Abstract
Fractionated partial or whole-brain irradiation (fWBI) is a widely used, effective treatment for primary and metastatic brain tumors, but it also produces radiation-induced brain injury, including cognitive impairment. Radiation-induced neural changes are particularly problematic for elderly brain tumor survivors who also experience age-dependent cognitive impairment. Accordingly, we investigated, i] radiation-induced cognitive impairment, and ii] potential biomarkers of radiation-induced brain injury in a rat model of aging. Fischer 344 × Brown Norway rats received fractionated whole-brain irradiation (fWBI rats, 40 Gy, 8 fractions over 4 wk) or sham-irradiation (Sham-IR rats) at 12 months of age; all analyses were performed at 26–30 months of age. Spatial learning and memory were measured using the Morris water maze (MWM), hippocampal metabolites were measured using proton magnetic resonance spectroscopy (1H MRS), and hippocampal glutamate receptor subunits were evaluated using Western blots. Young rats (7–10 month-old) were included to control for age effects. The results revealed that both Sham-IR and fWBI rats exhibited age-dependent impairments in MWM performance; fWBI induced additional impairments in the reversal MWM. 1H MRS revealed age-dependent decreases in neuronal markers, increases in glial markers, but no detectable fWBI-dependent changes. Western blot analysis revealed age-dependent, but not fWBI-dependent, glutamate subunit declines. Although previous studies demonstrated fWBI-induced changes in cognition, glutamate subunits, and brain metabolites in younger rats, age-dependent changes in these parameters appear to mask their detection in old rats, a phenomenon also likely to occur in elderly fWBI patients >70 years of age.
Keywords: MRS, fractionated whole brain irradiation, cognition
1. INTRODUCTION
Cognitive impairment is a profound impediment to the performance of activities in everyday life. Age-dependent declines in cognitive ability in the elderly pose a particular obstacle when compounded by central nervous system pathologies and therapeutic interventions. Fractionated partial or whole-brain irradiation (fWBI) is a widely used and effective treatment for primary and metastatic brain tumors, but evidence increasingly has linked fWBI to progressive and irreversible declines in cognitive function (Butler et al., 2006; Dietrich et al., 2008; Li et al., 2008). Because the incidence of primary tumors that are most likely to metastasize to the brain (i.e., breast, lung, and malignant melanoma) increases at middle age (Soffietti et al., 2002; Patchell and Regine, 2003), the effects of fWBI on cognitive function pose a greater potential problem for elderly brain tumor survivors who also are vulnerable to age-dependent cognitive decline.
Previously, we demonstrated fWBI-induced cognitive impairment in a middle-aged rodent model (Shi et al., 2006). In that study, rats that received fWBI at 12 months of age exhibited a significant impairment on the Morris water maze (MWM) test of spatial learning and memory at 24 months of age. MWM performance is dependent on the integrity of the hippocampus (Moser et al., 1993). We also found fWBI-induced changes in the glutamate receptor composition of the CA1 region of the hippocampus (Shi et al., 2006). However, quantitative analysis of hippocampal neuron number and myelin integrity in the same model indicated neither hippocampal neuron loss (Shi et al., 2008) nor changes in myelin integrity (Shi et al., 2009). These findings suggested that fWBI-induced brain injury in old rats that received fWBI at middle age involved more subtle molecular and/or cellular modifications.
Changes in brain metabolites have been detected using proton magnetic resonance spectroscopy (1H MRS) 12 months following fWBI delivered to 3–4 month old rats (Atwood et al., 2007). 1H MRS is a high resolution, noninvasive neuroimaging technique that can detect brain metabolite changes in a variety of neurological pathologies associated with cognitive impairment (Govindaraju et al., 2000; Manganas et al., 2007; Obenaus et al., 2008; Metastasio et al., 2006). In the present study, rats received fWBI (5 Gy twice a week for 4 weeks) at 12 months of age, cognitive testing with the MWM at 26 months, 1H MRS assessment of brain metabolites at 27 months, and Western blot measurement of hippocampal glutamate receptor subunit composition at 30 months; results were compared with those from age matched sham-irradiated (Sham-IR) and Young (7–10 month-old) rats. Our findings suggest that age-dependent changes are likely to prevent the detection of radiation-induced changes in cognition, brain metabolites, and glutamate receptors in elderly fWBI patients.
2. RESULTS
2.1 Age-dependent and fWBI-induced impairments in MWM performance
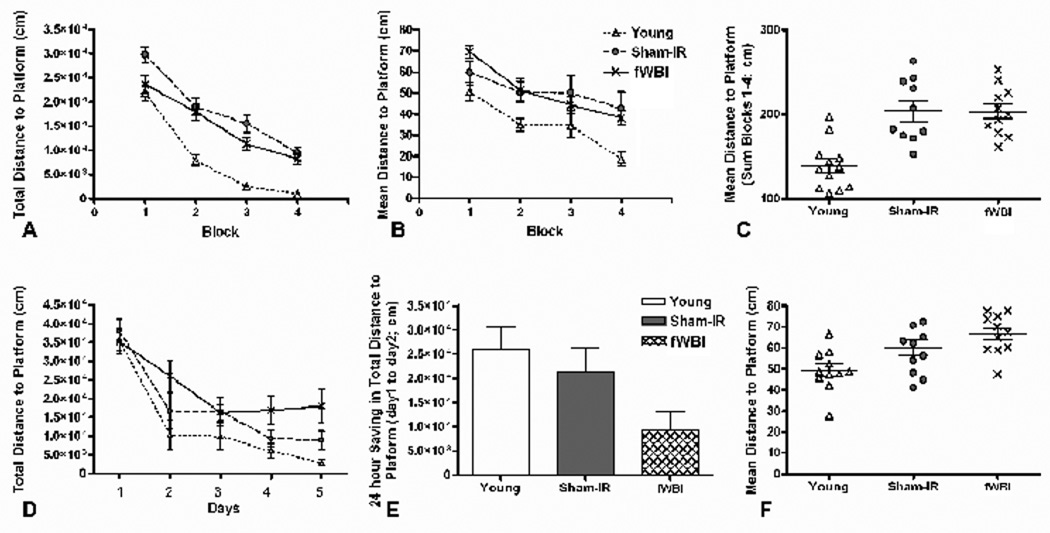
Spatial learning and memory was assessed using a “standard version” and a “reversal version” of the MWM. Sham-IR and fWBI rats were irradiated at 12 months of age, tested at 26 months (Figure 1A), and compared to 9 month-old Young rats. On the standard version of the MWM, rats were given one training trial per day to find a submerged platform. On the 6th day the platform was removed for a probe trial; the rats were given 30 sec to search for the platform. This sequence was repeated for 4 weeks (Figure 1B). Analysis of, i] total distance to the platform (F 3, 93 = 153, P < 0.001, Figure 2A), ii] path length (F 3, 93 = 134, P< 0.001, data not shown), and iii] escape latency (F 3, 93 = 113, P < 0.001, data not shown) on the training trials of the standard version of the MWM task revealed significant improvements across weeks, indicating that the rats in all groups learned the task. Examining the group by week interaction (P = 0.006) revealed significant differences in the path length and the total distance to platform between the Young group and both the Sham-IR and fWBI groups at old age (P’s < 0.05), but not between the Sham-IR and fWBI groups (P > 0.05). These data indicate a significant effect of age on these spatial learning measures, but no detectable effect of fWBI at old age.
