Abstract
Studies of the effects of disclosing stressful experiences among patients with rheumatoid arthritis (RA) have yielded inconsistent findings, perhaps due to different disclosure methods—writing or speaking—and various methodological limitations. We randomized adults with RA to a writing (n = 88) or speaking (to a recorder) sample (n = 93), and within each sample, to either disclosure or one of two control groups (positive or neutral events), which conducted 4, 20-minute, at-home sessions. Follow-up evaluations at 1, 3, and 6 months included self-reported, behavioral, physiological, and blinded physician-assessed outcomes. In both writing and speaking samples, the disclosure and control groups were comparably credible, and the linguistic content differed as expected. Covariance analyses at each follow-up point indicated that written disclosure had minimal effects compared to combined controls—only pain was reduced at 1 and 6 months, but no other outcomes improved. Spoken disclosure led to faster walking speed at 3 months, and reduced pain, swollen joints, and physician-rated disease activity at 6 months, but there were no effects on other outcomes. Latent growth curve modeling examined differences in the trajectory of change over follow-ups. Written disclosure improved affective pain and walking speed; spoken disclosure showed only a marginal benefit on sensory pain. In both analyses, disclosure's few benefits occurred relative to both positive and neutral control groups. We conclude that both written and spoken disclosure have modest benefits for patients with RA, particularly at 6 months, but these effects are limited in scope and consistency.
Keywords: rheumatoid arthritis, pain, emotional disclosure, expressive writing, stress, emotion, clinical trial
1. Introduction
Stressful experiences influence pain and adjustment [7,24,33], and having awareness and expression of emotions, rather than avoidance or inhibition, is thought to be adaptive [10,14]. To test this, Pennebaker and Beall [25] developed a paradigm in which participants are randomized to write for several 20-minute sessions about stressors and feelings (i.e., written emotional disclosure, or expressive writing) or about non-stressful control topics, and changes in health over subsequent months are examined. An early meta-analysis of healthy samples found a moderate benefit of disclosure [30], although recent meta-analyses of clinical samples [9] or that included more studies [8,11,19] revealed weaker effects.
Seven published studies have examined emotional disclosure in patients with rheumatoid arthritis (RA). The study of Smyth et al. [31] was most supportive, finding that disclosure improved physician-rated disease status; however, other studies have been less supportive. Danoff-Burg et al. [6] found that fatigue—but not pain, disability, or psychological functioning—improved after disclosure. Kelley et al. [13] reported improved affective and physical functioning, but no change in pain, joint condition, or behavior. Broderick et al. [5] found little or no benefit when disclosure occurred as a part of routine clinical practice. Wetherell et al. [36] noted better mood and less disease activity after disclosure, but the effects were due to unexpected worsening among controls. Van Middendorp et al. [34] found no effect of disclosure on clinical outcomes, but some evidence of improved immune markers. Finally, Keefe et al. [12] reported no benefits after either private or nurse-facilitated disclosure.
The available literature on emotional disclosure among people with RA has many limitations. In particular, speaking rather than writing has often been conducted. The study that demonstrated the most positive effects [31] used writing, whereas four studies used speaking into a recorder [12,13,34] or to a nurse [12] or permitted patients to choose the method [36]. The other two studies had additional limitations, including not verifying or obtaining patient writings [5] or using a mixed-diagnosis sample (RA and lupus) [6]. Also, most of these studies have not conducted manipulation checks, verified credibility of control conditions, or examined the content of the disclosures. Samples have often been quite small, such as 25 or fewer patients per condition [6,12,31,36], and follow-up periods have typically been 3 months or less [6,13,34,36]. Control groups are usually emotionally neutral, thereby controlling for time and effort, but failing to control for the personal, emotional relevance of the disclosure, such as might be obtained with a positive emotional disclosure exercise. Finally, no study has yet combined self-report, physician or clinical ratings, behavioral observations, and biomarker outcomes.
This study had two main goals: a) to examine the effects of both written and spoken disclosure separately among RA patients in a single study, and b) to improve upon prior studies by using multiple follow-up assessments over 6 months with a broad outcome battery, testing disclosure effects against both emotionally neutral and emotionally relevant controls, and conducting manipulation checks, credibility checks, and content analyses to inform interpretation of the results.
2. Methods
2.1 Participants
We recruited adults who met American College of Rheumatology criteria for non-juvenile RA from one of several urban or suburban rheumatology clinics. To ensure that the outcomes had room for improvement, included patients had to report experiencing pain or disability due to their RA in the preceding week. Exclusion criteria were a lack of RA-related pain and disability, physician-suspected or diagnosed cognitive impairment (dementia or psychosis), illiteracy or non-English speaking, the presence of another autoimmune rheumatic disease or other major medical condition for which they were receiving treatment, being physically unable to write or walk, participation in another clinical trial, or planning to leave the area within 6 months.
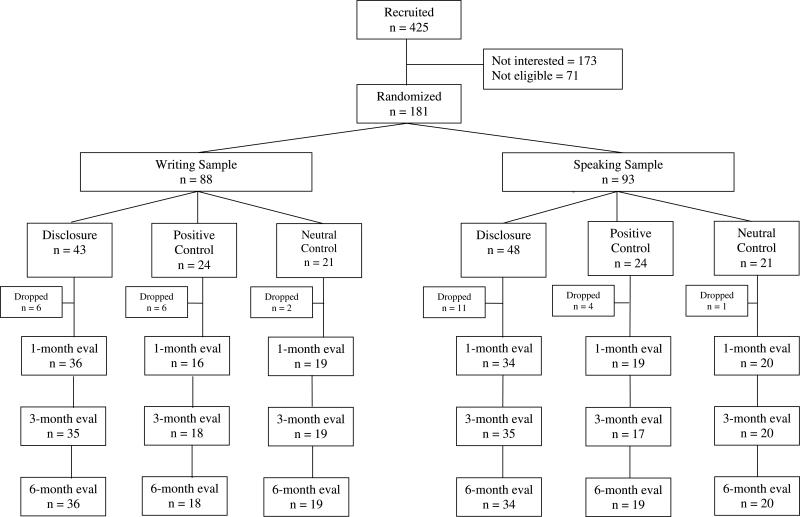
Figure 1 depicts patient flow through the study. Of 425 patients contacted for participation, 173 (40.7%) were not interested, and 71 (16.7%) were ineligible (primarily due to the presence of other medical conditions or lack of current symptoms). The remaining 181 patients (42.6%) completed the baseline assessment and were randomized, first to a sample (writing or speaking) and then to experimental conditions (disclosure or one of two control conditions). Table 1 presents demographic data for each sample (writing or speaking) and for each experimental condition within the two samples, including the two separate control groups and the combined controls. Overall, the 181 patients in the study were largely female (84%), included a substantial percentage of African Americans (44%), and averaged 54.6 years old (range of 20 to 74 years), 13.5 years of education (1.5 years post-high school), and 11.2 years since being diagnosed with RA.
Figure 1.
Flow of participants through the study.
Table 1.
Demographic Data for Each Experimental Condition, for Patients in the Writing Sample (Top) and Speaking Sample (Bottom)
| Sample / Variable |
Full Sample |
Disclosure |
Combined Control |
Positive Control |
Neutral Control |
|---|---|---|---|---|---|
| Writing Sample |
|
|
|
|
|
| Female n (%) | 74 (84%) | 38 (88.4%) | 36 (80%) | 19 (79%) | 17 (81%) |
| Male n (%) |
14 (16%) |
5 (11.6%) |
9 (20%) |
5 (21%) |
4 (19%) |
| Ethnicity n (%) | |||||
| European American | 46 (52%) | 24 (55.8%) | 22 (48.9%) | 11 (45.8%) | 11 (52.4%) |
| African American | 41 (47%) | 18 (41.9%) | 23 (51.1%) | 13 (54.2%) | 10 (47.6%) |
| Hispanic American |
1 (1%) |
1 (2.3%) |
0 (0%) |
0 (%) |
0 (0%) |
| Age M (SD) |
54.9 (10.8) |
55.4 (11.7) |
54.3 (10.0) |
53.1 (10.0) |
55.7 (10.0) |
| Education M (SD) |
13.6 (2.4) |
13.4 (2.4) |
13.8 (2.3) |
14.0 (2.7) |
13.6 (1.7) |
| RA Duration M (SD) |
13.2 (11.3) |
14.6 (11.4) |
12.0 (11.1) |
11.3 (9.3) |
12.8 (13.1) |
| Speaking Sample |
|
|
|
|
|
| Female n (%) | 78 (84%) | 40 (83.3%) | 38 (84.4%) | 20 (83.3%) | 18 (85.7%) |
| Male n (%) |
15 (16%) |
8 (16.7%) |
7 (15.6%) |
4 (16.7%) |
3 (14.3%) |
| Ethnicity n (%) | |||||
| European American | 54 (58%) | 28 (58.3%) | 26 (57.8%) | 13 (54.2%) | 13 (61.9%) |
| African American | 38 (41%) | 20 (41.7%) | 18 (40%) | 11 (45.8%) | 7 (33.3%) |
| Hispanic American |
1 (1%) |
0 (0%) |
1 (2.2%) |
0 (0%) |
1 (4.8%) |
| Age M (SD) |
54.3 (11.6) |
53.1 (11.3) |
55.5 (11.9) |
58.0 (12.2) |
52.6 (11.1) |
| Education M (SD) |
13.5 (2.6) |
13.1 (2.4) |
14.0 (2.7) |
14.5 (3.1) |
13.4 (2.1) |
| RA Duration M (SD) | 9.3 (8.4) | 9.0 (7.6) | 9.6 (9.3) | 10.2 (11.0) | 8.9 (7.1) |
2.2 Procedure
The protocol was approved by the Institutional Review Boards of all involved institutions. Enrollment into the study ran from December 1998 through August 2001, and follow-up assessments were completed in April 2002. Potential participants were identified on clinic schedules, and their rheumatologist sent them a letter that briefly described the study and invited participation. One week before their scheduled appointments, patients who had not declined (by telephoning their physician's office) were telephoned by an assistant who described the study, and potentially interested patients met with the assistant at the clinic before their regular medical appointment. There, participants provided written consent, were interviewed for demographics and medical history, and completed baseline measures including questionnaires, tests of grip strength and walking time, and videotaped pain behaviors. Patients then had their standard medical appointment, and their rheumatologist provided disease activity and inflammation data. Finally, before the patients left the clinic, the assistant consulted the randomization chart and informed them verbally and in writing of their assigned method and condition. Participants were instructed to write in a journal or speak into a cassette tape recorder (which was loaned to them) for 20 minutes daily for four days, preferably consecutively, at home in a quiet private place, in the week following their baseline appointment. At home, patients first rated the credibility of their condition and then wrote or spoke on their assigned topic for 4, 20-minute sessions, on different days, over the next week. Patients rated their mood before and after each session, and rated their writing or speaking on several scales after each session. Patients returned to the clinic for follow-up evaluations 1 month (at which point they returned their writings or recorders and session rating forms), 3 months, and 6 months after the baseline visit, and at each visit, they completed the same battery of health status measures. The assessing rheumatologist was blind to experimental condition throughout the study. Participants were paid $100 for completing all phases of the project.
