Abstract
Invadopodia are cellular structures that are thought to mediate tumor invasion. ASAP1, an Arf GTPase-activating protein (GAP) containing a BAR domain, is a substrate of Src. ASAP1 is required for the assembly of invadopodia and podosomes, which are Src-induced structures related to invadopodia in NIH 3T3 fibroblasts. The BAR domain of ASAP1 is required for the assembly of podosomes. Using two-hybrid screening, we have identified GEFH1, a guanine nucleotide exchange factor for RhoA, as a binding partner of the BAR domain of ASAP1. We validated the interaction of endogenous GEFH1 with ASAP1 by immunoprecipitation, and found GEFH1 colocalized with ASAP1 in podosomes. The overexpression of GEFH1 inhibited podosome assembly and ASAP1 catalytic activity as a GAP. A mutant of GEFH1 lacking the domain that binds to the BAR domain of ASAP1 was less effective. Reduced expression of GEFH1, achieved with siRNA treatment, did not affect matrix degradation by podosomes but increased the rate of podosome assembly. Based on these results, we conclude that GEFH1 is a negative regulator of podosomes.
Keywords: ASAP1, GEFH1, podosome, ArfGAP
Introduction
The ability of malignant cells to invade normal tissues underlies much of the pathology of cancer. The invasiveness of cells correlates with the presence of dynamic actin-rich structures called invadopodia [1; 2]. Invadopodia are similar in appearance and composition to structures called podosomes, which are found in osteoclasts, macrophages and Src-transformed fibroblasts [3]. Both invadopodia and podosomes are large macromolecular complexes that connect the extracellular matrix with the intracellular actin cytoskeleton. They are enriched with adhesion molecules, actin-modulating proteins, tyrosine kinases, matrix proteases, and tyrosine-phosphorylated proteins.
Arf GTPase-activating proteins (GAPs) induce hydrolysis of GTP bound to Arf family GTP-binding proteins. Arfs and Arf GAPs are critical regulators of the actin cytoskeleton and vesicle coat dynamics in membrane traffic [4; 5]. ASAP1 is an Arf GAP that is known to regulate the actin cytoskeleton [6]. ASAP1 has BAR, PH, ArfGAP, Ankyrin repeat, Proline-rich, D/ELPPKP repeat, and SH3 domains. ASAP1 is a substrate for the tyrosine kinase Src [7] and binds to variety of actin modulating proteins including cortactin [8; 9], which is a required component of invadopodia [10]. Previously, we reported that ASAP1 is required for invadopodia formation in a breast cancer cell line and for podosome formation in NIH 3T3 fibroblasts expressing active c-Src [9]. The reduction of ASAP1 expression prevented podosome formation[9]. We also found that the BAR domain of ASAP1 is required for podosome formation and that the overexpression of the BAR-PH domain of ASAP1 inhibited podosome formation. Taken together, these results are consistent with the hypothesis that the BAR-PH domains of ASAP1 bind to a target protein that regulates podosome assembly or disassembly. Here, we identified GEFH1 as a binding partner of the BAR domain of ASAP1 using two-hybrid screens. GEFH1 is a guanine nucleotide exchange factor (GEF) for RhoA GTP binding protein first identified as Lfc in mouse [11]. We confirmed that endogenous ASAP1 and GEFH1 bind and found that GEFH1 functions as an inhibitor of podosome formation in cells.
