Abstract
Pleasure-seeking deficits, including lack of libido, are a core feature of depression. Animal and preliminary clinical studies both suggest that phosphodiesterase 4 (PDE4) is a target for developing novel antidepressants. This study examined the potential involvement of PDE4 in the pathology of depression in both animal models and human postmortem brains. In humans, PDE4B and PDE4D levels were elevated in cingulate cortical tissue from individuals with major depressive disorder (MDD) compared to controls. Using the female urine smelling test (FUST), a recently refined method for monitoring sexual pleasure-seeking activity in mice, we found that icv infusion of novel, selective, and potent PDE4 inhibitors enhanced sexual pleasure-seeking activity in male mice that underwent the learned helplessness or serotonin depletion paradigms. The infusion also increased sexual pleasure-seeking activity in naïve male mice. The results suggest that PDE4 may be a plausible contributor to the sexual pleasure-seeking deficits seen in depressed patients; inhibiting PDE4 may restore these deficits.
Keywords: PDE4, postmortem human brain, depression, pleasure-seeking activity
1. Introduction
In humans, anhedonia is typically associated with deficits in pleasure-seeking or reward-seeking activities, and is commonly observed in conjunction with several psychiatric disorders, most notably major depressive disorder (MDD) (American Psychiatric Association, 2002). While anhedonia also typically encompasses symptoms of decreased libido, this issue is complicated by the fact that most currently available antidepressants frequently cause sexual dysfunction, which is one of the main reasons patients stop taking these medications (Kennedy and Rizvi, 2009, Schweitzer et al. , 2009). Therefore, novel medications that could relieve depressive symptoms without decreasing sexual pleasure-seeking activity would significantly improve quality of life for individuals suffering from MDD.
Our laboratory recently established a behavioral method for monitoring sexual pleasure-seeking activity in male rodents: the female urine sniffing test (FUST) (Malkesman et al. , 2010). The study showed that sniffing the urine of female rodents in estrus was a preferred activity compared to sniffing other odors (such as water). Urine sniffing was also reduced in mice that underwent the learned helplessness (LH) paradigm, a well-known animal model of depression; these reductions were alleviated by treatment with citalopram, a selective serotonin reuptake inhibitor. In addition, urine sniffing behavior was elevated in glutamatergic receptor 6 (GluR6) knockout (KO) mice. Notably, these mice display deficits related to the behavioral abnormalities associated with mania, and these deficits can be partially rescued by treatment with the classic mood stabilizer lithium. Thus, the FUST allows the study of sexual pleasure-seeking activity in rodent models of depression, though caution should naturally be used in interpreting results from just one test (Malkesman et al., 2010).
Phosphodiesterases (PDEs) are a superfamily of enzymes that regulate intracellular signaling by catalyzing the hydrolysis of the second messengers cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophospate (cGMP). Different PDE families have been identified. PDE4A, PDE4B, and PDE4D are widely expressed in the central nervous system. PDE4 was first suggested as a potential therapeutic target for treating depression more than two decades ago. Since then, significant results using PDE4 inhibitors such as rolipram have been obtained in animal behavioral paradigms sensitive to antidepressants (Mizokawa et al. , 1988, Wachtel, 1983), as well as in human clinical studies (Fleischhacker et al. , 1992, Hebenstreit et al. , 1989, Zeller et al. , 1984). The relationship between antidepressant activity and PDE4 with regard to the structure, brain distribution, and pharmacological properties of the different PDE4 isoforms was recently reviewed (Houslay et al. , 2005, Menniti et al. , 2006, Zhang, 2009).
Given the accumulating evidence implicating PDE4 in the molecular pathology of MDD and the development of novel therapeutics, we hypothesized that PDE4 alterations are part of the molecular pathology of depression and that PDE4 inhibitors would improve anhedonia-like sexual pleasure-seeking deficits in rodent models. A recent study from the NIH Chemical Genomics Center (NCGC) described new chemical entities with selectivity for PDE4 isoforms in vitro (Skoumbourdis et al. , 2009). In the present study, we examined PDE4 levels in postmortem brain tissues from individuals with MDD and non-psychiatric controls. We further assessed the behavioral effects of the newly described PDE4 inhibitors in several animal models. Taken together, the findings suggest that PDE4 may play a role in sexual pleasure-seeking activity, and novel compounds that target PDE4 could be used as adjunctive treatments for depression.
2. Material and Methods
2.1 Postmortem brain samples
Frozen postmortem brain samples of cingulate cortex from individuals with MDD and non-psychiatric controls were obtained from the Neuropathology Consortium of the SMRI (Rockville, MD, 15 samples per group). Samples were matched for age, gender, and postmortem interval (Table 1). The demographic and clinical characteristics of individual subjects have been extensively detailed. All samples were stored at −80°C until use.
Table 1.
Demographic characteristics of human postmortem brain samples from patients with major depressive disorder (MDD) and non-psychiatric control subjects.
| Subject | N | Gender | Ethnicity | Age (y) (Mean ± SD) |
PMI (h) (Mean ± SD) |
pH (Mean ± SD) |
|---|---|---|---|---|---|---|
| Control | 15 | 9M/6F | 14W/1AA | 48.1 ± 10.7 | 23.7 ± 9.9 | 6.3 ± 0.2 |
| MDD | 15 | 9M/6F | 15W | 46.5 ± 9.3 | 23.7 ± 10.7 | 6.2 ± 0.2 |
(AA, African American; MDD, major depressive disorder; PMI, post-mortem interval; W, white)
2.2 Tissue homogenization
Dissected brain tissue (100 mg approximately) was homogenized in 20 mM Tris-HCl, pH 7.5, 150mM NaCl, 2.5 mM sodium pyrophosphatase, 1 mM beta-glycerophosphate, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, and a protease inhibitor mixture (Sigma, St. Louis, MO) and then sonicated for 10 seconds. Homogenates were then centrifuged at 14,000 × g for 15 seconds, and supernatants were assayed for total protein concentration using the bicinchoninic acid method (Pierce, Rockford, IL).
