Abstract
Background
Most studies of catheter ablation for treatment of ventricular tachycardia (VT) are relatively small observational trials.
Objectives
To define the relative risk of VT recurrence in patients undergoing catheter ablation as an adjunct to medical therapy versus medical therapy alone in a pooled analysis of controlled studies.
Methods
Randomized and non-randomized controlled trials of patients who underwent adjunctive catheter ablation of VT versus medical therapy alone were sought. MEDLINE, EMBASE, the Cochrane Central register of controlled trials (CENTRAL), and Web of Science were searched from 1965 to July 2010. Supplemental searches included Internet resources, reference lists, and reports of arrhythmia experts. Three authors independently reviewed and extracted the data regarding baseline characteristics, ablation methodology, medical therapy, complications, VT recurrences, mortality, and study quality.
Results
Five studies were included totaling 457 participants with structural heart disease. Adjunctive catheter ablation was performed in 58% of participants whereas 42% received medical therapy alone for VT. Complications of catheter ablation included death (1%), stroke (1%), cardiac perforation (1%), and complete heart block (1.6%). Using a random effects model, a statistically significant 35% reduction in the number of patients with VT recurrence was noted with adjunctive catheter ablation (P < 0.001). There was no statistically significant difference in mortality.
Conclusion
Catheter ablation as an adjunct to medical therapy reduces VT recurrences in patients with structural heart disease and has no impact on mortality.
Keywords: Ventricular Tachycardia, Catheter Ablation, Medical Therapy, Meta-Analysis, Arrhythmia
INTRODUCTION
Ventricular tachycardia (VT) is a significant contributor to morbidity and mortality among patients with structural heart disease. Several clinical trials have proved the efficacy of implantable cardioverter defibrillators (ICD) in reducing the risk of sudden death from VT.1, 2 Despite their unquestionable benefits, ICD shocks are painful, can lead to decreased quality of life,3 and appear to be associated with increased mortality.4-7 Therefore, additional therapies for treatment of VT are often necessary. Antiarrhythmic medications have limited efficacy at reducing shocks and may result in severe adverse effects.8 Catheter ablation of VT is an alternative approach that targets the critical zone(s) in VT circuits. Catheter ablation for VT in the setting of structural heart disease is currently indicated for VT storm and symptomatic sustained VT that recurs despite antiarrhythmic drug therapy.9, 10 Most studies of catheter ablation for treatment of VT, however, are relatively small uncontrolled observational trials.11-14 In order to define the magnitude of benefit associated with catheter ablation of VT, we sought to perform a meta-analysis combining all prospective randomized controlled trials, and non-randomized trials with control groups. This meta-analysis tested the null hypothesis that the number of patients with VT recurrences is equal among those with structural heart disease undergoing catheter ablation as an adjunct to medical therapy versus medical therapy alone.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was used for our meta-analysis.15 Randomized controlled trials and non-randomized controlled trials evaluating the efficacy of catheter ablation as an adjunct to medical therapy versus medical therapy alone for suppression of VT were sought.
Data Sources and Searches
A comprehensive literature search was conducted in MEDLINE, EMBASE, the Cochrane Central register of controlled trials (CENTRAL) and Web of Science, using the MeSH terms “ventricular tachycardia” and “catheter ablation”. No limits of either language or date were applied to the search. Bibliographies of the retrieved articles and systematic reviews on the topic were searched for other relevant studies. The website www.theheart.org was used to search for related presentations at any of the major scientific meetings. The search was conducted in the third week of July 2010. The July issues of all major scientific journals were reviewed, as the latest articles might not be available in the databases. Arrhythmia experts were contacted to identify additional potential studies not identified by database searches. The National Institutes of Health Clinical Trials database was also searched for any recent completed trials comparing catheter ablation to medical therapy.
