Summary
Objective
To study temporomandibular joint (TMJ) involvement in an autoimmune murine model of rheumatoid arthritis (RA), a disease characterized by inflammatory destruction of the synovial joints. Although TMJ dysfunction is frequently found in RA, TMJ involvement in RA remains unclear, and TMJ pathology has not been studied in systemic autoimmune animal models of RA.
Methods
Proteoglycan (PG) aggrecan-induced arthritis (PGIA) was generated in genetically susceptible BALB/c mice. TMJs and joint tissues/cartilage were harvested for histological and immunohistochemical analyses and RNA isolation for quantitative polymerase chain reaction. Serum cytokine levels were measured in mice with acute or chronic arthritis, and in non-arthritic control animals.
Results
Despite the development of destructive synovitis in the limbs, little or no synovial inflammation was found in the TMJs of mice with PGIA. However, the TMJs of arthritic mice showed evidence of aggrecanase- and matrix metalloproteinase-mediated loss of glycosaminoglycan-containing aggrecan, and in the most severe cases, structural damage of cartilage. Serum levels of pro-inflammatory cytokines, including interleukin (IL)-1β, were elevated in arthritic animals. Expression of the IL-1β gene was also high in the inflamed limbs, but essentially normal in the TMJs. Local expression of genes encoding matrix-degrading enzymes (aggrecanases and stromelysin) was upregulated to a similar degree in both the limbs and the TMJs.
Conclusion
We propose that constantly elevated levels of catabolic cytokines, such as IL-1β, in the circulation (released from inflamed joints) create a pro-inflammatory milieu within the TMJ, causing local upregulation of proteolytic enzymes and subsequent loss of aggrecan from cartilage.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune joint disease affecting 1% of the human population. The disease starts with synovial inflammation in the peripheral joints, which ultimately leads to destruction of articular cartilage and complete loss of joint function1,2. Several animal models mimic one or more characteristics of the human disease. Cartilage proteoglycan (PG)-induced arthritis (PGIA) in BALB/c mice shares many similarities with RA, as indicated by genetic studies, clinical assessments, laboratory tests (presence of serum cytokines and autoantibodies, including rheumatoid factor and anti-citrullinated protein antibodies), x-rays and histopathology of diarthrodial joints3. Like RA, PGIA is a progressive joint disease, where the number of arthritic joints increases over time, and the affected joints ultimately become deformed due to loss of cartilage and erosion of bone4.
The temporomandibular joint (TMJ) is a synovial joint, in which the joint space is divided by a fibrocartilaginous disc (meniscus). TMJ dysfunction, which is relatively common in humans, is most frequently caused by meniscus displacement (internal derangement disorder) or is associated with the development of osteoarthritis (OA)-like degenerative changes of articular cartilage covering the mandibular condyle (reviewed in5,6). The estimated frequency of TMJ involvement in patients with RA ranges from 4 to 86 % depending on the patient selection and methods used for diagnostic evaluation7–9. The symptoms of TMJ dysfunction are different from those of arthritic joints, but very limited information is available about the histopathology of the TMJ in RA. One study reported complete replacement of cartilage by fibrous tissue in advanced RA10, and another described inflammatory and degenerative changes in the RA TMJ synovium obtained from arthroscopy material not containing cartilage11. The paucity of information regarding cartilage histopathology makes it uncertain whether TMJ involvement in RA is inflammatory or degenerative in nature.
Animal models have been developed to study the response of TMJ to inflammatory insults; however, in these models, the inflammation-inducing compounds, such as adjuvant or antigen, are injected directly into the TMJ12,13. TMJ involvement has not been studied in any of the systemic autoimmune animal models of RA, such as PGIA. Therefore, the goal of the present work was to identify pathologic changes in the TMJ in the context of arthritis development and progression in mice with PGIA.
