Abstract
Purpose of review
This article reviews recent advances in scaffold-based interventions for the treatment of vocal fold scarring, with a particular emphasis on atelocollagen sheet implantation in the vocal fold lamina propria.
Recent findings
Scaffold-based therapies have demonstrated therapeutic promise in both pre-clinical and early clinical studies. Recent research has begun to shed light on the interactions between scaffold material properties, encapsulated and infiltrating cells, stimulatory molecules such as growth factors, and external regulatory variables such as stress, strain and vibration. The atelocollagen sheet, a cross-linked collagen material with abundant micropores, has an established clinical track record as a scaffold for dermal and epidermal repair and exhibited potential therapeutic benefit in a recent study of patients with vocal fold scarring and sulcus vocalis.
Summary
Scaffolding is one of the useful tools in tissue engineering and atelocollagen sheet implantation has shown to be effective in vocal fold regeneration. However, many of the scaffold materials under investigation still await clinical translation, and those that have been investigated in human patients (such as the atelocollagen sheet) require additional research in appropriately powered placebo controlled studies.
Keywords: biologic scaffold, phonosurgery, regenerative medicine, tissue engineering
Introduction
Although the treatment of many voice disorders has improved alongside advances in voice therapy and phonosurgery, there remains no optimal therapeutic strategy for vocal fold scarring. Vocal fold scarring generally occurs following mucosal injury and inflammation, and is often induced by physical trauma, surgical resection, irradiation and infection [1, 2, 3]. It irrevocably changes the biomechanical properties of the native mucosa and impairs vibratory function for phonation, resulting in a severe, intractable dysphonia [4, 5]. Given the importance of voice to human communication and social interaction, scar-induced dysphonia can cause significant personal and professional handicap in many individuals.[6, 7] Consequently, there remains a pressing need for new and effective therapies for this condition.
Recent developments in the fields of regenerative medicine and tissue engineering have yielded promising new approaches to the restoration/replacement of disordered tissues. Early work in experimental models of vocal fold scarring suggests that these regenerative strategies hold therapeutic benefit and can lead to functional tissue improvement. The purpose of this review article is to summarize recent developments in this area with a focus on scaffold-based interventions, particularly the implantation of atelocollagen in the vocal fold lamina propria.
Vocal fold wound healing and scar formation
Any successful therapeutic approach to vocal fold scarring is dependant on a comprehensive understanding of its pathophysiology. Research conducted over the past 10 years has provided a detailed profile of the lamina propria extracellular matrix (ECM) changes that are characteristic of chronic scar, such as dense and/or disorganized collagen deposition, increased fibronectin abundance, and decreased elastin, decorin and hyaluronic acid (HA) abundance [3, 8]. These protein and glycan changes have been observed in both human and animal vocal fold mucosae [8, 9, 10, 11, 12]. Initial changes in the vocal fold mucosa following injury are predominantly directed by migratory inflammatory cells [13]; subsequent remodeling is directed by resident vocal fold fibroblasts (VFFs) and transdifferentated myofibroblasts [14]. Evidence from a number of biological systems, including the vocal fold, suggests that manipulating fibroblast-myofibroblast behavior is critical to improving scarring outcome [15, 16]. Mucosal repair culminating in mature scar formation takes approximately six months in humans [17], dogs [9] and rabbits [10]; two months in rats [11]; and four-to-six weeks in mice [12].
Traditional interventions and their limitations
Various behavioral, pharmacological and surgical interventions have been offered in an attempt to improve or regenerate scarred vocal fold mucosa. Voice therapy offers patients a means to maximize the efficiency of an imperfect vibratory glottal source, in addition to addressing behavioral issues such as compensatory hyperfunction [18]. Steroid injections are often used to reduce inflammation in the acute phase of wound healing [19]. These treatment approaches offer some benefit, but do not directly address the underlying ECM dysfunction in the chronically scarred vocal fold [1].