Materials and Methods
Reagents
A mammalian expression vector for constitutively active chicken Src (Src Y527F-pCEFL) was a kind gift from J. Silvio Gutkind (NIH, Bethesda, MD). The constructs of C-terminally diglutamate tagged mouse ASAP1 (ASAP1-EE) and N-terminally FLAG tagged BAR-PH (1-438) have been described [9; 12]. Wild type, Y393A, and C-terminal deletion of GEFH1 (1-572) constructs were kind gifts from Gary M Bokoch (The Scripps research institute, CA). The following antibodies were used: rabbit monoclonal antibody against GEFH1 for western blotting and immunofluorescence (Cell signal), rabbit polyclonal antibody against GEFH1 for immunoprecipitation (Bethyl laboratory), mouse monoclonal antibody against ASAP1 (BD), rabbit polyclonal antibody against ASAP1 (Rockland), mouse monoclonal antibody against diglutamate tag (anti-EE, Covance), mouse monoclonal antibody against cortactin (4F11) and Src (EC10, Millipore), rabbit polyclonal antibody against cortactin (Cell Signal). Sheep polyclonal antibody against mouse GEFH1 (Lfc) (Calbiochem). Rhodamin-labeled phalloidin was purchased from Invitrogen. An siRNA mixture of 4 sequences against GEFH1 (ARHGEF2) was purchased from Dharmacon. The 4 sequences are as follows; 5′-caacauugcuggacauuuc-3′, 5′-gcacugggaugcuggaaga-3′, 5′-guaccaaggucaagcagaa-3′, 5′-uggaaucccuuauugauga-3′.
Yeast two-hybrid screening
Yeast two-hybrid screening was carried out at Myriad Genetics (Salt Lake City, UT) using the BAR domain of human ASAP1 (aa 20–270) as bait with a mating-based method. The corresponding cDNA for ASAP1 BAR domain was cloned into pGBT.superB creating an open reading frame for ASAP1 fragments fused to the GAL4 DNA-binding domain. The bait plasmid was introduced into Myriad's ProNet yeast strain PNY200 (MATαura3-52 ade2-101 trp1-901 his3-Δ200 leu2-3112 gal4Δgal80Δ). The bait yeast cells were allowed to mate with Myriad's ProNet MATa yeast cells, BK100 (MATa ura3-52 trp1-901 his3-Δ200 leu2-3112 gal4Δ gal80ΔGAL2-ADE2 LYS2::GAL1-HIS3 met2::GAL7-lacZ) containing cDNA library from mouse embryo. After mating, at least 5 million diploid yeast cells were obtained from the library and selected on His- and Ade-lacking medium. The auxotrophy is suppressed if the bait and prey proteins interact. The prey plasmids were isolated from the positive colonies, and the interaction was confirmed by expression of third reporter gene (lacZ). cDNAs in the positive prey plasmids were sequenced.
Immunoprecipitation
For endogenous binding, 6×106 NIH 3T3 cells were transfected with pEGFP-C1 (Takara) or chicken Src Y527F using Lipofectamine 2000 (Invitrogen). After 18-22 hr, cells were washed twice with phosphate buffered saline (PBS) and lysed with 500 μl of assay buffer containing 25 mM Hepes-KOH, pH 7.0, 125 mM K-acetate, 2.5 mM Mg-acetate, 5 mM EGTA, 1% Triton X-100, 1 mM DTT and protease inhibitor cocktail (Roche). After centrifugation at 14,000 rpm at 4°C for 10 min, 30 μg of the lysate was taken as input, and the remaining lysate was incubated with either control rabbit IgG or anti-GEFH1 antibody together with γ-bind beads (GE Healthcare). Samples were rotated at 4°C for 1 ½ hrs, beads were washed twice with assay buffer and proteins bound to the beads were eluted into Laemmli's sample buffer. The eluted material was analyzed by immunoblotting using anti-ASAP1 and anti-Src antibodies. For experiments in which recombinant proteins were expressed, cells were transfected with plasmids directing expression of ASAP1-EE or FLAG-BAR-PH using Lipofectamine 2000 and incubated 18-22 hr prior to immunoprecipitating as above using mouse IgG, anti-EE or anti-FLAG conjugated beads (Sigma).