2.3 Quantitative Western blot
Immunoblotting was performed as previously described (Yuan et al. , 2001). Briefly, protein contents were adjusted to the same level for all samples. Samples were loaded on the gels within the linear range and each sample was run in duplicate. Molecular weight markers and a pooled sample were run on each gel for quality control and interblot normalization. After electrophoresis, proteins were transferred to nitrocellulose membranes (Invitrogen, CA). Primary antibodies to PDE4B, PDE4D, and lactate dehydrogenase (LDH) (Santa Cruz Biotechnology, Santa Cruz, CA) were used. A secondary antibody (GE Healthcare, Piscataway, NJ) was used and the immune-complex was detected using chemiluminiscence (ECL, GE Healthcare, Piscataway, NJ). Quantification of the immunoreactive bands was performed by optical densitometric scanning using an image analysis system from Syngene (Frederick, MD). The Western blot results were normalized with LDH.
Animals and treatments
129Sv mice (8–10 weeks old) were used for the animal experiments. In order to maintain a constant supply of each drug over 13 days, an infusion cannula was placed into the third ventricle and attached to an osmotic minipump filled with saline, citalopram (25 µg/d), PDE4 inhibitor NCGC00167114 (1 ng/d), PDE4 inhibitor NCGC00168459 (1 ng/d), or the inactive control compound NCGC00165288 (1 ng/d) which belongs to the same series of compounds. Animals underwent the forced swim test (10 days post-operation), and the FUST (different days post-operation). The FUST was used to assess the effects of the PDE4 inhibitors by themselves, as well as their effects on mice that had undergone the LH and serotonin depletion paradigms. For these experiments, osmotic minipumps filled with saline, citalopram, NCGC00167114, NCGC00168459, or NCG00165288. The compounds were synthesized together with the inactive control compound at the NIH Chemical Genomics Center (NCGC). All experimental procedures were approved by the Animal Care and Use Committee of the National Institute of Mental Health and followed National Institutes of Health guidelines.
2.4 PCPA-induced monoamine depletion
On post-operative Day 13, mice received a single injection of PCPA, a tryptophan hydroxylase inhibitor (300 mg/kg, i.p.); mice underwent the FUST on Day 14, roughly 24 hours later.
2.5 The female urine sniffing test (FUST)
The FUST was conducted as previously described (Malkesman et al., 2010). Briefly, mice were transferred to a dark room (~3 lux lighting). One hour before the test, they were habituated to a sterile cotton tip applicator adhered to their home-cage. The test had three phases: (1) one exposure (three minutes) to a sterile cotton tip applicator dipped in sterile water, during which sniffing duration was measured; (2) an interval of 45 minutes during which no applicator was presented to the animal; and (3) one exposure (three minutes) to a sterile cotton tip applicator infused with fresh urine collected from females in estrus, during which length of time spent sniffing was measured.
2.6 The learned helplessness (LH) paradigm
The LH paradigm was conducted using the Gemini Avoidance System (San Diego Instruments, San Diego, CA) as previously described (Maeng et al. , 2008). Induction profile: 120 inescapable shocks (0.45 mA, 15-second duration, at random intervals with an average of 45 seconds); screening profile: 30 trials (0.45 mA; three-second duration for conditioned stimulus and three-second duration for unconditioned stimulus, conducted at random intervals with a mean of 45 seconds); testing profile: 30 trials (0.3 mA; three-second duration for conditioned stimulus and 24-second for unconditioned stimulus, conducted at random intervals with a mean of 45 seconds). The number of escape failures and latency to escape were recorded for each mouse (n = 18–19 in each group). Mice were said to have developed helplessness when they showed at least 20 failures to escape. Those mice that had developed helplessness were treated with either the inactive control compound, NCGC00168459, or saline for 14 days. Mice then underwent the FUST.
2.7 Forced Swim Test
Transparent Plexiglas cylinders, 50 cm high with a diameter of 20 cm, were filled with tap water at 22–25°C approximately 25 cm high so that mice were unable to touch the floor or otherwise escape, as previously described (Shaltiel et al. , 2008). Normal overhead fluorescent lighting of approximately 400 lux was used during the test. Individual mice were placed in the water for a six-minute session, and their behavior was videotaped using CaptureStar video recording software (Clever Systems Inc., Reston, VA) for later analysis. At the end of each session, mice were dried with a paper towel and returned to their home cage. Water was replaced after each trial (n = 7–8 in each group). Immobility time, defined as a lack of activity except movements needed to keep the nose above water, was scored during the last four minutes of each session using ForcedSwimScan software (Clever Systems Inc., Reston, VA).
2.8 Tail suspension test
The tail suspension test was conducted as previously described (Gould et al., 2008) with minor modifications. A 15 cm length of tape 1.9 cm in width (TimeMed Labeling Systems, Inc, Burr Ridge, IL) was positioned with approximately 2 mm of tail protruding. This short distance of the tape from the end of the tail and long length of tape utilized prevented the mice from being able to balance themselves in preparation for any climbing behavior. Mice were suspended by their tails for 6 minutes during video-taped sessions. Mobility was defined as movement of the hind legs. A blind observer scored the videotapes for the full 6 minutes.
2.8 Statistical analysis
Statistical analyses were performed by two-way ANOVA (or repeated measures ANOVA, where indicated) for the behavioral and immunoblot experiments, and with Bonferroni post-hoc tests where appropriate. Data are expressed as mean ± SEM; significance was evaluated at p < 0.05, two-tailed.