Study Selection
Three reviewers (J.M., G.N., and S.N.) independently reviewed all identified publications and abstracts for inclusion using predetermined criteria. The inclusion criteria were 1) randomized controlled trials, and 2) non-randomized controlled studies, where catheter ablation was used to treat VT and a control group received medical therapy only. Given the limited number of studies, there were no exclusions based on patient inclusion criteria, mapping and ablation methodology, or type of catheter used.
Data Extraction and Quality Assessment
Three independent authors (J.M., G.N., and S.N.) assessed the potential for systematic bias in studies selected for inclusion. Each reviewer assessed every candidate study for risk of bias and methodological quality. The specific criteria to assess the risk of bias are summarized below: 1) study design, 2) inclusion criteria, 3) length of follow up, 4) definition of VT recurrences and method for primary outcome assessment, 5) proportionate and acceptable amount of loss to follow up, and 6) study funding. The answers to specific questions in each category were tabulated (Table 1). The tables from each reviewer were compared and disagreements were resolved by discussion.
Table 1.
Study Quality Assessment
| Epstein et al, 1998 21 | Schreieck et al, 2004 22 | Reddy et al, 2007 19 | Niwano et al, 2008 23 | Kuck et al, 2010 20 | |
|---|---|---|---|---|---|
| Study design | Randomized multicenter controlled trial |
Randomized controlled trial |
Randomized multicenter controlled trial |
Observational controlled trial |
Randomized multicenter controlled trial |
| Study site | United States | Germany | United States | Japan | Europe |
| Publication status | Abstract | Abstract | Manuscript | Manuscript | Manuscript |
| Inclusion criteria | Patients with structural heart disease and 1) ≥ 2 episodes of sustained VT within 2 months of enrollment; 2) hemodynamically stable spontaneous VT; and 3) failure of at least 2 anti- arrhythmic agents (extracted from parent study11) |
Patients with post myocardial infarction VT |
Patients with post myocardial infarction VT |
Patients with history of congestive heart failure and spontaneous sustained or non-sustained (> 5 beats) VT/ventricular fibrillation |
Patients aged 18-80 years with stable clinical VT, previous myocardial infarction, and left ventricular ejection fraction ≤ 50% |
| Follow-up period in months, mean (standard deviation) |
6 | 11.3 (8.9) | 22.5 (5.5) | 31 (22) | 22.5 (9) |
| Outcome assessment |
Not provided | ICD arrhythmia logs | ICD arrhythmia logs | ICD arrhythmia logs and sudden deaths in patients without ICDs |
ICD arrhythmia logs |
| Lost to follow up, No. (%) |
Not provided | Not provided | 2 (1.6) | Not provided | 1 (0.9) |
| Funding Source | Cardiac Pathways Corporation, Sunnyvale, CA (extracted from parent study11) |
Not provided | National Institutes of Health |
Not provided | St. Jude Medical Corporation, St. Paul, MN |
Data from included studies were extracted independently by two reviewers (J.M., G.N.) and confirmed in duplicate by a third reviewer (S.N.). Extracted data included baseline patient characteristics, number of patients screened and enrolled, VT mapping and ablation technique and equipment, number of patients allocated to each group, complications, number of patients with VT recurrences, electrical storm events, and deaths.
The primary outcome was the number of patients with VT recurrences. VT recurrences were most commonly defined as ICD interventions that included both appropriate ICD shocks and anti-tachycardia pacing. In studies where only VT recurrences were mentioned, we made the assumption that VT recurrences and ICD interventions for VT represent the same information. The secondary outcomes were complications, electrical storm events, and all cause mortality.
Data Synthesis and Analysis
Intention-to-treat data were used for all randomized studies. The principle summary measure was relative risk. Pooled relative risks with 95% confidence intervals were calculated using fixed effects models with Mantel-Haenszel and Inverse Variance weighting. If the fixed effects models showed statistical significance, DerSimonian Laird random effects analyses were also performed. Results obtained from random effects analysis were then compared with results of the fixed effects model to ascertain the effect of heterogeneity. I2 statistics were calculated to further assess for heterogeneity. Repeating the analysis with data only from the randomized clinical trials was used to assess sensitivity to non-randomized results. All statistical analyses were performed using STATA 10 statistical software (StataCorp, College Station, Texas). Two-sided P values less than 0.05 were considered statistically significant.