2.3 Randomization and Group Assignment
Before recruitment, randomization schemes were developed from a random numbers table by a person not associated with patient recruitment and assessment. Randomization was conducted separately (i.e., stratified) for both genders and for two ethnic groups (European American or other) to ensure equal representations of these groups across conditions. Patients were assigned to conditions in blocks of 8 to one of two samples (writing or speaking) in a 1:1 ratio, and to either the disclosure group or one of the two control groups in a 2:1:1 ratio. (This stratification and blocking scheme still yielded slightly different sizes of the writing or talking samples because we were not able to complete the recruitment of full blocks of 8 patients within each gender and ethnicity stratum.)
All participants were given the same general instructions, and the disclosure and both control topics were presented as possible stress management techniques. Patients were told that grammar and spelling (for the writing sample) were not important, and they should try to write or speak for the entire time allotted. The topics were as follows:
2.3.1 Disclosure group
Instructions were consistent with those used in most prior studies of emotional disclosure. Participants were asked to identify a stressful or traumatic event or experience, preferably one that remains unresolved, and to write (or speak) about this stressful experience, incorporating both facts and deepest feelings. They were encouraged to make their memories as vivid as possible, including mental images, emotions and bodily sensations. The instructions also noted that patients may experience strong emotions, but reassured them that that was both normal and potentially beneficial, and encouraged them to identify and name their feelings. Participants were also encouraged to revisit and process one stressful event over the four sessions; however, if a particular topic was no longer upsetting, they were instructed to address another stressful experience.
Control patients in each sample were randomized to one of two non-stress control conditions.
2.3.2 Positive control group
Half of the control patients were instructed to write (or speak) about positive emotional events in their lives, including both facts and feelings, and to describe their memories as vividly as possible. If their recollections were negative, participants were to look at positive aspects and avoid dwelling on negative features. This condition controlled not only for time, effort, and expectations, but also for the personal, emotional relevance of the topic.
2.3.2 Neutral control group
The other half of the control patients were instructed to write (or speak) about their daily activities over four different time intervals: Day 1, the previous week; Day 2, the previous day; Day 3, their plans for the next day; and Day 4, their plans for the next week. They were instructed to avoid discussing feelings, concerns or opinions regarding their activities. This condition controlled for time, effort, and expectations.
2.4 Measures
We included several manipulation check measures and measures of disclosure content. We also assessed a broad range of outcome measures including self-reports, behavioral, clinical, and physiological measures. Our primary outcome was self-reported pain; the other outcome measures were secondary.
2.4.1 Manipulation Check and Content Measures
Intervention credibility
After participants learned about their writing or speaking assignment and its rationale, they completed the Credibility Scale [3] to assess how much they thought their assignment was credible as a stress management technique. Items were rated from 0 to 6 and averaged; higher scores indicate greater credibility.
Immediate mood
Immediately before and after each session, participants rated how they felt “right now” on specific moods using an abbreviated version of the Positive and Negative Affect Schedule-Expanded Version [PANAS-X; 35], which has been used elsewhere [16]. Participants rated anger, fear, sadness, and guilt from 0 (not at all) to 6 (very much).
Post-session ratings
At the end of each session, patients rated their writing or speaking from 0 (not at all) to 6 (very much) regarding: a) how personal it was; b) how much they revealed their emotions; c) how meaningful it was; and d) how much they had inhibited or actively held back from talking to others about it.
Linguistic content
All writings and audiotapes were transcribed, and the transcripts were submitted to the Linguistic Inquiry and Word Count-2007 [LIWC; 26]. This software uses a number of dictionaries and calculates the percentage of all words in a transcript that are in various language categories. For this study, we recorded total word count, first person pronouns, positive emotion, negative emotion, cognitive mechanisms, insight, causality, and health-related words.
2.4.2 Outcome Measures
Affective and sensory pain
The McGill Pain Questionnaire-Short Form [MPQ-SF; 21] assessed these two dimensions of pain, as currently experienced by the patient. Each of the 11 items was rated on a 0 (none) to 3 (severe) scale, and ratings were averaged.
Self-reported physical and psychological functioning
Patients completed the Arthritis Impact Measurement Scales–2 [AIMS2; 20], which surveys the effects of arthritis on multiple domains of functioning during the previous month. Items were rated on a scale of 1 to 5 with respect to the frequency (number of days in a week) that a particular behavior or symptom was experienced, and ratings were averaged; higher scores indicated greater dysfunction. We analyzed two scales: a) physical dysfunction, which assesses dysfunction in mobility, walking/bending, hand and finger function, arm functioning, ability to perform household tasks, and self-care; and b) affective disturbance, which assesses both anxious and depressive symptoms. The AIMS2 scales have excellent internal consistency, have been widely used and validated, and are recommended by the American College of Rheumatology for clinical trials.
Pain behavior
A structured observation system [18], which was designed for RA patients, assessed overt pain behavior. At each evaluation, patients were videotaped in the examination room for 10 minutes by a camera in the doorway while they engaged in four standardized maneuvers (walking, sitting, standing, and reclining), which were presented in a random order. The research assistant operated the camera and refrained from interacting with the patient other than to give directions for the next behavior. Raters were trained to code these videotapes by the developer of the system (Francis J. Keefe) and achieved high inter-rater reliability during training. Next, these raters, blind to experimental condition, reviewed study videotapes for the presence of seven pain behaviors--guarding, bracing, grimacing, sighing, rigidity, passive rubbing, and active rubbing. The 10-minute tapes were divided into 20, 30-second epochs, the presence or absence of each pain behavior during each epoch was recorded, and a total score of all behaviors across all epochs was calculated. Previous studies have shown this system has very high inter-observer reliability (> 90%), is valid when compared to global judgments of physicians, and is sensitive to intervention-induced change [1,2].
Walking speed and grip strength
The assistant instructed patients to walk “as quickly as possible, but safely” down a 50-foot corridor, and recorded the time to do so in seconds. Higher values mean slower walking. Also, patients’ grip strength was assessed by having them squeeze as firmly as possible a sphygmomanometer bulb, and the pressure generated was recorded from two trials with each hand; all four values were averaged to a single score. Unlike all other measures in the study, higher grip strength values indicate better functioning.
Joint counts
Joint swelling reflects local inflammation and limited motion in affected areas. The patient's rheumatologist, blind to the patient's experimental condition, evaluated 16 joints bilaterally (5 interphalangeal and 5 metacarpal phalangeal joints in addition to shoulder, elbow, wrist, knee ankle and metatarsals, for a total of 32 joints), and the presence or absence of swelling was recorded for each joint.
Physician's global rating of disease activity
The evaluating physician rated patients’ overall disease activity on a 0 to 100 visual analog, with anchors of 0 = “no activity,” to 100 = “most activity.”
Erythrocyte sedimentation rate (ESR)
At the end of each examination, blood was obtained and assayed for ESR, which is a common index of inflammation. Values higher than 20 mm/hr indicate elevated inflammation and disease activity. This measure was not always requested by the physician, or the patient was not able to go to the lab; therefore, ESR was missing on approximately 20% of the patients at each visit.