3. Results
3.1 PDE4B and PDE4D protein levels in postmortem cingulated cortical tissues from controls and individuals with MDD
We first examined PDE4 levels in postmortem cingulate cortical tissues from non-psychiatric controls and individuals with MDD. The samples were obtained from the Neuropathology Consortium of the Stanley Medical Research Institute (SMRI). Demographic data, clinical characteristics, postmortem interval, and tissue condition for these samples have been published previously and can also be founded on the SMRI website: (http://www.stanleyresearch.org/dnn/Default.aspx?tabid=89).
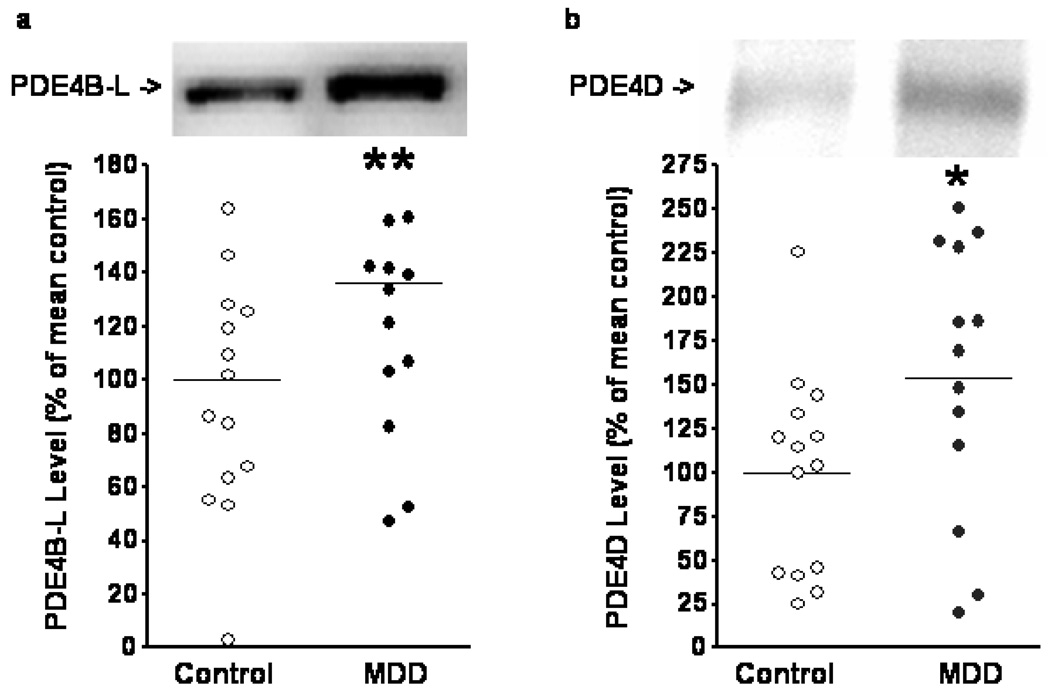
PDE4B and PDE4D antibodies used in the experiments described in Figure 1 produced reliable and quantifiable results. We noted a statistical trend towards elevation for PDE4B-L (the long form of PDE4B) in individuals with MDD compared to controls (Figure 1a) (unpaired t-test, t(28)=1.905, p=0.0671). PDE4D levels were significantly elevated in individuals with MDD compared to controls (Figure 1b) (t(26)=2.242, p=0.0337). Western blotting results were normalized with LDH levels.
Figure 1.
Levels of PDE4 subtypes in cingulate cortical tissue from non-psychiatric controls and individuals with MDD. Tissues were processed and blotted for PDE4B-L (long form) (a) and PDE4D (b) as described in the Methods. Samples (one from each group) that did not show bands with expected molecular weight were excluded from the final analysis. There was a statistical trend toward elevation of PDE4B-L levels in the MDD group (t(28)=1.905, p=0.0671). Significantly elevated PDE4D levels were seen in the MDD group (t(25)=2.069, p=0.0491). Data are mean ± SD. *: p<0.05. **: p<0.1.
3.2 Effects of PDE4 inhibitors and citalopram on sexual pleasure-seeking behavior in the serotonin depletion paradigm
We further speculated that if PDE4 elevations did indeed play a role in anhedonia and depression, PDE4 inhibitors would improve anhedonia-like pleasure-seeking deficits in animal models of depression. To investigate this issue, we used the serotonin depletion paradigm. Notably, serotonin depletion is known to cause depressive episodes in vulnerable individuals (Shopsin et al., 1976). Para-chlorophenylalanine (PCPA) has been used to induce serotonin depletion in rodents; while PCPA treatment alone does not alter immobility in the forced swim test, it blocks the effects of SSRIs in this paradigm. A recent study from our laboratory found that PCPA treatment caused a 40% reduction in brain serotonin levels in rodents, an effect accompanied by reductions in time spent sniffing urine, in the FUST (under revision).
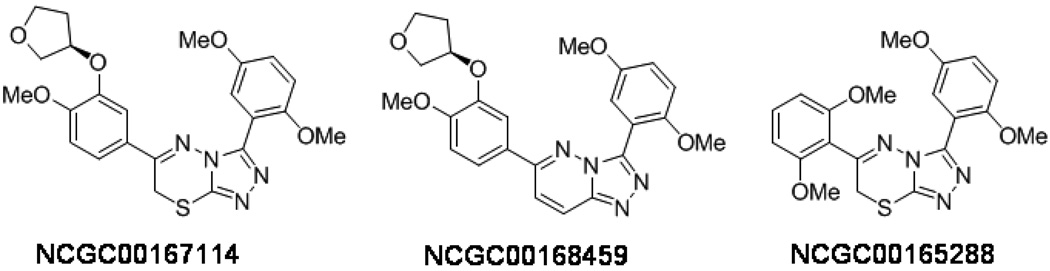
The ability of the PDE4 inhibitors NCGC00167114 and NCGC00168459 (Skoumbourdis et al., 2009) (Figure 2) to penetrate the blood brain barrier is largely unknown; therefore, we conducted this proof-of principle study using direct icv infusion to deliver the control and experimental agents to the third ventricle of the brain. PDE4 inhibitor concentrations were empirically selected based on the assumption that, over a 24-hour period, the infusion would result in whole brain concentrations of the inhibitors at 2.2 nM similar to the IC50s of PDE4 inhibition in vitro (2 nM) (Skoumbourdis et al., 2009). The concentration of citalopram used for this infusion was based on a previous study (Thakker et al. , 2005). Male mice received a continuous icv infusion of vehicle, citalopram, NCGC00167114, or NCGC00168459 for 13 days.