Immunofluorescence
NIH3T3 cells were grown on coverslips coated with 10 μg/ml fibronectin at 37°C for 1 hr. Cells were transfected with chicken SrcY527F using Lipofectamine 2000 (Invitrogen). GEFH1 DNAs were double transfected as indicated. For siRNA transfections, Dharmafect 4 (Dharmacon) was used. After 18-22 hr, cells were washed with PBS and fixed with 4% paraformaldehyde (PFA) for 15 min and quenched with 50 mM NH4Cl. Cells were incubated with blocking solution (0.4% saponin, 10% fetal bovine serum, in PBS) for 30 min. Primary and secondary antibodies were diluted with blocking solution and incubated with cells for 30 min each. Slides were prepared using mounting solution (DakoCytomation). A confocal microscope (Zeiss 510 meta) was used for capturing images. The cells with podosomes judged by phalloidin and cortactin staining were counted and expressed as percentage of the total number of Src-transfected cells.
Matrix degradation assay
Matrix solution was prepared with the mixture of 0.2% gelatin from porcine skin (Sigma), 20 μg/ml fibronectin (sigma), and 0.2 % oregon-green gelatin (Invitrogen) in phosphate buffered saline (PBS). Oregon-green gelatin was mixed in 1:4 dilution of the mix. Coverslips were incubated with the matrix for 10 min at room temperature, and washed twice in PBS. NIH 3T3 cells were transfected with siRNAs for 48 hr and subsequently with Src Y527F for 24 hr, then plated on coated cover slips at a density of 75×103 cells per coverslip. Cells were grown for 3 hr at 37° C and fixed. Podosomes were detected by rhodamin-labeled phalloidin, and images were captured using a Zeiss 510 meta.
GAP assay
NIH 3T3 fibroblasts were grown in 6 well plates and transfected with plasmids for expression of ASAP1 and GEFH1 as indicated. Lysates from 480×103 cells were prepared in 100 μl of lysis buffer (50mM Tris-HCL, pH 7.5, 100mM NaCl, 2mM MgCl2, 1mM DTT, protease inhibitor complete EDTA free (Roche), 0.2% TritonX-100). The lysates were diluted more than 10 fold with the GAP assay buffer and GAP activity was determined using Arf1•GTP as a substrate as described [7].
Statistics
Statistical analysis was performed using GraphPad Prism 5. Specific analysis are described in the figure legends.
Results
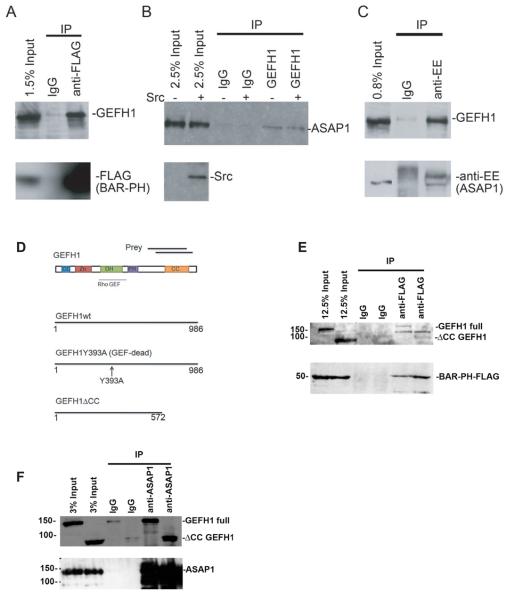
To find binding partners of the BAR domain of ASAP1, we used two-hybrid screening with the BAR domain of ASAP1 (amino acids 20-270) as a bait. cDNAs encoding KIF15, Cdc42BP, Dynamin1, PACSIN2, Annexin IV, GEFH1 were identified. To confirm binding, we performed immunoprecipitation with an anti-FLAG antibody using cells overexpressing FLAG-tagged BAR-PH domain. Among these binding candidates, we found GEFH1 binds to ASAP1 (Figure 1A). To determine if endogenous proteins associated, we immunoprecipitated proteins using an anti-GEFH1 antibody from NIH 3T3 fibroblasts expressing constitutively active Src (SrcY527F) (Figure 1B). ASAP1 was detected in precipitates with or without Src expression, consistent