Materials and methods
ANTIGEN, ANIMALS, IMMUNIZATION, AND ASSESSMENT OF PERIPHERAL ARTHRITIS
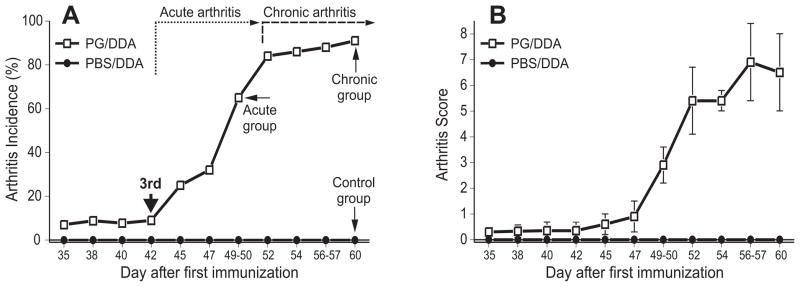
The collection of cartilage from consenting patients undergoing total joint replacements was approved by the Institutional Review Board of Rush University Medical Center (Chicago, IL). PG (aggrecan) was extracted from cartilage and purified as described4. Female BALB/c mice (12–16 weeks old) were purchased from Charles River Laboratories (Kingston Colony). Animals were immunized intraperitoneally with an emulsion of PG (100 μg of protein in 100 μl phosphate-buffered saline, PBS) and 2 mg dimethyl dioctadecyl-ammonium bromide (DDA) adjuvant (Sigma-Aldrich, St. Louis, MO) on days 0, 21, and 424. Control mice received an emulsion of PBS and DDA (without antigen) under the same schedule. All animal procedures were conducted under a protocol approved by the Institutional Animal Care and Use Committee of Rush University Medical Center. Immunized mice were examined 2–3 times a week for clinical symptoms of arthritis after the second immunization. The time of onset and incidence of arthritis were recorded, and severity was scored based upon swelling and redness of each paw on a scale ranging from 0 to 4, yielding a potential maximum severity score of 16 per mouse4,14. Mice assigned to the “acute” group (N=10) were sacrificed during the clinically determined acute phase of PGIA (see Fig. 1A, dotted-line arrow), i.e., 6–7 days after the onset of arthritis, when swelling and redness were steadily increasing in the distal joints of the limbs. The “chronic” group (N=12) was sacrificed during the chronic phase of the disease (Fig. 1A, dashed-line arrow), i.e., approximately two weeks after the onset of arthritis (day 60 after the first PG/DDA injection), when swelling was still present, but hardening of periarticular soft tissue, joint deformities and ankylosis dominated the clinical picture3,14. PBS/DDA-injected (non-arthritic) control mice (N=8) were sacrificed with the chronic group on day 60.
Fig. 1.
Incidence and severity of proteoglycan-induced arthritis (PGIA) in BALB/c mice used in this study. (A) Arthritis incidence is shown as the % of arthritic animals among all cartilage PG-immunized (PG/DDA-injected, N = 22) or adjuvant (PBS/DDA)-injected (N = 8) mice. Thick solid arrow points to the time of the 3rd PG/DDA or PBS/DDA injection. The approximate time frames of clinically determined acute and chronic phases of arthritis are indicated by dotted-line and dashed-line arrows, respectively. Thin solid arrows depict the time points at which groups of mice with acute (N = 10) and chronic (N = 12) arthritis, and non-arthritic control mice (N = 8) were sacrificed. (B) Disease severity in the same groups is expressed as a cumulative inflammation score. The data shown are the means (incidence) or the means ± 95% confidence levels (CI 95%) of disease severity scores.
MEASUREMENT OF CYTOKINES IN SERUM
Blood was collected from control (PBS/DDA-injected) and arthritic mice at the time of sacrifice. Concentrations of pro-inflammatory cytokines, including interleukin (IL)-1β, tumor necrosis factor (TNF)α, IL-6, and IL-17, in serum were measured using capture enzyme-linked immunosorbent assays (ELISA) according to the manufacturers’ instructions (BD Biosciences, San Diego, CA, or R&D Systems, Minneapolis, MN). Results were expressed as picograms (pg) of cytokine per ml serum.
HISTOLOGY, HISTOCHEMISTRY, AND IMMUNOHISTOCHEMISTRY
Hind limbs and heads were dissected from euthanized mice on day 49 or 50 (for mice with acute arthritis of peripheral joints) and from mice in the chronic arthritis and control groups on day 60. Heads were cut in half in the sagittal direction before fixation in 10% neutral buffered formalin (Protocol Formalin, Fisher Scientific, Kalamazoo, MI). Both the limbs and heads were acid-decalcified and embedded in paraffin. The heads were carefully oriented in the paraffin blocks so that the sectioning blade could run parallel to the posterior mandible and the mandibular head. Deparaffinized sagittal serial sections (6–8 μm thick) of paws and TMJs were stained with hematoxylin and eosin (Sigma) to evaluate structural integrity, or with safranin O and fast green (Acros Organics, NJ) to assess cartilage PG aggrecan loss15, using standard histochemical methods described earlier16. Sections from all experimental groups were stained simultaneously.