Figure 2.
Chemical structure of the PDE4 inhibitors NCGC00167114 and NCGC00168459, and of the inactive compound NCGC00165288.
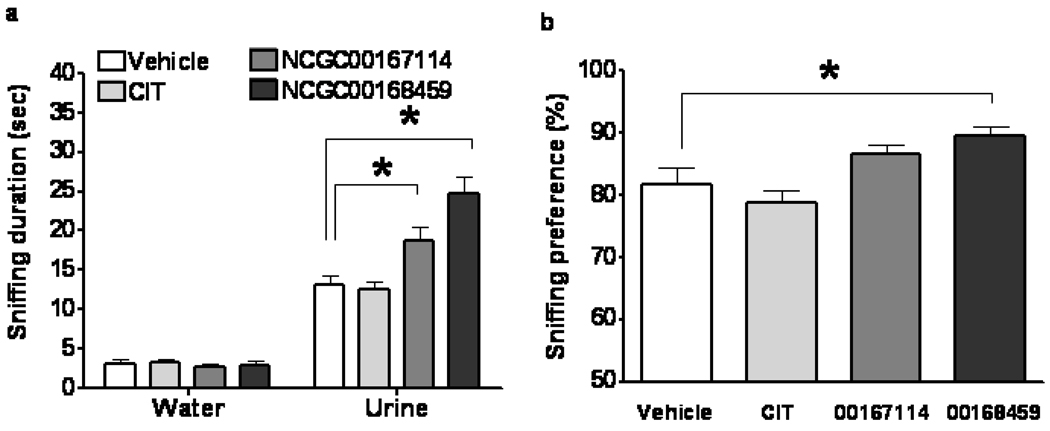
A single PCPA or vehicle (PBS) injection was given the morning of infusion Day 13. The FUST was conducted on Day 14. We found that odor (water versus urine), treatment (vehicle, citalopram, NCGC00167114, or NCGC00168459), and interaction significantly affected time spent sniffing (Figure 3a) (two-way repeated measures ANOVA, p<0.05). Post-hoc tests revealed that treatment type did not alter time spent sniffing water compared to the vehicle group. Treatment with either PDE4 inhibitor, but not with citalopram, significantly increased time spent sniffing urine in mice that received PCPA (Bonferroni post-tests, p< 0.05) compared to the vehicle group. Treatment with NCGC00168459, but not with citalopram or NCGC00167114, significantly increased preference for sniffing urine compared to the vehicle group (Figure 3b) (one-way ANOVA, p<0.05).
Figure 3.
Effect of PDE4 inhibitors and citalopram on pleasure-seeking behavior in mice treated with PCPA in the FUST. Male mice received icv infusion of vehicle, citalopram, PDE4 inhibitor NCGC00167114, or PDE4 inhibitor NCGC00168459 for 14 days. Odor (water vs. urine), treatment (vehicle, citalopram, or PDE4 inhibitors), and interaction significantly affected time spent sniffing (a) (two-way repeated measures ANOVA, F-odor(1,34)=362.5, p<0.0001; F-treatment( 3,34)=9.074, p=0.0001; F-interaction(3,34)=15.84, p<0.0001). Citalopram had no significant effect (a) on time spent sniffing water (Bonferroni post-test, t=0.1187, p>0.05) or urine (Bonferroni post-test, t=0.2267, p>0.05). PDE4 inhibitor NCGC00167114 infusion (a) significantly increased time spent sniffing urine (Bonferroni post-test, t=2.937, p<0.05), but not water (Bonferroni post-test, t=0.2129, p>0.05). PDE4 inhibitor NCGC00168459 infusion (a) significantly increased time spent sniffing urine (Bonferroni post-test, t=6.135, p<0.001), but not water (Bonferroni post-test, t=0.09466, p>0.05). Treatment significantly influenced sniffing preference (b) (one-way ANOVA, F(3,37)=6.416, p=0.0015). Infusion of NCGC00168459, but not citalopram or NCGC00167114, significantly increased sniffing preference (b) (Bonferroni's Multiple Comparison Test; citalopram, t=1.013, p>0.05; NCGC00167114, t=1.742, p>0.05; NCGC00168459, t=2.782, p<0.05). Data are mean ± SD. *: p<0.05.
