Abstract
Hox transcription factors provide positional information during patterning of the anteroposterior axis. Hox transcription factors can co-operatively bind with PBC-class co-factors, enhancing specificity and affinity for their appropriate binding sites. The nuclear localisation of these co-factors is regulated by the Meis-class of homeodomain proteins. During development of the zebrafish hindbrain, Meis3 has previously been shown to synergise with Hoxb1 in the autoregulation of Hoxb1. In Xenopus XMeis3 posteriorises the embryo upon ectopic expression. Recently, an early temporally collinear expression sequence of Hox genes was detected in Xenopus gastrula mesoderm (see intro. P3). There is evidence that this sequence sets up the embryo's later axial Hox expression pattern by time-space translation. We investigated whether XMeis3 is involved in regulation of this early mesodermal Hox gene expression. Here, we present evidence that XMeis3 is necessary for expression of Hoxd1, Hoxb4 and Hoxc6 in mesoderm during gastrulation. In addition, we show that XMeis3 function is necessary for the progression of gastrulation. Finally, we present evidence for synergy between XMeis3 and Hoxd1 in Hoxd1 autoregulation in mesoderm during gastrulation.
Introduction
During the development of most animal species studied, Hox transcription factors specify positional information along the anterior - posterior axis [1]–[5]. Hox genes comprise a subfamily of the homeobox containing gene family, and are organised in four clusters, each located on a different chromosome. The homeobox encodes a DNA binding motif called the homeodomain. A strict control of the expression and function of these Hox genes is essential.
It has been shown that Pbx family members, and their Drosophila melanogaster counterpart Extradenticle (Exd), function as cofactors for Hox proteins; they can enhance their binding specificity and affinity for specific target sequences in DNA [6]–[10]. Pbx/Exd family members are part of a particular subfamily of the homeodomain containing proteins, namely the TALE-class. This class is characterised by having a three amino acid loop extension between the first and second helices of the homeodomain [11]. It has been proposed that cooperative binding of Hox and Pbx/Exd proteins can lead to transactivation while binding of the individual factors leads to repression on the same promoter elements [12]. When Hox proteins bind to DNA cooperatively with a Pbx/Exd family member, the main protein-protein interaction consists of binding of the hexapeptide motif of the Hox protein to a pocket formed by the atypical homeodomain of PBC family members [13]–[15] This pocket is composed of the three amino acid loop extension of the PBC homeodomain, residues in the third helix of the homeodomain, and a residue in the C-terminal helix of PBC homeodomains [15]. The nuclear localisation of Pbx/Exd proteins is controlled by competing nuclear import and export signals [16]. When members of the Meis family, or their Drosophila counterpart Homothorax (Hth), also members of the TALE-class of homeodomain proteins, are present in the cytoplasm they can interact with Pbx/Exd family members in such a way that the nuclear export signal of the Pbx/Exd family member is shielded, resulting in a net influx of Pbx/Exd into the nucleus, sometimes influencing the function of Hox proteins present [9], [17], [18]. However, Pbx/Exd and Meis/Hth proteins are not used exclusively as cofactors for Hox proteins. The myogenic bHLH factors [19] and Engrailed [20] also depend on the activity of Pbx and Meis members for proper functioning.
For Hox paralog group 1 members, autoregulation dependent on Pbx/Exd and Meis/Hth has been shown in the neurectoderm of mouse embryos [21], [22], in C. elegans [23] and in endoderm of Drosophila embryos [17], [24]. Binding of Hox and Pbx family members to bipartite Hox-Pbx binding sites is essential for autoregulation [17], [21], [24], [25]. Meis proteins have been shown to be indispensable as mediators of this process [17], [24], [25].
In Xenopus, a member of the Meis family, XMeis3, is a posteriorising factor in the neurectoderm of Xenopus laevis, and is required for hindbrain patterning [26], [27]. Recent findings also show that neurectodermal XMeis3 mediates the posteriorising action of XWnt3A in the developing CNS [28]. In zebrafish embryos, similar functions have been reported for Meis3 and other Meis family members [29]–[31]. Expression of XMeis3 is reported as being initiated in a stripe in the neural plate of early-mid neurula embryos. During neurula and early-tailbud stages, expression is mainly localised to rhombomeres (r's) 2, 3, and 4, and the anterior spinal cord, while posterior rhombomeres show some ventral expression [26]. Expression of XMeis3 overlaps with neurectodermal expression of Hoxd1 (r4 and r5) [32], Hoxb4 (r7, r8, and the anterior spinal cord) [33], and Hoxc6 (anterior spinal cord) [34], [35]. These overlaps are consistent with the idea that XMeis3 is involved in controlling the function of the Hox proteins with which it is co-expressed. These studies do, however, leave many questions unanswered. They pay little attention to when and where Meis cofactors actually interact with Hox proteins at different stages during the early AP patterning process. These details are likely to be crucial for understanding the mechanism at hand. Studies of vertebrate Hox expression and function have already delivered strong evidence that AP patterning depends on a specific early spatiotemporal sequence of Hox gene expression. Expression of each Hox gene is initiated in a specific mesodermal domain in the gastrula embryo and then undergoes an establishment phase during which this expression domain changes to a gene specific AP zone in axial mesoderm and the neural plate and finally a maintenance phase during which this AP zone is consolidated. This sequence is employed universally in mammals, birds, fish and amphibians and shows generic features in these different species [36]–[40]. A recent study analysed the early Hox expression patterns in Xenopus, and this revealed temporally colinear initiation of expression of a sequence of Hox genes within a horseshoe-shaped domain of ventrolateral marginal zone mesoderm with the tips of the horseshoe facing dorsal at different stages during gastrulation and then sequential dorsalisation of each Hox expression zone corresponding with its translation into a stable AP pattern zone in axial mesoderm and the neural plate [40], [41]. This sequence reflects timed interactions between an early ventrolateral mesodermal Hox cascade and the Spemann organiser that are probably imperative for AP axis formation.
We set out to investigate whether early expression of Hox genes depends on the activity of XMeis3 and whether XMeis3 is involved in regulation of expression of these Hox genes in mesoderm during gastrulation. In order for XMeis3 to be able to regulate Hox expression in mesoderm, it and Hox genes need to be co-expressed there. We performed whole mount in situ hybridisation to study the detailed early expression of XMeis3 and compared it to the early expression patterns of Hoxd1, Hoxb4, and Hoxc6 and found significant co-expression in lateral regions of marginal zone mesoderm, early during gastrulation. This is the first time that Xmeis3 expression has been reported in gastrula mesoderm. To gain further insight into the early functions of XMeis3, we followed a gain- and a loss-of-function strategy. In the gain-of-function strategy synthetic XMeis3 mRNA was microinjected into early blastomeres and expression of Hox genes was studied. These experiments showed that ectopic expression of XMeis3 during gastrulation is capable of inducing expression of the Hox genes assayed in mesoderm as well as in ectoderm. In the loss-of-function strategy we made use of an antisense morpholino oligonucleotide (reviewed in [42] and references therein) to inhibit the translation of XMeis3 mRNA (MOXMeis3). Injection of MOXMeis3 leads to a reduction in expression of Hoxd1, Hoxb4, and Hoxc6 in mesoderm and ectoderm during gastrulation, and to severe patterning defects. Finally we show synergy between Hoxd1 and XMeis3 and show that the mesodermal expression of Hoxd1 during early gastrulation is already dependent on XMeis3 mediated autoregulation.