For immunohistochemistry (IHC), deparaffinized sections were rehydrated in 50 mM Tris-acetate buffer (pH 7.4). To unmask the protein epitopes in cartilage, the sections were digested with 0.5 units/ml chondroitinase ABC (Seikagaku Corp., Japan) dissolved in 50 mM Tris-acetate buffer for 60 min16. Non-specific protein binding sites were blocked with 10% normal goat serum in PBS and then stained with rabbit antibodies specific to cartilage PG (aggrecan) neoepitopes. These antibodies were raised against the neoepitopes -VDIPEN341 and -NVTEGE373 (NITEGE in human) as described16. Neoepitope -VIDIPEN is generated mostly by stromelysin (matrix metalloproteinase [MMP]-3) by cleaving the interglobular domain (IGD) of the core protein of cartilage PG aggrecan. NITEGE/NVTEGE is another cleavage product of the IGD by aggrecanase-1 (also known as a disintegrin and metalloproteinase with thrombospondin type 1 motif-4, ADAMTS-4), or aggrecanase-2 (ADAMTS-5)17,18. After the cleavage of the IGD of aggrecan by these enzymes, the neoepitopes (bound to hyaluronan via the G1 domain) become available for the respective antibodies16,17. The core protein released by the enzymes (the rest of the PG molecule), with negatively charged glycosaminoglycan (GAG) side chains, diffuses out of the cartilage. As a result, the negatively charged GAGs, stained red with safranin O15, are lost. The rabbit antibodies were used at a 1:40 dilution, and the binding of the primary antibodies was detected with peroxidase-conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA). Digestion with chondroitinase ABC was sufficient for these antibodies to access the epitopes in the paraffin sections, and no antigen retrieval was necessary. Peroxidase reactions were developed with diaminobenzidine (DAB) chromogen and H2O2 substrate (Vector Laboratories, Burlingame, CA) as described16. All slides were exposed to DAB for the same length of time, and the specificity of immunostaining was ensured by replacement of the first antibodies with normal rabbit serum in one set of slides. All tissue sections were viewed under a Nikon Microphot-FXA (Nikon, Melville, NY) bright-field microscope. TIF images (in 24-bit color RGB mode) were generated using MetaView image acquisition software (Molecular Devices, Sunnyvale, CA).
MORPHOMETRIC ANALYSIS OF SAFRANIN O AND IHC STAINING
Morphometric analyses of the densities of safranin O staining and IHC (DAB staining) were performed by a blinded investigator, using a modified version of a previously published Adobe Photoshop-based protocol for quantitative image analysis19. In brief, TIF images of tissue sections were opened in Adobe Photoshop (version 6, Adobe Systems, San Jose, CA), and 3 rectangular areas (corresponding to full-thickness cartilage in the middle and 2 lateral regions of the mandibular or distal tibial cartilage) were selected from each section. Using the “magic wand” tool, a small group of pixels of positive staining (red for safranin O, and dark brown for DAB staining, respectively) was selected within each area. Using “Similar” under the Selection command, the pixels were extended to regions containing color pixels of similar intensity. The data of the image histogram (mean intensity and pixel number of the selected color) were exported to Microsoft Excel. The image was then converted to grayscale, and the gray pixel values of the histogram of the entire field (total pixels) were also exported to Excel. The percentage of pixels of positive staining, relative to the total pixels of each area, was calculated by dividing the selected color pixels by the total (gray) pixels of the entire area, and then multiplying by 100. Quantitative comparison of positively stained areas of control and arthritic specimens was carried out using the same main color intensity values for all tissue sections stained with the same reagent. Average of data from left-side and right-side joints from the same mouse was handled as a single value from a single animal.