3.3 Effects of PDE4 inhibitor NCGC00168459 on outcome measures in the LH paradigm
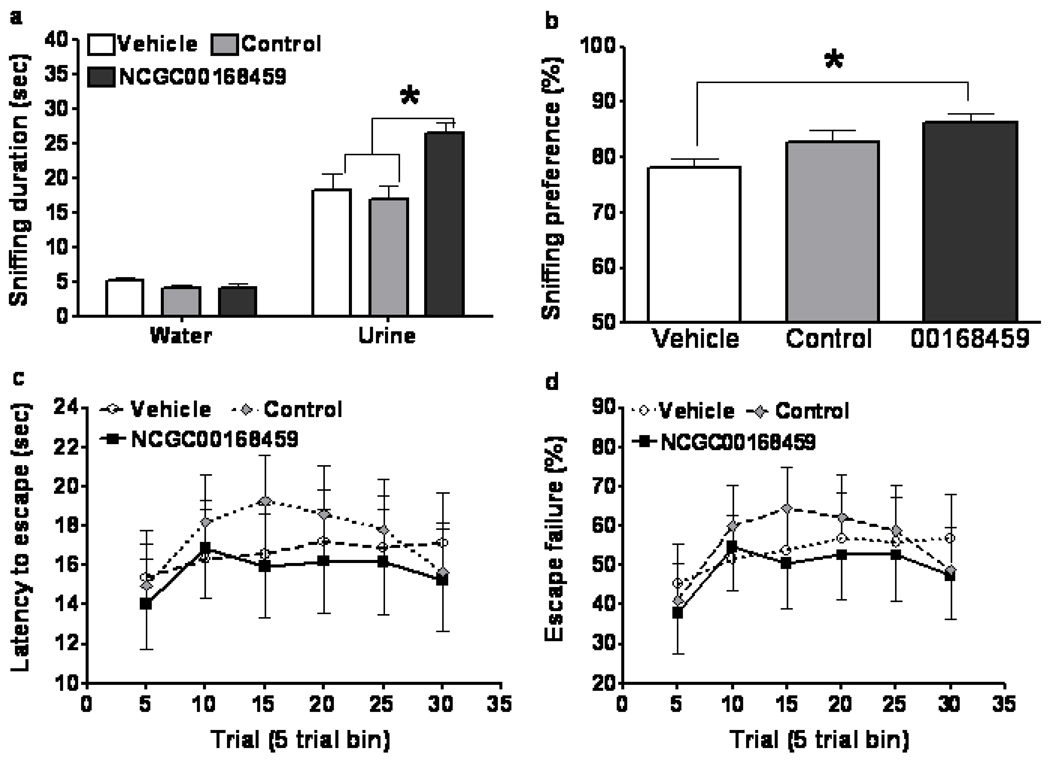
The LH paradigm is a robust, antidepressant-sensitive stress paradigm used in animal models of depression and post-traumatic stress disorder (PTSD). The paradigm has three sequential sections: the induction of helplessness, helplessness screening, and helplessness re-test after spontaneous or treatment-induced recovery. The classic measures of the paradigm include latency to escape and number of escape failures. In the present study, a cohort of mice underwent the induction and screening portions of the LH paradigm. Mice identified as having developed helplessness then received vehicle or NCGC00168459 icv infusions. A negative control compound in the same series of PDE4 inhibitors but that does not inhibit PDE4 (NCGC00165288) (Figure 2) was also studied in this set of experiments. On Day 4 of the continuous infusion, mice underwent the FUST. The test showed that, compared to the vehicle group, treatment with NCGC00168459, but not the control compound, significantly increased time spent sniffing urine in the mice that had developed helplessness (Figure 4a) (two-way repeated measures ANOVA, p<0.05). NCGC00168459 treatment also significantly increased urine sniffing preference (Figure 4b) (one-way ANOVA, p<0.05). On continuous infusion Day 14, the mice underwent the helplessness re-test. Unexpectedly, the test revealed that NCGC00168459 did not significant affect escape latency (Figure 4c) (two-way repeated measures ANOVA, p>0.05) or escape failures compared to the vehicle group (Figure 4d) (two-way repeated measures ANOVA, p>0.05).
Figure 4.
Effect of PDE4 inhibitor NCGC00168459 on pleasure-seeking behavior as assessed by the FUST at Day 3 in mice that developed helplessness after undergoing the LH paradigm. Mice identified as having developed helplessness received an icv infusion of vehicle, negative control compound, or NCGC00168459 for 14 days. At Day 3, odor (water vs. urine), treatment (vehicle, or NCGC00168459), and interaction were all found to significantly affect sniffing duration (a) (two-way repeated measures ANOVA, F-odor(1,53)=216.3, p<0.0001; F-treatment(2,53)=5.993, p=0.0045; F-interaction(2,53)=7.912, p=0.0010). PDE4 inhibitor NCGC00168459 infusion significantly increased length of time spent sniffing urine compared to vehicle (Bonferroni post-test, t=4.010, p<0.001) and negative control (Bonferroni post-test, t=4.606, p<0.001). Treatment significantly influenced sniffing preference (b) (one-way ANOVA, F(2,53)=4.404, p=0.0172). Infusion of NCGC00168459, but not negative control, significantly increased sniffing preference (b) (Bonferroni's Multiple Comparison Test; negative control, t=1.665, p>0.05; PDE4 inhibitor II, t=2.960, p>0.01). Mice were also tested for recovery from helplessness using the active avoidance test. Treatment had no significant effect on latency to escape (c) or on number of escape failures (d). Data are mean ± SD. *: p<0.05.
3.4 Effects of NCGC00168459 on immobility duration in the forced swim and tail suspension tests
The forced swim and tail suspension tests are thought to measure goal-directed behavior and behavioral despair in rodents; both are commonly used to assess the behavioral actions of antidepressants (Chen et al. , 2010 ). Because NCGC00168459 did not produce classic antidepressant-like effects in the LH paradigm, we used these two tests to further examine whether the PDE4 inhibitors produced “classic” antidepressant-like actions in rodents. Mice received infusions as described above. The tail suspension test was conducted on infusion Day 7, and the forced swim test was conducted on infusion Day 10. Type of treatment had no significant effect on immobility duration in either test (one-way ANOVA, p>0.05).