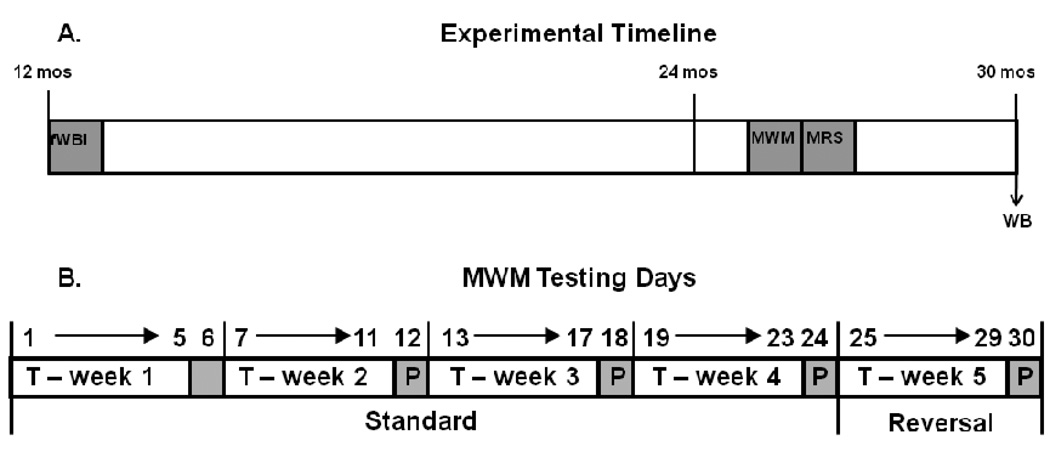
Figure 1.
(A) Fischer 344 × Brown Norway rats received fWBI (5Gy twice a week for 4 weeks) or Sham-IR at 12 months, were tested on the Morris water maze (MWM) at 26 months, had their brain metabolites assessed by magnetic resonance spectroscopy (MRS) at 27 months, and had their hippocampal glutamate receptor subunits measured by Western blots (WB) at 30 months. Results were compared with those from 7–10 month old rats. (B) The Morris water maze (MWM) testing schedule for determining the spatial learning, reference memory, and spatial reversal learning ability of old rats that received either fWBI or sham-irradiation at middle age; 7–8 month-old unirradiated rats were tested at the same time as the old rats. For the “standard” version of the MWM, each week consisted of training the rats for 5 days (T-week) to locate the escape platform followed by a single probe trial (P) on the 6th day with the platform lowered beyond their reach. This schedule was repeated 4 times (T-weeks 1–4) over 4 weeks. For the “reversal” version of the MWM, the platform was moved to the opposite quadrant, and the rats trained for 5 days (T) to locate the escape platform followed by a single probe trial on the 6th day with the platform lowered beyond their reach.
Figure 2.
Performance of Young, Sham-IR, and fWBI groups on the standard (A–C) and reversal (D–F) versions of the MWM. (A) The total distance to platform during the training trials of the standard MWM version revealed an age effect, but no fWBI effect at old age. The rate of learning was similar in all 3 groups of rats. (B) The mean distance to platform during the probe trials of the standard MWM version again revealed an age effect, but no fWBI effect at old age. (C) Summing the mean distance to platform for probe trials 1–4 of the standard MWM also revealed an age effect, but no fWBI effect at old age. (D) The total distance to platform during the training trials of the reversal MWM revealed an effect of fWBI at old age on Days 4 and 5. (E) The 24-hour saving for the total distance to platform on the reversal MWM also revealed an effect of fWBI. (F) The mean distance to platform on the probe trial of the reversal MWM revealed an age effect, but no fWBI effect at old age.
Reference memory was measured with the probe trial of the standard MWM test (Figures 2B and 2C). Analysis revealed significant differences in the mean distance to platform among the groups (F 2, 91 = 14.6, P < 0.001) and across the weeks (F 3, 91 = 14.2, P < 0.001). Post-hoc tests revealed significant differences in the mean distance to platform between the Young and both the Sham-IR and the fWBI groups at old age (P’s < 0.05), but not between the Sham-IR and fWBI groups (P > 0.05). The results were similar when the mean distance to platform was summed over weeks 1–4 (Figure 2C). Thus, these data indicate a significant effect of age on reference memory, but no detectable effect of fWBI at old age.
In the 1 week reversal version of the MWM, rats were given one training trial per day to find a submerged platform in a new location. On the 6th day the platform was removed for a probe trial, and the rat was given 30 seconds to search for the platform (Figure 1B). The reversal version of the MWM measures the ability to use the strategy learned during the standard MWM to find the platform in a new location. In training trials on this version (Figure 2D), there was a significant group effect (F 2, 31 = 5.74, P = 0.008), but post-hoc tests revealed statistically significant differences in the total distance to platform only between Young rats and fWBI rats at old age (P = 0.0020). The results were similar for measurements of the path length (F 2, 124 = 5.39, P = 0.006, data not shown) and escape latency (F 2, 124 = 7.75, p = 0.0007, data not shown). Post-hoc comparisons examining the group effect for each day indicated that the old fWBI rats performed marginally worse on the total distance to platform measure than old Sham-IR rats on Day 4 (P = 0.06) and statistically worse on Day 5 (P = 0.04), suggesting an effect of fWBI on reversal learning at old age.
Another sensitive parameter for evaluating learning is the 24-h saving, a measure of the improvement between Day 1 and Day 2, on the reversal version that assesses how rapidly the new platform location is learned. The 24-h saving for the total distance to the platform (Figure 2E) differed significantly only between the Young and fWBI rats at old age (P = 0.01), although the difference between the old Sham-IR and old fWBI groups approached significance (P = 0.06). Because Young and Sham-IR groups did not differ (P = 0.45), we compared pooled data from those unirradiated groups to the fWBI group and found a significantly lower 24-h saving on the total distance to platform for the fWBI group (P = 0.01), indicating an effect of fWBI on this measure. Analysis of the mean distance to platform on the probe trial of the reversal version indicated a significant difference (P < 0.05) in the mean distance to platform only between Young rats and fWBI rats at old age (Figure 2F).
Finally, there were no significant group differences in sensory or motor performance as assessed by the latency to reach a visible platform (P >0.05). Taken together, the results of the standard MWM testing indicated a significant age-dependent decline in spatial learning and reference memory, but no effect of fWBI at old age. Only the more challenging reversal version of the MWM task was able to detect a significant effect of fWBI in the old rats.