QUANTITATIVE REAL-TIME POLYMERASE CHAIN-REACTION (QRT-PCR)
To isolate RNA, mandibular cartilage and adjacent synovial tissue (without bone) were dissected from euthanized animals (experimental groups similar to those shown in Fig. 1) under a preparative stereo microscope in RNAse-free conditions. The tissue pieces were collected in RNALater solution and homogenized in TRI Reagent (Sigma-Aldrich). Tissue samples from at least two animals (4-6 TMJs) per group (non-arthritic controls, mice with acute PGIA and mice with chronic PGIA) were pooled, and RNA was isolated from three independent pools. RNA was also isolated similarly from normal and inflamed paw homogenates, which contained cartilage and soft tissue dissected from the ankle and mid-foot area, but not bone. The quality of RNA samples was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies, Foster City, CA). High quality samples were used in reverse transcription reactions. cDNAs were synthesized using an oligo-dT reverse primer and the SuperScript First Strand Synthesis kit (Invitrogen, Carlsbad, CA). QRT-PCR was carried out with the iQ5 real-time PCR detection system (Bio-Rad, Hercules, CA). Gene-specific PCR primers and FAM-labeled TaqMan probes for mouse IL-1β, stromelysin [MMP-3], ADAMTS-4 and ADAMTS-5 were purchased from Applied Biosystems (Foster City, CA). TaqMan gene expression assays were carried out according to the manufacturer’s instructions (Applied Biosystems). QRT-PCR data were analyzed with the iQ5 system’s software package. Expression of the GAPDH housekeeping gene was used to normalize the data within each pooled sample. Quantification of gene expression among pooled samples (relative to control) was done employing the 2−ΔΔCT method20, and results were expressed as fold changes relative to the corresponding control samples.
STATISTICAL ANALYSES
Statistical analyses were performed using SPSS software (version 16; SPSS Inc., Chicago, IL). Descriptive statistics were used to determine group means and 95% confidence intervals (mean ± CI 95%). Statistically significant differences among the groups were assessed using ANOVA with post-hoc Dunnett’s t test. A p value of <0.05 was considered statistically significant.
Results
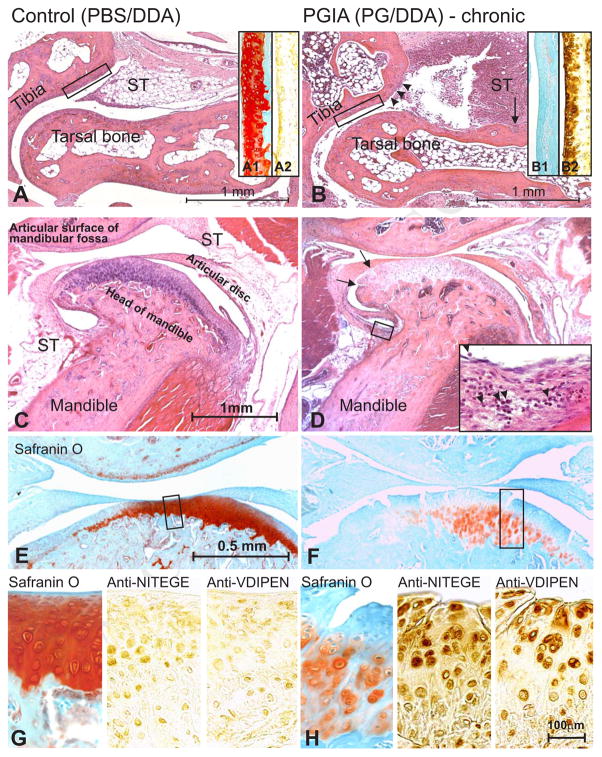
We investigated TMJ involvement in PGIA at the acute (10 mice) and subacute/chronic (12 mice) phases of peripheral joint inflammation compared with age-matched, (8 PBS/DDA-injected) non-arthritic, control animals. Arthritis developed after the third injection of PG in DDA [Fig. 1 (A, thick solid arrow)]. This initial acute phase [Fig. 1 (A, dotted-line arrow)] was characterized by increasing swelling and redness of the paws. Approximately 10 days after arthritis onset (~day 52 of immunization), the animals entered the chronic phase of PGIA [Fig. 1(A), dashed-line arrow], characterized by hardening of the periarticular soft tissue, deformities and ankylosis3,14. By the end of the experimental period (day 60), 11 of the 12 remaining PG-immunized animals had chronic arthritis involving the limbs, while none of the control animals showed any signs of joint disease [Fig. 1(A-B)]. To monitor the progression of inflammation or cartilage destruction in the TMJs (if any), mice with acute and chronic arthritis were sacrificed on days 49–50 and day 60, respectively [Fig. 1 (A, thin solid arrows)], and their TMJs and ankle joints were compared with each other or with the corresponding tissues of non-arthritic control mice. Figures 2(A) and 2(B) show representative examples of hematoxylin-eosin-stained histological sections prepared from the ankle joints of control and arthritic animals sacrificed on day 60. The ankle joints of the control mice did not exhibit any signs of structural damage [Fig. 2(A)], but inflammatory cell infiltration of the synovial tissue and massive joint destruction were evident in the ankles of the PG-immunized