2.5 Data Analyses
Sample size was determined via power analysis, estimated using a calculation developed by Borm et al. [4] for 2-group ANCOVA (disclosure vs. combined controls) in the context of randomized trials. We powered the study to detect a moderate effect size (0.50 SD), which was consistent with a prior meta-analysis [30] and which we deemed to be clinically meaningful. Calculations indicated that a sample size of approximately 90 would achieve a power of .8 using 2-tailed tests at an alpha of .05.
All analyses were conducted separately on the writing sample and the speaking sample so that conclusions could be drawn about these two methods of disclosure. Our original plan was to compare disclosure to the two control groups combined together, which is reflected in the randomization strategy of a 1:1 disclosure: control ratio. We also sought to explore secondarily whether the type of control group (emotionally positive or neutral) mattered. Therefore, our primary analyses were disclosure vs. the combined controls, but secondary analyses compared disclosure to each of the two control groups separately. The latter comparisons had less statistical power because each control group was, by design, half the size of the disclosure group.
Analyses proceeded as follows. First, t-tests or chi-squares examined the success of randomization in creating equivalent disclosure and control groups on demographics and baseline levels of the outcome measures. Next, attrition analyses compared those who dropped from the study to those who provided follow-up data. We next analyzed the manipulation checks and processes of disclosure. We compared disclosure and control groups (via t-tests) on credibility of the conditions, changes in immediate mood, post-session ratings, and linguistic content—these latter three variables were averaged over the four writing or speaking days.
The primary analyses compared disclosure and control groups on the outcome measures, for the writing and speaking samples separately. Primary analyses were conducted in two complementary ways. First, we used analysis of covariance (ANCOVA) to compare the two groups on each outcome measure at each follow-up point separately, controlling for the baseline level of the measure. For each significant effect, we present partial eta squared (pη2), which estimates the proportion of variance in the outcome related to the group factor (disclosure vs. control) while holding constant baseline scores. Values of pη2 of .01, .06, and .14 are considered to be small, medium, and large, respectively. In addition, significant group effects were followed by paired t-tests within each group, to determine how each group changed over time.
In addition to ANCOVAs, we used a second, more sophisticated approach—latent growth curve (LGC) modeling [37]—to estimate group differences in slopes or rates of change across the baseline and three follow-up periods for each outcome measure. This is a type of multilevel modeling and is well-suited for the evaluation of clinical interventions in randomized trials, where data are collected longitudinally and attrition occurs [22]. This approach involves the use of constrained structural equation models in which common variance across the repeated measures is captured in a number of growth factors. This approach complements the ANCOVAs because it examines change over time, rather than effects at each separate follow-up point. In addition, LGC modeling includes all cases, including those with only baseline data, and it estimates missing values. This produces less biased estimates than analyses treating missing data with deletion methods [28], and it accomplishes intent-to-treat analyses by analyzing all randomized patients. Also, both mean-structure change (continuity) and variance-covariance structure (stability) are tested simultaneously, and LGC modeling explicitly incorporates time into the model, whereas traditional analysis models have strong limitations in the measurement of change [29]. Finally, LGC approaches model change explicitly at the individual level such that variance can be decomposed into fixed effects (i.e., aggregate level effects) and random effects (intra-individual variation in growth processes. This allows for a greater degree of accuracy in estimation of the impact of clinical interventions. We tested growth models using Mplus version 5.01 [23]. Because the data were determined to be missing at random, we used the EM maximum likelihood estimation procedure in Mplus to estimate the growth curves utilizing all randomized cases [28].
3. Results
3.1 Baseline Comparisons of Randomized Groups
Table 1 presents the demographic data for each experimental condition within each sample. To determine the success of randomization, we compared disclosure and control groups on demographics (age, sex, ethnicity [European American vs. minority—African American and Hispanic combined], duration of RA, and education) and baseline levels of the health status measures. Among the 88 patients in the writing sample, the 43 disclosure patients did not differ from the 45 combined controls (all p > .28) on demographics and all but one of the baseline health measures; disclosure writers had higher baseline ESR (M = 48.86, SD = 22.10) than combined controls (M = 34.37, SD = 22.79), (p = .007), and the neutral controls in particular (M = 30.44, SD = 21.04), p = .005. Otherwise, disclosure writers did not differ from either positive or neutral controls.
Among the 93 patients in the speaking sample, the 48 disclosure patients did not differ from the 45 combined controls on any demographic or baseline health variable (all p > .12). Similarly, the disclosure speakers did not differ from the positive control speakers on any health variable, although disclosure speakers were slightly less educated (M = 13.14 years, SD = 2.40) than positive controls (M = 14.50, SD = 3.14), p = .04. Disclosure speakers were not different from neutral control speaker on any baseline measure, except disclosure speakers had lower physician's global rating of disease activity (M = 27.61, SD = 17.16) than neutral controls (M = 38.56, SD = 19.85), p = .02.
3.2 Patient Flow, Attrition, and Adherence
As shown in Figure 1, drop-outs occurred almost exclusively immediately after the randomization. Because patients learned fully about the study, consented, did the baseline assessment, and were randomized during one session, some patients simply waited until they got home before informing us that they did not want to participate. In the writing sample, 14 of the 88 patients (15.9%) dropped from the study, and drop-out did not differ between disclosure (14%) and combined controls (17.8%), positive controls (25%), or neutral controls (9.5%). However, dropping from the writing sample was associated with being male, χ2 = 4.88, p = .03 (35.7% of men vs. 12.2% of women dropped) or ethnic minority, χ2 = 6.35, p = .01 (26.2% of minorities vs. 6.5% of European Americans dropped), and marginally associated with having RA for fewer years (7.9 vs. 14.3 years), t(85) = 1.96, p = .054. Other demographics (age, education) and most health status measures were unrelated to attrition (all p > .18); however, those who dropped had higher affective pain at baseline (M = 1.0, SD = 1.06) than those who remained in the study (M = 0.40, SD = 0.57), t(84) = 2.92, p = .004.
In the speaking sample, 16 of the 93 patients (17.2%) dropped, and drop-out did not differ significantly between disclosure (22.9%) and combined control groups (11.1%,), positive controls (16.9%), or neutral controls (4.8%). Drop-out from the speaking sample was marginally related to being male, χ2 = 3.23, p = .07 (33.3% of men vs. 14.1% of women), but was unrelated to all other demographic and baseline measures, including ethnicity (17.9% of minorities, and 16.7% of European Americans).
Patients who remained in the study returned their writings or audiotapes, which were reviewed for adherence and content. Of the 74 patients retained in the writing sample, all but two completed all four days of writing; one of the 37 disclosure writers completed two days, and one of the 37 control writers (a neutral control patient) completed three days. Of the 77 patients retained in the speaking sample, 37 had been assigned to disclosure, and of them, 32 did all four days, two patients did three days, and one patient each completed two, one, and zero days. Of the 40 control speakers, 38 did all four days, one (a positive control) did three days, and one (a positive control) did two days. Thus, adherence to the intervention sessions was quite high among both writing groups and the speaking groups.
3.3 Credibility
For the writing sample, disclosure (M = 3.64, SD = 1.49) did not differ in credibility from combined controls (M = 3.59, SD = 1.87, p = .89), positive control (M = 3.99, SD = 1.53, p = .43), or neutral control (M = 3.21, SD = 2.12, p = .39). Similarly, for the speaking sample, disclosure (M = 3.32, SD = 1.52) did not differ in credibility from the combined controls (M = 3.83, SD = 1.56, p = .22); however, the spoken disclosure condition was viewed as less credible than the positive control (M = 4.30, SD = 1.46, p = .02), and very similar to the neutral control (M = 3.33, SD = 1.55, p = .98). Thus, for both samples, the control condition(s) were viewed at least as, if not more credible than, the disclosure condition as a stress management technique. It is notable, however, that all conditions received only moderate credibility ratings (i.e., the mid-point of the 1 to 7 scale).
3.4 Mood and Post-Session Ratings
Table 2 presents the mean change in each of the four moods (post-session minus pre-session) as well as the post-session ratings, averaged across all days for each patient, for both the writing sample (top half of the table) and speaking sample (bottom half). In the writing sample, compared to combined controls, disclosure led to significantly larger increases in anger, sadness, and guilt, but not fear, during the sessions. These effects were due largely to differences between the disclosure and positive control groups, but not the neutral control group. Post-session ratings showed that disclosure patients rated their writing as significantly more personal, revealing, and meaningful, than did the combined controls, but all of these differences were due solely to comparisons between disclosure and the neutral controls; as intended, positive controls were similar to the disclosure condition on these measures. Also, disclosure writers reported higher levels of inhibition than both positive and neutral controls, supporting the expectation that disclosure dealt with relatively inhibited topics.
Table 2.