Traditional surgical approaches to improving vocal fold scar include injection laryngoplasty, medialization thyroplasty, scar excision and/or lysis [1, 2, 20]. Injection laryngoplasty and medialization thyroplasty provide vocal fold augmentation that generally improves vocal fold closure and entrainment for phonation; however, neither approach is able to regenerate the optimal vibratory properties of the native mucosa. Further, the majority of injectates with desirable viscoelastic properties are readily absorbed over time. Scar excision involves resecting fibrotic tissue with the expectation that subsequent wound healing is regenerative; scar lysis attempts to improve vibratory function by shortening longitudinal scar bands into smaller segments. Both of these approaches are generally reserved for severe cases and outcomes are highly dependant upon individual patient healing capacity.
Emerging scaffold-based interventions
Tissue engineering is focused on the regeneration of biological structures and systems using a combination of scaffolds, cells and growth factors under appropriate conditions [21]. This therapeutic paradigm has been adopted by a number of vocal fold researchers in an attempt to prevent, attenuate and reverse vocal fold scarring. Although cell and growth factor-based therapies have shown some restorative effects in experimental studies, combining these approaches with an appropriately matched scaffold environment may bring these therapies even closer to achieving complete regeneration. In general, a restorative scaffold should be biocompatible and biodegradable, match the volume dimensions of the target structure, and provide a permissive environment for cell infiltration, proliferation and regenerative behavior.
HA is the most well-studied synthetic scaffold material employed in human and animal vocal fold scarring research. As the unmodified HA molecule is rapidly degraded in vivo, most recent studies have utilized cross-linking or other forms of chemical modification to extend HA residence time. Hallen et al. [22] injected cross-linked HA into the uninjured rabbit vocal fold. They identified residual HA material up to 12 months post-injection with progressive fibroblast infiltration, connective tissue ingrowth and no evidence of foreign body reaction. In series of studies, Thibeault et al. [23, 24, 25] injected different formulations of a chemically modified HA gelatin hydrogel into rabbit vocal folds at the time of injury. Hydrogel injected vocal folds exhibited improved viscoelastic properties compared to saline injected controls at both early and late time points, supporting the regenerative potential of this scaffold material when introduced to the acute wound environment. In a study focused on intervention during the remodeling phase of wound healing, Jahan-Parwar et al. [26] reported therapeutic benefit following injection of cross-linked HA into canine vocal folds one month following laser-induced injury, compared to a single saline injected control. Finck et al. [27, 28] reported ongoing gradual improvement in vocal function following lamina propria implantation of esterified HA after microflap-based benign lesion excision in human patients. A control group subjected to the same microflap procedure without HA implantation failed to demonstrate equivalent benefit.
Collagen has been a popular injection laryngoplasty material since the mid-1980s [29], and has also recently been investigated as a scaffold for vocal fold tissue engineering. Kanemaru et al. [30*] injected cultured autologous mesenchymal stem cells (MSCs) encapsulated in 1% hydrochloric acid atelocollagen into injured canine vocal folds and reported therapeutic benefit. Although the relative contribution of the MSCs and atelocollagen scaffold was not investigated in this experiment, the cells appeared to proliferate within the scaffold in vivo, leading the authors to conclude that atelocollagen is a suitable scaffold material for MSC-based vocal fold tissue engineering.
An alternative approach to using engineered synthetic scaffolds such as the HA and collagen derivatives described above involves autologous, allogeneic and xenogeneic tissue materials, either in native or decellularized states. Tsunoda et al. [31] performed autologous temporalis fascia transplantation in a series of sulcus vocalis patients and noted gradual improvement in maximum phonation time, vibratory closure pattern and mucosal wave excursion over time. Immunohistochemical examination of herniated fascia removed from a single patient seven days post-transplantation revealed actively proliferating (Ki-67 positive) cells in the scaffold. Additional unpublished data suggests that both autologous fascia transplantation and allogeneic (cadaveric) decellularized dermis (AlloDerm; LifeCell Corporation, Branchburg, NJ) implantation are helpful for the treatment of sulcus vocalis in certain patients [20].