2.2 Glutamate receptor subunits in CA1 exhibit an age effect, but not an fWBI effect in old rats
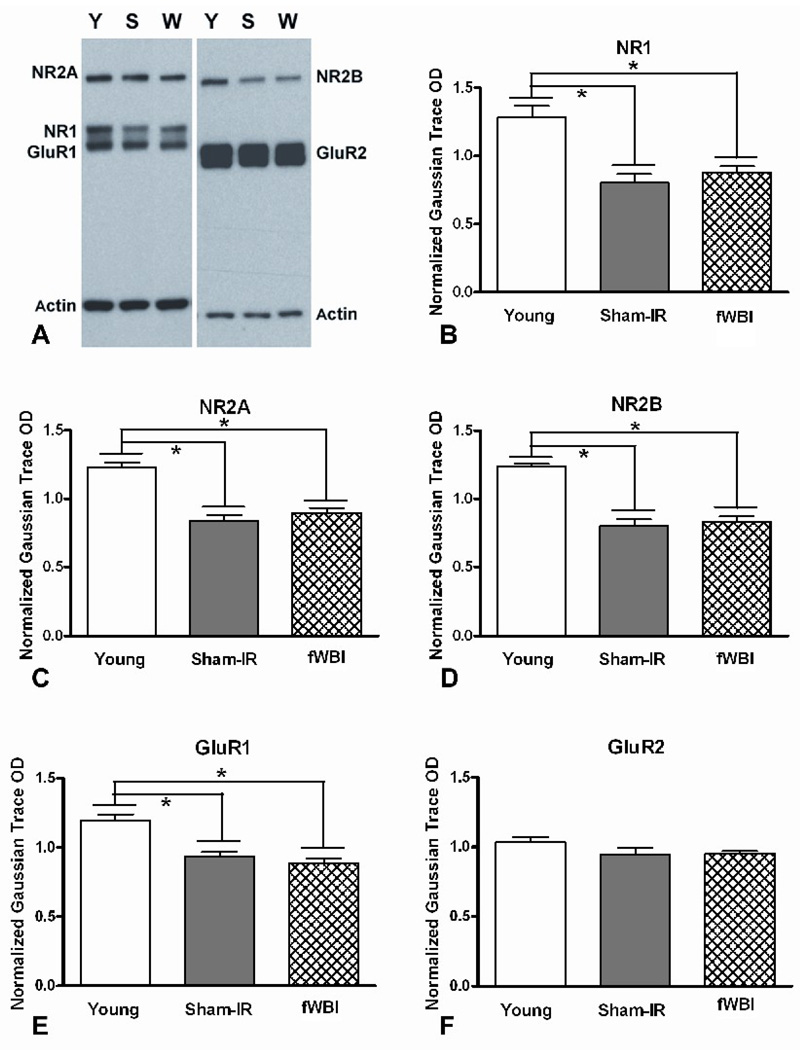
The present study evaluated the effect of age and fWBI on the relative protein levels of NMDA and AMPA subunits of the glutamate receptor in CA1 of the hippocampus. Western blot analysis was performed on tissue harvested at 30 months of age from the fWBI and Sham-IR rats tested on the MWM (Figure 1A) and from 7 month old Young rats. A representative immunoblot and graphic representations of the results are shown in Figures 3A-F. There were significant age-dependent decreases in the CA1 levels of the NMDA subunits, NR1 (F(1,18)=54, P <0.001), NR2A (F(1,18)=27, P <0.001), and NR2B (F(1,18)=63, P <0.001) as well as in the CA1 levels of the AMPA subunit, GluR1 (F(1,18)=22, P <0.001). No age-dependent decrease was detected in the AMPA subunit, GluR2 (F(1,18)=2.50, P=0.13). In contrast, none of the NMDA or AMPA subunits differed between the Sham-IR and fWBI rats (P’s>0.05) at old age. These findings indicate a significant age-dependent decline in all of the subunits except GluR2, but no detectable fWBI-induced change in any of these NMDA and AMPA subunits at old age.
Figure 3.
Western blot analysis of the glutamate receptor subtypes. (A) Representative immunoblots of the NMDA subunits, NR1, NR2A, and NR2B, as well as the AMPA subunits, GluR1 and GluR2, from Young (Y), Sham-IR (S), and fWBI (W) rats; actin was used as the loading control. Quantitation of the individual bands in the gels revealed an age effect, but no fWBI effect at old age for NR1 (B), NR2A (C), NR2B (D), and GluR1 (E). There was no age or fWBI effect for GluR2 (F).
2.3 Hippocampal metabolites exhibit an age effect, but not an fWBI effect in old rats
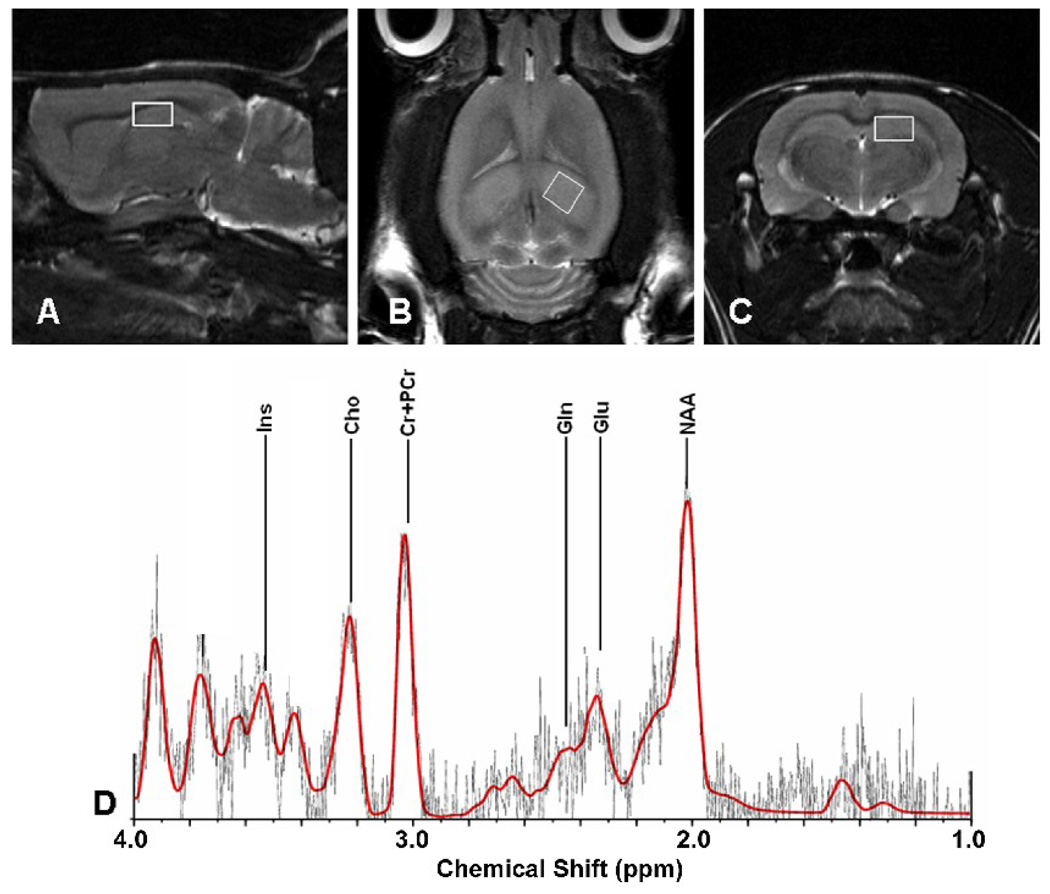
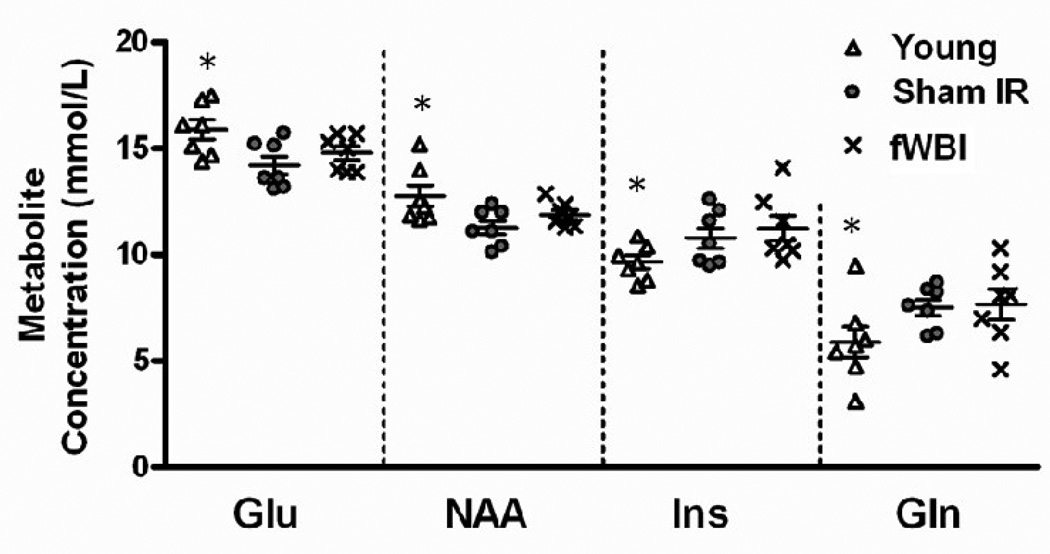
Brain metabolites were compared in the hippocampus of 27 month old fWBI and Sham-IR rats and 10 month-old Young rats that were previously tested on the MWM (Figure 1A). Placement of voxels (2.5 mm × 2.5 mm × 2.5 mm) to obtain 1H MR spectra in the hippocampus was accomplished using T2 MR images (Figures 4A–C). The positions of glutamate (Glu), N-acetyl-aspartic acid (NAA), myo-inositol (Ins), and Glutamine (Glu) in the spectra were identified (Figure 4D) and their concentrations determined from the area under the peak using calibration curves constructed with known concentrations of each metabolite (Figure 5). Because the creatine (Cr), and phosphocreatine (PCr) peaks are difficult to separate (Figure 4D), total creatine (tCr) was determined by summing the area under their peaks. The tCr concentration is unaffected by age or fWBI (Atwood et al, 2007), so comparisons among the Sham-IR, fWBI, and Young rat groups are valid if the tCr concentrations in the brain are relatively constant and fall with the range of published values. Our values of 12.8 mmol/L, 13.4 mmol/L, and 13.9 mmol/L, respectively, are both relatively constant and fall within the range of previously published values for rats (Macri et al., 2006).