Results
The expression of XMeis3 overlaps with Hox gene expression in mesoderm
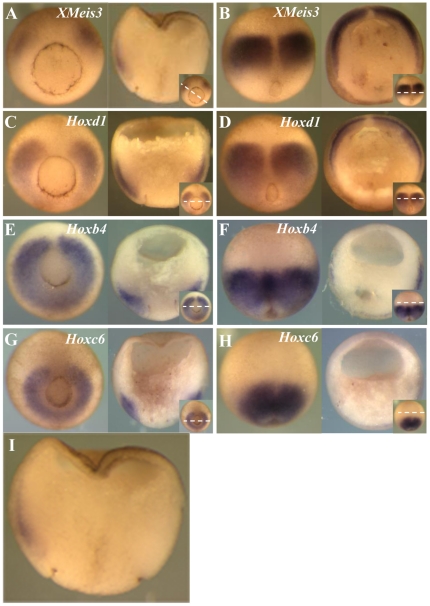
To determine whether XMeis3 is co-expressed with Hox genes in the mesoderm of gastrula embryos, whole mount in situ hybridisation was performed for XMeis3, Hoxd1, Hoxb4, and Hoxc6 (Fig. 1). Expression of XMeis3 is initiated in a horseshoe-shaped domain in ventrolateral marginal zone mesoderm of the early gastrula (st. 10.5) (the tips of the horseshoe face dorsal). By stage 11, expression is lost in the ventralmost tissue, resulting in two lateral expression domains, one on either side of the organiser in mesoderm of early gastrula stage embryos (Fig. 1A). Expression thus becomes localised to mesoderm lateral to the midline and to a very low extent also possibly to the overlying ectoderm (Fig. 1A). Expression later, at the beginning of neurulation (st.13) is primarily in neurectoderm, as has been reported previously [52] but there is also remaining expression in dorsolateral mesoderm (Fig. 1B). Early expression of Hoxd1, Hoxb4, and Hoxc6 is initiated in ventrolateral mesoderm and each of these genes follows a similar spatiotemporal expression sequence but with specific timing [40]. During early phases of gastrulation mesodermal expression of Hoxd1 (Fig. 1C), Hoxb4 (Fig. 1E), and Hoxc6 (Fig. 1G) overlaps with expression of XMeis3 in the dorsolateral domains of these Hox genes (compare Fig. 1A to 1C, 1E, and 1G). At the end of gastrulation, the overlap between mesodermal expression of Hoxd1 (Fig. 1D) and XMeis3 (Fig. 1B) in mesoderm is maintained, and the newly initiated expression of both genes in the neurectoderm also overlaps. At the same time, the more posteriorly expressed Hoxb4 (Fig. 1F) and Hoxc6 (Fig. 1H) only partially overlap XMeis3 expression (Fig. 1B) in involuted mesoderm. Hoxb4 expression also partially overlaps expression of XMeis3 in overlying ectoderm (compare Fig. 1F to 1B). These results show that there is indeed an overlap in expression of XMeis3 and of early Hox genes in mesoderm during gastrulation, and that expression of XMeis3 also overlaps with Hoxd1, and to some degree Hoxb4, in neurectoderm.
Figure 1. Expression of XMeis3, Hoxd1, Hoxb4, and Hoxc6 during gastrulation.
Embryos were analysed by whole-mount in situ hybridisation for expression of XMeis3 (A and B), Hoxd1 (C and D), Hoxb4 (E and F), and Hoxc6 (G and H). Whole mounts are shown on the left side of each panel, sections of these embryos are shown on the right side of each panel, in the inset, on the bottom right corner of every panel, the dotted line indicates the plane of sectioning. Spemann's organiser is clearly visible in Figs 1A,C,E, as the gap in the Hox or Meis expression domain, facing up in the left hand panels . Embryos shown are at stage 11, vegetal views with dorsal up (A, C, E, and G) and at stage 13, dorsal views with anterior up (B, D, F, and H). XMeis3 expression overlaps with dorsolateral expression of Hoxd1, Hoxb4, and Hoxc6 in mesoderm at stage 11 (A, C, E, and G). XMeis3 expression in ectoderm at stage 13 overlaps with expression of Hoxd1 but not with expression of Hoxb4 and Hoxc6 (B, D, F, and H). At stage 11, Hox and Meis expression is limited by a sharp boundary, running parallel to the outside of the embryo. This boundary is Brachy's cleft, the boundary between involuted mesoderm and external ectoderm Brachy's cleft runs from the blastopore to the upper limit of the involuted mesoderm (and is actually visible as a cleft in the upper part of the right panel of Fig 1C). All early Hox expression is known to be inside this cleft at this stage (mesodermal, not ectodermal) and thus marks the position of the cleft. The early XMeis3 expression shows the same pattern. It is mesodermal. At a later stage (st.13, Fig 1B), [40] XMeis 3 expression is also outside Brachy's cleft (ectodermal).