RESULTS
Search Results
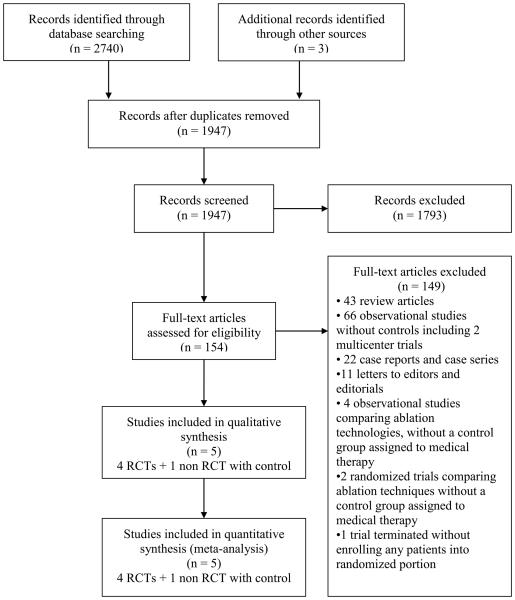
Among a total of 1944 studies that were obtained using the MeSH terms from different databases and 3 other studies selected from bibliographies of scientific meetings and discussion with arrhythmia experts, 154 potential studies were selected by review of titles (Figure 1). Out of the total 154 potential studies, 149 studies were excluded after assessment of the full text. Review articles, letters to editors, observational studies without a control group of patients, case reports, case series, and studies comparing ablation technologies without a group of patients allocated to medical therapy were excluded. Of the randomized trials, 2 were excluded because their objective was to compare electroanatomic versus standard fluoroscopic mapping and no patients were assigned to medical therapy.16, 17 Another registry and planned randomized study from Poland was excluded due to failure to enroll patients into the randomized portion of the study.18 The studies selected for final meta-analysis included 2 published randomized clinical trials,19, 20 2 randomized trials that are only available in abstract format,21, 22 and an observational study with a patient group that did not undergo ablation.23
Figure 1.
Flow diagram of literature search and study selection process.
Studies of Catheter Ablation as an Adjunct to Medical Therapy versus Medical Therapy Alone for Ventricular Tachycardia
Table 1 provides an outline of the quality assessment for each study. Four studies were randomized clinical trials.19-22 The first randomized trial, conducted by Epstein et al, was a sub-study (available in abstract format only) of the Cooled RF Multi-Center Investigators Group,11 comparing adjunctive catheter ablation to medical therapy alone.21 The other unpublished (abstract format only) randomized trial, conducted by Schreieck et al, was a single center study in Germany comparing adjunctive catheter ablation to medical therapy alone.22 The third randomized trial, conducted by Reddy et al, was a 3-center study done in the United States randomizing patients to catheter ablation as an adjunct to beta-blockers versus medical therapy with beta-blockers alone.19 The latest randomized trial, conducted by Kuck et al and published in 2010, was a 16-center trial from Europe, which randomized patients to adjunctive catheter ablation versus medical therapy alone with antiarrhythmic therapy left to investigator discretion in both arms.20 The non-randomized study, conducted by Niwano et al, was a single center observational study conducted in Japan with five study groups: 1) acute successful ablation, 2) acute failed ablation, 3) ablation not attempted, 4) ventricular fibrillation, and 5) no inducible VT/ventricular fibrillation groups. 23 For the purposes of our analysis, the acute successful and failed ablation groups (groups 1 and 2) were combined as the arm allocated to adjunctive catheter ablation and their long-term outcomes compared with the ablation not attempted group (group 3). Both groups received medical therapy with beta-blockers. Sensitivity to non-randomized results was assessed by performing the meta-analysis with and without the results of this trial. All studies enrolled patients with structural heart disease and were thus immune to bias resulting from enrollment of patients with idiopathic and focal VT. All studies reported at least 180 days of follow up. All studies reported outcome assessment through ICD arrhythmia logs with the exception of Epstein et al and Niwano et al's studies, 23 where not all participants had ICDs. Loss to follow up was minimal when reported, but unavailable in 3 studies.21-23
Data of included studies are outlined in Table 2. A total of 457 participants were enrolled across all 5 studies. Of these, 266 (58%) patients were in the intervention group and 191 (42%) in the control group. The mean age was between 60-70 years in all trials. The majority of patients in all studies were male. Three studies enrolled only patients with ischemic cardimyopathy.19, 20, 22 However, the population of Epstein et al and Niwano et al's studies included patients with both ischemic and non-ischemic cardiomyopathy.21, 23 The average ejection fraction for all studies was approximately 30-35%.