Mood Change and Post-session Ratings for the Disclosure and Control Groups (Combined, Positive, and Neutral) for the Writing Sample (Top) and Speaking Sample (Bottom)
| Sample / Variable | Disclosure M (SD) | Combined Control M (SD) | Disclosure v. Combined Control p-value | Positive Control M (SD) | Disclosure v. Positive Control p-value | Neutral Control M (SD) | Disclosure v. Neutral Control p-value |
|---|---|---|---|---|---|---|---|
| Writing Sample | |||||||
| Anger change | 0.40 (0.99) | -0.19 (0.93) | .009 | -0.36 (1.00) | .009 | -0.02 (0.84) | .12 |
| Fear change | 0.08 (1.05) | -0.06 (0.47) | .45 | -0.18 (0.53) | .31 | 0.06 (0.38) | .94 |
| Sadness change | 0.47 (1.12) | -0.08 (0.60) | .01 | -0.14 (0.60) | .03 | 0.00 (0.61) | .10 |
| Guilt change | 0.14 (1.00) | -0.28 (0.74) | .04 | -0.45 (0.76) | .03 | -0.11 (0.69) | .34 |
| Personal nature | 5.08 (0.90) | 4.17 (1.92) | .01 | 4.94 (1.30) | .63 | 3.40 (2.14) | <.001 |
| Revealing | 4.88 (0.88) | 3.83 (1.86) | .003 | 4.70 (1.25) | .54 | 3.00 (2.00) | <.001 |
| Meaningful | 4.79 (1.05) | 3.84 (1.87) | .008 | 4.98 (0.94) | .52 | 2.75 (1.90) | <.001 |
| Inhibited | 3.02 (1.57) | 1.92 (1.62) | .004 | 1.91 (1.68) | .02 | 1.93 (1.61) | .02 |
| Speaking Sample | |||||||
| Anger change | 0.16 (1.16) | -0.10 (1.15) | .34 | -0.07 (1.60) | .55 | -0.13 (0.48) | .31 |
| Fear change | 0.04 (0.93) | -0.05 (0.93) | .66 | -0.04 (1.00) | .77 | -0.07 (0.88) | .67 |
| Sadness change | 0.36 (1.12) | -0.18 (1.07) | .03 | -0.09 (1.46) | .19 | -0.26 (0.44) | .02 |
| Guilt change | -0.05 (1.08) | -0.15 (0.93) | .66 | -0.18 (1.20) | .69 | -0.13 (0.56) | .78 |
| Personal nature | 4.63 (1.31) | 4.22 (1.05) | .13 | 4.70 (0.84) | .83 | 3.78 (1.04) | .01 |
| Revealing | 4.64 (1.19) | 3.82 (1.10) | .02 | 4.20 (0.63) | .39 | 3.48 (1.32) | .005 |
| Meaningful | 4.21 (1.35) | 3.94 (1.27) | .36 | 4.65 (0.96) | .22 | 3.30 (1.18) | .01 |
| Inhibited | 2.71 (1.55) | 1.82 (1.88) | .03 | 1.54 (2.12) | .02 | 2.08 (1.65) | .15 |
In the speaking sample, disclosure led to more sadness than the combined controls (and the neutral control), but disclosure did not differ from the combined controls (or positive or neutral controls) on the other three moods. Disclosure led to significantly higher ratings of revealing and previous inhibition than combined controls, to greater inhibition than positive controls, and to greater personal nature, revealing, and meaningfulness than neutral controls.
3.5 Content Analyses
Table 3 presents the various linguistic indices provided by LIWC for each condition. For the writing sample, compared with the combined controls, the disclosure group generated a significantly greater proportion of words that were categorized as first person, negative emotion, cognitive mechanism (including insight and causal thinking), and health-related; and these differences were found in comparison to both positive and neutral control conditions. As expected, disclosure writing led to less positive emotion word use than the positive control group. Disclosure did not differ from the positive control in the total number of words written, but the disclosure group wrote marginally more words than the neutral control group. For the speaking sample, the findings were generally similar to the writing sample, although first person words did not differ between disclosure and control conditions.
Table 3.
Linguistic Content for the Disclosure and Control Groups (Combined, Positive, and Neutral) for the Writing Sample (Top) and Speaking Sample (Bottom)
| Sample / Variable | Disclosure M (SD) | Combined Control M (SD) | Disclosure v. Combined Control p-value | Positive Control M (SD) | Disclosure v. Positive Control p-value | Neutral Control M (SD) | Disclosure v. Neutral Control p-value |
|---|---|---|---|---|---|---|---|
| Writing Sample | |||||||
| Word count | 377 (342) | 265 (133) | .06 | 305 (146) | .39 | 225 (108) | .07 |
| I / first person | 9.38 (2.89) | 6.82 (3.15) | <.001 | 7.21 (2.87) | .009 | 6.43 (3.43) | .001 |
| Positive emotion | 2.54 (0.92) | 3.51 (1.82) | .004 | 4.93 (1.28) | <.001 | 2.09 (0.94) | .09 |
| Negative emotion | 3.16 (1.23) | 0.87 (0.91) | <.001 | 1.07 (0.91) | <.001 | 0.66 (0.88) | <.001 |
| Cognitive mechanisms | 18.15 (2.56) | 15.22 (3.56) | <.001 | 16.18 (2.56) | .008 | 14.26 (4.19) | <.001 |
| Insight | 2.63 (0.89) | 1.43 (1.17) | <.001 | 1.94 (0.72) | .005 | 0.91 (1.30) | <.001 |
| Causal | 1.45 (0.50) | 1.18 (0.60) | .03 | 1.41 (0.57) | .76 | 0.95 (0.55) | .001 |
| Health | 2.20 (1.46) | 1.15 (0.99) | <.001 | 0.86 (0.62) | <.001 | 1.43 (1.21) | .05 |
| Speaking Sample | |||||||
| Word count | 1620 (930) | 1278 (800) | .09 | 1524 (925) | .72 | 1045 (593) | .02 |
| I / first person | 8.66 (2.23) | 8.59 (2.21) | .88 | 8.39 (2.28) | .67 | 8.78 (2.18) | .85 |
| Positive emotion | 2.34 (0.70) | 3.22 (1.18) | <.001 | 3.92 (1.05) | <.001 | 2.57 (0.89) | .30 |
| Negative emotion | 2.24 (0.85) | 0.98 (0.54) | <.001 | 1.05 (0.43) | <.001 | 0.91 (0.63) | <.001 |
| Cognitive mechanisms | 20.02 (1.99) | 18.08 (2.28) | <.001 | 18.55 (2.38) | .02 | 17.63 (2.14) | <.001 |
| Insight | 2.63 (0.80) | 1.60 (0.73) | <.001 | 1.97 (0.76) | .004 | 1.24 (0.50) | <.001 |
| Causal | 1.58 (0.41) | 1.21 (0.43) | <.001 | 1.27 (0.42) | .01 | 1.15 (0.44) | <.001 |
| Health | 1.37 (0.64) | 0.91 (0.70) | .005 | 0.84 (0.59) | .004 | 0.99 (0.80) | .06 |
A doctoral student read all of the stress disclosure transcripts (both writing and speaking) and categorized the stressful topic(s) disclosed for each day. For both samples, a substantial portion of the daily transcripts focused on the patient's own health problems, typically RA (writing: 34%, speaking: 41%), followed by relationship conflicts, such as marital difficulties, divorce, or conflicts with friends (writing: 29%, speaking: 23%), and the death or illness of loved ones (writing: 24%, speaking: 20.5%). Other disclosed topics were much less common: experiences often linked with shame, such as having an affair, having an abortion, being arrested or jailed, bankruptcy, personal drug or alcohol problems (writing: 6%, speaking: 7%); childhood physical or sexual abuse (writing: 3%, speaking: 0%), family of origin problems such as parental divorce or drug use (writing: 1%, speaking: 0%), adult sexual or physical victimization (writing: 1%, speaking: 0%), and random stressors such as natural disasters, fires, accidents, being robbed, or witnessing violence (writing: 1%, speaking: 3%).
3.6 Primary Outcome Analyses for the Writing Sample: ANCOVAs
Table 4 presents the primary outcome data for the writing sample, comparing the disclosure to both the combined controls as well as the positive and neutral controls, on all outcome measures at all four time-points (baseline and three follow-up points). The p-values for each follow-up time point are from ANCOVAs, comparing the disclosure group with each control group individually, covarying the baseline. (Note that baseline values in Tables 3 and 4 are from only those participants who were retained in the study; that is, who provided any follow-up data.)
Table 4.