with constitutive association of GEFH1 and ASAP1. We also determined if we could detect GEFH1 in complex with ASAP1 when immunoprecipitating ASAP1. Di-glutamate (EE) tagged mouse ASAP1 was expressed in NIH 3T3 fibroblasts and immunoprecipitated using an anti-EE antibody (Figure 1C). GEFH1 co-precipitated with EE-tagged ASAP1. These results support the conclusion that GEFH1 binds to ASAP1 in vivo. We further defined the binding site, comparing the binding of wild type GEFH1 with [ΔCC]GEFH1, which contains amino acids 1-572 of GEFH1. From the two-hybrid screening, 2 clones of C-terminal region of human GEFH1, from amino acid 660 to 858, and 704 to 924, respectively, were identified (Figure 1D). This indicates that C-terminal region of GEFH1, which has a coiled-coil domain, mediates binding to the BAR domain of ASAP1. We compared the binding of full length and [ΔCC]GEFH1 to the BAR-PH domains of ASAP1 (Figure 1E). We found that wild type and [ΔCC]GEFH1 coprecipitated with BAR-PH domain of ASAP1; however, there was nonspecific immunoprecipitation of [ΔCC]GEFH1 with IgG. We did not observe an increase in coprecipitation of [ΔCC]GEFH1 with BAR-PH, using an antibody to the Flag epitope, as compared with the amount precipitated with the control IgG. These results are consistent with the coiled-coil domain of GEFH1 binding to the BAR domain of ASAP1. We also examined the binding of full length ASAP1 to wild type GEFH1 and [ΔCC]GEFH1 (Figure 1F). More [ΔCC]GEFH1 coprecipitated with full length ASAP1, using an antibody to the EE epitope, than with the control IgG, indicating that there may be additional binding sites between the proteins.
Figure 1. GEFH1 binds to ASAP1 in vivo.
FLAG-tagged BAR-PH was expressed in HEK293 cells and immunoprecipitated with FLAG antibody conjugated beads. GEFH1 was detected in the precipitate by immunoblotting with an anti-GEFH1 antibody. (B) NIH 3T3 cells were transfected with expression vectors for active Src or GFP. Proteins from the cell lysates were precipitated with anti-GEFH1 antibody and ASAP1 was detected in the precipitate by immunoblotting. (C) EE-tagged ASAP1 was expressed in NIH 3T3 fibroblasts and was immunoprecipitated with anti-EE antibody. GEFH1 was detected in the precipitate by immunoblotting. (D) The recombinant GEFH1 proteins used in this paper. The two-hybrid clones obtained were indicated at the top as prey. CC; coiled-coil, Zn; Zinc finger, DH; Dbl-homology, PH; Plecstrin homology domain. (E) Flag-tagged [1-438]ASAP1 (BAR-PH) was expressed in NIH 3T3 fibroblasts with full length GFP-tagged GEFH1 or [1-572]GEFH1 (ΔCC GEFH1) and proteins were immunoprecipitated with either nonspecific IgG or an anti-Flag antibody. GEFH1 was detected by immunoblotting. (F) EE-tagged full length ASAP1 was coexpressed with either full length GEFH1 or ΔCC GEFH1. Proteins were immunoprecipitated with a nonspecific IgG or with an antibody to the EE epitope tag. GEFH1 was detected by anti-GFP antibody.