Table 2.
Data of Included Studies
| Epstein et al, 1998 21 | Schreieck et al, 2004 22 | Reddy et al, 2007 19 | Niwano et al, 2008 23 | Kuck et al, 2010 20 | |
|---|---|---|---|---|---|
| Number screened | Not provided | Not provided | Not provided | 864 | Not provided |
|
| |||||
| Number allocated to | |||||
| Ablation group | 73 | 19 | 64 | 58 (success and failure groups) |
52 |
| Control group | 32 | 20 | 64 | 20 | 55 |
|
| |||||
| Age, mean (standard deviation) |
|||||
| Ablation group | 62.5 (19.8) | 65 (9) | 67 (9) | Success: 64 (8) Failure: 66 (9) |
67.7 (8.3) |
| Control group | 66.7 (19.8) | 66 (10) | 62 (10) | 64.4 (8.2) | |
|
| |||||
| Female, % | |||||
| Ablation group | 8 | Not provided | 8 | Success: 46.5 Failure: 33.3 |
4 |
| Control group | 16 | 19 | 34.9 | 9 | |
|
| |||||
| Ischemic cardiomyopathy, % |
|||||
| Ablation group | 83 | 100 | 100 | Success: 60 Failure: 40 |
100 |
| Control group | 91 | 45 | |||
|
| |||||
| Ejection fraction, mean (standard deviation) |
|||||
| Ablation group | 31 (13) | 33 (14) | 30.7 (9.5) | Success: 37 (7) Failure: 35 (5) |
34 (9.6) |
| Control group | 29 (12) | 32.9 (8.5) | 35 (8) | 34.1 (8.8) | |
|
| |||||
| ICD, % | |||||
| Ablation group | 70 | 100 | 100 | Not Provided | 100 |
| Control group | 75 | ||||
|
| |||||
| Mapping technique | Not provided | Electroanatomic voltage (Carto, n=11) or non- contact mapping (Ensite, n=8) and exit sites determined by pace- mapping |
Electroanatomic voltage mapping (Carto) and pace-mapping of exit sites |
Electroanatomic voltage mapping (system not specified) and pace- mapping of exit sites |
Electroanatomic voltage (Carto, n=32), non-contact (Ensite, n=11), or conventional mapping (n=2) |
|
| |||||
| Ablation catheter | Internally cooled radiofrequency |
Cooled or large tip radiofrequency |
Standard 4 mm tip or 3.5 mm irrigated radiofrequency |
Not provided | Not provided |
|
| |||||
| Medical therapy | |||||
| Ablation Group | Not provided | Not provided | Beta-blocker (94%) | Beta-blocker (78%) | Beta-blocker (75%) and Amiodarone (35%) |
| Control Group | Beta-blocker (% Not provided) |
Beta-blocker (98%) | Beta-blocker (70%) | Beta-blocker (75%) and Amiodarone (35%) |
|
|
| |||||
| Complications | |||||
| Ablation group | 7 (2 deaths, 1 stroke, 1 perforation, and 3 third degree atrioventricular block) |
Not provided | (1 pericardial effusion, 1 congestive heart failure exacerbation, 1 deep vein thrombosis |
Not provided | 6 (2 from ablation: 1 transient ST elevation, 1 transient ischemic attack; 4 from ICD: 2 lead dislodgement, 2 generator defects) 9 (all from ICD: 2 lead dislodgement, 3 generator defects, 2 dislodged ICD generators, 1 T-wave over-sensing, 1 lead insulation damage, 1 twiddler's syndrome, 1 system infection) |