Writing Sample: Baseline and Follow-up Values of Outcome Measures for the Disclosure and Control Groups (Combined, Positive, Neutral), and Results of Analysis of Covariance at Each Follow-up Point
| Variable / Time Point | Disclosure M (SD) | Combined Control M (SD) | Disclosure v. Combined Control p-value | Positive Control M (SD) | Disclosure v. Positive Control p-value | Neutral Control M (SD) | Disclosure v. Neutral Control p-value |
|---|---|---|---|---|---|---|---|
| Sensory Pain | |||||||
| Baseline | 0.75 (0.53) | 0.83 (0.68) | 0.91 (0.68) | 0.75 (0.69) | |||
| 1-month | 0.56 (0.57) | 0.89 (0.72) | .04 | 0.90 (0.85) | .21 | 0.88 (0.61) | .045 |
| 3-month | 0.71 (0.59) | 0.68 (0.63) | .64 | 0.80 (0.74) | .89 | 0.56 (0.50) | .36 |
| 6-month | 0.59 (0.52) | 0.83 (0.76) | .17 | 0.99 (0.84) | .09 | 0.68 (0.65) | .59 |
| Affective Pain | |||||||
| Baseline | 0.41 (0.52) | 0.39 (0.63) | 0.39 (0.69) | 0.39 (0.59) | |||
| 1-month | 0.40 (0.59) | 0.55 (0.62) | .23 | 0.53 (0.68) | .43 | 0.57 (0.58) | .28 |
| 3-month | 0.31 (0.43) | 0.41 (0.57) | .35 | 0.52 (0.76) | .16 | 0.30 (0.28) | .96 |
| 6-month | 0.24 (0.35) | 0.51 (0.66) | .02 | 0.60 (0.75) | .02 | 0.43 (0.56) | .09 |
| Physical dysfunction | |||||||
| Baseline | 2.14 (0.61) | 2.33 (0.74) | 2.35 (0.78) | 2.32 (0.73) | |||
| 1-month | 2.12 (0.65) | 2.06 (0.77) | .09 | 2.05 (0.69) | .14 | 2.07 (0.85) | .15 |
| 3-month | 2.03 (0.65) | 2.13 (0.77) | .81 | 2.20 (0.89) | .99 | 2.07 (0.65) | .74 |
| 6-month | 1.94 (0.61) | 2.22 (0.81) | .16 | 2.25 (0.77) | .22 | 2.19 (0.87) | .26 |
| Affective dysfunction | |||||||
| Baseline | 2.46 (0.90) | 2.41 (0.68) | 2.44 (0.67) | 2.37 (0.71) | |||
| 1-month | 2.36 (0.81) | 2.18 (0.66) | .37 | 2.27 (0.70) | .72 | 2.12 (0.65) | .29 |
| 3-month | 2.43 (0.88) | 2.28 (0.69) | .42 | 2.44 (0.72) | .90 | 2.13 (0.65) | .16 |
| 6-month | 2.39 (0.86) | 2.35 (0.66) | .95 | 2.44 (0.73) | .70 | 2.26 (0.59) | .62 |
| Pain Behavior | |||||||
| Baseline | 14.68 (9.30) | 13.40 (8.96) | 12.60 (9.43) | 14.16 (8.68) | |||
| 1-month | 13.17 (8.86) | 12.00 (8.27) | .81 | 11.52 (8.12) | .72 | 12.43 (8.62) | .92 |
| 3-month | 10.55 (7.28) | 12.02 (6.66) | .17 | 10.60 (7.28) | .62 | 13.21 (6.03) | .12 |
| 6-month | 14.83 (10.11) | 14.53 (7.48) | .90 | 15.50 (8.09) | .57 | 13.61 (6.95) | .75 |
| Grip strength | |||||||
| Baseline | 219.11 (119.61) | 223.14 (123.76) | 238.79 (111.54) | 208.32 (135.67) | |||
| 1-month | 226.93 (120.78) | 232.24 (123.27) | .73 | 243.06 (111.84) | .88 | 223.13 (134.49) | .72 |
| 3-month | 245.56 (117.66) | 225.10 (129.80) | .07 | 223.68 (123.77) | .02 | 226.45 (138.66) | .45 |
| 6-month | 232.04 (117.54) | 223.11 (113.19) | .44 | 235.83 (109.64) | .53 | 211.05 (118.14) | .47 |
| Walking time | |||||||
| Baseline | 15.92 (7.80) | 15.88 (5.54) | 15.40 (6.40) | 16.33 (4.72) | |||
| 1-month | 15.24 (6.78) | 15.16 (5.28) | .96 | 15.10 (6.46) | .93 | 15.23 (4.08) | .99 |
| 3-month | 14.98 (6.08) | 15.94 (6.96) | .37 | 16.27 (8.77) | .35 | 15.56 (4.13) | .57 |
| 6-month | 13.70 (3.44) | 16.50 (5.07) | .14 | 15.26 (3.60) | .88 | 17.43 (5.89) | .05 |
| Swollen joint count | |||||||
| Baseline | 4.47 (5.17) | 4.92 (4.90) | 4.39 (4.53) | 5.42 (5.29) | |||
| 1-month | 3.00 (4.08) | 4.21 (5.07) | .32 | 2.47 (2.95) | .59 | 5.58 (5.98) | .08 |
| 3-month | 2.89 (3.95) | 3.64 (4.78) | .54 | 2.28 (3.97) | .55 | 5.00 (5.22) | .13 |
| 6-month | 3.61 (4.22) | 3.92 (3.86) | .81 | 4.39 (3.97) | .49 | 3.47 (3.81) | .80 |
| Physician global rating | |||||||
| Baseline | 30.10 (19.30) | 29.77 (18.25) | 25.93 (15.23) | 33.41 (20.45) | |||
| 1-month | 24.60 (21.60) | 24.52 (20.75) | .98 | 18.67 (15.02) | .55 | 29.39 (23.86) | .70 |
| 3-month | 19.44 (14.91) | 22.09 (18.51) | .42 | 19.56 (18.92) | .76 | 24.76 (18.25) | .31 |
| 6-month | 23.69 (21.58) | 25.46 (17.69) | .70 | 30.65 (18.21) | .20 | 20.56 (16.25) | .52 |
| Erythrocyte sed rate | |||||||
| Baseline | 46.06 (20.56) | 35.15 (23.73) | 39.75 (25.66) | 30.82 (21.63) | |||
| 1-month | 49.03 (22.79) | 37.48 (29.40) | .56 | 41.23 (31.26) | .66 | 34.44 (28.46) | .96 |
| 3-month | 40.00 (21.72) | 31.65 (27.38) | .96 | 38.14 (30.61) | .57 | 26.29 (24.03) | .41 |
| 6-month | 46.37 (18.19) | 30.50 (23.57) | .27 | 40.09 (29.73) | .66 | 23.47 (15.33) | .008 |
Note: Baseline data are from only those participants who provided follow-up data (n = 74 participants in the writing sample).
In the writing sample, disclosure led to significantly lower sensory pain at 1-month follow-up (pη2 = .06), and less affective pain at 6-month follow-up (pη2 = .08), than did the combined controls. For both variables, the disclosure group showed reductions in pain, in comparison to no change or slight increases in the combined controls. There were no significant effects of disclosure vs. combined control writing on any of the other health outcomes at any follow-up point.
Compared to the positive controls, there were two significant effects, both favoring disclosure. Disclosure writing led to less affective pain at 6-month follow-up (pη2 = .11), and greater grip strength at 3-month follow-up (pη2 = .11). Compared to the neutral control condition, there were three significant effects. Disclosure writing led to less sensory pain at 1-month follow-up (pη2 = .08) and faster walking speed at 6-month follow-up (pη2 = .09). There was one unexpected finding, however; the neutral control group had better erythrocyte sedimentation rate than the disclosure group at 6-month follow-up only (pη2 = .18).
3.7 Primary Outcome Analyses for the Speaking Sample: ANCOVAs
Table 5 presents the primary outcome data for the speaking sample. Compared to the combined controls, disclosure speaking led to significantly less sensory pain (pη2 = .07) at 6-month follow-up, which was due to both improvement in the disclosure group and worsening in combined controls. Disclosure also led to significantly less affective pain (pη2 = .06) at 6-month follow-up than combined control, which was due solely to worsening in the control condition. In addition to these effects on pain, disclosure speaking led to a faster walking time (pη2 = .06) at 3-month follow-up, due to improvement in the disclosure group; fewer swollen joints (pη2 = .05) and an improved physician's global rating (pη2 = .09) at 6-month follow-up, due in both cases to much greater improvement in the disclosure than the combined control group.
Table 5.
