164/antibiotics.56.871. [DOI] [PubMed] [Google Scholar]
- Kawakami SK, Gledhill M, Achterberg EP. Production of phytochelatins and glutathione by marine phytoplankton in response to metal stress. J Phycol. 2006;42:975–89. doi: 10.1007/s10534-005-5115-6. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Graham DW, DiSpirito AA, Alterman MA, Galeva N, et al. Methanobactin, a copper-acquisition compound from methane-oxidizing bacteria. Science. 2004;305:1612–15. doi: 10.1126/science.1098322. [DOI] [PubMed] [Google Scholar]
- Kupper FC, Carrano CJ, Kuhn JU, Butler A. Photoreactivity of iron(III)-aerobactin: photoproduct structure and iron(III) coordination. Inorg Chem. 2006;45:6028–33. doi: 10.1021/ic0604967. [DOI] [PubMed] [Google Scholar]
- La Roche J, Boyd PW, McKay ML, Geider RJ. Flavodoxin as an in situ marker for iron stress in phytoplankton. Nature. 1996;382:802–5. [Google Scholar]
- Laglera LM, Van Den Berg CMG. Copper complexation by thiol compounds in estuarine waters. Mar Chem. 2003;82:71–89. [Google Scholar]
- Lane TW, Saito MA, George GN, Pickering IJ, Prince RC, Morel FMM. A cadmium enzyme from a marine diatom. Nature. 2005;435:42. doi: 10.1038/435042a. [DOI] [PubMed] [Google Scholar]
- Lankford CE. Bacterial assimilation of iron. Crit Rev Microbiol. 1973;2:273–331. [Google Scholar]
- Ledyard KM, Butler A. Structure of putrebactin, a new dihydroxamate siderophore produced by Shewanella putrefaciens. J Biol Inorg Chem. 1997;2:93–97. [Google Scholar]
- Lee JG, Ahner BA, Morel FMM. Export of cadmium and phytochelatin by the marine diatom Thalassiosira weissflogii. Environ Sci Tech. 1996;30:1814–21. [Google Scholar]
- Lewis BL, Holt PD, Taylor SW, Wilhelm SW, Trick CG, et al. Voltammetric estimation of iron(III) thermodynamic stability constants for catecholate siderophores isolated from marine bacteria and cyanobacteria. Mar Chem. 1995;50:179–88. [Google Scholar]
- Lorenzo MD, Stork M, Alice AF, Lopez CS, Crosa JH. Vibrio. In: Crosa JH, Mey AR, Payne SM, editors. Iron Transport in Bacteria. Washington, DC: ASM Press; 2004. pp. 241–55. [Google Scholar]
- Luther GW, Rozan TF, Witter A, Lewis B. Metal-organic complexation in the marine environment. Geochem Trans. 2001;2:65. doi: 10.1186/1467-4866-2-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrellis HM, Trick CG, Rue EL, Smith G, Bruland KW. Collection and detection of natural iron-binding ligands from seawater. Mar Chem. 2001;76:175–87. [Google Scholar]
- Martin JD, Ito Y, Homann VV, Haygood MG, Butler A. Structure and membrane affinity of new amphiphilic siderophores produced by Ochrobactrum sp. SP18. J Biol Inorg Chem. 2006;11:633–41. doi: 10.1007/s00775-006-0112-y. [DOI] [PubMed] [Google Scholar]
- Martin JH, Coale KH, Johnson KS, Fitzwater SE, Gordon RM, et al. Testing the iron hypothesis in ecosystems of the equatorial Pacific-Ocean. Nature. 1994;371:123–29. [Google Scholar]
- Martin JH, Gordon RM, Fitzwater S, Broenkow WW. Vertex: phytoplankton iron studies in the Gulf of Alaska. Deep-Sea Res Oceanogr Res Papers. 1989;36:649–80. [Google Scholar]