| Control group | Not provided | Not provided | |||
|
| |||||
| Number of patients with VT recurrence (%) |
|||||
| Ablation group | 36 (49%) | 9 (47%) | 8 (12%) | 14 (24%) | 26 (50%) |
| Control group | 24 (75%) | 12 (60%) | 21 (33%) | 10 (50%) | 38 (69%) |
|
| |||||
| Number of electrical storm events (%) |
|||||
| Ablation group | Not provided | Not provided | 4 (6%) | Not provided | 13 (25%) |
| Control group | 12 (19%) | 17 (31%) | |||
|
| |||||
| Number of Deaths (%) | |||||
| Ablation group | Not provided | Not provided | 6 (9%) | 9 (16%) | 5 (10%) |
| Control group | 11 (17%) | 4 (20%) | 4 (7%) | ||
Carto – Electroanatomic mapping system, Biosense Webster, Diamond Bar, CA; Ensite – Non-contact mapping system, St Jude Medical, St Paul, MN
Complications of Catheter Ablation for Ventricular Tachycardia
Data regarding complications related to catheter ablation was available from three studies and has been detailed in Table 2.19-21 Pooling data from all three studies resulted in a 6.3% complication rate as a result of catheter ablation for VT. Major complications included death (1%), stroke/transient ischemic attack (1%), cardiac perforation (1%), and third degree atrioventricular block (1.6%).
Ventricular Tachycardia Recurrences
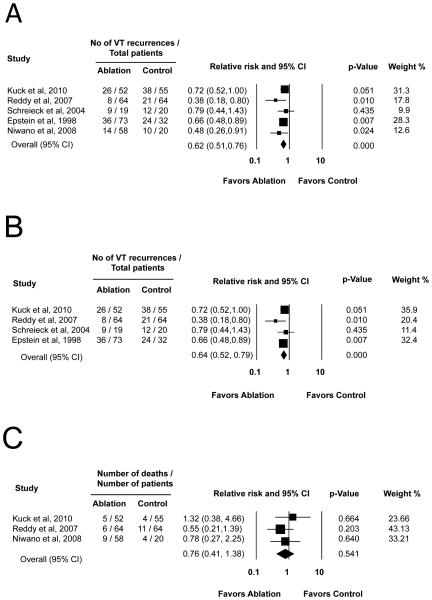
During a follow-up period ranging from 6 to 22.5 months (Table 1), 93 of 266 (35%) patients with catheter ablation experienced VT recurrence compared to 105 of 191 (55%) patients in the control group. The pooled relative risk using a Mantel-Haenszel fixed effects model revealed a statistically significant 38% reduction in the number of patients with VT recurrence associated with adjunctive catheter ablation (relative risk 0.62; 95% confidence interval: 0.51 – 0.76, p < 0.001). The estimated relative risk was similar when using Inverse Variance fixed and DerSimonian Laird random effects models (relative risk for both models 0.65; 95% confidence interval: 0.54 – 0.79, P < 0.001). Figure 2A shows the pooled relative risk of VT recurrence after adjunctive catheter ablation compared with medical therapy alone with all 5 studies included. The results were similar in magnitude and direction, when the analysis was repeated with only randomized controlled trials excluding the single non-randomized trial (Mantel-Haenszel pooled relative risk 0.64, 95% confidence interval: 0.52 – 0.79, p < 0.001; Inverse-Variance pooled relative risk 0.67, 95% confidence interval: 0.55 -0.82, p<0.001; and DerSimonian Laird random effects pooled relative risk 0.67, 95% confidence interval: 0.55 -0.82, p<0.001; Figure 2B).