Speaking Sample: Baseline and Follow-up Values of Outcome Measures for the Disclosure and Control Groups (Combined, Positive, Neutral), and Results of Analysis of Covariance at Each Follow-up Point
| Variable / Time Point | Disclosure M (SD) | Combined Control M (SD) | Disclosure v. Combined Control p-value | Positive Control M (SD) | Disclosure v. Positive Control p-value | Neutral Control M (SD) | Disclosure v. Neutral Control p-value |
|---|---|---|---|---|---|---|---|
| Sensory Pain | |||||||
| Baseline | 0.84 (0.75) | 0.78 (0.58) | 0.69 (0.54) | 0.88 (0.62) | |||
| 1-month | 0.70 (0.58) | 0.72 (0.50) | .73 | 0.60 (0.35) | .70 | 0.84 (0.60) | .43 |
| 3-month | 0.77 (0.64) | 0.85 (0.64) | .36 | 0.94 (0.74) | .05 | 0.77 (0.56) | .90 |
| 6-month | 0.68 (0.63) | 0.93 (0.59) | .03 | 1.01 (0.59) | .02 | 0.86 (0.59) | .23 |
| Affective Pain | |||||||
| Baseline | 0.40 (0.67) | 0.53 (0.55) | 0.49 (0.48) | 0.56 (0.62) | |||
| 1-month | 0.34 (0.53) | 0.52 (0.63) | .28 | 0.41 (0.65) | .71 | 0.62 (0.60) | .12 |
| 3-month | 0.46 (0.62) | 0.70 (0.89) | .28 | 0.76 (0.97) | .18 | 0.64 (0.84) | .52 |
| 6-month | 0.39 (0.47) | 0.72 (0.78) | .046 | 0.75 (0.85) | .06 | 0.69 (0.73) | .10 |
| Physical dysfunction | |||||||
| Baseline | 2.15 (0.75) | 2.15 (0.69) | 2.14 (0.76) | 2.15 (0.64) | |||
| 1-month | 1.98 (0.71) | 2.05 (0.67) | .80 | 2.12 (0.76) | .43 | 1.99 (0.60) | .78 |
| 3-month | 1.96 (0.77) | 2.14 (0.79) | .21 | 2.34 (0.93) | .06 | 1.96 (0.62) | .87 |
| 6-month | 2.01 (0.72) | 2.04 (0.66) | .84 | 2.16 (0.77) | .41 | 1.92 (0.62) | .59 |
| Affective dysfunction | |||||||
| Baseline | 2.42 (0.73) | 2.46 (0.76) | 2.47 (0.90) | 2.46 (0.62) | |||
| 1-month | 2.37 (0.68) | 2.23 (0.70) | .18 | 2.23 (0.80) | .26 | 2.24 (0.62) | .26 |
| 3-month | 2.45 (0.78) | 2.46 (0.79) | .86 | 2.42 (0.83) | .61 | 2.49 (0.79) | .83 |
| 6-month | 2.40 (0.76) | 2.44 (0.73) | .90 | 2.32 (0.73) | .47 | 2.57 (0.73) | .40 |
| Pain Behavior | |||||||
| Baseline | 11.90 (7.16) | 12.52 (8.57) | 14.04 (8.64) | 10.99 (8.44) | |||
| 1-month | 12.32 (8.36) | 11.56 (8.64) | .41 | 11.98 (10.06) | .42 | 11.17 (7.29) | .56 |
| 3-month | 14.22 (9.38) | 10.90 (8.10) | .11 | 10.57 (8.41) | .15 | 11.17 (8.06) | .31 |
| 6-month | 12.63 (10.63) | 12.60 (7.89) | .96 | 13.83 (8.96) | .72 | 11.43 (6.77) | .79 |
| Grip strength | |||||||
| Baseline | 261.53 (126.00) | 251.50 (119.17) | 238.64 (128.02) | 264.36 (111.41) | |||
| 1-month | 280.00 (125.59) | 276.06 (118.36) | .94 | 262.46 (139.86) | .96 | 289.66 (94.05) | .80 |
| 3-month | 283.10 (123.02) | 277.70 (126.20) | .93 | 241.37 (151.40) | .39 | 308.58 (93.22) | .32 |
| 6-month | 293.80 (124.37) | 277.57 (116.79) | .46 | 253.59 (130.85) | .22 | 300.35 (99.68) | .96 |
| Walking time | |||||||
| Baseline | 14.95 (8.22) | 15.54 (5.52) | 15.07 (5.11) | 16.01 (5.99) | |||
| 1-month | 14.16 (5.75) | 15.91 (6.41) | .32 | 14.69 (3.96) | .96 | 17.12 (8.12) | .15 |
| 3-month | 13.27 (4.79) | 15.44 (6.27) | .049 | 14.78 (4.73) | .20 | 16.03 (7.46) | .06 |
| 6-month | 13.33 (5.00) | 14.94 (5.27) | .32 | 15.65 (3.89) | .11 | 14.32 (6.31) | .93 |
| Swollen joint count | |||||||
| Baseline | 4.59 (5.91) | 5.45 (6.20) | 4.70 (6.03) | 6.20 (6.43) | |||
| 1-month | 4.33 (5.49) | 3.79 (4.99) | .39 | 4.58 (6.06) | .72 | 3.05 (3.72) | .08 |
| 3-month | 3.55 (4.24) | 3.00 (3.46) | .32 | 2.94 (3.96) | .78 | 3.05 (3.10) | .31 |
| 6-month | 1.85 (2.97) | 3.87 (4.69) | .053 | 4.39 (4.60) | .01 | 3.40 (4.83) | .27 |
| Physician global rating | |||||||
| Baseline | 27.53 (16.70) | 33.76 (19.75) | 27.78 (18.46) | 39.74 (19.60) | |||
| 1-month | 28.11 (17.82) | 27.00 (19.91) | .51 | 24.06 (18.17) | .70 | 29.63 (21.48) | .45 |
| 3-month | 26.74 (20.07) | 23.21 (19.60) | .32 | 22.13 (18.49) | .55 | 24.11 (15.33) | .36 |
| 6-month | 16.59 (11.58) | 26.89 (19.72) | .01 | 26.82 (19.34) | .03 | 26.95 (20.55) | .02 |
| Erythrocyte sed rate | |||||||
| Baseline | 34.77 (20.01) | 42.03 (27.62) | 46.88 (32.93) | 37.72 (21.97) | |||
| 1-month | 38.11 (19.69) | 39.39 (23.05) | 42.82 (27.09) | .17 | 35.75 (17.99) | .40 | |
| 3-month | 36.70 (21.67) | 37.91 (23.87) | .93 | 49.14 (25.91) | .24 | 29.63 (18.91) | .18 |
| 6-month | 35.19 (18.98) | 43.58 (27.05) | .60 | 47.69 (34.94) | .88 | 40.61 (20.18) | .48 |
Note: Baseline data are from only those participants who provided follow-up data (n = 77 participants in the speaking sample).
Compared to the positive controls, there were several significant effects, all favoring disclosure. Disclosure speaking led to less sensory pain at 3-month (pη2 = .08) and 6-month (pη2 = .11) follow-ups, and to fewer swollen joints (pη2 = .13) and improved physician's global rating (pη2 = .10) at the 6-month follow-up. Compared to the neutral control condition, there was only one effect: disclosure speaking led to improved physician's global rating at 6 months (pη2 = .10).
3.8 Primary Outcome Analyses: Latent Growth Curve Modeling
Latent growth curve analyses were then conducted for all outcome measures in order to obtain a more robust estimate of the influences of the experimental condition over time. Preliminary unconditional linear growth models were specified prior to testing the effects of disclosure versus control to confirm that a linear trajectory provided an optimal fit to the data and that there was significant variance in the slope term to warrant further conditional models. These preliminary analyses yielded adequately fitting models using the standard criteria of a non-significant model χ2, CFI > .9 and an RMSEA <.06 across all outcome variables (except for swollen joint count for comparisons with positive and neutral controls). We then estimated conditional growth models by regressing the experimental condition (disclosure vs. control) on each outcome variable. The path coefficients associated with these predictor variables assess the relationship of experimental condition with variability in the degree of change in the outcome over time.
The results are presented in Table 6, and the slope estimate and its p-value are the key indices. For the writing sample, disclosure led to significant improvements in affective pain and walking speed over time compared to combined controls. These effects stemmed from a marginally significant improvement in affective pain compared to the positive controls, and a significant improvement in walking time compared to the neutral controls, which were the only effects of disclosure when compared to the two specific control groups.
Table 6.
Results of Latent Growth Curve Modeling of Disclosure vs. Combined Control Groups, Positive Control, and Neutral Control for Patients in the Writing Sample (Top) and Speaking Sample (Bottom)
| Disclosure vs. Combined Controls | Disclosure vs. Positive Controls | Disclosure vs. Neutral Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample / Outcome Measure | Path Estimate1 | SE | t | p | Path Estimate | SE | t | p | Path Estimate | SE | t | p |
| Writing Sample | ||||||||||||
| Sensory pain | 0.04 | 0.04 | 0.84 | .40 | 0.08 | 0.05 | 1.49 | .14 | -0.01 | 0.03 | -0.17 | .86 |
| Affective pain | 0.05 | 0.02 | 2.04 | .04 | 0.05 | 0.03 | 1.78 | .09 | 0.01 | 0.01 | 0.41 | .68 |
| Physical dysfunction | 0.03 | 0.02 | 1.70 | .09 | 0.04 | 0.02 | 1.57 | .12 | 0.02 | 0.01 | 1.54 | .13 |
| Affective dysfunction | 0.01 | 0.02 | 0.33 | .74 | 0.02 | 0.03 | 0.60 | .55 | -0.00 | 0.02 | -0.07 | .94 |
| Pain behavior | 0.41 | 0.40 | 1.02 | .31 | 0.02 | 0.05 | 0.32 | .75 | -0.02 | 0.03 | -0.67 | .51 |
| Grip strength | -3.34 | 3.35 | -1.00 | .32 | -5.06 | 3.44 | -0.15 | .14 | -2.44 | 1.71 | -1.43 | .15 |
| Walking time | 0.39 | 0.20 | 1.99 | .047 | 0.11 | 0.19 | 0.57 | .57 | 0.22 | 0.10 | 2.35 | .02 |
| Swollen joint count | 0.03 | 0.07 | 0.41 | .68 | NA2 | NA | ||||||
| Physician global rating | 0.84 | 0.91 | 0.93 | .35 | 0.61 | 0.45 | 1.33 | .18 | 0.08 | 0.23 | 0.35 | .73 |
| Erythrocyte sed rate | -0.41 | 0.70 | -0.58 | .56 | 0.41 | 0.79 | 0.53 | .60 | -0.49 | 0.39 | -1.24 | .21 |
| Speaking Sample | ||||||||||||
| Sensory pain | 0.09 | 0.05 | 1.91 | .06 | 0.16 | 0.06 | 2.79 | .005 | 0.02 | 0.03 | 0.58 | .56 |
| Affective pain | 0.03 | 0.03 | 1.13 | .26 | 0.07 | 0.03 | 2.74 | .006 | 0.02 | 0.01 | 1.36 | .17 |
| Physical dysfunction | 0.01 | 0.03 | 0.35 | .73 | 0.03 | 0.02 | 1.23 | .22 | 0.01 | 0.00 | -0.49 | .62 |
| Affective dysfunction | 0.01 | 0.03 | 0.33 | .74 | 0.01 | 0.03 | 0.41 | .68 | 0.02 | 0.02 | 1.04 | .30 |
| Pain behavior | 0.01 | 0.35 | 0.02 | .98 | 0.03 | 0.05 | 0.59 | .56 | 0.02 | 0.03 | 0.68 | .49 |
| Grip strength | -3.58 | 2.76 | -1.30 | .20 | -1.94 | 3.02 | -0.64 | .52 | -0.30 | 1.63 | -0.19 | .85 |
| Walking time | 0.13 | 0.15 | 0.91 | .36 | 0.39 | 0.19 | 2.01 | .04 | 0.00 | 0.09 | 0.04 | .96 |
| Swollen joint count | 0.43 | 0.40 | 1.06 | .29 | NA | NA | ||||||
| Physician global rating | 1.44 | 0.94 | 1.54 | .12 | -0.03 | 0.48 | -0.06 | .95 | 0.05 | 0.21 | 0.21 | .84 |
| Erythrocyte sed rate | 0.68 | 0.83 | 0.82 | .41 | 0.13 | 0.99 | 0.13 | .90 | 0.62 | 0.40 | 1.54 | .12 |
Path estimates are the unstandardized coefficients of the relationship between experimental group and slope estimate variance.