- Martinez JS, Butler A. Marine amphiphilic siderophores: marinobactin structure, uptake, and microbial partitioning. J Inorg Biochem. 2007;101:1692–98. doi: 10.1016/j.jinorgbio.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JS, Carter-Franklin JN, Mann EL, Martin JD, Haygood MG, Butler A. Structure and membrane affinity of a suite of amphiphilic siderophores produced by a marine bacterium. Proc Natl Acad Sci USA. 2003;100:3754–59. doi: 10.1073/pnas.0637444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JS, Haygood MG, Butler A. Identification of a natural desferrioxamine siderophore produced by a marine bacterium. Limnol Oceanogr. 2001;46:420–24. [Google Scholar]
- Martinez JS, Zhang GP, Holt PD, Jung HT, Carrano CJ, et al. Self-assembling amphiphilic siderophores from marine bacteria. Science. 2000;287:1245–47. doi: 10.1126/science.287.5456.1245. [DOI] [PubMed] [Google Scholar]
- Matzanke BF. Structures, coordination chemistry and functions of microbial iron chelates. In: Winkelmann G, editor. CRC Handbook of Microbial Chelates. Boca Raton: CRC Press; 1991. pp. 15–65. [Google Scholar]
- McCormack P, Worsfold PJ, Gledhill M. Separation and detection of siderophores produced by marine bacterioplankton using high-performance liquid chromatography with electrospray ionization mass spectrometry. Anal Chem. 2003;75:2647–52. doi: 10.1021/ac0340105. [DOI] [PubMed] [Google Scholar]
- Moffett JW, Brand LE. Production of strong, extracellular Cu chelators by marine cyanobacteria in response to Cu stress. Limnol Oceanogr. 1996;41:388–95. [Google Scholar]
- Moffett JW, Dupont C. Cu complexation by organic ligands in the subarctic NW Pacific and Bering Sea. Deep-Sea Res Part I-Oceanogr Res Papers. 2007;54:586–95. [Google Scholar]
- Moffett JW, Zika RG, Brand LE. Distribution and potential sources and sinks of copper chelators in the Sargasso Sea. Deep-Sea Res. 1990;37:27–36. [Google Scholar]
- Morel FMM, Price NM. The biogeochemical cycles of trace metals in the oceans. Science. 2003;300:944–47. doi: 10.1126/science.1083545. [DOI] [PubMed] [Google Scholar]
- Okbamichael M, Sanudo-Wilhelmy SA. A new method for the determination of Vitamin B12 in seawater. Anal Chim Acta. 2004;517:33–38. [Google Scholar]
- Rawls R. Ironing the ocean. Chem Eng News. 1996;74:40–43. [Google Scholar]
- Reid RT, Butler A. Investigation of the mechanism of iron acquisition by the marine bacterium, Al-teromonas luteoviolacea: characterization of siderophore production. Limnol Oceanogr. 1991;36:1783–92. [Google Scholar]
- Reid RT, Live DH, Faulkner DJ, Butler A. A siderophore from a marine bacterium with an exceptional ferric ion affinity constant. Nature. 1993;366:455–58. doi: 10.1038/366455a0. [DOI] [PubMed] [Google Scholar]
- Roy EG, Wells ML, King DW. Persistence of iron(II) in surface waters of the western subarctic Pacific. Limnol Oceanogr. 2008;53:89–98. [Google Scholar]
- Rue EL, Bruland KW. Complexation of iron(III) by natural ligands in the Central North Pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method. Mar Chem. 1995;50:117–38. [Google Scholar]
- Rue EL, Bruland KW. The role of organic complexation on ambient iron chemistry in the equatorial Pacific Ocean and the response of a mesoscale iron addition experiment. Limnol Oceanogr. 1997;42:901–10. [Google Scholar]
- Saito MA, Moffett JW. Cobalt speciation in the equatorial Pacific and Peru upwelling region: Sources and chemical properties of natural cobalt ligands. Presented at Am. Soc. Limnol. Oceanogr. Meet; New Mexico. 2001a. [Google Scholar]
- Saito MA, Moffett JW. Complexation of cobalt by natural organic ligands in the Sargasso Sea as determined by a new high-sensitivity electrochemical cobalt speciation method suitable for open ocean work. Mar Chem. 2001b;75:49–68. [Google Scholar]