Figure 4.
T2 proton magnetic resonance images of a Sham-IR rat brain showing the voxel placement for obtaining the 1H MR spectra in (A) sagittal, (B) horizontal, and (C) coronal images. The voxels (2.5 mm × 2.5 mm × 2.5 mm) were placed to include the dorsal hippocampus. (D) A representative 1H MR spectrum from a Sham-IR rat with the peaks for the neuronal markers, glutamate (Glu) and N-acetyl-aspartic acid (NAA) and for the glial markers, myo-inositol (Ins) and glutamine (Gln) identified. Quantitation was performed for the metabolites whose profiles have an SD <20% (Glu, NAA, Ins), or an SD >20%, but <50% (Gln).
Figure 5.
Concentrations of the hippocampal metabolites detected by 1H MRS. The neuronal markers, glutamate (Glu) and N-acetyl-aspartic acid (NAA), revealed a decrease with age, but no effect of fWBI at old age. The glial markers, myo-inositol (Ins) and glutamine (Gln), revealed an increase with age, but no effect of fWBI at old age. *P < 0.05 for comparison of the fWBI and Sham-IR groups.
For the metabolite analysis, the percent standard deviation (SD), defined by the Cramer-Rao lower bounds criteria (Cavassila et al., 2001) was determined for each hippocampal metabolite as an index of quantification reliability. As in previous studies (Provencher, 1993, 2001; Atwood et al., 2007), an SD ≤ 20% was taken as the acceptable level for including the data in our final analyses. Three metabolites, glutamate (Glu), N-acetyl-aspartic acid (NAA), and myo-inositol (Ins), had SD values < 20%. A fourth metabolite, glutamine (Gln), had an SD > 20%, but < 50%, and was included because of its functional relevance; glutamate and NAA are neuronal markers, whereas Ins and Gln are glial markers (Govindaraju et al., 2000; Manganas et al., 2007; Kaiser et al., 2005b; den Heijer et al., 2006). Analysis of the data in Figure 5 revealed an age-dependent decrease in the hippocampal concentrations of Glu (P = 0.01) and NAA (P = 0.02), but no significant difference between the Sham-IR and fWBI groups at old age for either metabolite (P = 0.31 and P = 0.26, respectively). Further analysis revealed significant increases in the hippocampal concentrations of Ins (P = 0.03) and Gln (P = 0.04) with age, but no significant difference between the Sham-IR and fWBI groups at old age for either metabolite (P = 0.48 and P = 0.87, respectively). Taken together, these 1H MRS findings show age-dependent decreases in the levels of Glu and NAA, metabolites associated with neuronal activity, and age-dependent increases in the levels of Ins and Gln, metabolites associated with glial activity, but no effect of fWBI on the levels of any of these metabolites at old age.