Figure 2.
A) Forest plot of relative risk of VT recurrences after catheter ablation versus medical therapy, all studies included. B) Forest plot of relative risk of VT recurrences after catheter ablation versus medical therapy, randomized controlled trials only. C) Forest plot of relative risk of mortality after catheter ablation versus medical therapy. Relative risks in this figure were calculated using the fixed effects Mantel-Haenszel method.
Electrical Storm Events
The incidence of electrical storm was reported in 2 studies.19, 20 Electrical storm occurred in 17 of 116 (15%) patients assigned to adjunctive catheter ablation and 29 of 119 (24%) patients assigned to medical therapy. The pooled relative risk using a fixed effects model revealed a trend toward reduction of electrical storm among patients allocated to adjunctive catheter ablation (Mantel-Haenszel pooled relative risk 0.61, 95% confidence interval: 0.36 – 1.03, p = 0.066; Inverse-Variance pooled relative risk 0.65, 95% confidence interval: 0.38 – 1.11, p=0.115).
Mortality
Data regarding long-term mortality after catheter ablation of VT were available from three studies.19, 20, 23 Mortality occurred in 20 of 174 (12%) patients allocated to adjunctive catheter ablation compared to 19 of 139 patients (14%) in the control group. The pooled relative risk using a fixed effects model revealed reduced mortality with catheter ablation; however, this difference was not statistically significant (Mantel-Haenszel pooled relative risk 0.76; 95% confidence interval: 0.41 – 1.38, p = 0.37; Inverse-Variance pooled relative risk 0.76; 95% confidence interval: 0.41 – 1.40, p = 0.37; Figure 2C). Causes of death included congestive heart failure (ablation 4, control 7), arrhythmic deaths (ablation 5, control 1), non-cardiac causes (ablation 7, control 5) and unknown (ablation 4, control 6).
DISCUSSION
To the best of our knowledge, this is the first meta-analysis of studies regarding catheter ablation for VT. To avoid risks associated with publication bias, we conducted a rigorous search with a detailed protocol to include both published and unpublished studies. We also assessed the sensitivity of our analysis to inclusion of non-randomized studies. The results of this meta-analysis show that among patients with structural heart disease and VT, catheter ablation as an adjunct to medical therapy reduces the number of patients with VT recurrences during follow up and has no impact on mortality.
Recurrent VT episodes may result in sudden death despite the presence of an ICD,24 and recurrent shocks can reduce quality of life 3, 25 and appear to be associated with increased mortality.4-7 Anti-arrhythmic medications have limited efficacy and can have severe adverse effects. With the availability of new technologies and strategies for ablation of hemodynamically unstable VT during sinus rhythm, catheter ablation appears to be a promising technique for prevention of VT recurrences. Based upon the results of prior studies included in this meta-analysis,19, 20, 22 some experts favor the use of “prophylactic” catheter ablation at the time of ICD implantation for secondary prevention of VT. Such an approach appears very promising and may be considered in individuals with high likelihood of VT recurrence. However, given the small number of controlled trials, limited follow-up data, and relatively high procedural complication rate (6.3%), current recommendations to reserve catheter ablation for patients experiencing multiple VT recurrences that are not responsive to anti-arrhythmic medications, appear well justified.9, 10 The results of this meta-analysis, however, underscore the utility of adjunctive catheter ablation when patients experience VT recurrences despite medical therapy.