animals [Fig. 2(B)]. To determine the extent of inflammation-induced loss of cartilage aggrecan, we stained adjacent sections of the ankle joints of normal and arthritic mice with safranin O and fast green. Safranin O detects the negatively charged GAG side chains of aggrecan in cartilage15. As a result, the extent of red staining is proportional to the amount of GAG-containing (presumably intact) aggrecan molecules. Cartilage of the distal tibia of the control mouse showed intense red staining with safranin O [Fig. 2(A1)], whereas very little red staining was seen in the tibial cartilage of the mouse with chronic arthritis in the ankle joint [Fig. 2(B1)]. Considering that the loss of GAG-decorated aggrecan fragments from cartilage is the result of cleavage of the core protein by aggrecanases (ADAMTS-4 and ADAMTS-5) or stromelysin (MMP-3)17,21, we sought to determine whether the aggrecan neoepitopes generated by ADAMTSs or MMP-3 (-NITEGE and -VDIPEN, respectively) were detectable in the cartilage of arthritic ankles. As compared to the normal cartilage, which showed essentially negligible immunostaining with the anti-NITEGE antibody [Fig. 2(A2)], staining for this aggrecan neoepitope was very strong in the cartilage of arthritic ankle [Fig. 2(B2)]. Similar differences were observed when sections of control and arthritic ankle joints were reacted with the anti-VDIPEN antibody [Table I and results not shown].
Fig. 2.
Histochemical and immunohistochemical features of the limb (ankle) joints and temporomandibular joints (TMJ) in mice with PGIA. (A) A hematoxylin-eosin-stained tissue section from the ankle (tibio-tarsal) joint from a PBS/DDA injected control mouse shows normal structure. The articular cartilage surface is smooth, and the synovial tissue (ST) is free of inflammatory cell infiltrates. (A1) The articular cartilage of the distal tibia (section adjacent to the boxed area in A) shows strong and fairly even staining with safranin O (red), but (A2) negligible immunostaining for aggrecan neoepitopes with anti-NITEGE antibody (or anti-VDIPEN; not shown). (B) The ankle joint from a mouse with chronic arthritis shows massive infiltration of synovial tissue (ST) by leukocytes and loss of superficial zone chondrocytes at the joint margins where inflammatory cells are in contact with cartilage (also known as “pitting”; depicted here by arrowheads). Bone erosion by the invasive synovium is also evident (arrow). (B1) The cartilage of distal tibia shows generalized loss of safranin O staining, associated with (B2) strong positive immunostaining for aggrecan neoepitopes (anti-NITEGE is shown). (C) Normal TMJ structure from the control mouse. (D) The TMJ of the mouse with chronic PGIA (ankle shown in B). The TMJ does not exhibit severe structural damage, except for the lighter staining of the mandibular cartilage than the control TMJ, and a few fissures at the cartilage margin (arrows). A small collection of inflammatory cells is also present in the synovium (box and insert at 6x magnification). (E) The cartilage of the normal TMJ shows strong and homogenous staining with safranin O (red), which indicates intact aggrecan content. (F) Safranin O staining is weak and restricted to a few chondrocytes in the TMJ cartilage of a mouse with chronic PGIA. The cartilage also shows evidence of structural damage (fissures) and chondrocyte clustering (boxed area, magnified in H). (G) Adjacent sections of the control TMJ were stained with safranin O (left-hand panel) and with antibodies against the aggrecan neoepitopes -NITEGE (middle panel) and -VDIPEN (right-hand panel). Weak positive staining for both neoepitopes is visible around some chondrocytes. (H) The area of the TMJ from the arthritic mouse that stains weakly with safranin O (left-hand panel) shows strong immunostaining for both -NITEGE and -VDIPEN neoepitopes (middle and right-hand panels, respectively).
Table I.