Recent research focused on xenogeneic decellularized scaffold implantation in the lamina propria has employed bovine vocal fold mucosa [32, 33] and porcine liver stroma [34]. Xu et al. implanted decellularized bovine vocal fold mucosa in the cartilaginous rat vocal fold following injury and observed elevated collagen and glycosaminoglycan (GAG) abundance compared to non-implanted injured controls [32]. Complete scaffold turnover occurred by three months post-implantation. Follow-up work using the same experimental model demonstrated the potential of this scaffold as a delivery system for the anti-fibrotic molecule hepatocyte growth factor (HGF) [33]. Gilbert et al. [34] utilized porcine liver-derived ECM for vocal fold reconstruction following injury in a canine model, selected in part because the native liver is rich in HGF. No significant differences in elastin and GAG abundance were observed between scaffold-treated and non-treated control samples; however, the scaffold-treated group exhibited an elevated ratio of type III to type I collagen, in addition to elevated overall collagen deposition. The authors concluded that although complete regeneration was not achieved, their scaffold-based strategy promoted collagen subtype accumulation approximating that of the normal vocal fold and is therefore worthy of additional investigation.
Effects of scaffolds on cells
The majority of candidate scaffolds proposed for vocal fold tissue engineering were initially selected based on favorable physical and biological features, such as biocompatibility, biodegradability and viscoelasticity; however, recent work has given additional attention to the effect of the scaffold material on resident cell phenotype. Regardless of whether cells are encapsulated in a scaffold prior to implantation, or infiltrate from surrounding tissues following implantation, interaction between the scaffold and various cell types is a critical parameter in the optimization of therapeutic outcome. A number of studies have demonstrated the ability of both synthetic and decellularized scaffolds to support VFF proliferation and synthesis of appropriate ECM constituents in three-dimensional culture [35, 36, 37, 38, 39]. Recent reports have also suggested that engineered scaffold parameters such as mesh size, gelatin concentration and HA molecular weight directly influence ECM synthesis by resident VFFs [25, 40, 41]. Further, external biomechanical stimuli including stress, strain and vibration appear to be important co-modulators of cell behavior within the scaffold environment [42, 43].
Recent work by Badylak et al. [44] highlights the potential impact of scaffold composition on infiltrating cell phenotype following in vivo implantation. Rat abdominal wall tissue was excised and replaced with either decellularized porcine small intestinal submucosa (SIS), carbodiimide cross-linked SIS, or an autologous tissue graft. Infiltrating macrophage phenotype and eventual repair outcome varied as a function of scaffold type, suggesting that the scaffold manufacturing process can have a significant influence upon initial host cell response and subsequent remodeling events. This study highlights the necessity of considering both therapeutically delivered and infiltrating cells when attempting to optimize tissue engineered constructs, and is an important area for ongoing investigation.
Atelocollagen sheet implantation
Researchers at Kyoto University recently reported on the therapeutic potential of a commercial atelocollagen sheet (Terudermis, Olympus Terumo Biomaterials Corporation, Tokyo, Japan) as a candidate scaffold for vocal fold tissue regeneration [45**]. This material, which differs from the 1% hydrochloric acid atelocollagen formulation utilized for MSC delivery by Kanemaru et al. [30*], is biocompatible and biodegradable in humans and has previously been used to promote healing of postsurgical dermal and epidermal defects [46]. The sheet is composed of dehydrothermally cross-linked fibrillar atelocollagen and heat-denatured atelocollagen derived from bovine skin, and is 3 mm thick with abundant 50–500 μm diameter micropores. Its physical composition confers collagenase resistance, a prolonged residence time in vivo, and sufficient porosity for cellular infiltration. Initial scaffold development and early animal experiments confirmed maintenance of scaffold structure and successful cell infiltration four weeks following implantation [47].
Atelocollagen sheet implantation in the vocal fold lamina propria is a straightforward procedure consisting of four steps (Figure 1): Elevation of a microflap, microdissection and excision of underlying scar tissue, implantation of the collagen sheet, and wound closure. In contrast with injection-based interventions, this procedure involves the physical removal of scar tissue prior to placement of the therapeutic scaffold. The hypothesis of this treatment approach is that presence of the atelocollagen sheet facilitates regenerative ECM synthesis and remodeling within the lamina propria. Kishimoto et al. [45**] reported the outcome of atelocollagen sheet implantation in six patients with post-cordectomy scar and sulcus vocalis. The majority of patients exhibited gradual improvement in aerodynamic, acoustic and videostroboscopic parameters six months following implantation, although inter-patient variation was observed. Although this study reported on a small number of patients and did not include a control group, these findings suggest that atelocollagen sheet implantation holds therapeutic potential in certain patients with vocal fold scarring and is worthy of additional research.