XMeis3 gain-of-function upregulates Hox gene expression in mesoderm and ectoderm
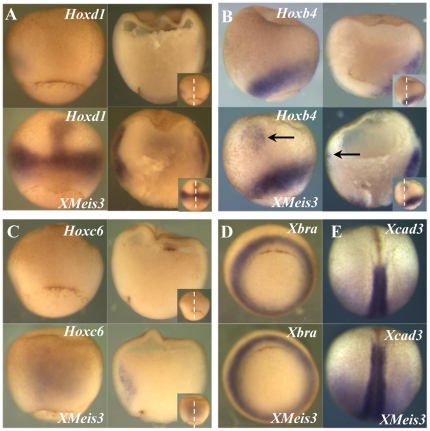
To investigate whether XMeis3 is capable of contributing to the regulation of Hox gene expression, 2 ng of synthetic mRNA containing the full-length coding region of XMeis3 was injected into the animal pole of embryos at the one-cell stage. The amount of 2 ng was chosen because this was shown to lead to posteriorisation of injected embryos [26]. The effects on expression of Hoxd1, Hoxb4, Hoxc6, Xbra, and the posterior marker Xcad3 in gastrula stages were assayed by in situ hybridisation (Fig. 2). The ectopic expression of Hoxd1 (Fig. 2A) in injected embryos is remarkable because it is found in the region harbouring the Spemann organiser, tissue that normally does not express Hox genes. The horseshoe-shaped domain of expression is also expanded and expression levels appear to be enhanced. Furthermore expression can be found in ectoderm of the animal cap and in the mesoderm underlying it, in the form of a streak of expression in contact with the expanded ring of expression around the blastopore (Fig 2A). Hoxb4 also shows ectopic expression in animal cap ectoderm and expansion of the endogenous expression domain (Fig. 2B), but no closure of the dorsal expression gap neither in organiser mesoderm nor in overlying ectoderm can be observed. Interestingly, induced expression of Hoxc6 can already be found in dorsal mesoderm at stage 10.25 (Fig. 2C), significantly earlier than its endogenous initiation of expression (st11) and like ectopic Hoxd1 expression, this occurs in dorsal mesoderm. In later stages an expansion of the endogenous horseshoe-shaped expression domain is also found (data not shown). Expression of the mesodermal marker Xbra appears unaltered in injected embryos (Fig. 2D), suggesting that changes in Hox expression domains are not due to changes in induction of mesoderm, but rather to its patterning. The previously described posteriorising effect of XMeis3 on neurectoderm is confirmed by anterior expansion of expression of the posterior marker Xcad3 (Fig. 2E).
Figure 2. XMeis3 gain-of-function.
Embryos were injected into the animal hemisphere at the one-cell stage with 2 ng synthetic mRNA containing the full-length coding region of XMeis3, and analysed by whole-mount in situ hybridisation. In each panel, control embryos are shown on top, the XMeis3 injected embryos are shown on the bottom. Each letter indicates at least a pair of images: one embryo injected with XMeis3 mRNA (experimental, labeled XMeis3), one not (control, unlabelled). The label above on each image indicates the gene being assayed; the label below, if present, indicates XMeis3 injection (or no injection, if not present). For D and E, there are only images of intact embryos processed for whole mount in situ hybridization. For A, B, and C, two whole mounts are shown on the left hand side, and sections of these embryos are shown on the right hand side of each panel. Each of these letters thus represents four images. The plane of sectioning is depicted by the dotted line in the insets of A, B, and C. (A) Expression of Hoxd1, whole mounts are shown in dorsal view, with anterior to the top, at stage 10.5. Lateral expression of Hoxd1 in injected embryos is stronger and in a broader domain, the gap in expression on the dorsal mesoderm is closed and a streak of expression in dorsal mesoderm is observed. (B) Expression of Hoxb4, whole mounts are shown in lateral view, with dorsal to the left, at stage 11. Lateral expression of Hoxb4 is not affected by injection of XMeis3, the black arrow points to a patch of ectopic expression in ectoderm. This is joined to the mesodermal expression domain by a very faint streak of expression. (C) Expression of Hoxc6, whole mounts are shown in dorsal view, with anterior to the top, at stage 10.5. Injected embryos show extensive early ectopic expression of Hoxc6 in dorsal mesoderm, prior to initiation of endogenous expression of Hoxc6. Please note that this early induced expression of the Hox genes is clearly mesodermal (internal to Brachy's cleft) and not ectodermal (surface expression) (D) Expression of Xbra, embryos at stage 10.5 are shown in vegetal view with dorsal to the top. No change can be observed in the expression of the mesodermal marker Xbra as a result of injection of XMeis3. (E) Expression of Xcad3, embryos at stage 17 are shown in dorsal view with anterior to the top. The anterior expression boundary of the posterior marker Xcad3 is shifted to a more anterior position following injection of XMeis3. Spemann's organizer is indicated by the crescent stripe, bottom centre, in the upper left panels of Figs. 2A and 2C.
XMeis3 loss-of-function downregulates expression of Hox genes and arrests gastrulation
To determine whether XMeis3 function is necessary for initiation and/or establishment of Hoxd1, Hoxb4, and Hoxc6 expression, an antisense morpholino oligonucleotide directed against XMeis3 mRNA (MOXMeis3) was injected into the animal hemisphere of embryos at the one-cell stage. XMeis3 loss-of-function leads to a loss of trunk structures and defects in axis specification, in a concentration dependent manner. When 12 ng MOXMeis3 was injected a loss of trunk structures and defects in head development and tail formation can be observed, while the anteriormost structure, the cement gland, remains present (Fig. 3B). When 24 ng MOXMeis3 was injected, an enlargement of the cement gland was visible accompanied by a stronger loss of trunk structures (Fig. 3C) In half the injected embryos spina bifida's are observed, suggesting that the embryos suffer from gastrulation problems. When 32 ng or more MOXMeis3 were injected, the embryos arrested during gastrulation at stage 11 (Fig. 3D). Embryos injected with this high dose of MOXMeis3 appear unaffected and posses normal looking blastopores until the moment of arrest. This is unlike what would be expected if the arrest was caused by toxicity of an injected agent, this would generally generate a much larger spread in stages at which embryos die or arrest, accompanied by irregular formation of the blastopore. Removal of the vitelline membrane revealed that cells have lost cell-cell contact, but appear round and intact (not shown). This suggests that the observed effect is the result of a strong knockdown of XMeis3 function and not an aspecific effect of MOXMeis3. Injection of the same amount of a control morpholino (MOcontr), in sequence unrelated to MOXMeis3, has no outward effects on embryos (data not shown). These findings support the idea that the gastrulation arrest phenotype is a true result of XMeis3 loss-of-function and that XMeis3 is required for patterning (a part of) the primary axis in Xenopus embryos. Actually, this result is perhaps not so surprising because: a recent result shows that EMT timing during internalisation of mesoderm into the gastrula is regulated (delayed) by hox genes [43] and because we present evidence (below) that the important function of Meis3 in the gastrula is to mediate mesodermal autoregulation of Hox genes.
Figure 3. Effects of XMeis3 MO loss-of-function on embryonic development and the rescue of MOXMeis3.
Embryos at the one-cell stage were injected into the animal hemisphere with MOXMeis3 in amounts of 12 ng (B), 24 ng (C), and 36 ng (D), and allowed to develop until the control embryos (A) reached tadpole stages. This treatment disturbs development of the embryonic axis. At the highest concentration, the embryo is blocked during gastrulation (fig. 3D) and then disintegrates to a mass of dissociated cells contained within the vitelline membrane (not shown). The specificity of MOXMeis3 is shown by the rescue with XMeis3 synthetic mRNA. Embryos were injected with 32 ng of MOXMeis3 and 125 pg synthetic mRNA for XMeis3 and allowed to develop until the control embryos reached the tad pole stage (E), In the majority of the embryos a large part of the axis was rescued (F), in a small number of embryos the phenotype could even be reversed, not only is the axis fully rescued but the embryo shown in (G) even possesses additional trunk structures as was revealed by the presence of somites in the axis outgrowth (not shown). The most extreme MO treatment thus produced a gastrulation block. Other treated embryos were allowed to develop to comparable stages (¬ 40–45) as shown by development of stage specific structures, for example the cement gland (seen best in Figs 3A, B, C, F G as the black spot at the lower front end of each embryo. Front ends are left in 3A, B, E, F, G. Various directions in 3C.