Path estimates not available for swollen joint count for analyses of disclosure vs. specific control groups because the model did not converge or adequately fit.
For the speaking sample, disclosure led only to marginal improvements in sensory pain when compared to the combined controls. However, disclosure led to significantly improved sensory pain, affective pain, and walking time when compared with the positive controls. There were no differences between disclosure and the neutral controls.
4. Discussion
We found that both written and spoken methods of disclosure, conducted at home for four sessions, provided some health benefits for people with RA, compared to both emotionally positive or neutral control conditions. These effects, however, were somewhat limited in scope—for only a few outcomes—and consistency—for only certain follow-up time points.
The findings differed somewhat by the data analytic method used—ANCOVAs at each of the three follow-up time points on available patients, or latent growth curve modeling of the trajectory of change over the 6-month period using data from all randomized participants. ANCOVAs indicated that written disclosure led to lower sensory and affective pain than the combined controls, although these effects were at different time points (1-month for sensory and 6-month for affective pain). No other effects at single time points occurred, although latent growth modeling identified a benefit of written disclosure on affective pain and walking time across the follow-up period. Overall, these analyses indicate that written emotional disclosure in RA improves the pain, particularly the affective dimension, and walking time, but not a number of other measures, including self-reported physical or affective dysfunction, pain behavior, physician's assessment of disease and joint count, and inflammation.
Spoken emotional disclosure showed more effects in ANCOVAs, but fewer effects in latent growth curve modeling. Compared to combined controls, spoken disclosure led to improvements in sensory and affective pain, swollen joints, and disease activity at 6-month follow-up, and improved walking time at 3-month follow-up. Although these differences were found at specific times, there were few clear trends over the three follow-up points, and the increased variability in trajectory direction and magnitude led to a less sanguine picture for spoken disclosure when analyzed via latent growth curve modeling. These analyses found only a marginally significant effect for sensory pain. Thus, spoken emotional disclosure had a mixed picture, with apparent benefits in pain and joint and disease status at six months, but this is tempered by the fact that the trajectories over time are quite variable and inconsistent.
Although the primary analyses compared disclosure with the combined controls, the effects were clarified when disclosure was compared to both specific control conditions. Thus, although disclosure's benefits were generally limited, they occurred in comparison not only to an emotionally neutral control—consistent with prior studies—but also to an emotionally positive control, which was as personal, meaningful, and revealing as the disclosure condition. Indeed, latent growth curve modeling indicated that spoken disclosure surpassed the positive emotional controls on several variables, but did not surpass the neutral controls. Overall, these results suggest that disclosure specifically about negative or stressful experiences—rather than just emotionally relevant experiences—is important. Writing or speaking about positive experiences had a temporary mood benefit but did not induce longer-lasting, more adaptive changes, which are sometimes seen when an enhanced positive task is used, such as writing about one's “best possible self” [15].
Other analyses also support the validity of the findings. The control groups were viewed as or more credibly as the disclosure group as stress reduction techniques; thus, disclosures’ better outcomes on select variables cannot be attributed to differential expectations. In addition, compliance was quite good with both disclosure and control writing and speaking, at least among patients who decided to continue the study once they learned fully about it. Furthermore, the language used in both written and spoken disclosure and control conditions contained the elements of emotion and cognition that confirm a successful manipulation.
On the other hand, a close examination of the process and content of the disclosures sheds a different light that might clarify how patients approached these techniques and the rather limited effects that were found. First, neither type of disclosure was viewed as a very credible stress reduction technique—the sample scored only at the mid-point of the credibility scale. Second, the content of the disclosures were typically not the highly personal, secret, and unresolved traumatic experiences that this intervention was originally developed to target. Rather, up to 40% of the disclosures dealt with health problems—typically the stress of having RA. Relationship conflicts, such as divorce or marital difficulties accounted for another quarter of the disclosures, and the death or illness of loved ones accounted for a fifth or more of the disclosures. In contrast, shameful experiences including childhood abuse, family of origin problems, and adult victimization were rarely disclosed. Although such events may simply not have happened to these patients, we think it is likely that patients chose relatively safe, publicly known, and socially validated stressors to disclose. Thus, the disclosures we obtained likely led to weaker effects than might have been found with more powerful, unresolved stressors because sharing frustration about one's health is rather common , particularly in medical settings, and disclosing about the death of loved ones has been found to have no benefits [32].
The rate of non-participation was similar among patients assigned to either the writing or the speaking sample (16% to 17%), but men were more likely than women to withdraw from both samples. Yet, there were some differences in approaches and reactions to writing and speaking. Ethnic minorities (almost all were African American) were more likely than European Americans to drop from the writing sample, but not from the speaking sample. Because experience with writing is linked to higher education, it is likely that ethnic minorities, who had less formal education than the European Americans in this study, were more likely to find writing uncomfortable. Speaking, in contrast, is likely less threatening and, therefore, had comparable rates of participation. However, speaking appeared to engage patients less strongly. Negative mood did not reliably increase after spoken disclosure sessions, as it did with written disclosure, and such activation of negative emotions is thought to be an indicator of a more powerful disclosure experience. Thus, spoken disclosure was probably less powerful than written disclosure, which may have contributed to the less consistent benefits across the follow-up period.
These findings advance earlier studies of disclosure in RA. The finding that written disclosure has some health benefits—particularly after a few months, is consistent with the study of Smyth et al. [31]. In contrast, Broderick et al. [5] did not find benefits of written disclosure in RA, but that study was an effectiveness trial and sought to reflect real world practice by not having the writings submitted to the researchers, whereas submitting the writings may strengthen disclosure's effect [26]. Other studies of written disclosure in RA showed benefits on just one of several measures [6] or had results that were hard to interpret, such as worsening of controls [36], and some studies also had mixed patient samples [6] or mixed disclosure methods [36].
Regarding spoken disclosure, our results generally support those of Kelley et al. [13] who found benefits on a few outcomes, but the current study used more credible control conditions than did Kelley et al., who had control patients describe landscape pictures. Two other studies found no clinical benefits of spoken disclosure, although the sample sizes were smaller in one [12], and some benefits of immune parameters were found in the other [34].
Overall, the findings suggest modest or limited support for both written and spoken emotional disclosure among people with RA, with effects limited to a few outcomes and generally later rather than earlier time points, such as 6 months after disclosure. Nonetheless, it is noteworthy that such a simple and low-cost intervention has any benefit at all. The findings also remind us that stress and negative emotions are potentially important intervention targets for some patients with RA, and encouraging patients to approach rather than avoid such emotions can be beneficial.
Yet, the effects of written or spoken emotional disclosure are limited enough in both magnitude and breadth of outcomes that this technique likely can serve only a supplemental role, if any, in the care of patients with RA. Also, disclosure does not appear to be for everyone. Many men withdrew, and only about one-third of potential patients took part in this study—a figure that is very consistent with final participation rates in studies that recruited using similar [13] or different [5,31] methods. It may be that the majority of patients are not interested in this sort of intervention, and future research might explore ways to increase participation, such as motivational enhancement. Even among interested patients, this intervention is probably indicated for only a subset. It has been hypothesized that patients who can acknowledge unresolved stressors but who inhibit their feelings and have little social support for disclosure might benefit the most from disclosure [17], but little research has tested this hypothesis, and supplemental analyses of the current sample (not shown here) failed to identify baseline moderators. Even with appropriately targeted patients, however, emotional disclosure by itself may have limited effects unless patients are also given skills to help manage or regulate their emotions and stressful relationships. It would be valuable to test the benefits of combining disclosure with emotion regulation and interpersonal skills training.