3. DISCUSSION
The goals of the present study were to investigate, i] the detection of radiation-induced brain injury at old age following fWBI at middle age, and ii] the neurobiological correlates of this radiation-induced brain injury. We evaluated cognition, glutamate receptor subunits, and hippocampal metabolites 14–18 months after fWBI at 12 months of age. By including Sham-IR and fWBI groups of old rats and Young unirradiated rats, we were able to determine effects of both age and fWBI. Our results demonstrate significant age-dependent, i] cognitive impairments in spatial learning, reference memory, and spatial reversal learning, ii] modifications in NMDA and AMPA receptor subunits in the hippocampus, and iii] alterations in hippocampal metabolites. fWBI exacerbated the age-dependent deficits in spatial reversal learning, but had no additional, detectable effect on other measures of cognition, hippocampal levels of glutamate receptor subunits, or hippocampal levels of brain metabolites in old rats.
The age-dependent deficit in spatial reversal learning was exacerbated in fWBI rats at old age as indicated by, i] the increased distance required to find the new platform location on Days 4 and 5 of the task (Figure 2D), and ii] the smaller 24–h saving parameter between Days 1 and 2 of the training trials (Figure 2E). Spatial reversal learning requires the rats to refrain from responding to the previously learned platform location and to establish a new response by finding the platform in a novel location. Performance on the reversal MWM task depends not only on the acquisition of new information, but also on the active suppression of previously acquired experiences. This process is defined as “inhibitory learning” (Xu et al., 2009), and is a very sensitive measure of the cognitive flexibility associated with the executive functions (Vorhees and Williams, 2006) that are crucial to the quality of life in the elderly. The high sensitivity of the reversal MWM task is likely to be responsible for its ability to detect fWBI-induced cognitive impairment in old rats. These higher order learning and memory processes are associated with the prefrontal cortex and, in the case of spatial reversal learning, the hippocampus (Moser et al., 1993; Xu et al., 2009; Majdi et al., 2009). Consequently, the decline in reversal learning performance after fWBI in our old rats may well indicate changes in neural processing in these brain regions.
Age-dependent declines in spatial learning memory have been reported across rat strains using different MWM protocols (Fordyce and Wehner, 1993; Moser et al., 1995; Markowska and Savonenko, 2002; Robitsek et al., 2008; Bizon et al., 2009). The data presented here are the first to demonstrate age-related spatial learning and reference memory impairments using a one training trial per day protocol with interspersed probe trials. Although fWBI-induced spatial learning and memory impairments were not detected here at old age, we previously observed fWBI-induced cognitive impairment in 24 month-old rats using a MWM protocol with one trial per day and a single probe trial at the end of training (Shi et al., 2006). The difference in findings between these two studies may be due to, i] the difference in the MWM protocols, ii] the age of the rats at testing (24 vs 26–27 months), or iii] the length of time after fWBI when the testing occurred (12 vs 14–15 months). The extra months at the end of the rat’s short lifespan could have profound effects on the results. This point is particularly relevant since recent studies of cancer patients suggest that patients > 70 years of age demonstrate distinctly more negative treatment outcomes than patients < 70 years of age (Klepin and Hurd, 2006). Importantly, the present observations suggest that age-dependent cognitive impairments late in the life span may make it difficult to detect the cognitive consequences of fWBI or other treatments/interventions in very old animals or humans.
As the predominant excitatory neurotransmitter in the hippocampus, glutamate plays an essential role in the neural processing associated with spatial learning and memory. In particular, the NMDA and AMPA subunits of the glutamate receptor have been shown to be integrally related to hippocampal synaptic plasticity (Clark et al., 1992; Kullmann et al., 2000; Clayton et al., 2002; Riedel et al,. 2003). The present study detected significant age-dependent decreases in the CA1 levels of the NR1, NR2A, and NR2B subunits of the NMDA receptor and the GluR1 subunit of the AMPA receptor (Figure 3B–E). However, no fWBI-induced changes were detected in glutamate receptor subunits. These age-dependent decreases in NMDA and AMPA subunits are consistent with our previous findings (Shi et al., 2007; Adams et al., 2008). Numerous reports have provided strong support for the concept that these decreases can lead to diminished neural transmission, synaptic plasticity, learning, and memory across lifespan (e.g. Clark et al., 1992; Magnusson, 1998; Adams et al., 2001; Clayton et al., 2002). In contrast to the age-dependent decreases in NR1 and NR2, we previously found increases in NR1 and NR2A subunits at 24 months of age, 12 months following fWBI (Shi et al., 2006). In the present study, the age of the rats (30 months) and the time after fWBI (18 months) was 6 months greater than in that previous study. If, as expected, fWBI produces the same radiation damage and, therefore, the same small increases in glutamate receptor subunits as in the previous study, it is very likely that the much larger age effect in the opposite direction (Figure 3) would mask the small fWBI effect in old rats.
Recent reports suggest that changes in brain metabolites detected with 1H MRS may provide information on the biochemical alterations associated with cognitive changes including spatial learning and memory impairments (Govindaraju et al., 2000; den Heijer et al., 2006). In the present study, we observed significant changes in several hippocampal metabolites during aging, but did not detect fWBI-induced changes in old rats that were irradiated at middle age. Two metabolites associated with neuronal activity, Glu and NAA, were present at significantly lower levels in both groups of old rats when compared to Young rats (Figure 5). Glu is concentrated in neurons and age-dependent decreases in hippocampal Glu levels have been associated with cognitive decline in humans (Kaiser et al., 2005a, 2005b). NAA has been used as a marker of neuron density, integrity, and metabolic activity (den Heijer et al., 2006; Metastasio et al., 2006; Manganas et al. 2007) and hippocampal levels of NAA have been found to be a sensitive indicator of cognitive function (Adalsteinsson et al., 2000). Consistent with the age-dependent decrease in NAA reported here, reduced NAA levels have been associated with neuron dysfunction (Govindaraju et al., 2000; Malloy, 2001) in the elderly, particularly those with Alzheimer’s disease (Firbank et al., 2002; Kaiser et al., 2005b; den Heijer et al., 2006). In contrast to the neuronal markers, levels of the glial markers, Gln and Ins (Brand et al. 1993), exhibited aged-dependent increases (Figure 5). Gln is a precursor of Glu found predominantly in glia (Govindaraju et al., 2000). Elevated Gln levels during normal aging have been associated with increased glial proliferation, specifically astrocyte proliferation (Kaiser et al,. 2005a, 2005b). Similarly, Ins is well-established as a glial marker in 1H MRS studies (Brand et al., 1993). Notably, increased levels of Ins have been reported during both normal aging (Brand et al., 1993; Govindaraju et al., 2000; Kaiser et al., 2005b) and in Alzheimer patients (Firbank et al., 2002). Thus, the age-dependent alterations in hippocampal metabolites likely reflect functional changes that contribute to the cognitive impairments observed in old rats.