Figure 1.
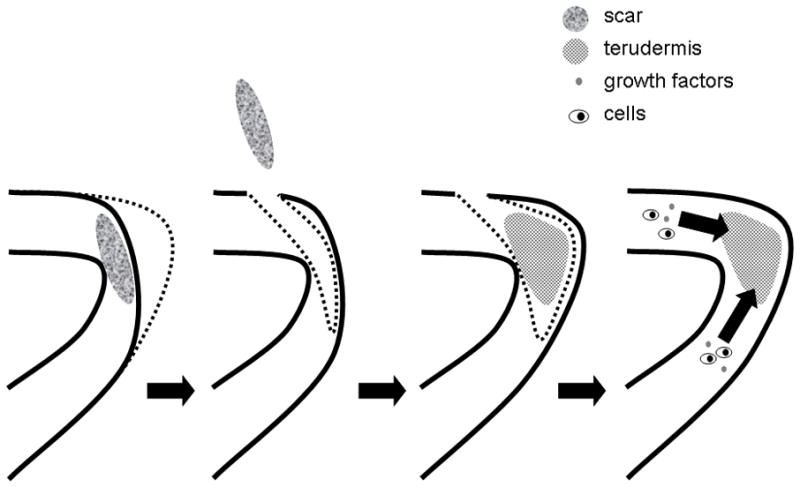
Schematic illustrating atelocollagen sheet implantation. An incision is made lateral to the scarred site and underlying scar tissue is dissected with a microdissector. Following the removal of scar tissue, the atelocollagen sheet is implanted in a subepithelial pocket. The microflap is put back into its original position and the pocket is closed with a suture or fibrin glue to prevent dislocation of the material. The sheet holds an extended residence time and is infiltrated by cells and possibly growth factors from surrounding tissues. Adapted from [45]
In anticipation of future cell-scaffold applications, Ohno et al. [48*] examined the ability of the atelocollagen sheet to support bone marrow derived stromal cells (BMSCs) in three-dimensional culture. Seeded BMSCs adhered to the sheet, proliferated, and synthesized ECM proteins. Although ECM production capacity represents just one aspect of cell phenotype, it appears that the atelocollagen sheet is biocompatible with this cell type and may be suitable as a scaffold to support the delivery of a number of therapeutic cell populations.
Future directions in scaffold-based therapy
Scaffold-based therapies for the treatment of vocal fold scarring remain in their infancy, however this area of tissue engineering is gaining momentum with increasing representation in the wider literature. In addition to ongoing technological advances in materials science, detailed investigation of the influence of scaffolding materials on cells, the turnover and replacement of scaffolds by cells, and the role of growth factors and other molecules in these processes are still needed. Many of the scaffold materials under investigation still await clinical translation, and those that have been investigated in human patients (such as the atelocollagen sheet) require additional research in appropriately powered placebo controlled studies. Further, the majority of published experimental work in animal models has focused on the attenuation and prevention of scar formation by introducing scaffold materials at the time of injury. Future research should give additional attention to the applicability of scaffold-based therapies to the chronically scarred vocal fold.
Conclusions
Scaffold-based therapies for vocal fold scarring are under investigation by an increasing number of research groups and show therapeutic promise in both pre-clinical and early clinical studies. Recent research has begun to shed light on the interactions between scaffold material properties, encapsulated and infiltrating cells, stimulatory molecules such as growth factors, and external regulatory variables such as stress, strain and vibration. The ateolocollagen sheet has an established clinical track record as a scaffold for dermal and epidermal repair and exhibited potential therapeutic benefit in a recent study of patients with vocal fold scarring and sulcus vocalis. Significant additional research with this candidate scaffold is required prior to its endorsement for widespread clinical use.