3.5 Effects of NCGC00168459 on measures of the FUST in naïve mice
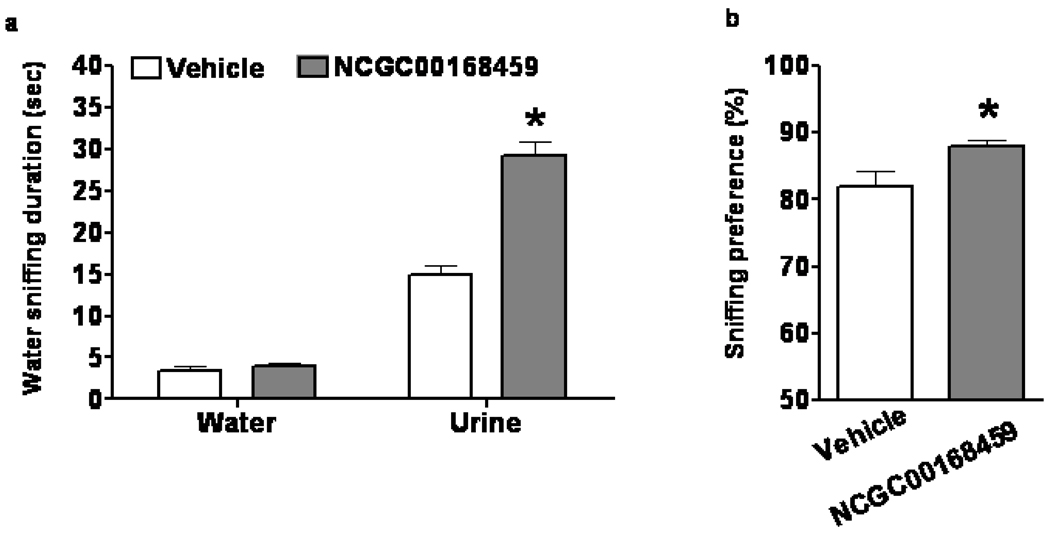
Because the PDE4 inhibitors did not produce “classic” antidepressant-like effects in either the LH paradigm or the immobility tests, we suspected that the ability of PDE4 inhibitors to enhance sexual pleasure-seeking activity in PCPA-treated or helpless mice did not occur solely as a result of antidepressant-like effects. Therefore, we used the FUST to examine the effects of PDE4 inhibitors in naïve mice who had undergone no prior behavioral or chemical manipulations. We found that infusion of NCGC00168459 significantly increased the time these mice spent sniffing urine, but not water (Figure 5a) (two-way repeated measures ANOVA and post-hoc tests, p<0.05). The infusion also increased urine sniffing preference in these mice (Figure 5b) (unpaired t-test, p<0.05).
Figure 5.
Effect of NCGC00168459 on pleasure-seeking behavior in the FUST. Naïve 129S1/SVImJ male mice received an icv infusion of vehicle or NCGC00168459 for seven days and then underwent the FUST. Odor (water vs. urine), treatment (vehicle vs. NCGC00168459), and interaction significantly affected length of time spent sniffing (two-way repeated measures ANOVA, F-odor(1,28)=392.3, p<0.0001; F-treatment(1,28)=36.36, p<0.0001; F-interaction( 1,28)=54.15, p<0.0001). Treatment had no significant effect on time spent sniffing water (a) (Bonferroni post-test, t=0.2758, p>0.05). Infusion with NCGC00168459 significantly increased time spent sniffing urine (b) (Bonferroni post-test, t=8.282, p<0.001). PDE4 inhibitor NCGC00167114 infusion also significantly increased sniffing preference (c) (t(28)=2.352, p=0.058). Data are mean ± SD. *: p<0.05.
4. Discussion
The present study found significant elevations of cortical PDE4B-L and PDE4D levels in individuals with MDD. This novel observation supports the heretofore suspected role of PDE4 overexpression in the etiology of MDD. In addition, we found that NCGC00168459, a PDE4 inhibitor, enhanced sexual pleasure-seeking activity in naïve, stressed, and serotonin-depleted animals, suggesting that PDE4 regulates this pleasure-seeking activity. The latter findings raise the novel notion that PDE4 inhibitors may be potentially effective in treating the sexual deficits associated with severe stress and depression.
Cyclic nucleotide signaling pathways are critical regulators of neural function and plasticity. By virtue of the metabolic inactivation of cAMP and cGMP, PDEs are key regulators of cyclic nucleotide signaling pathways. Previous studies demonstrated that PDE4D is regulated by repeated treatment with drugs that produce antidepressant effects via different neuropharmacological mechanisms, including selective norepinephrine reuptake inhibitors (SNRIs), SSRIs, and the PDE4 inhibitor rolipram (Dlaboga et al. , 2006). PDE4D knockout mice have been found to behave similarly to wild-type mice treated with antidepressants(O'Donnell and Zhang, 2004), suggesting that the PDE4D subtype may be a particularly important antidepressant target. In addition, previous preclinical studies found that the PDE4 inhibitor rolipram exerted significant antidepressant effects in the forced swim test, an antidepressant-sensitive behavioral paradigm(Zhang et al. , 2006). However, whether PDE4 inhibitors improved pleasure-seeking deficits related to anhedonia remained unknown. The present study therefore tested the effects of PDE4 inhibitors on anhedonia-like deficits in two animal models of depression: the serotonin depletion paradigm and the LH paradigm. Two new PDE4 inhibitors were selected for the study because of their higher (roughly 10-fold) potency over rolipram (Skoumbourdis et al., 2009).
Recently, we adapted and refined a rodent serotonin depletion model of depression, and showed that PCPA treatment reduced serotonin in the brain and caused anhedonia-like deficits in sexual pleasure-seeking activity (under revision). Recent clinical data show that ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist, produces rapid and robust antidepressant effects even in treatment-resistant patients (Maeng and Zarate, 2007). We recently found that in this serotonin depletion model of depression, antidepressants alone did not restore pleasure-seeking deficits, but ketamine treatment did (under revision). In the present study, we found that infusion of PDE4 inhibitors, but not citalopram, increased the time that PCPA-treated mice spent sniffing urine, but not water. Treatment with PDE4 inhibitors also increased urine sniffing preference. Thus, our results suggest that, like ketamine, PDE4 inhibitors may induce positive effects on anhedonia-like deficits.