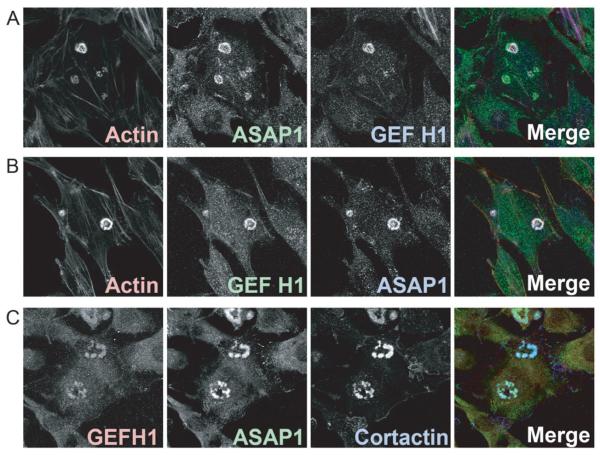
As a first step to determine if GEFH1 functions with ASAP1 in podosomes, we examined GEFH1 localization in NIH 3T3 fibroblasts expressing active Src. 40 % of the cells with Src had podosomes, which often formed circular patterns called rosettes. Endogenous GEFH1 was detected in the podosomes, colocalizing with ASAP1 (Figure 2A). To exclude the possibility that the rhodamine-labeled actin fluorescence bled into the far red channel used to detect GEFH1, we used an Alexa 488 labeled secondary antibody for GEFH1. Again, we found that the signal for GEFH1 localized in podosomes with ASAP1 (Figure 2B). To confirm these results, we used another antibody raised against GEFH1. The antibody also stained podosomes, with signal colocalizing with ASAP1 and cortactin (Figure 2C). Based on these results, we conclude that GEFH1 colocalizes with ASAP1 in podosomes and further considered a role of GEFH1 in regulation of podosomes.
Figure 2. GEFH1 associates with podosomes.
NIH 3T3 fibroblasts were plated on fibronectin-coated coverslips and transfected with vector for the expression of active Src to form podosomes. (A) Cells were triple stained with phalloidin (Actin), mouse monoclonal antibody against ASAP1, and rabbit monoclonal antibody against GEFH1. (B) Cells were incubated with the same primary antibodies as in A. Different secondary antibodies were used for GEFH1 and ASAP1. (C) Experiment was similar to (A) but a sheep polyclonal antibody was used to detect GEFH1, a rabbit polyclonal antibody to detect ASAP1 and a mouse monoclonal to cortactin was used to mark podosomes.
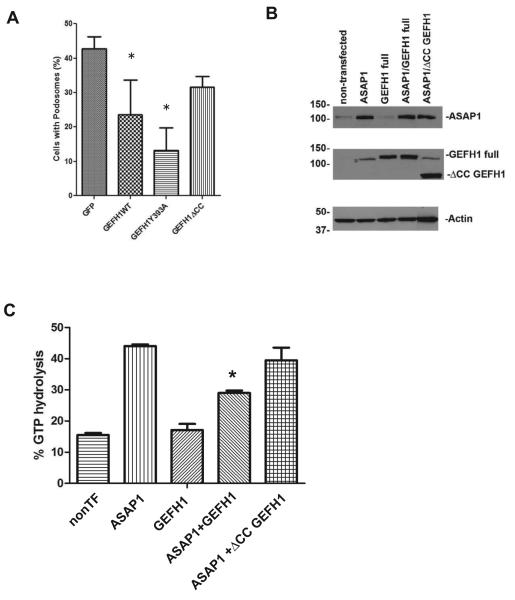
To examine if GEFH1 is involved in podosome formation, we overexpressed 3 recombinant proteins derived from GEFH1; wild type GEFH1, a GEF-dead mutant of GEFH1 (Y393A), and [ΔCC]GEFH1 (Figure 1D). We double transfected plasmids directing expression of these proteins with plasmids for active Src into NIH 3T3 cells and examined the number of cells with podosomes, which were detected by staining actin with phalloidin and staining with an antibody against cortactin. The expression of wild type and Y393A GEFH1 reduced podosome formation, while [ΔCC]GEFH1 was less effective compared with wild type GEFH1 (Figure 3A). These results support the idea that GEFH1 inhibits podosomes formation and the effect is partially dependent on the C-terminal region.
Figure 3. GEFH1 overexpression inhibits podosome assembly and ArfGAP activity.