Previous 1H MRS studies have reported fWBI-induced changes in both animal models and patients. In the clinic, significant metabolite changes were detected with 1H MRS in the brains of glioma patients that received a 60 Gy total dose of fWBI (Adalsteinsson et al. 2000; Rutkowski et al. 2003). A recent 1H MRS study revealed a small, but statistically significant, increase in the Glu+Gln/tCr) and NAA/tCr ratios and a small, but statistically significant decrease in the Ins/tCr ratio measured in central thalamus one year following fWBI of male Fisher 344 rats at 3–4 months of age with the same dose and schedule as that used in the present study (Atwood et al., 2007). Those fWBI-induced changes in metabolite concentrations are in the opposite direction to the age-dependent changes reported here. Although differences in voxel placement and rat strain could contribute to the difference between the two studies, the age of the rat when scanned likely played the greatest role in the difference. Even though the post-fWBI period was one year in both studies, rats in the previous study were scanned at 15–16 months of age compared to 27 months of age here. If, as expected, fWBI produces the same radiation damage and, therefore, the same metabolite changes in middle-aged rats as in young rats, it is likely that the much larger significant age effect in the opposite direction measured here (Figure 5) masks the small fWBI effect in old rats.
4. EXPERIMENTAL PROCEDURES
4.1. Animals and fWBI
Middle–aged male Fischer 344 × Brown Norway (F344×BN) rats (Harlan Industries, Indianapolis, IN, USA) were assigned randomly to Sham-IR or fWBI groups at 12 months of age. As previously described (Shi et al., 2006), fWBI rats were lightly anesthetized (26.5 mg/kg ketamine, Fort Dodge Animal Health, Fort Dodge, Iowa 50501, USA, 5.4 mg/kg xylazine, VEDCO, Inc., St. Joseph, MO 64507, USA) and irradiated in a self-shielded 137Cs irradiator using lead and Cerrobend devices to collimate the beam and irradiate the whole brain, including the brain stem. A total dose of 40 Gy in 8 fractions over 4 weeks was delivered to alternate sides of the head on alternate days to ensure that each rat received the same midline dose. Sham-IR rats were anesthetized, but not irradiated. In addition, 7–10 month-old (Young) male F344×BN rats were included for comparison. The animal protocol for this study conforms to National Institute of Health guidelines and was approved by the Animal Care and Use Committee of Wake Forest University Health Sciences. Rats were housed singly in a climate-controlled environment with a 12-hour light/dark cycle and provided food and water ad libitum. Weight was monitored weekly; fWBI rats continued to gain weight, but at a slower rate than the Sham-IR rats. The body weight difference between groups stabilized by several months post-irradiation with fWBI rats weighing approximately 100 g less than Sham-IR rats. During the course of experiment, one fWBI rat and one Sham-IR rat were euthanized for health reasons unrelated to the WBI.
4.2 Morris water maze
Spatial learning, reference memory, and spatial reversal learning were evaluated in 26 month-old Sham-IR (n = 11), 26 month-old fWBI (n = 11), and 9 month-old Young (n = 12) rats using the Morris water maze (MWM) (Morris, 1984). As previously described (Shi et al., 2006), rats were placed in a circular white plastic tank filled with opacified water and surrounded by dark geometric cues affixed to white curtains. The tank was divided into 4 imaginary quadrants (quadrants 1, 2, 3, and 4) with an escape platform 2 cm under the water surface in the middle of one quadrant. MWM testing consisted of two phases, a “standard version” and a “reversal version”, illustrated schematically in Figure 1B.
During the standard version of the MWM, spatial learning and reference memory were measured. The escape platform was placed in quadrant 1, the rats were introduced into the quadrants in a systematically random pattern, and each rat’s performance was recorded using an automated tracking system (Ethovision Observer, Nodulus, Leesburg, VA, USA). One training trial was given each day for 5 days followed by 1 probe trial on the 6th day. During each training trial, the rat was allowed to search for 90 sec to locate the platform, and the total distance to the platform (same parameter as cumulative distance in Gallagher et al., 1993), the path length to the platform, and the escape latency were measured as described previously (Shi et al., 2006). During the probe trial, the platform was lowered beyond reach, the rat was allowed to search for 30 sec, and the mean distance to the site where the platform was located (same parameter as average proximity in Gallagher et al., 1993) was measured. This schedule was repeated for 4 weeks (Figure 1B).
In the reversal version of the MWM, the platform location was moved to the opposite quadrant (quadrant 3) with the visual cues remaining in the same positions. This task required the rats to inhibit their previously learned response and form a new strategy to find the escape platform in a novel location. Reversal training trials were carried out for 5 consecutive days, followed by a single probe trial on the 6th day. The same parameters were measured in the reversal version that had been measured in the standard version of the MWM. After the reversal learning task was completed, 6 consecutive trials were conducted using a visible platform in a new position for each trial in order to evaluate each rat’s sensory and motor performance.
4.3 SDS–PAGE and Western blot analysis
Western blots were used to assess the protein levels of NMDA and AMPA subunits of glutamate receptors in the hippocampus of 30 month-old Sham-IR (n = 7), 30 month-old fWBI (n=7), and 7 month-old Young (n = 7) rats as previously described (Shi et al., 2006; Shi et al., 2007). Rats were deeply anesthetized (intraperitoneal sodium pentobarbital, 150 mg/kg, Ovation Pharmaceuticals, Inc., Deerfield, IL 60015, USA) and decapitated. The CA1 region from the dorsomedial hippocampus was dissected on ice, weighed, and stored at − 80° C (Newton et al. 2005). Samples were homogenized using 50 µL/mg of a modified Laemmli buffer consisting of 60 mM Tris base-HCl (Fisher Scientific, Fair Lawn, NJ 07410, USA), 10% glycerol (Fluka, Sigma-Aldrich, Co., St Louis, MO 63103, USA), 2% SDS (Fisher Scientific, Fair Lawn, NJ 07410, USA) and protease inhibitors consisting of 2 mM EDTA and 1:250 Protease Inhibitor Cocktail (both from Sigma-Aldrich, Co., St Louis, MO 63103, USA). Homogenates were heated, centrifuged, and protein concentrations determined from aliquots of the soluble supernatant using a BCA protein assay (Pierce Technology, Rockford, IL, USA).
For analysis of the NMDA and AMPA subunits, 7.5 µg of CA1 supernatant protein were loaded into 15 well 10% Tris–HCl Ready gels (Bio-Rad Laboratories, Hercules, CA, USA). A sample from each group was run in duplicate on each gel, and the gels were duplicated to produce 4 values for each rat. Samples were separated under reducing conditions using SDS–PAGE and transferred electrophoretically onto Immobilon membranes (Millipore, Bedford, MA, USA). Rabbit polyclonal antibodies to NR1 (0.6 µg/mL, Millipore, formerly Chemicon, Temecula, CA, USA), NR2A (0.07 µg/mL, Millipore/Chemicon), NR2B (0.07 µg/mL, Millipore/Chemicon), GluR1 (0.01 µg/mL, Millipore/Chemicon) and GluR2 (0.25 µg/mL, Millipore/Chemicon) were used for immunodetection. Peroxidase-conjugated donkey anti-rabbit IgG (10 ng/mL, Jackson ImmunoResearch, West Grove, PA, USA) and anti-mouse IgG (10 ng/mL, Millipore/Chemicon) secondary antibodies were used with the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL, USA) to visualize individual proteins. The loading control was actin stained with a mouse monoclonal antibody (0.002 µg/mL, Millipore/Chemicon). Blots were exposed on Kodak Biomax film (PerkinElmer Life and Analytical Sciences, Shelton, CT, USA) and individual bands were quantified with Bio-Rad VersaDoc and Quantity One software (Bio-Rad Laboratories). The Gaussian trace optical densities of individual bands were normalized, and the 4 normalized values of each subunit were averaged to derive the mean for each rat.