Acknowledgments
This work was supported by grants R01 DC004428 and R01 DC010777 from the National Institute on Deafness and Other Communicative Disorders, and partly by grant from Takeda Science Foundation.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Hirano S. Current treatment of vocal fold scarring. Curr Opin Otolaryngol Head Neck Surg. 2005;13:143–7. doi: 10.1097/01.moo.0000162261.49739.b7. [DOI] [PubMed] [Google Scholar]
- 2.Benninger MS, Alessi D, Archer S, et al. Vocal fold scarring: current concepts and management. Otolaryngol Head Neck Surg. 1996;115:474–82. doi: 10.1177/019459989611500521. [DOI] [PubMed] [Google Scholar]
- 3.Hansen JK, Thibeault SL. Current understanding and review of the literature: vocal fold scarring. J Voice. 2006;20:110–20. doi: 10.1016/j.jvoice.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Welham NV, Montequin DW, Tateya I, et al. A rat excised larynx model of vocal fold scar. J Speech Lang Hear Res. 2009;52:1008–20. doi: 10.1044/1092-4388(2009/08-0049). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirano S, Bless DM, Nagai H, et al. Growth factor therapy for vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol. 2004;113:777–85. doi: 10.1177/000348940411301002. [DOI] [PubMed] [Google Scholar]
- 6.Welham NV, Dailey SH, Ford CN, et al. Voice handicap evaluation of patients with pathologic sulcus vocalis. Ann Otol Rhinol Laryngol. 2007;116:411–7. doi: 10.1177/000348940711600604. [DOI] [PubMed] [Google Scholar]
- 7.Rosen CA, Lee AS, Osborne J, et al. Development and validation of the voice handicap index-10. Laryngoscope. 2004;114:1549–56. doi: 10.1097/00005537-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Hirano S, Minamiguchi S, Yamashita M, et al. Histologic characterization of human scarred vocal folds. J Voice. 2009;23:399–407. doi: 10.1016/j.jvoice.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Rousseau B, Hirano S, Scheidt TD, et al. Characterization of vocal fold scarring in a canine model. Laryngoscope. 2003;113:620–7. doi: 10.1097/00005537-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Rousseau B, Hirano S, Chan RW, et al. Characterization of chronic vocal fold scarring in a rabbit model. J Voice. 2004;18:116–24. doi: 10.1016/j.jvoice.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Tateya T, Tateya I, Sohn JH, et al. Histologic characterization of rat vocal fold scarring. Ann Otol Rhinol Laryngol. 2005;114:183–91. doi: 10.1177/000348940511400303. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita M, Bless DM, Welham NV. Morphological and Extracellular Matrix Changes following Vocal Fold Injury in Mice. Cells Tissues Organs. 2010;192:262–71. doi: 10.1159/000315476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ling C, Yamashita M, Waselchuk EA, et al. Alteration in cellular morphology, density and distribution in rat vocal fold mucosa following injury. Wound Repair Regen. 18:89–97. doi: 10.1111/j.1524-475X.2009.00550.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwano M, Fischer A, Okada H, et al. Conditional abatement of tissue fibrosis using nucleoside analogs to selectively corrupt DNA replication in transgenic fibroblasts. Mol Ther. 2001;3:149–59. doi: 10.1006/mthe.2000.0251. [DOI] [PubMed] [Google Scholar]
- 15.Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143–79. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- 16.Vyas B, Ishikawa K, Duflo S, et al. Inhibitory effects of hepatocyte growth factor and interleukin-6 on transforming growth factor-beta1 mediated vocal fold fibroblast-myofibroblast differentiation. Ann Otol Rhinol Laryngol. 2010;119:350–7. doi: 10.1177/000348941011900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishimoto Y, Hirano S, Tateya I, et al. Temporal changes in vocal functions of human scarred vocal folds after cordectomy Laryngoscope. in press. [DOI] [PubMed] [Google Scholar]