As noted above, the LH paradigm is a well-known stress model of depression and PTSD (Nestler et al. , 2002). In this paradigm, rodents receive a series of uncontrollable and unavoidable foot shocks, after which they develop deficits in the active avoidance test. Those animals with severe deficits (e.g. failure to escape at least 70% of escape trials) are considered to have developed helplessness. Chronic treatment with antidepressants (Gambarana et al. , 2001) as well as a single injection of ketamine (Maeng et al., 2008) have both been found to facilitate recovery from helplessness. Mice that have developed helplessness, also show sexual pleasure-seeking deficits in the FUST. These deficits can be improved by treatment with citalopram. In the present study, mice that had developed helplessness received an infusion of PDE4 inhibitor; such treatment significantly affected urine sniffing preference, as well as the length of time mice spent sniffing urine, indicating that the inhibitors, like citalopram, affect anhedonia-like deficits.
Latency to escape and the number of escape failures are the traditional outcome measures in the LH paradigm (Maeng et al., 2008). Unexpectedly, we failed to demonstrate that PDE4 inhibitors significantly improved these measures. It is unlikely that the concentration of the inhibitor was insufficient for behavioral effects in general, since our data did show that NCGC00168459 affected performance in the FUST. One possible explanation is that the spontaneous recovery measured by the active avoidance test has a ceiling effect. Also unexpectedly, PDE4 inhibitors did not improve immobility measures in the forced swim and tail suspension tests, both commonly used to assess the behavioral actions of acute and chronic antidepressants. Using PDE4D wild-type, heterozygous knockout, and homozygous knockout mice, Zhang and colleagues (Zhang et al. , 2002) showed that PDE4D had gene-dosage effects in the tail suspension and forced swim tests. The same investigators found that compared to wild-type mice, PDE4B homozygous knockout mice displayed immobility reductions in the forced swim test, but a tendency toward immobility increases in the tail suspension test (Zhang et al., 2002). Alternately, the negative results on immobility measures observed in the present study could be due to insufficient inhibition or to the differential regulation of immobility in the tail suspension test by PDE4 subtypes.
The unexpected and somewhat conflicting findings from the FUST, the active avoidance test, and the immobility tests led us to suspect that PDE4 inhibitors are more likely to affect sexual pleasure-seeking activity than classic behavioral measures typically affected by antidepressants in rodent models. We therefore tested the effects of NCGC00168459 on measures of the FUST in naïve male mice. Infusion with the PDE4 inhibitor NCGC00168459 increased urine sniffing duration and preference in naïve male mice. This finding is particularly interesting because studies have shown that urine sniffing is associated with increased dopamine release in the nucleus accumbens. Several intracellular signaling pathways exist downstream of dopamine receptors. Furthermore, behavioral pharmacology data suggest that the D1 receptor, which couples to the cAMP-protein kinase A (PKA) pathway, mediates sexual desire (Pfaus et al. , 2009). To date, limited information exists on the intracellular signaling mechanisms underlying sexual pleasure-seeking activity. However, our PDE4 inhibitor data support the role of the dopamine – D1 receptor – cAMP – PKA pathway in pleasure-seeking activity.
It is important to note that clinical studies also suggest the beneficial effects of PDE4 inhibitors in treating depressive symptoms (Zhang, 2009). Despite the fact that PDE4 inhibitors have been tested in animal models of depression as well as in clinical trials, whether and how PDE4 alterations are involved in the etiology of MDD remains to be elucidated. Fatemi and colleagues reported reduced cerebellar PDE4A levels in individuals with bipolar disorder, but not in those with schizophrenia or MDD (Fatemi et al. , 2008). In the present study, we found higher levels of PDE4B-L and PDE4D in cingulate cortical tissue from individuals with MDD, providing the first evidence that PDE4 overexpression may play a role in depression; altered PDE4 levels in other brain regions are possible and should be further investigated. However, the mechanisms underlying PDE4 subtype overexpression in the depressed brain remain unclear. Medication effects cannot be discarded as most of the depressed patients used in this study (n=12) were receiving antidepressant treatment at the time of their death, and only three of them were not medicated. The small number of subjects complicates any possible comparison between depressed, non-medicated subjects, and control subjects.
In summary, we found that protein levels of PDE4B-L and PDE4D were elevated in the postmortem cingulate cortical tissue of individuals with MDD compared to non-psychiatric controls. In a variety of animal models of depression, PDE4 inhibitors improved sexual pleasure-seeking activity. Interestingly, NCGC00168459 also enhanced sexual pleasure-seeking activity in naïve animals. However, the PDE4 inhibitors did not reduce immobility measures in the forced swim and tail suspension tests, nor did they alter classic helplessness measures in modeled mice. These data suggest a novel role for PDE4 in the regulation of “general” sexual pleasure-seeking behavior. The data further imply that PDE4 alterations can contribute to the lack of libido associated with anhedonia. Further studies that target PDE4 for adjunctive treatment of depression are warranted.
Acknowledgements
This study was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services (IRP-NIMH-NIH-DHHS). Ioline Henter provided invaluable editorial assistance.
Role of Funding Source
This study was supported by the Intramural Research Program of the National Institute of Mental Health (Bethesda, Maryland). The NIMH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors gratefully acknowledge the support of the Intramural Research Program of the National Institute of Mental Health (NIMH) and have no conflict of interest to disclose, financial or otherwise. Dr. Chen is now a full-time employee of Johnson and Johnson Pharmaceutical Research and Development; this work was conducted while he was employed by the NIMH.
Contributor Information
Peixiong Yuan, Email: PeixiongYuan@mail.nih.gov.
Tyson Tragon, Email: tragont@mail.nih.gov.
Menghang Xia, Email: mxia@mail.nih.gov.
Christopher A. LeClair, Email: leclairc@mail.nih.gov.
Amanda P. Skoumbourdis, Email: Amanda.skoumbourdis@gmail.com.
Wei Zheng, Email: wzheng@mail.nih.gov.
Craig J. Thomas, Email: craigt@mail.nih.gov.
Ruili Huang, Email: huangru@mail.nih.gov.