4.4 Magnetic resonance imaging and spectroscopy
Magnetic resonance imaging (MRI) and 1H MRS scans were performed on 27 month-old Sham-IR (n = 7), 27 month-old fWBI (n = 7), and 10 month-old Young (n = 7) rats. Details of the 1H MRS procedure have been described previously (Atwood et al., 2007). During all imaging procedures, the rats were held in place with a headholder, the anesthesia was maintained using an isoflurane + O2 system (Surgivet, Smiths Medical, Waukesha, WI, USA), and the heart rate, breathing rate, and body temperature were monitored (SA Instruments, Stony Brook, NY, USA). All MRI/1H MRS scans, gated on the heart and breathing signals, were performed on a horizontal 7T magnet (Bruker BioSpin, Billerica, MA, USA) interfaced with a digital spectrometer operating at a resonant frequency of 300 MHz. The system was equipped with a 12 cm actively-shielded gradient coil with a maximum gradient strength of 400 mT/m. The radiofrequency excitation and signal reception was accomplished with a 38 mm inside diameter litzcage coil (Doty Scientific, Columbia, SC, USA).
A rapid acquisition with relaxation enhancement (RARE) sequence (TE = 61 msec, TR = 2500 msec, slice thickness = 1.0 mm, field of view = 3.0 cm, matrix = 256 × 256, NEX = 4) was used to acquire T2-weighted images of the rat brains. These high-resolution T2 images were used to position the spectroscopic voxels in the hippocampus (Figure 4A–C). Anatomical coronal sections were acquired in the axial plane. All MR spectra were acquired using a single-voxel, point-resolved, double spin-echo spectroscopy (PRESS) sequence, with TE = 20 msec and TR = 2500 msec, voxel size = 2.5 × 2.5 × 2.5 mm. The total number of acquisitions was 1024. From each PRESS voxel, one MR spectrum with no water suppression and one spectrum using variable pulse power and optimized relaxation delays (VAPOR) water suppression were acquired (Figure 4D). Prior to each spectral acquisition, localized voxel shimming was performed using the FASTMAP technique (Gruetter, 1993).
For quantitative measurement of the brain metabolites, the 1H MRS raw data were transferred to a Linux workstation, and the spectra analyzed using the LCModel (Provencher, 1993, 2001; Cavassila et al., 2001) as previously described (Atwood et al. 2007). The replace-and-match method (Jansen et al., 2006) was used to convert in vivo metabolite concentrations to mmol concentrations.
4.5 Statistical analysis
For analysis of standard MWM training trials, a two-way repeated measures mixed model approach was used that considered individual rats as random effects, and the weeks and groups as fixed effects to assess the outcomes of total distance to the platform, path length, and escape latency. For each outcome, the week by group interaction was tested to determine if the group effects were different by week. If the interaction was significant, differences among groups for each week were examined separately using a “least squares means” approach that estimated the predicted value for the outcome of interest, conditional on the specific levels of the factors of interest (i.e., for specific week and group combinations). If the interaction was not significant, then overall group comparisons were made, adjusting for all weeks simultaneously. Similar models were fitted to examine the outcome of mean distance to the platform in the probe trials.
For the MWM reversal trials, we first examined group differences with one-way ANOVA models without repeated measures where the outcome was averaged across the 5 days of trials. If the difference in performance between Young and Sham-IR rats was not significant, those groups were combined to test for an overall radiation effect. Next, a two-way repeated measures mixed model ANOVA was used to examine group by day interactions with each of the individual days included in the model. If the interaction was significant, differences among groups for each day were examined separately. Finally, the visible platform escape latency was compared among the 3 groups of rats using a one way ANOVA. For all comparisons, the two-tailed significance level for each outcome was held at 0.05. All analyses were performed using SAS Version 9.1 software.
For the 1H MRS data, one-way ANOVA models were used to determine if there was an overall difference in the metabolite levels among the Young, Sham-IR, and fWBI groups. If the overall test was significant, then a two-step procedure was used. First, the Sham-IR and fWBI rats were compared to determine if there was a significant fWBI effect at old age. Next, if there was no significant fWBI effect at old age, a specific contrast was tested which compared the Young group to a combined old group of Sham-IR and fWBI rats to determine if there was an age effect. For all comparisons, a two-tailed significance level for each of the main effects was held at 0.05.
For the Western blot data, the effects of the two independent variables, age and fWBI, on each of the dependent variables derived from the Gaussian trace of the optical densities for NR1, NR2A, NR2B, GluR1, and GluR2, were determined using a two-way ANOVA. The age by condition interaction was examined for each outcome. If the term was significant, it was included in the model; if non-significant, it was removed. A two-tailed significance level for each of the main effects was held at 0.05.
SUMMARY
Aging is associated with impaired hippocampal-dependent spatial learning and reference memory. The present study revealed age-dependent impairments in both standard and reversal MWM tasks accompanied by significant age-dependent hippocampal changes in glutamate receptor subunits and metabolites. These age-dependent subunit and metabolite changes were in the opposite direction from the fWBI-induced changes reported in younger rats (Shi et al., 2006, Atwood et al., 2007), suggesting that fWBI-induced neurobiological changes in old rats may be masked by the substantial molecular and metabolic changes that occur during aging. Consequently, i] detecting radiation-induced brain injury using 1H MRS, and/or ii] determining the underlying causes of radiation-induced brain injury in elderly fWBI patients >70 years old are likely to be more difficult than in younger fWBI patients.