Acknowledgments
This research was funded by a Clinical Science Award from the Arthritis Foundation, and supported by NIH grants AR049059 and AR057808. These data were presented, in part, at the annual meetings of the American Psychosomatic Society (2003) and Society of Behavioral Medicine (2007).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors has any conflict of interest with the research presented here.
References
- 1.Anderson KO, Bradley LA, McDaniel LK, Young LD, Turner RA, Agudelo CA, Gaby NS, Keefe FJ, Piski EJ, Synder RM, Semble EL. The assessment of pain in rheumatoid arthritis: Disease differentiation and temporal stability of a behavioral observation method. J Rheumatol. 1987;14:700–704. [PubMed] [Google Scholar]
- 2.Anderson KO, Bradley LA, McDaniel LK, Young LD, Turner RA, Agudelo CA, Keefe FJ, Piski EJ, Synder RM, Semble EL. The assessment of pain in rheumatoid arthritis. Validity of a behavioral observation method. Arthritis Rheum. 1987;30:36–43. doi: 10.1002/art.1780300105. [DOI] [PubMed] [Google Scholar]
- 3.Borkovec TJ, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry. 1972;3:257–260. [Google Scholar]
- 4.Borm GF, Fransen J, Lemmens AJ G. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60:1234–1238. doi: 10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Broderick JE, Stone AA, Smyth JM, Kaell AT. The feasibility and effectiveness of an expressive writing intervention for rheumatoid arthritis via home-based videotaped instructions. Ann Behav Med. 2004;27:50–59. doi: 10.1207/s15324796abm2701_7. [DOI] [PubMed] [Google Scholar]
- 6.Danoff–Burg S, Agee JD, Romanoff NR, Kremer JM, Strosberg JM. Benefit finding and expressive writing in adults with lupus or rheumatoid arthritis. Psychol Health. 2006;21:651–665. [Google Scholar]
- 7.Davis MC, Zautra AJ, Smith BW. Chronic pain, stress, and the dynamics of affective differentiation. J Pers. 2004;72:1133–1159. doi: 10.1111/j.1467-6494.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frattaroli J. Experimental disclosure and its moderators: A meta-analysis. Psychol Bull. 2006;132:823–865. doi: 10.1037/0033-2909.132.6.823. [DOI] [PubMed] [Google Scholar]
- 9.Frisina PG, Borod JC, Lepore SJ. A meta-analysis of the effect of written disclosure on the health outcomes of clinical populations. J Nerv Ment Dis. 2004;192:629–634. doi: 10.1097/01.nmd.0000138317.30764.63. [DOI] [PubMed] [Google Scholar]
- 10.Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 11.Harris AH. Does expressive writing reduce health care utilization? A meta-analysis of randomized trials. J Consult Clin Psychol. 2006;74:243–252. doi: 10.1037/0022-006X.74.2.243. [DOI] [PubMed] [Google Scholar]
- 12.Keefe FJ, Anderson T, Lumley M, Caldwell D, Stainbrook D, McKee D, Waters S, Connelly M, Affleck G, Pope MS, Weiss M, Riordan PA, Uhlin BD. A randomized controlled trial of emotional disclosure in rheumatoid arthritis: Can clinician assistance enhance the effects? Pain. 2008;137:164–172. doi: 10.1016/j.pain.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley JE, Lumley MA, Leisen JCC. Health effects of emotional disclosure in rheumatoid arthritis patients. Health Psychol. 1997;16:331–334. doi: 10.1037//0278-6133.16.4.331. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy-Moore E, Watson JC. Expressing Emotion: Myths, Realities, and Therapeutic Strategies. Guilford; New York: 1999. [Google Scholar]
- 15.King LA. The health benefits of writing about life goals. Personality & Social Psychology Bulletin. 2001;27:798–807. [Google Scholar]
- 16.Labouvie-Vief G, Lumley MA, Jain E, Heinze H. Age and gender differences in cardiac reactivity and subjective emotion responses to emotional autobiographical memories. Emotion. 2003;3:115–126. doi: 10.1037/1528-3542.3.2.115. [DOI] [PubMed] [Google Scholar]
- 17.Lumley MA, Tojek TM, Macklem DJ. The effects of written emotional disclosure among repressive and alexithymic people. In: Lepore SJ, Smyth JM, editors. The writing cure: How expressive writing promotes health and emotional well-being. American Psychological Association; Washington, DC: 2002. pp. 75–95. [Google Scholar]
- 18.McDaniel LK, Anderson KO, Bradley LA, Young LD, Turner RA, Agudelo CA, Keefe FJ. Development of an observation method for assessing pain behavior in rheumatoid arthritis patients. Pain. 1986;24:165–154. doi: 10.1016/0304-3959(86)90039-4. [DOI] [PubMed] [Google Scholar]
- 19.Meads CA, Nouwen A. Does emotional disclosure have any effects? A systematic review of the literature with meta-analyses. Int J Technol Assess Health Care. 2005;21:153–164. [PubMed] [Google Scholar]
- 20.Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2: The content and properties of a revised and expanded Arthritis Impact Measurement Scale Health Status Questionnaires. Arthritis Rheum. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 21.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 22.Murray DM, Varnell SP, Blitstein JL. Design and analysis of group-randomized trials: A review of recent methodological developments. Amer J Public Health. 2004;94:423–432. doi: 10.2105/ajph.94.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muthén B, Muthén L. Mplus version 5.1 [Computer Software] Muthén & Muthén; Los Angeles: 2007. [Google Scholar]
- 24.Quartana P, Burns JW. The painful consequences of anger suppression. Emotion. 2007;7:400–414. doi: 10.1037/1528-3542.7.2.400. [DOI] [PubMed] [Google Scholar]
- 25.Pennebaker JW, Beall SK. Confronting a traumatic event: Toward an understanding of inhibition and disease. J Abnorm Psychol. 1986;95:274–81. doi: 10.1037//0021-843x.95.3.274. [DOI] [PubMed] [Google Scholar]
- 26.Pennebaker JW, Booth RJ, Francis ME. Linguistic Inquiry and Word Count: LIWC 2007. LIWC Inc.; Austin, TX: 2007. [Google Scholar]
- 27.Radcliffe AM, Lumley MA, Stevenson J, Beltran J. Written emotional disclosure: Testing whether social disclosure matters. J Soc Clin Psychol. 2007;26:362–384. doi: 10.1521/jscp.2007.26.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 29.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press; New York, NY: 2003. [Google Scholar]
- 30.Smyth JM. Written emotional expression: Effect sizes, outcome types, and moderating variables. J Cons Clin Psychol. 1998;66:174–184. doi: 10.1037//0022-006x.66.1.174. [DOI] [PubMed] [Google Scholar]
- 31.Smyth JM, Stone AA, Hurewitz A, Kaell A. Effects of writing about stressful experiences on symptom reduction in patients with asthma or rheumatoid arthritis: A randomized trial. JAMA. 1999;281:1304–1309. doi: 10.1001/jama.281.14.1304. [DOI] [PubMed] [Google Scholar]
- 32.Stroebe M, Schut H, Stroebe W. Who benefits from disclosure? Exploration of attachment style differences in the effects of expressing emotions. Clin Psychol Rev. 2006;26:66–85. doi: 10.1016/j.cpr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Taylor GJ, Bagby RM, Parker JDA. Disorders of Affect Regulation: Alexithymia in Medical and Psychiatric Illness. Cambridge University Press; Cambridge UK: 1997. [Google Scholar]
- 34.van Middendorp H, Geenen R, Sorbi M, van Doornen LJP, Bijlsma JWJ. Health and physiological effects of an emotional disclosure intervention adapted for application at home: A randomized clinical trial in rheumatoid arthritis. Psychol Psychother. 2009;78:145–151. doi: 10.1159/000206868. [DOI] [PubMed] [Google Scholar]
- 35.Watson D, Clark LA. The PANAS-X: Manual for the positive and negative affect schedule-Expanded Form. University of Iowa; Iowa City: 1994. [Google Scholar]
- 36.Wetherell MA, Byrne-Davis L, Dieppe P, Donovan J, Brookes S, Byron M, Vedhara K, Horne R, Weinman J, Miles J. Effects of emotional disclosure on psychological and physiological outcomes in patients with rheumatoid arthritis: An exploratory home-based study. J Health Psychol. 2005;10:277–285. doi: 10.1177/1359105305049778. [DOI] [PubMed] [Google Scholar]
- 37.Willett JB. Investigating individual change and development: The multilevel model for change and the method of latent growth modeling. Res Human Develop. 2004;1:37–57. [Google Scholar]
- 38.Watson D, Clark LA. The PANAS-X. Manual for the Positive and Negative Affect Schedule-Expanded Form. The University of Iowa; Iowa City, IA: 1994. [Google Scholar]