Our meta-analysis has several limitations. First, a small number of studies were included. This reflects the paucity of controlled trials assessing the efficacy of catheter ablation for VT. There are only two published randomized controlled trials to date. Two additional randomized trials included in the current meta-analysis were only published in abstract format. Inclusion of data reported in conference abstracts has potential limitations such as lack of rigorous peer-review required for manuscript format publications. However, greater than half of trials reported in conference abstracts never reach full publication, and those that are eventually published are more likely to show a positive association between the subject under study and outcome.26 Therefore, randomized studies in abstract format have been included to reduce the risk of publication bias in this meta-analysis. Second, it is important to note that the included studies were conducted among different populations and two studies included patients with ischemic and non-ischemic cardiomyopathy. There were also differences in VT mapping strategies, ablation technologies, methodologies of collecting information regarding VT recurrences, and length of follow-up. Importantly, given the variation in the length of follow-up between studies we had to make the assumption that the relative risk for VT recurrence is stable over time. Meta-regression or stratification by study characteristics could not be performed due to the low number of available studies. However, random effects analyses did not significantly alter the results of the fixed effects models, and the between study heterogeneity was not significant with respect to VT recurrence (I2 = 0%; p = 0.42) or mortality (I2 = 0%; p = 0.54) endpoints. Therefore, although the included studies had different characteristics, the pooled relative risk estimates are likely reasonable. Finally, the studies were primarily performed in tertiary care centers; therefore, the reported efficacy and complications rates may not be generalizable to other settings and populations not included in the meta-analysis.
Data regarding the efficacy of catheter ablation for VT are limited and many questions remain unanswered. Despite finding a reduction in VT recurrences, in the one study where quality of life measures were collected, no difference was found between the ablation and control groups.20 Also, data regarding mortality after catheter ablation of VT are sparse. Since mortality was not the primary outcome of included studies,19, 20, 23 and arrhythmic death was likely prevented by ICDs in the majority of included patients, additional studies are necessary to assess this important endpoint. These unresolved issues clearly indicate the need for more sufficiently powered randomized trials and multi-center registry studies of catheter ablation for VT.
In summary, catheter ablation of VT as an adjunct to medical therapy reduces the number of patients with structural heart disease that experience VT recurrences. No statistically significant mortality benefit or detriment was associated with catheter ablation. Future randomized controlled studies to assess mortality and quality of life associated with catheter ablation of VT are warranted.
Acknowledgments
Funding: Dr. Nazarian is funded by a National Heart, Lung, and Blood Institute Career Development Award (K23HL089333).
Abbreviations
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- CENTRAL
Cochrane Central register of controlled trials
- VT
Ventricular Tachycardia
- ICD
Implantable Cardioverter Defibrillator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors report no conflicts of interest related to this article.
REFERENCES
- 1.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverterdefibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 3.Kamphuis HC, de Leeuw JR, Derksen R, Hauer RN, Winnubst JA. Implantable cardioverter defibrillator recipients: quality of life in recipients with and without ICD shock delivery: a prospective study. Europace. 2003;5(4):381–9. doi: 10.1016/s1099-5129(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 4.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351(24):2481–8. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg I, Moss AJ, Hall WJ, et al. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation. 2006;113(24):2810–7. doi: 10.1161/CIRCULATIONAHA.105.577262. [DOI] [PubMed] [Google Scholar]