To further test the specificity of the MOXMeis3, 125 pg of synthetic XMeis3 mRNA, lacking most of the sequence that the MOXMeis3 is complementary to, was co-injected with 32 ng MOXMeis3 into the animal hemisphere of embryos at the one-cell stage (Fig. 3F). The exogenous XMeis3 was able to largely rescue the MOXMeis3 phenotype (compare Fig. 3D to 3E, and 3F). In a small number of the co-injected embryos a full recovery of the axis can be observed, sometimes accompanied by a secondary axial outgrowth out of the primary axis, containing somites (Fig. 3G).
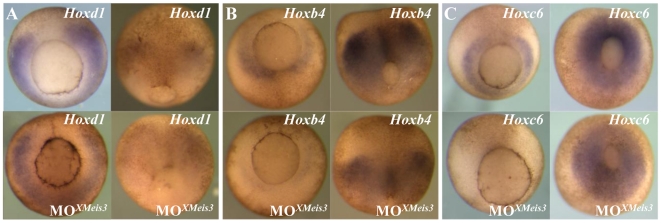
The effect of XMeis3 loss-of-function on Hox expression was studied by injecting 16 ng MOXMeis3 into the animal hemisphere of embryos at the one-cell stage followed by in situ hybridisation at appropriate stages. To be able to analyse marker expression in late gastrula stage embryos, the arrest in gastrulation, observed after injection of a high amount of MOXMeis3, was avoided, by the injection of 16 ng. The XMeis3 loss-of-function leads to downregulation of expression of Hoxd1 (Fig. 4A), Hoxb4 (Fig. 4B), and Hoxc6 (Fig. 4C), early in mesoderm and later in neurectoderm. This led to our conclusion that XMeis3 is necessary for Hox gene expression in marginal zone mesoderm, and neural plate ectoderm.
Figure 4. XMeis3 loss-of-function.
Embryos were injected at the one-cell stage with 16 ng of the MOXMeis3, and analysed by whole mount in situ hybridisation at stage 10.5/11, shown on the left side of each panel, and at stage 12, shown at the right side of each panel. Injected embryos are shown at the bottom of each panel, untreated embryos are shown on top. Shown are vegetal views with dorsal to the top. Expression of Hoxd1 (A), Hoxb4 (B), and Hoxc6 (C) is downregulated in mesoderm of injected embryos at early gastrula stages. A reduction in neurectodermal expression of the three Hox genes studied, is also observed in injected embryos at stage 12.
Synergy between Hoxd1 and XMeis3
Autoregulation is known to occur among labial type Hox genes in murine hindbrain neurectoderm [21], [44], in endoderm of Drosophila embryos [17], [25], and in C. elegans [23] For a number of these cases it has been shown that this autoregulation is dependent on a Pbx/Hox bipartite binding site in the Hox promoters [17], [21], [23], [25]
Because nuclear localisation of Pbx family members is dependent on the action of Meis family members and because XMeis3 loss-of-function led to a significant downregulation of Hoxd1 expression in mesoderm and ectoderm, we suspected that XMeis3 might be involved in Hoxd1 autoregulation. To test our idea that XMeis3 may mediate autoregulation of labial type Hox genes in Xenopus development, we co-injected relatively small amounts of synthetic mRNA for XMeis3 and Hoxd1 and also injected them separately using double the amount of mRNA. Small amounts of mRNA were used to be able to observe compound phenotypes in co-injected embryos. If a strong effect was generated in embryos injected with only a single messenger this would not have been possible. The embryos injected with only a single synthetic messenger show little or no phenotypic effect, while co-injected embryos show a significant retardation in head development (Fig. 5). This points towards a synergistic relation between Hoxd1 and XMeis3.
Figure 5. Synergistic effect between Hoxd1 and XMeis3 in ectopic expression.
Embryos at the one-cell stage were injected into the animal hemisphere with either 100 pg Hoxd1 mRNA, 100 pg Xmeis3 mRNA, or 50 pg of both mRNA's. A single injection of 100 pg of either factor is not sufficient to induce a phenotypic effect. The combination of half the amount of Hoxd1 and XMeis3, results in posteriorisation, shown by a clear reduction of eye formation, and an anterior shift of the eye.
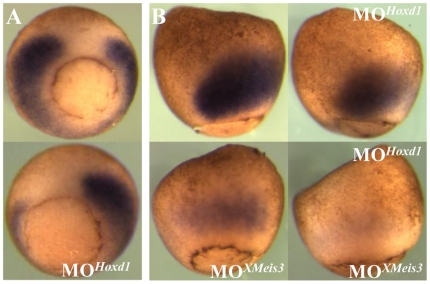
To further test this synergy, and to test whether XMeis3-mediated Hoxd1 autoregulation is involved in the establishment of Hoxd1 expression, we wished to investigate the necessity of Hoxd1 for maintaining Hoxd1 expression in mesoderm. If XMeis3 activity is needed in early gastrula mesoderm to enhance or alter the function of Hoxd1, then Hoxd1 loss-of-function should generate the same effect on Hoxd1 expression as XMeis3 loss-of-function. To test whether this is the case, 32 ng MOHoxd1 [53] was injected into the equatorial region of the 2 blastomeres making up the presumptive left side of 4-cell stage embryos. The other half of the embryos served as an internal control. This results in a downregulation of expression of Hoxd1 in mesoderm on the injected side (Fig. 6A). This finding extends our recent investigation of the effect of MO knockdown of labial Hox genes on neurectodermal Hox gene expression [53]. To further test whether establishment of expression of Hoxd1 needs both Hoxd1 and XMeis3, sub optimal amounts of morpholinos against both messengers were co-injected and injected separately. Embryos were harvested at stage 11 and assayed for Hoxd1 expression (Fig 6B). Sub optimal morpholino amounts were used to allow different levels of reduction in Hoxd1 expression, thus allowing possible synergistic effects to be observed. A downregulation of Hoxd1 expression in embryos injected with a single morpholino and a strong additional reduction by injection of both morpholinos is visible (Fig. 6B). This suggests that there is indeed a synergistic effect of Hoxd1 and XMeis3 on establishment of Hoxd1 expression in marginal zone mesoderm during gastrulation.
Figure 6. Synergistic effects in loss-of-function of Hoxd1 and XMeis3.