Christopher P. Austin, Email: austinc@mail.nih.gov.
Guang Chen, Email: GChen13@its.jnj.com.
Xavier Guitart, Email: guitartx@mail.nih.gov.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; Washington, DC: American Psychiatric Press; 2002. [Google Scholar]
- Chen G, Henter ID, Manji HK. Translational research in bipolar disorder: emerging insights from genetically based models. Mol Psychiatry. 2010;15:883–895. doi: 10.1038/mp.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlaboga D, Hajjhussein H, O'Donnell JM. Regulation of phosphodiesterase-4 (PDE4) expression in mouse brain by repeated antidepressant treatment: comparison with rolipram. Brain Res. 2006;1096:104–112. doi: 10.1016/j.brainres.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Lee S. Phosphodiesterase-4A expression is reduced in cerebella of patients with bipolar disorder. Psychiatr Genet. 2008;18:282–288. doi: 10.1097/YPG.0b013e3283060fb8. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Hinterhuber H, Bauer H, Pflug B, Berner P, Simhandl C, et al. A multicenter double-blind study of three different doses of the new cAMP-phosphodiesterase inhibitor rolipram in patients with major depressive disorder. Neuropsychobiology. 1992;26:59–64. doi: 10.1159/000118897. [DOI] [PubMed] [Google Scholar]
- Gambarana C, Scheggi S, Tagliamonte A, Tolu P, De Montis MG. Animal models for the study of antidepressant activity. Brain Res Brain Res Protoc. 2001;7:11–20. doi: 10.1016/s1385-299x(00)00056-8. [DOI] [PubMed] [Google Scholar]
- Gould TD, O' Donnell KC, Dow ER, Du J, Chen G, Manji HK. Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology. 2008;54:577–587. doi: 10.1016/j.neuropharm.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebenstreit GF, Fellerer K, Fichte K, Fischer G, Geyer N, Meya U, et al. Rolipram in major depressive disorder: results of a double-blind comparative study with imipramine. Pharmacopsychiatry. 1989;22:156–160. doi: 10.1055/s-2007-1014599. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10:1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Rizvi S. Sexual dysfunction, depression, and the impact of antidepressants. J Clin Psychopharmacol. 2009;29:157–164. doi: 10.1097/JCP.0b013e31819c76e9. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA., Jr The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G, et al. The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol Psychiatry. 2010;67:864–871. doi: 10.1016/j.biopsych.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menniti FS, Faraci WS, Schmidt CJ. Phosphodiesterases in the CNS: targets for drug development. Nat Rev Drug Discov. 2006;5:660–670. doi: 10.1038/nrd2058. [DOI] [PubMed] [Google Scholar]
- Mizokawa T, Kimura K, Ikoma Y, Hara K, Oshino N, Yamamoto T, et al. The effect of a selective phosphodiesterase inhibitor, rolipram, on muricide in olfactory bulbectomized rats. Jpn J Pharmacol. 1988;48:357–364. doi: 10.1254/jjp.48.357. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, et al. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- O'Donnell JM, Zhang HT. Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4) Trends Pharmacol Sci. 2004;25:158–163. doi: 10.1016/j.tips.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Wilkins MF, Dipietro N, Benibgui M, Toledano R, Rowe A, et al. Inhibitory and disinhibitory effects of psychomotor stimulants and depressants on the sexual behavior of male and female rats. Horm Behav. 2009 doi: 10.1016/j.yhbeh.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Schweitzer I, Maguire K, Ng C. Sexual side-effects of contemporary antidepressants: review. Aust N Z J Psychiatry. 2009;43:795–808. doi: 10.1080/00048670903107575. [DOI] [PubMed] [Google Scholar]
- Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T, et al. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13:858–872. doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shopsin B, Friedman E, Gershon S. Parachlorophenylalanine reversal of tranylcypromine effects in depressed patients. Arch Gen Psychiatry. 1976;33:811–819. doi: 10.1001/archpsyc.1976.01770070041003. [DOI] [PubMed] [Google Scholar]
- Skoumbourdis AP, Leclair CA, Stefan E, Turjanski AG, Maguire W, Titus SA, et al. Exploration and optimization of substituted triazolothiadiazines and triazolopyridazines as PDE4 inhibitors. Bioorg Med Chem Lett. 2009;19:3686–3692. doi: 10.1016/j.bmcl.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker DR, Natt F, Husken D, van der Putten H, Maier R, Hoyer D, et al. siRNA-mediated knockdown of the serotonin transporter in the adult mouse brain. Mol Psychiatry. 2005;10:782–789. doi: 10.1038/sj.mp.4001687. [DOI] [PubMed] [Google Scholar]
- Wachtel H. Neurotropic effects of the optical isomers of the selective adenosine cyclic 3',5'-monophosphate phosphodiesterase inhibitor rolipram in rats in-vivo. J Pharm Pharmacol. 1983;35:440–444. doi: 10.1111/j.2042-7158.1983.tb04318.x. [DOI] [PubMed] [Google Scholar]
- Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK, Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- Zeller E, Stief HJ, Pflug B, Sastre-y-Hernandez M. Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry. 1984;17:188–190. doi: 10.1055/s-2007-1017435. [DOI] [PubMed] [Google Scholar]
- Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des. 2009;15:1688–1698. doi: 10.2174/138161209788168092. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Jin SL, Frith SA, Suvarna N, Conti M, et al. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology. 2002;27:587–595. doi: 10.1016/S0893-133X(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Zhao Y, Huang Y, Deng C, Hopper AT, De Vivo M, et al. Antidepressant-like effects of PDE4 inhibitors mediated by the high-affinity rolipram binding state (HARBS) of the phosphodiesterase-4 enzyme (PDE4) in rats. Psychopharmacology (Berl) 2006;186:209–217. doi: 10.1007/s00213-006-0369-4. [DOI] [PubMed] [Google Scholar]