- 6.Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359(10):1009–17. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweeney MO, Sherfesee L, DeGroot PJ, Wathen MS, Wilkoff BL. Differences in effects of electrical therapy type for ventricular arrhythmias on mortality in implantable cardioverter-defibrillator patients. Heart Rhythm. 7(3):353–60. doi: 10.1016/j.hrthm.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ, Dorian P, Roberts RS, et al. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. Jama. 2006;295(2):165–71. doi: 10.1001/jama.295.2.165. [DOI] [PubMed] [Google Scholar]
- 9.Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA) Heart Rhythm. 2009;6(6):886–933. doi: 10.1016/j.hrthm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Natale A, Raviele A, Al-Ahmad A, et al. Venice Chart International Consensus Document on Ventricular Tachycardia/Ventricular Fibrillation Ablation. J Cardiovasc Electrophysiol. 2010 doi: 10.1111/j.1540-8167.2009.01686.x. [DOI] [PubMed] [Google Scholar]
- 11.Calkins H, Epstein A, Packer D, et al. Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a prospective multicenter study. Cooled RF Multi Center Investigators Group. J Am Coll Cardiol. 2000;35(7):1905–14. doi: 10.1016/s0735-1097(00)00615-x. [DOI] [PubMed] [Google Scholar]
- 12.Carbucicchio C, Santamaria M, Trevisi N, et al. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation. 2008;117(4):462–9. doi: 10.1161/CIRCULATIONAHA.106.686534. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson WG, Wilber DJ, Natale A, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118(25):2773–82. doi: 10.1161/CIRCULATIONAHA.108.788604. [DOI] [PubMed] [Google Scholar]
- 14.Tanner H, Hindricks G, Volkmer M, et al. Catheter Ablation of Recurrent Scar-Related Ventricular Tachycardia Using Electroanatomical Mapping and Irrigated Ablation Technology: Results of the Prospective Multicenter Euro-VT-Study. J Cardiovasc Electrophysiol. 2010;21:47–53. doi: 10.1111/j.1540-8167.2009.01563.x. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 16.Earley MJ, Showkathali R, Alzetani M, et al. Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. Eur Heart J. 2006;27(10):1223–9. doi: 10.1093/eurheartj/ehi834. [DOI] [PubMed] [Google Scholar]
- 17.Sporton SC, Earley MJ, Nathan AW, Schilling RJ. Electroanatomic versus fluoroscopic mapping for catheter ablation procedures: a prospective randomized study. J Cardiovasc Electrophysiol. 2004;15(3):310–5. doi: 10.1111/j.1540-8167.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- 18.Szumowski L, Przybylski A, Maciag A, et al. Outcomes of a single centre registry of patients with ischaemic heart disease, qualified for an RF ablation of ventricular arrhythmia after ICD intervention. Kardiol Pol. 2009;67(2):123–7. discussion 128-9. [PubMed] [Google Scholar]
- 19.Reddy VY, Reynolds MR, Neuzil P, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357(26):2657–65. doi: 10.1056/NEJMoa065457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuck KH, Schaumann A, Eckardt L, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010;375(9708):31–40. doi: 10.1016/S0140-6736(09)61755-4. [DOI] [PubMed] [Google Scholar]
- 21.Epstein AE, Wilber DJ, Calkins H, et al. Randomized controlled trial of ventricular tachycardia treatment by cooled tip catheter ablation vs. drug therapy. J Am Coll Cardiol. 1998;31(2 suppl A):118A. [Google Scholar]
- 22.Schreieck J, Michael A, Schneider E, et al. Preventive ablation of post infarction ventricular tachycardias: Results of a prospective randomized study. Heart Rhythm May. 2004;1(1; Supplement 1):S35–S37. [Google Scholar]
- 23.Niwano S, Fukaya H, Yuge M, et al. Role of electrophysiologic study (EPS)-guided preventive therapy for the management of ventricular tachyarrhythmias in patients with heart failure. Circ J. 2008;72(2):268–73. doi: 10.1253/circj.72.268. [DOI] [PubMed] [Google Scholar]
- 24.Anderson KP. Sudden cardiac death unresponsive to implantable defibrillator therapy: an urgent target for clinicians, industry and government. J Interv Card Electrophysiol. 2005;14(2):71–8. doi: 10.1007/s10840-005-4547-9. [DOI] [PubMed] [Google Scholar]
- 25.Sears SE, Jr., Conti JB. Understanding implantable cardioverter defibrillator shocks and storms: medical and psychosocial considerations for research and clinical care. Clin Cardiol. 2003;26(3):107–11. doi: 10.1002/clc.4960260303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scherer RW, Langenberg P, von Elm E. Full publication of results initially presented in abstracts. Cochrane Database Syst Rev. 2007;(2):MR000005. doi: 10.1002/14651858.MR000005.pub3. [DOI] [PubMed] [Google Scholar]