ACKNOWLEDGEMENTS
This work was supported by grants CA119990 and CA112593 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adalsteinsson E, Sullivan EV, Kleinhans N, Spielman DM, Pfefferbaum A. Longitudinal decline of the neuronal marker N-acetyl aspartate in Alzheimer's disease. Lancet. 2000;355:1696–1697. doi: 10.1016/s0140-6736(00)02246-7. [DOI] [PubMed] [Google Scholar]
- Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, Newton IG, Carter CS, Sonntag WE, Brunso-Bechtold JK. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp. Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Smith TD, Moga D, Gallagher M, Wang Y, Wolfe BB, Rapp PR, Morrison JH. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J. Comp. Neurol. 2001;432:230–243. doi: 10.1002/cne.1099. [DOI] [PubMed] [Google Scholar]
- Atwood T, Payne VS, Zhao W, Brown WR, Wheeler KT, Zhu JM, Robbins ME. Quantitative magnetic resonance spectroscopy reveals a potential relationship between radiation-induced changes in rat brain metabolites and cognitive impairment. Radiat. Res. 2007;168:574–581. doi: 10.1667/RR0735.1. [DOI] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol. Aging. 2009;30:646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev. Neurosci. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiat. Res. 2005;164:662–668. doi: 10.1667/rr3453.1. [DOI] [PubMed] [Google Scholar]
- Butler JM, Rapp SR, Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr. Treat. Options Oncol. 2006;7:517–523. doi: 10.1007/s11864-006-0026-5. [DOI] [PubMed] [Google Scholar]
- Cavassila S, Deval S, Huegen C, van Ormondt D, Graveron-Demilly D. Cramer-Rao bounds: an evaluation tool for quantitation. NMR Biomed. 2001;14:278–283. doi: 10.1002/nbm.701. [DOI] [PubMed] [Google Scholar]
- Clark AS, Magnusson KR, Cotman CW. In vitro autoradiography of hippocampal excitatory amino acid binding in aged Fischer 344 rats: relationship to performance on the Morris water maze. Behav. Neurosci. 1992;106:324–335. doi: 10.1037//0735-7044.106.2.324. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J. Neurosci. 2002;22:3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer T, Sijens PE, Prins ND, Hofman A, Koudstaal PJ, Oudkerk M, Breteler MM. MR spectroscopy of brain white matter in the prediction of dementia. Neurology. 2006;66:540–544. doi: 10.1212/01.wnl.0000198256.54809.0e. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13:1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, Harrison RM, O'Brien JT. A comprehensive review of proton magnetic resonance spectroscopy studies in dementia and Parkinson's disease. Dement Geriatr. Cogn. Disord. 2002;14:64–76. doi: 10.1159/000064927. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Wehner JM. Effects of aging on spatial learning and hippocampal protein kinase C in mice. Neurobiol. Aging. 1993;14:309–317. doi: 10.1016/0197-4580(93)90116-s. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav. Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn. Reson. Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240:318–332. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol. Aging. 2005a;26:665–672. doi: 10.1016/j.neurobiolaging.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Scyllo-inositol in normal aging human brain: 1H magnetic resonance spectroscopy study at 4 Tesla. NMR Biomed. 2005b;18:51–55. doi: 10.1002/nbm.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepin HD, Hurd DD. Autologous transplantation in elderly patients with multiple myeloma: are we asking the right questions? Bone Marrow Transplant. 2006;38:585–592. doi: 10.1038/sj.bmt.1705486. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F, Walker MC. The role of mammalian ionotropic receptors in synaptic plasticity: LTP, LTD and epilepsy. Cell. Mol. Life Sci. 2000;57:1551–1561. doi: 10.1007/PL00000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2008;71:64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- Macri MA, D'Alessandro N, Di Giulio C, Di Iorio P, Di Luzio S, Giuliani P, Bianchi G, Esposito E. Regional changes in the metabolite profile after long-term hypoxia-ischemia in brains of young and aged rats: a quantitative proton MRS study. Neurobiol. Aging. 2006;27:98–104. doi: 10.1016/j.neurobiolaging.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. The aging of the NMDA receptor complex. Front. Biosci. 1998;3:e70–e80. doi: 10.2741/a368. [DOI] [PubMed] [Google Scholar]
- Majdi M, Ribeiro-da-Silva A, Cuello AC. Variations in excitatory and inhibitory postsynaptic protein content in rat cerebral cortex with respect to aging and cognitive status. Neuroscience. 2009;159:896–907. doi: 10.1016/j.neuroscience.2008.11.034. [DOI] [PubMed] [Google Scholar]
- Malloy CR. Correlation of cerebral metabolites with clinical outcome among patients with severe congestive heart failure. Circulation. 2001;103:2771–2772. doi: 10.1161/01.cir.103.23.2771. [DOI] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Maletic-Savatic M. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko A. Retardation of cognitive aging by life-long diet restriction: implications for genetic variance. Neurobiol. Aging. 2002;23:75–86. doi: 10.1016/s0197-4580(01)00249-4. [DOI] [PubMed] [Google Scholar]
- Metastasio A, Rinaldi P, Tarducci R, Mariani E, Feliziani FT, Cherubini A, Pelliccioli GP, Gobbi G, Senin U, Mecocci P. Conversion of MCI to dementia: Role of proton magnetic resonance spectroscopy. Neurobiol. Aging. 2006;27:926–932. doi: 10.1016/j.neurobiolaging.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J. Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc. Nat. Acad. Sci. USA. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenaus A, Huang L, Smith A, Favre CJ, Nelson G, Kendall E. Magnetic resonance imaging and spectroscopy of the rat hippocampus 1 month after exposure to 56Fe-particle radiation. Radiat. Res. 2008;169:149–161. doi: 10.1667/RR1135.1. [DOI] [PubMed] [Google Scholar]
- Patchell RA, Regine WF. The rationale for adjuvant whole brain radiation therapy with radiosurgery in the treatment of single brain metastases. Technol. Cancer Res. T. 2003;2:111–115. doi: 10.1177/153303460300200206. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H. Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J. Neurosci. 2008;28:8945–8954. doi: 10.1523/JNEUROSCI.1893-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski T, Tarnawski R, Sokol M, Maciejewski B. 1H-MR spectroscopy of normal brain tissue before and after postoperative radiotherapy because of primary brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2003;56:1381–1389. doi: 10.1016/s0360-3016(03)00327-4. [DOI] [PubMed] [Google Scholar]
- Shi L, Linville MC, Iversen E, Molina DP, Yester J, Wheeler KT, Robbins ME, Brunso-Bechtold JK. Maintenance of white matter integrity in a rat model of radiation-induced cognitive impairment. J. Neurol. Sci. 2009;285:178–184. doi: 10.1016/j.jns.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Adams MM, Linville MC, Newton IG, Forbes ME, Long AB, Riddle DR, Brunso-Bechtold JK. Caloric restriction eliminates the aging-related decline in NMDA and AMPA receptor subunits in the rat hippocampus and induces homeostasis. Exp. Neurol. 2007;206:70–79. doi: 10.1016/j.expneurol.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, Nicolle MM, Robbins M, D'Agostino R, Jr, Brunso-Bechtold JK. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat. Res. 2006;166:892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- Shi L, Molina DP, Robbins ME, Wheeler KT, Brunso-Bechtold JK. Hippocampal neuron number is unchanged 1 year after fractionated whole-brain irradiation at middle age. Int. J. Radiat. Oncol. Biol. Phys. 2008;71:526–532. doi: 10.1016/j.ijrobp.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffietti R, Ruda R, Mutani R. Management of brain metastases. J. Neurol. 2002;249:1357–1369. doi: 10.1007/s00415-002-0870-6. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J. Neurosci. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]