- 18.Ramig LO, Verdolini K. Treatment efficacy: voice disorders. J Speech Lang Hear Res. 1998;41:S101–16. doi: 10.1044/jslhr.4101.s101. [DOI] [PubMed] [Google Scholar]
- 19.Mortensen M, Woo P. Office steroid injections of the larynx. Laryngoscope. 2006;116:1735–9. doi: 10.1097/01.mlg.0000231455.19183.8c. [DOI] [PubMed] [Google Scholar]
- 20.Dailey SH, Ford CN. Surgical management of sulcus vocalis and vocal fold scarring. Otolaryngol Clin North Am. 2006;39:23–42. doi: 10.1016/j.otc.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 22.Hallen L, Johansson C, Laurent C. Cross-linked hyaluronan (Hylan B gel): a new injectable remedy for treatment of vocal fold insufficiency--an animal study. Acta Otolaryngol. 1999;119:107–11. doi: 10.1080/00016489950182043. [DOI] [PubMed] [Google Scholar]
- 23.Thibeault SL, Klemuk SA, Chen X, et al. In Vivo Engineering of the Vocal Fold ECM With Injectable HA Hydrogels-Late Effects on Tissue Repair and Biomechanics in a Rabbit Model. J Voice. 2011;25:249–53. doi: 10.1016/j.jvoice.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen JK, Thibeault SL, Walsh JF, et al. In vivo engineering of the vocal fold extracellular matrix with injectable hyaluronic acid hydrogels: early effects on tissue repair and biomechanics in a rabbit model. Ann Otol Rhinol Laryngol. 2005;114:662–70. doi: 10.1177/000348940511400902. [DOI] [PubMed] [Google Scholar]
- 25.Duflo S, Thibeault SL, Li W, et al. Effect of a synthetic extracellular matrix on vocal fold lamina propria gene expression in early wound healing. Tissue Eng. 2006;12:3201–7. doi: 10.1089/ten.2006.12.3201. [DOI] [PubMed] [Google Scholar]
- 26.Jahan-Parwar B, Chhetri DK, Ye M, et al. Hylan B gel restores structure and function to laser-ablated canine vocal folds. Ann Otol Rhinol Laryngol. 2008;117:703–7. doi: 10.1177/000348940811700913. [DOI] [PubMed] [Google Scholar]
- 27.Finck C, Lefebvre P. Implantation of esterified hyaluronic acid in microdissected Reinke's space after vocal fold microsurgery: first clinical experiences. Laryngoscope. 2005;115:1841–7. doi: 10.1097/01.mlg.0000173158.22274.8d. [DOI] [PubMed] [Google Scholar]
- 28.Finck CL, Harmegnies B, Remacle A, et al. Implantation of Esterified Hyaluronic Acid in Microdissected Reinke's Space After Vocal Fold Microsurgery: Short- and Long-Term Results. J Voice. 2010;24:626–635. doi: 10.1016/j.jvoice.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Ford CN, Bless DM. A preliminary study of injectable collagen in human vocal fold augmentation. Otolaryngol Head Neck Surg. 1986;94:104–12. doi: 10.1177/019459988609400117. [DOI] [PubMed] [Google Scholar]
- 30*.Kanemaru S, Nakamura T, Omori K, et al. Regeneration of the vocal fold using autologous mesenchymal stem cells. Ann Otol Rhinol Laryngol. 2003;112:915–20. doi: 10.1177/000348940311201101. In this study, the atelocollagen was used in combination with cell therapy for the treatment of injured vocal fold. [DOI] [PubMed] [Google Scholar]
- 31.Tsunoda K, Kondou K, Kaga K, et al. Autologous transplantation of fascia into the vocal fold: long-term result of type-1 transplantation and the future. Laryngoscope. 2005;115:1–10. doi: 10.1097/01.mlg.0000183966.72921.31. [DOI] [PubMed] [Google Scholar]