(A) Embryos were injected with 362 ng of MOHoxd1 into the lateral marginal zone on the left side of embryos, rendering the un-injected side an internal control. Embryos were allowed to develop until control stage 11 and assayed by in situ hybridisation for expression of Hoxd1. Embryos are shown in vegetal view, with dorsal up. Expression of Hoxd1 is reduced on the left side of injected embryos (shown on the bottom of the panel). (B) To investigate whether there is synergy between Hoxd1 and XMeis3, 16 ng MOXMeis3 and 16 ng MOHoxd1 were injected, together and separately, into the animal hemisphere of one-cell stage embryos. The embryos were harvested at st 11 and assayed for expression of Hoxd1 by in situ hybridisation. Embryos are shown in lateral view, with dorsal to the left. Injection of either MOHoxd1 or MOXMeis3 separately leads to a reduction in the early mesodermal expression of Hoxd1. Their co-injection leads to a further reduction in early mesodermal Hoxd1 expression as compared to injection of either MOXMeis3 or MOHoxd1 separately. This suggests that Hoxd1 and XMeis3 work synergistically in mediating establishment of Hoxd1 expression in mesoderm during early gastrula stages.
Discussion
XMeis3 expression overlaps early Hox expression
Much effort has been put into finding out details about the relation between Hox proteins and their cofactors Pbx/Exd and Meis/Hth. Although much has been accomplished, many questions remain. In Xenopus embryos, it has been shown that XMeis3 has a function in hindbrain patterning [26]–[28], these results are corroborated by recent reports concerned with Meis function in hindbrain formation in zebrafish embryos [29]–[31]. We show here that XMeis3 is expressed in marginal zone mesoderm significantly earlier than previously described [26], [28]. We went on to show that an overlap is found between expression of XMeis3 and of early Hox genes in ventral and lateral and dorsolateral mesoderm during gastrulation. At st. 11, the overlap is restricted to dorsolateral mesoderm. This co-localisation with early Hox genes is compatible with a role for XMeis3 in the regulation of Hox gene expression in mesoderm during the early phases of gastrulation.
Ectopic XMeis3 enhances Hox expression in mesoderm
By gain-of-function experiments we showed that ectopic XMeis3 is capable of inducing expression of Hoxd1, Hoxb4, and Hoxc6, expanding the endogenous expression domains of these genes in early mesoderm, and ectopically initiating expression in dorsal mesoderm. Interestingly, this induction of Hox expression by ectopic XMeis3 can only be found as expansions of the endogenous expression domains or in streaks of expression still in contact with the expanded endogenous domains of expression. This is most obvious for ectopic expression of Hoxd1 in dorsal mesoderm, expanding into more animally located mesoderm and ectoderm. This suggests that ectopic XMeis3 only enhances the expression of the assayed Hox genes, requiring factors already present in their endogenous Hox expression domains rather than inducing expression de novo. We suspect that the endogenous factors required are the Hox proteins themselves. These patterns are consistent with our idea (below) that XMeis3 enhances Hox autoregulation in mesoderm of Xenopus embryos.
XMeis3 is necessary for Hox expression in mesoderm and ectoderm
The injection of MOXMeis3 led to a downregulation of mesodermal expression of all three Hox genes assayed. For Hoxd1 and Hoxb4 this held true for mesoderm and ectoderm, in the case of Hoxc6, mesodermal expression partially recovers during later phases of gastrulation, but ectodermal expression could not be observed. This indicates that XMeis3 protein is necessary, in ventral and lateral mesoderm and in neurectoderm during gastrulation, for proper establishment and maintenance of Hox expression.
XMeis3 loss-of-function using small amounts of MOXMeis3 already led to a strong phenotype, indicating the necessity of XMeis3 function in anteroposterior patterning. This phenotype corroborates the results of Dibner and co-workers [27]. The sudden arrest in gastrulation at stage 11, caused by injecting a high amount of MOXMeis3 is very striking. We show by coinjecting a limited amount of XMeis3 mRNA that the observed effect is not aspecific. We note that there is published evidence that Hox genes regulate cell movement and EMT's during gastrulation [43] and suspect that this XMeis3 effect is connected with this. This is possibly due to an effect on Hox/Meis synergy: See below. The phenotype observed after injection of less morpholino, namely loss of trunk structures, head defects, and retarded tail formation described in this report and by Dibner and co-workers [27], is therefore most likely a result of reduced XMeis3 function, not a complete loss of function. We cannot be certain that the phenotype caused by injection of 32 ng MOXMeis3 represents the complete loss-of-function phenotype, but it suggests the need for XMeis3 in two processes during early development: the progression of gastrulation and Hox expression and patterning in the early mesoderm and hindbrain.
Synergy between Hoxd1 and XMeis3
We suspected that Meis3 is important for Hox expression because it mediates Hox autoregulation so we tested whether Hoxd1 and Meis3 synergise in early gastrula mesoderm. The synergistic effects we have observed in the gain-of-function experiment by injection of synthetic XMeis3 and Hoxd1 mRNA together show that these two factors, when co-expressed can indeed generate a phenotype that cannot be accomplished by injecting double the amount of either factor separately. These results recall the findings of Vlachakis and co-workers [29], who have shown that in zebrafish embryos, Meis3, Pbx4, and Hoxb1 synergise to promote hindbrain fate. Combined Hoxd1 and XMeis3 loss-of-function also indicates synergy; while sub optimal amounts of either morpholino against Hoxd1 or XMeis3 led to a reduction of Hoxd1 expression, the combination led to a much stronger reduction. This adds to the evidence for a synergistic relation between Hoxd1 and XMeis3. Taken together our results show that XMeis3 is necessary in marginal zone mesoderm to establish the early expression of Hox genes. This XMeis3-mediated mesodermal Hox cascade is of vital importance for axis formation and AP patterning.