(A) NIH 3T3 cells were double transfected with plasmids directing expression of Src and the indicated recombinant GEFH1. The number of Src-transfected cells which had podosomes is expressed as the percentage of the total number of cells expressing Src. The data are the summary of three experiments. *, indicates p<0.05 by ANOVA analysis with a Dunnett post test comparing each condition to the GFP control. (B) Expression level of ASAP1 and GEFH1 or ΔCC GEFH1 in cell lysates used for GAP assays were examined. Proteins in the cell lysates were detected by immunoblotting. * in GEFH1 blot indicates faint band of ASAP1 after stripping of ASAP1 antibody from the membrane of upper panel. (C) GAP activity in cell lysates. GAP activity was determined as previously described. The data presented are the summary of 4 experiments. The data were analyzed by ANOVA and a Bonferoni post test comparing activity in the three samples containing ASAP1. *, p<0.05.
Inhibition of podosome formation by Y393A GEFH1 indicated that the inhibitory effect of GEFH1 on podosome formation could be independent of Rho GEF activity. We determined whether GEFH1 affected the Arf GAP activity of ASAP1 (Figure 3C). In these experiments we expressed GEFH1 alone, ASAP1 alone and ASAP1 with either GEFH1 or with [ΔCC]GEFH1 in NIH3T3 fibroblasts. ASAP1 expression in each cell lysate was determined by western blotting (Figure 3B). GAP activity in the cell lysates using Arf1•GTP as a substrate was determined. Activity was higher in cells expressing ASAP1 than in those without the plasmid for ASAP1. We found that GEFH1 but not [ΔCC]GEFH1 inhibited activity due to ASAP1 expression.
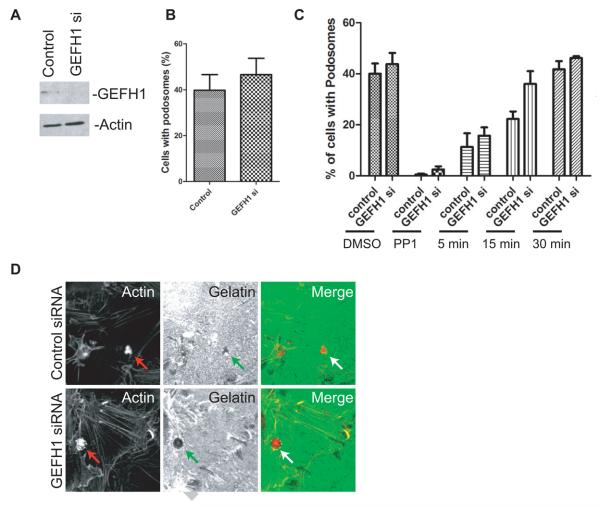
To further analyze the GEFH1 function in podosome formation, we reduced GEFH1 expression by treating cells with siRNA, which reduced protein expression by greater than 70 % (Figure 4A). Consistent with the results of experiments in which GEFH1 was overexpressed, podosome formation was slightly increased in cells with reduced GEFH1 expression (Figure 4B). To determine if GEFH1 affected podosome assembly, cells were treated with the src inhibitor PP1, which results in the disassembly of podosomes. PP1 was then washed from the cells and the reassembly of podosomes was followed. Podosomes formed more quickly in cells with reduced GEFH1 expresssion. We also examined matrix degradation activity of podosomes formed in cells with reduced expression of GEFH1 (Figure 4D). Matrix degradation occurs similarly to control siRNA treated cells. Taken together, these results support the idea that GEFH1 is a negative regulator of podosome formation.
Figure 4. Reduced GEFH1 expression increases podosome formation.