- Saito MA, Moffett JW. Temporal and spatial variability of cobalt in the Atlantic Ocean. Geochim Cosmochim Acta. 2002;66:1943–53. [Google Scholar]
- Saito MA, Moffett JW, Chisholm SW, Waterbury JB. Cobalt limitation and uptake in Prochlorococcus. Limnol Oceanogr. 2002;47:1629–36. [Google Scholar]
- Saito MA, Moffett JW, DiTullio GR. Glob Biogeochem Cycles. Vol. 18. 2004. Cobalt and nickel in the Peru upwelling region: a major flux of labile cobalt utilized as a micronutrient; p. GB4030. [Google Scholar]
- Saito MA, Rocap G, Moffett JW. Production of cobalt binding ligands in a Synechococcus feature at the Costa Rica upwelling dome. Limnol Oceanogr. 2005;50:279–90. [Google Scholar]
- Sakamotoarnold CM, Hanson AK, Huizenga DL, Kester DR. Spatial and temporal variability of cadmium in gulf-stream warm-core rings and associated waters. J Mar Res. 1987;45:201–30. [Google Scholar]
- Sunda WG, Huntsman SA. Cobalt and zinc interreplacement in marine phytoplankton: biological and geochemical implications. Limnol Oceanogr. 1995;40:1404–17. [Google Scholar]
- Tortell PD, Maldonado MT, Granger J, Price NM. Marine bacteria and biogeochemical cycling of iron in the oceans. FEMS Microbiol Ecol. 1999;29:1–11. [Google Scholar]
- Tsuda A, Takeda S, Saito H, Nishioka J, Nojiri Y, et al. A mesoscale iron enrichment in the western Subarctic Pacific induces a large centric diatom bloom. Science. 2003;300:958–61. doi: 10.1126/science.1082000. [DOI] [PubMed] [Google Scholar]
- Van Den Berg CMG. Determination of the complexing capacity and conditional stability constants of complexes of copper(II) with natural organic ligands in seawater by cathodic stripping voltammetry of copper-catechol complex ions. Mar Chem. 1984;15:1–18. [Google Scholar]
- Van Den Berg CMG. Evidence for organic complexation of iron in seawater. Mar Chem. 1995;50:139–57. [Google Scholar]
- Van Den Berg CMG, Merks AGA, Duursma EK. Organic complexation and its control of the dissolved concentrations of copper and zinc in the Scheldt Estuary. Estuar Coast Shelf Sci. 1987;24:785–97. [Google Scholar]
- Van Den Berg CMG, Nimmo M. Determination of interactions of nickel with dissolved organic material in seawater using cathodic stripping voltammetry. Sci Total Environ. 1987;60:185–95. [Google Scholar]
- Vong L, Laes A, Blain S. Determination of iron-porphyrin-like complexes at nanomolar levels in sea-water. Anal Chim Acta. 2007;588:237–44. doi: 10.1016/j.aca.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Witter AE, Hutchins DA, Butler A, Luther GW. Determination of conditional stability constants and kinetic constants for strong model Fe-binding ligands in seawater. Mar Chem. 2000;69:1–17. [Google Scholar]
- Wong CS, Johnson WK, Sutherland N, Nishioka J, Timothy DA, et al. Iron speciation and dynamics during SERIES, a mesoscale iron enrichment experiment in the NE Pacific. Deep-Sea Res II Top Stud Oceanogr. 2006;53:2075–94. [Google Scholar]
- Wu J, Luther GW. Complexation of Fe(III) by natural organic ligands in the Northwest Atlantic Ocean by a competitive ligand equilibration method and a kinetic approach. Mar Chem. 1995;50:159–77. [Google Scholar]
- Xu G, Martinez JS, Groves JT, Butler A. Membrane affinity of the amphiphilic marinobactin siderophores. J Am Chem Soc. 2002;124:13408–15. doi: 10.1021/ja026768w. [DOI] [PubMed] [Google Scholar]