Autoregulation by Hoxd1 is necessary for establishment of its expression in marginal zone mesoderm
Autoregulation dependent on Pbx has been shown for Hox paralog group 1 and 4 members in neurectoderm [17], [21]–[24], [44]. This suggests that the the regulation of Hox expression by XMeis3 that we have demonstrated could take place at the level of Hox autoregulation. Indeed, injection of MOHoxd1 led to a reduction in Hoxd1 mRNA expression. The expectation is that this is the result of a reduction in Hoxd1 translation, leading to a reduced amount of Hoxd1 protein and we suspect that this causes a reduction in availability of Hoxd1 mRNA because of autoregulation. This suggests that Hoxd1 autoregulation is an essential step in the establishment (but not initiation), and not only the maintenance (as in neurectoderm), of Hoxd1 expression in mesoderm during gastrulation in Xenopus embryos. We do not yet know whether this autoregulation is direct or indirect and have no evidence as to the mechanism. However, the involvement of Meis3 suggests that it is by the known mechanism [17], [21]–[25], [44]. The observed reduction of Hoxd1 expression could also be explained if binding of MOHoxd1 to mRNA led directly to destabilisation of the Hoxd1 messenger, however this effect has, to our knowledge, not been reported and our findings (above) of the necessity of Meis for mesodermal Hox expression and for synergy between Hoxd1 and Meis3 also point strongly to autoregulation via the known Meis dependent mechanism [17], [21]–[25], [44]. The necessity for Hoxd1 autoregulation in mesoderm is a remarkable discovery considering that vertebrate Hox autoregulation has previously only been shown in the hindbrain We note that Hoxd1 loss-of-function is clearly not fully, if at all, rescued by the other labial type Hox genes; : Hoxa1 and Hoxb1 that are normally co-expressed during gastrulation. Either Hoxa1 and Hoxb1 are not capable of inducing the expression of Hoxd1, which seems unlikely taking into account the redundant functions of these paralog group members (reviewed in [45] and references therein), or expression of Hoxa1 and Hoxb1 is reduced or prevented by Hoxd1 loss-of-function. This second idea would suggest the necessity of Hoxd1 to induce the two other labial homologous (which are expressed slightly later) during gastrulation in Xenopus embryos. Additional experiments are needed to distinguish between the two possibilities but whatever the outcome, this finding sheds new light on the initiation and establishment of expression of the early gastrula Hox cascade. Obviously, auto and cross regulation can not be involved in initiating the very first expression of Hox genes. We conclude that autoregulation is involved only in the establishment and maintenance phases of Hox expression and not initiation. In fact, we have evidence that Hox expression in the Xenopus gastrula is initiated by Wnt8, which directly induces expression of Hoxd1 and of its paralogues but not of other Hox genes [54].
Concluding Remarks
Our investigations shed new light on the roles of Meis3 and of Hox genes in early embryonic development and axial patterning. We made four main findings which relate to the role of the early gastrula non organiser mesoderm which has recently been shown to be very important in early embryonic patterning [40], [41]. This early mesoderm is important because it is the first embryonic tissue to express Hox genes. It has a temporally collinear sequence of Hox gene expression that is used to ste up the spatially collinear Hox sequence in the later embryo's axial pattern by time- space translation [40], [41] We show here that Xmeis3 and Meis-Hox synergy are needed for setting up this early mesodermal Hox sequence
1/ We showed for the first time that Meis3 starts to be expressed earlier in the early Xenopus embryo than preiously reported: in the non organiser mesoderm at the early gastrula stage St 10.5 rather than the early neurula stage, after gastrulation. This early mesodermal Meis expression overlaps with the early mesodermal expression of the Hox genes.
2/ We showed for the first time that artificial ectopic expression of Meis3 causes ectopic expression of Hox genes in the early gastrula non organiser mesoderm as well as in embryonic neurectoderm. This ectopic expression occurs only in tissue that is in contact with non organiser mesoderm expressing the Hox gene in question or another Hox gene, indicating the need for additional endogenous factors for ectopic expression. We speculate that these may be the Hox proteins themselves. This finding constituted our first piece of evidence suggesting that Meis3 may be needed for early gastrula Hox expression.
3/ We showed for the first time that Meis3 loss of function via antisense oligonucleotide morpholinos blocks or downregulates Hox gene expression in early gastrula non organiser mesoderm. This is evidence that mesodermal Meis is indeed needed for mesodermal Hox expression.
4/ We showed for the first time that endogenous and ectopic Meis3 and Hoxd1 can and do synergise to induce Hoxd1 expression in early gastrula mesoderm. This is evidence that synergy between Meis and Hox mediates mesodermal expression of at least one Hox gene. We believe that this reveals a detail of how Meis3 regulates Hoxd1 expression.
Materials and Methods
Xenopus embryos and microinjections
Pigmented Xenopus laevis embryos were obtained by in vitro fertilisation, and after dejelling in a 2% cysteine solution (pH 8.0), cultured in 0.1× Marc's Modified Ringers's (MMR) [46] containing 50 µg/ml gentamycin at 14–21°C. Embryos were injected in 1× MMR+4% ficoll and afterwards transferred to 1× MMR+1% Ficoll, and cultured in this medium for 1 to 7 hours, after which they were transferred and to 0.1× MMR in which they were cultured until harvesting. Staging of the embryos was performed according to Nieuwkoop and Faber [47]. Embryos at the one-cell stage were injected into the animal pole with synthetic mRNA dissolved in water. The synthetic capped mRNA was made using the Ambion mMessage mMachine Kit with CS2-XMeis3, or CS2-Hoxd1, linearised with NotI, as template. CS2-XMeis3 was constructed by cloning the full-length coding region of XMeis3, obtained by PCR using stage 15 cDNA as template and the following primers: f: 5′-gcgggatccatggcacaaaggtatgatgag, r: 5′–cgcctcgagcatgtagtgccactgcccctcc, containing an BamHI or a XhoI restriction site respectively, in the CS2+ vector [48] using the restriction sites in the primers. CS2-Hoxd-1 contains the complete coding sequence of XHoxd1 in CS2+, kindly provided by W. Van den Akker.
MOXMeis3 Gene Tools, LLC, (directed against the XMeis3 mRNA's 5′ region) has the sequence: 5′-cctttgtgccattccgagttgggtc, and was injected in amounts of 8 to 36 ng. in a concentration of 8 ng/nl. MOcontr, supplied by Gene Tools, LLC, has the licence: 5′-cctcttacctcagttacaatttata and was injected using the same amounts and concentrations as MOXMeis3.
Whole mount in situ hybridisation and antisense probes
Whole mount in situ hybridisations were performed according to Harland (1991), with minor modifications. The antisense RNA probes were generated by run off in vitro translation using DIG RNA labelling mix (Roche), and T7 or Sp6 RNA polymerase (Promega). The probes were generated using the following templates: Hoxd1: [49], Hoxb4: a 708 bp fragment containing the complete Hoxb-4 ORF cloned in pGEMTE, Hoxc6: a 998 bp Hoxc-6 fragment in pGEM1 containing a part of the homeodomain and extending into the 3′ UTR, Xcad3: [50]; Xbra: pSP73Xbra [51].
Ethics
Our work uses early Xenopus embryos. It is carried out according to national and EU guidelines and regulations. The animal work is covered by a ‘DEC’ licence, No. 513, covering ethical aspects, issued by the University of Leiden's Animal Experimentation Committee (Dier Experimenten Commissie) to A.J.Durston. The molecular work is covered by an existing COGEM licence No. GGO 02-055, issued to H.P.Spaink. This work requires no other licence.