- 32.Xu CC, Chan RW, Weinberger DG, et al. Controlled release of hepatocyte growth factor from a bovine acellular scaffold for vocal fold reconstruction. J Biomed Mater Res A. 2010;93:1335–47. doi: 10.1002/jbm.a.32632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu CC, Chan RW, Weinberger DG, et al. A bovine acellular scaffold for vocal fold reconstruction in a rat model. J Biomed Mater Res A. 2010;92:18–32. doi: 10.1002/jbm.a.32279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert TW, Agrawal V, Gilbert MR, et al. Liver-derived extracellular matrix as a biologic scaffold for acute vocal fold repair in a canine model. Laryngoscope. 2009;119:1856–63. doi: 10.1002/lary.20575. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Thibeault SL. Biocompatibility of a synthetic extracellular matrix on immortalized vocal fold fibroblasts in 3-D culture. Acta Biomater. 2010;6:2940–8. doi: 10.1016/j.actbio.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hahn MS, Teply BA, Stevens MM, et al. Collagen composite hydrogels for vocal fold lamina propria restoration. Biomaterials. 2006;27:1104–9. doi: 10.1016/j.biomaterials.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 37.Luo Y, Kobler JB, Zeitels SM, et al. Effects of growth factors on extracellular matrix production by vocal fold fibroblasts in 3-dimensional culture. Tissue Eng. 2006;12:3365–74. doi: 10.1089/ten.2006.12.3365. [DOI] [PubMed] [Google Scholar]
- 38.Kutty JK, Webb K. Mechanomimetic hydrogels for vocal fold lamina propria regeneration. J Biomater Sci Polym Ed. 2009;20:737–56. doi: 10.1163/156856209X426763. [DOI] [PubMed] [Google Scholar]
- 39.Xu CC, Chan RW, Tirunagari N. A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria. Tissue Eng. 2007;13:551–66. doi: 10.1089/ten.2006.0169. [DOI] [PubMed] [Google Scholar]
- 40.Liao H, Munoz-Pinto D, Qu X, et al. Influence of hydrogel mechanical properties and mesh size on vocal fold fibroblast extracellular matrix production and phenotype. Acta Biomater. 2008;4:1161–71. doi: 10.1016/j.actbio.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munoz-Pinto DJ, Jimenez-Vergara AC, Gelves LM, et al. Probing vocal fold fibroblast response to hyaluronan in 3D contexts. Biotechnol Bioeng. 2009;104:821–31. doi: 10.1002/bit.22436. [DOI] [PubMed] [Google Scholar]
- 42.Kutty JK, Webb K. Vibration stimulates vocal mucosa-like matrix expression by hydrogel-encapsulated fibroblasts. J Tissue Eng Regen Med. 2010;4:62–72. doi: 10.1002/term.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Titze IR, Hitchcock RW, Broadhead K, et al. Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses. J Biomech. 2004;37:1521–9. doi: 10.1016/j.jbiomech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Badylak SF, Valentin JE, Ravindra AK, et al. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–42. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 45**.Kishimoto Y, Hirano S, Kojima T, et al. Implantation of an atelocollagen sheet for the treatment of vocal fold scarring and sulcus vocalis. Ann Otol Rhinol Laryngol. 2009;118:613–20. doi: 10.1177/000348940911800902. The only study discussing the effects of atelocollagen sheet implantation on vocal fold scarring and sulcus vocalis. [DOI] [PubMed] [Google Scholar]
- 46.Lee JW, Jang YC, Oh SJ. Use of the artificial dermis for free radial forearm flap donor site. Ann Plast Surg. 2005;55:500–2. doi: 10.1097/01.sap.0000183789.00146.c6. [DOI] [PubMed] [Google Scholar]
- 47.Koide M, Osaki K, Konishi J, et al. A new type of biomaterial for artificial skin: dehydrothermally cross-linked composites of fibrillar and denatured collagens. J Biomed Mater Res. 1993;27:79–87. doi: 10.1002/jbm.820270111. [DOI] [PubMed] [Google Scholar]
- 48*.Ohno S, Hirano S, Tateya I, et al. Atelocollagen sponge as a stem cell implantation scaffold for the treatment of scarred vocal folds. Ann Otol Rhinol Laryngol. 2009;118:805–10. This study shows the potential of the atelocollagen sheet as stem cell implantation scaffolding for the treatment of vocal fold scarring. [PubMed] [Google Scholar]