(A) NIH 3T3 fibroblasts were transfected with siRNA for GEFH1. 10μg of lysate was electrophoresed and immunoblotted to assess endogenous GEFH1 expression. There was more than a 70% reduction in expression. Actin was used as internal control. (B) NIH 3T3 fibroblasts were transfected with siRNA against GEFH1 and podosome formation was analyzed after cells were transfected with a plasmid directing expression of active Src. (C) GEFH1 affects rate of podosome assembly. NIH 3T3 fibroblasts were treated with siRNA targeting GEFH1 with a consequent greater than 70% decrease in GEFH1 expression. The cells were transfected with [Y527F]c-Src to induce podosome formation. Podosomes were disassembled with the Src inhibitor PP1 (30μM, dissolved in DMSO) for 10 min at 37°C. PP1 was subsequently washed from the cells and podosome formation was determined after 5, 15, and 30 min at 37°C. The data were analyzed by 2 way ANOVA. GEFH1 affected the rate of assembly with a p=0.01. (D) Matrix degradation was examined in cells transfected with siRNAs of control and GEFH1. Podosomes were detected by actin (red) and the matrix with gelatin (green). Podosomes and areas of matrix degradation were indicated by arrows.
Discussion
In this paper, we identified GEFH1 as a binding partner of the BAR domain of ASAP1 and found that GEFH1 associated with podosomes. Functional analyses supported the idea that GEFH1 is negative regulator of podosome formation.
Overexpression of GEFH1 reduced the number of Src-transformed NIH 3T3 fibroblasts that have podosomes, whereas a mutant GEFH1 with a C-terminal deletion was less effective (Figure 3A) and reducing GEFH1 expression increased the rate of podosome formation (Figure 4C). The c-terminus of GEFH1 interacts with the BAR domain of ASAP1 (Figure 1E). Taken together, the results are consistent with the role of GEFH1 binding to the BAR domain of ASAP1 for inhibiting podosome formation. ASAP1 has GAP activity against Arf1 and Arf5, converting Arf•GTP to Arf•GDP [7]. The deletion of BAR domain is known to increase GAP activity of ASAP1 [13]. Similarly, FIP3, an endosomal protein bound to ASAP1 BAR domain, also increased GAP activity of ASAP1 [12]. Our preliminary data suggested that activated Arf is involved in podosomes formation (Shiba, unpublished results). One possible mechanism for the effect of GEFH1 on podosomes is that it increases GAP activity of ASAP1, leading to decrease Arf•GTP levels in cells, thereby decreasing podosomes formation. However, we observed that GAP activity of ASAP1 was decreased in cells overexpression GEFH1. The inhibition was dependent on the C-terminus of GEFH1, which binds the BAR domain of ASAP1 (Figure 1E and 3C). One possible explanation is that binding of GEFH1 to BAR domain inhibits ASAP1 binding to Arf, preventing ASAP1 function of promoting podosome formation downstream of activated Arf.
Although [ΔCC]GEFH1 was less active than wild type GEFH1 in reducing podosome formation, it, nevertheless, had a partial effect (Figure 3A). Although this result could be consequent to the fragment of GEFH1 competing with full length GEFH1 for a site of action, the result could also be interpreted as the presence of a domain in the 1-572 region of GEFH1 that is inhibitory to podosome formation. GEFH1 is a guanine nucleotide exchange factor (GEF) for RhoA [11; 14]. The GEF domain is included in 1-572 region. The precise function of RhoA in podosome and invadopodia assembly is unclear. Of the Rho family proteins, only Cdc42 has consistently been found to be critical for podosome and invadopodia formation [15; 16]. Our siRNA data showed that GEFH1 is not required for podosome formation. It is plausible that Rho may antagonize the effect of Cdc42 and be involved in invadopodia disassembly. RhoA is known to be important for podosome belt disassembly. For instance, in osteoclasts, microtubule disruption leads to disruption of the podosome belt [17]. RhoA inhibition prevents podosome belt disruption following microtubule depolymerisation by nocodazole [18]. The GEF activity of GEFH1 is activated by microtubule disruption [19], raising a possibility that GEFH1 is responsible for the increased RhoA activity necessary for podosome belt disassembly. Microtubule disruption also leads to increased Rho activity in other cell types, such as NIH 3T3 fibroblasts. Although microtubule regulation in NIH 3T3 fibroblasts and osteoclasts may be different (RhoA inhibition destabilized microtubule in NIH 3T3 fibroblasts, whereas RhoA inhibition stabilized microtubule in osteoclasts), microtubule disruption has the same effect on Rho in both cell types. Future studies will address whether RhoA can antagonize Cdc42 in the formation of podosomes/invadopodia and if GEFH1 is the responsible GEF.