Acknowledgments
We thank W. van den Akker for kindly providing the CS2-Hoxd1 plasmid prior to publication and C. McNulty, G. Mainguy, and S. Wacker for critically reading the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by EU grant FP6 No. LSHM-CT-2003-504468. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bürglin TR, Ruvkun G. The Caenorhabditis elegans homeobox gene cluster. Curr Opin Genet Dev. 1993;3:615–620. doi: 10.1016/0959-437x(93)90097-9. [DOI] [PubMed] [Google Scholar]
- 2.Bürglin TR, Ruvkun G, Coulson A, Hawkins NC, McGhee JD, et al. Nematode homeobox cluster. Nature. 1991;351:703–706. doi: 10.1038/351703a0. [DOI] [PubMed] [Google Scholar]
- 3.Manak JK, Scott MP. A class act: conservation of homeodomain protein functions. Development . 1994;Suppl.:61–77. [PubMed] [Google Scholar]
- 4.Lawrence PA, Morata G. Homeobox genes: their function in Drosophila segmentation and pattern formation. Cell. 1994;78:181–189. doi: 10.1016/0092-8674(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 5.McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- 6.Knoepfler PS, Kamps MP. The Pentapeptide Motif of Hox Proteins Is Required for Cooperative DNA Binding with Pbx1, Physically Contacts Pbx1, and Enhances DNA binding by Pbx1. Mol Cell Biol. 1995;15:5811–5819. doi: 10.1128/mcb.15.10.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang CP, Shen WF, Rozenfeld S, Lawrence HJ, Largman C, et al. Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox Proteins. Genes Dev. 1995;9:663–674. doi: 10.1101/gad.9.6.663. [DOI] [PubMed] [Google Scholar]
- 8.Neuteboom STC, Murre C. Pbx raises the DNA binding specificity but Not the Selectivity of Antennapedia Hox Proteins. Mol Cell Biol. 1997;17:4696–4706. doi: 10.1128/mcb.17.8.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryoo HD, Mann RS. The control of trunk Hox specificity and activity by Extradenticle. Genes Dev. 1999;13:1704–1716. doi: 10.1101/gad.13.13.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dijk A, Murre C. extradenticle Raises the DNA Binding Specificity of Homeotic Selector Gene Products. Cell. 1994;78:617–624. doi: 10.1016/0092-8674(94)90526-6. [DOI] [PubMed] [Google Scholar]
- 11.Bürglin TR. Analysis of TALE superclass homeobox genes (Meis, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acid Res. 1997;25:4173–4180. doi: 10.1093/nar/25.21.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinsonneault J, Florence B, Vaessin H, McGinnis W. A model for extradenticle function as a switch that changes HOX proteins from repressors to activators. EMBO J. 1997;16:2032–2042. doi: 10.1093/emboj/16.8.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabet C, Gitti R, Summers MF, Wolberger C. NMR Studies of the Pbx1 TALE Homeodomain Protein Free in Solution and Bound to DNA: Proposal for a Mechanism of HoxB1-Pbx1-DNA Complex Assembly. J Mol Biol. 1999;291:521–530. doi: 10.1006/jmbi.1999.2983. [DOI] [PubMed] [Google Scholar]
- 14.Passner JM, Ryoo HD, Shen L, Mann RS, Aggarwal AK. Structure of a DNA-bound Ultrabithorax-Extradenticle homeodomain complex. Nature. 1999;397:714–719. doi: 10.1038/17833. [DOI] [PubMed] [Google Scholar]
- 15.Piper DE, Batchelor AH, Chang C, Cleary ML, Wolberger C. Structure of a HoxB1-Pbx1 Heterodimer Bound to DNA: Role of the Hexapeptide and a Fourth Homeodomain Helix in Complex Formation. Cell. 1999;96:587–597. doi: 10.1016/s0092-8674(00)80662-5. [DOI] [PubMed] [Google Scholar]
- 16.Abu-Shaar M, Ryoo HD, Mann RS. Control of the nuclear localization of Extradenticle by competing nuclear import and export signals. Genes Dev. 1999;13:935–945. doi: 10.1101/gad.13.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryoo HD, Marty T, Casares F, Affolter M, Mann RS. Regulation of Hox target genes by a DNA bound Homothorax/Hox/Extradenticle complex. Development. 1999;126:5137–5148. doi: 10.1242/dev.126.22.5137. [DOI] [PubMed] [Google Scholar]
- 18.Jaw TJ, You LR, Knoepfler PS, Yao LC, Pai CY, et al. Direct interaction of two homeoproteins, Homothorax and Extradenticle, is essential for EXD nuclear localization and function. Mech Dev. 2000;91:279–291. doi: 10.1016/s0925-4773(99)00316-0. [DOI] [PubMed] [Google Scholar]
- 19.Berkes CA, Bergstrom DA, Penn BH, Seaver KJ, Knoepfler PS, et al. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol Cell. 2004;14(4):465–77. doi: 10.1016/s1097-2765(04)00260-6. [DOI] [PubMed] [Google Scholar]
- 20.Peltenburg LTC, Murre C. Engrailed and Hox homeodomain proteins contain a related Pbx interaction motif that recognizes a common structure present in Pbx. EMBO J. 1996;15:3385–3393. [PMC free article] [PubMed] [Google Scholar]
- 21.Pöpperl H, Bienz M, Struder M, Chan SK, Aparicio S, et al. Segmental expression of Hoxb1 is Controlled by a Highly conserved Autoregulatory Loop Dependent upon exd/pbx. Cell. 1995;81:1031–1042. doi: 10.1016/s0092-8674(05)80008-x. [DOI] [PubMed] [Google Scholar]
- 22.Ferretti E, Marshall H, Pöpperl H, Maconochie M, Krumlauf R, et al. Segmental expression of Hoxb2 in r4 requires two separate sites that integrate cooperative interactions between Prep1, Pbx and Hox proteins. Development. 2000;127:155–166. doi: 10.1242/dev.127.1.155. [DOI] [PubMed] [Google Scholar]
- 23.Streit A, Kohler R, Marty T, Belfiore M, Takacs-Vellai K, et al. Conserved Regulation of the Caenorhabditis elegans labial/Hox1 gene ceh-13. Dev Biol. 2002;232:96–108. doi: 10.1006/dbio.2001.0544. [DOI] [PubMed] [Google Scholar]
- 24.Marty T, Vigano MA, Ribeiro C, Nussbaumer U, Grieder NC, et al. HOX complex, a repressor element and a 50 bp sequence confer regional specificity to a DPP-responsive enhancer. Development. 2001;128:2833–2845. doi: 10.1242/dev.128.14.2833. [DOI] [PubMed] [Google Scholar]
- 25.Grieder NC, Marty T, Ryoo HD, Mann RS, Affolter M. Synergistic activation of a Drosophila enhancer by HOM/EXD and DPP signaling. EMBO J. 1997;16:7402–7410. doi: 10.1093/emboj/16.24.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salzberg A, Elias S, Nachaliel N, Bonstein L, Henig C, et al. A Meis family member protein caudalizes neural cell fates in Xenopus. Mech Dev. 1999;80:3–13. doi: 10.1016/s0925-4773(98)00187-7. [DOI] [PubMed] [Google Scholar]
- 27.Dibner C, Elias S, Frank D. XMeis3 protein is required for proper hindbrain patterning in Xenopus laevis embryos. Development. 2001;128:3415–3426. doi: 10.1242/dev.128.18.3415. [DOI] [PubMed] [Google Scholar]
- 28.Elkouby YM, Elias S, Casey ES, Blythe SA, Tsabar N, et al. Mesodermal Wnt signaling organizes the neural plate via Meis3. Development. 2010;137(9):b1531–41. doi: 10.1242/dev.044750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vlachakis N, Choe SK, Sagerström CG. Meis3 synergizes with Pbx4 and Hoxb1b in promoting hindbrain fates in the zebrafish. Development. 2001;128:1299–1312. doi: 10.1242/dev.128.8.1299. [DOI] [PubMed] [Google Scholar]
- 30.Waskiewicz AJ, Rikhof HA, Hernandez RE, Moens CB. Zebrafish Meis functions to stabilize Pbx proteins and regulate hindbrain patterning. Development. 2001;128:4139–4151. doi: 10.1242/dev.128.21.4139. [DOI] [PubMed] [Google Scholar]
- 31.Choe SK, Vlachakis N, Sagerström CG. Meis family proteins are required for hindbrain development in the zebrafish. Development. 2001;128:585–595. doi: 10.1242/dev.129.3.585. [DOI] [PubMed] [Google Scholar]
- 32.Kolm PJ, Sive HL. Regulation of the Xenopus labial Homeodomain Genes, HoxA1 and HoxD1: Activation by Retinoids and Peptide Growth Factors. Dev Biol. 1995;167:34–49. doi: 10.1006/dbio.1995.1005. [DOI] [PubMed] [Google Scholar]
- 33.Harvey RP, Melton DA. Microinjection of synthetic Xhox-1A homeobox mRNA disrupts somite formation in developing Xenopus embryos. Cell. 1988;53:687–697. doi: 10.1016/0092-8674(88)90087-6. [DOI] [PubMed] [Google Scholar]
- 34.Oliver G, Wright CV, Hardwicke J, De Robertis EM. Differential antero-posterior expression of two proteins encoded by a homeobox gene in Xenopus and mouse embryos. EMBO J. 1988;7:3199–3209. doi: 10.1002/j.1460-2075.1988.tb03187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Robertis EM, Oliver G, Wright CVE. Determination of Axial Polarity in the vertebrate Embryo: Homeodomain Proteins and Homeogenetic Induction. Cell. 1989;57:189–191. doi: 10.1016/0092-8674(89)90954-9. [DOI] [PubMed] [Google Scholar]
- 36.Duboule D, Dolle P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989;8:1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham A, Papalopulu N, Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989;57:367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- 38.Gaunt SJ, Strachan L. Temporal colinearity in expression of anterior Hox genes in developing chick embryos. Dev Dyn. 1996;207:270–280. doi: 10.1002/(SICI)1097-0177(199611)207:3<270::AID-AJA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 39.Deschamps J, van den Akker E, Forlani S, de Graaff W, Oosterveen T, et al. Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. Int J Dev Biol. 1999;43:635–650. [PubMed] [Google Scholar]
- 40.Wacker SA, Jansen HJ, McNulty CL, Houtzager E, Durston AJ. Timed interactions between the Hox expressing non-organiser mesoderm and the Spemann organiser generate positional information during vertebrate gastrulation. Dev Biol. 2004;268(1):207–19. doi: 10.1016/j.ydbio.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 41.Durston AJ, Jansen HJ, Wacker SA. Review: Time-Space Translation Regulates Trunk Axial Patterning In The Early Vertebrate Embryo. 2010. Genomics: In press. [DOI] [PubMed]
- 42.Heasman J. Morpholino Oligos: Making Sense of Antisense? Dev Biol. 2002;243:209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- 43.Iimura T, Pourquie O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature. 2006;442(7102):568–71. doi: 10.1038/nature04838. [DOI] [PubMed] [Google Scholar]
- 44.Gould A, Morrison A, Sproat G, White RA, Krumlauf R. Positive cross-regulation and enhancer sharing: two mechanisms for specifying overlapping Hox expression patterns. Genes Dev. 1997;11:900–913. doi: 10.1101/gad.11.7.900. [DOI] [PubMed] [Google Scholar]
- 45.Morrison AD. 1+1 = r4 and much much more. Bioessays. 1998;20:794–797. doi: 10.1002/(SICI)1521-1878(199810)20:10<794::AID-BIES4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 46.Sive HL, Grainger RM, Harland RM. Cold Spring Harbor Laboratory press; 2000. Early development of Xenopus laevis A laboratory manual. [Google Scholar]
- 47.Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Amsterdam: North Holland; 1967. [Google Scholar]
- 48.Rupp RAW, Snider L, Weitraub H. Xenopus embryos regulate the nuclear localisation of XMyoD. Genes Dev. 1994;8:1311–1323. doi: 10.1101/gad.8.11.1311. [DOI] [PubMed] [Google Scholar]
- 49.Sive HL, Cheng PF. Retinoic acid perturbs the expression of Xhox.lab genes and alters mesodermal determination in Xenopus laevis. Genes Dev. 1991;5:1321–1332. doi: 10.1101/gad.5.8.1321. [DOI] [PubMed] [Google Scholar]
- 50.Pownall ME, Tucker AS, Slack JMW, Isaacs HV. eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development. 1996;122:3881–3892. doi: 10.1242/dev.122.12.3881. [DOI] [PubMed] [Google Scholar]
- 51.Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate- early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- 52.Godsave SF, Koster CH, Getahun A, Mathu M, Hooiveld M, et al. Graded retinoid responses in the developing hindbrain. Dev Dyn. 1998;213(1):39–49. doi: 10.1002/(SICI)1097-0177(199809)213:1<39::AID-AJA4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 53.McNulty C, Peres J, van den Akker W, Bardine N, Durston A. Knockdown of the complete Hox paralogous group 1 leads to dramatic hindbrain and neural crest defects. Development. 2005;132(12):2861–71. doi: 10.1242/dev.01872. [DOI] [PubMed] [Google Scholar]
- 54.In der Rieden PMJ, Lloret Vilaspasa F, Durston A. Xwnt8 directly initiates expression of labial Hox genes. Dev Dynamics. 2010;29:226–239. doi: 10.1002/dvdy.22020. [DOI] [PubMed] [Google Scholar]