Acknowledgment
This work was supported by the intramural program of the National Cancer Institute, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 2.Albiges-Rizo C, Destaing O, Fourcade B, Planus E, Block MR. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J Cell Sci. 2009;122:3037–3049. doi: 10.1242/jcs.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jurdic P, Saltel F, Chabadel A, Destaing O. Podosome and sealing zone: specificity of the osteoclast model. Eur J Cell Biol. 2006;85:195–202. doi: 10.1016/j.ejcb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Inoue H, Randazzo PA. Arf GAPs and their interacting proteins. Traffic. 2007;8:1465–1475. doi: 10.1111/j.1600-0854.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- 5.Nie Z, Randazzo PA. Arf GAPs and membrane traffic. J Cell Sci. 2006;119:1203–1211. doi: 10.1242/jcs.02924. [DOI] [PubMed] [Google Scholar]
- 6.Randazzo PA, Inoue H, Bharti S. Arf GAPs as regulators of the actin cytoskeleton. Biol Cell. 2007;99:583–600. doi: 10.1042/bc20070034. [DOI] [PubMed] [Google Scholar]
- 7.Brown MT, Andrade J, Radhakrishna H, Donaldson JG, Cooper JA, Randazzo PA. ASAP1, a phospholipid-dependent arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol Cell Biol. 1998;18:7038–7051. doi: 10.1128/mcb.18.12.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onodera Y, Hashimoto S, Hashimoto A, Morishige M, Mazaki Y, Yamada A, Ogawa E, Adachi M, Sakurai T, Manabe T, Wada H, Matsuura N, Sabe H. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 2005;24:963–973. doi: 10.1038/sj.emboj.7600588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharti S, Inoue H, Bharti K, Hirsch DS, Nie Z, Yoon HY, Artym V, Yamada KM, Mueller SC, Barr VA, Randazzo PA. Src-dependent phosphorylation of ASAP1 regulates podosomes. Mol Cell Biol. 2007;27:8271–8283. doi: 10.1128/MCB.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 11.Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem. 1998;273:34954–34960. doi: 10.1074/jbc.273.52.34954. [DOI] [PubMed] [Google Scholar]
- 12.Inoue H, Ha VL, Prekeris R, Randazzo PA. Arf GTPase-activating protein ASAP1 interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor-positive recycling endosome. Mol Biol Cell. 2008;19:4224–4237. doi: 10.1091/mbc.E08-03-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jian X, Brown P, Schuck P, Gruschus JM, Balbo A, Hinshaw JE, Randazzo PA. Autoinhibition of Arf GTPase-activating protein activity by the BAR domain in ASAP1. J Biol Chem. 2009;284:1652–1663. doi: 10.1074/jbc.M804218200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glaven JA, Whitehead IP, Nomanbhoy T, Kay R, Cerione RA. Lfc and Lsc oncoproteins represent two new guanine nucleotide exchange factors for the Rho GTP-binding protein. J Biol Chem. 1996;271:27374–27381. doi: 10.1074/jbc.271.44.27374. [DOI] [PubMed] [Google Scholar]
- 15.Ory S, Brazier H, Pawlak G, Blangy A. Rho GTPases in osteoclasts: orchestrators of podosome arrangement. Eur J Cell Biol. 2008;87:469–477. doi: 10.1016/j.ejcb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 17.Destaing O, Saltel F, Geminard JC, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell. 2003;14:407–416. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Destaing O, Saltel F, Gilquin B, Chabadel A, Khochbin S, Ory S, Jurdic P. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J Cell Sci. 2005;118:2901–2911. doi: 10.1242/jcs.02425. [DOI] [PubMed] [Google Scholar]
- 19.Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4:294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]