Quantitative analysis of Safranin O staining of GAGs and immunohistochemical staining of aggrecan neoepitopes (NITEGE and VDIPEN) of the cartilage of TMJ and ankle joints in mice with acute or chronic PGIA and in non-arthritic controls
| Joint/Staining (% positive) | Control (N = 8) | Acute PGIA (N = 10) | p (acute vs. control) | Chronic PGIA (N = 11) | p (chronic vs. control) | p (chronic vs. acute) |
|---|---|---|---|---|---|---|
| TMJ/Safranin O | ||||||
| Mean (CI 95%) | 54.2 (46.2 – 62.2) | 49.1 (43.0 – 55.2) | 0.1911 | 6.4 (4.5 – 8.3) | 0.0014 | 0.0027 |
| Ankle/Safranin O | ||||||
| Mean (CI 95%) | 47.9 (40.6 – 55.2) | 12.6 (9.3 – 15.9) | 0.0033 | 1.9 (1.0 – 2.8) | 0.0001 | 0.0079 |
| TMJ/NITEGE | ||||||
| Mean (CI 95%) | 2.1 (1.6 – 2.6) | 2.6 (1.8 – 3.4) | 0.2565 | 16.7 (13.2 – 20.2) | 0.0299 | 0.0357 |
| Ankle/NITEGE | ||||||
| Mean (CI 95%) | 0.7 (0.3 – 1.1) | 15.5 (11.7 – 19.3) | 0.0016 | 26.3 (22.2 – 30.4) | 0.0004 | 0.0425 |
| TMJ/VDIPEN | ||||||
| Mean (CI 95%) | 0.5 (0.2 – 0.8) | 1.1 (0.6 – 1.6) | 0.0450 | 13.9 (10.3 – 17.5) | 0.0001 | 0.0003 |
| Ankle/VDIPEN | ||||||
| Mean (CI 95%) | 0.7 (0.2 – 1.2) | N.D. | 19.0 (14.7 – 23.3) | 0.0001 | ||
The percent of positively stained area (% positive) within each TMJ or ankle cartilage section was quantitatively determined using Adobe Photoshop-based image analysis, as described in the Methods. The values shown are the means and 95% confidence levels (CI 95%). Statistically significant differences (p<0.05) are indicated in italics. N: number of mice; N.D.: not determined.
When hematoxylin-eosin-stained sections of the TMJs from control and arthritic mice were compared, the most notable difference was the diminished basophilic staining in the mandibular cartilage of arthritic mice [Fig. 2(C) and (D)]. In the most severe cases of chronic PGIA, there was structural evidence of cartilage damage (fibrillation or fissures) in the TMJ [Fig. 2 (D, arrows), or small collections of inflammatory cells could be seen in the TMJ synovium [Fig. 2 (D, insert)]. To determine if the apparent loss of hematoxylin staining from the TMJ cartilage of the arthritic mouse could reflect a loss of aggrecan, we stained adjacent sections of the corresponding TMJs with safranin O and fast green. Indeed, the control TMJ cartilage showed strong and nearly homogenous staining with safranin O [Fig. 2(E) and (G)] whereas significant loss of red staining was observed in the TMJ of the arthritic mouse [Fig. 2(F) and (H)]. In the latter case, safranin O staining was mainly found around the chondrocytes and was absent from the inter-territorial matrix. In addition, the chondrocytes appeared to form clusters in the TMJ of the arthritic mouse [Fig. 2(H)]. Immunostaining for aggrecan neoepitopes revealed light staining for both -NITEGE and -VDIPEN around some of the chondrocytes in the healthy TMJ [Fig. 2 (G, middle and right-side panels, respectively)], which is consistent with the normal turnover of aggrecan in the cartilage. In comparison with the control mice, immunostaining for -NITEGE and -VDIPEN was very strong in the TMJ cartilage of the arthritic mouse [Fig. 2(H)], which indicated that aggrecan fragments were generated more extensively in the TMJ of the mouse affected with peripheral joint inflammation than in the one without arthritis.
While the presence of inflammatory cells in the TMJs was not typical, diminished safranin O staining and enhanced aggrecan neoepitope immunostaining was found in the TMJs of most animals at the chronic phase of PGIA and in a few TMJs in the acute arthritic group. To quantitatively assess the extent of aggrecan degradation in the different joints, we performed morphometric analyses of safranin O staining and IHC (anti-NITEGE and anti-VDIPEN) reactions using tissue sections from the TMJs and ankle joints of control mice and of animals with acute and chronic PGIA. As shown in Table I, neither safranin O staining nor immunostaining for the NITEGE neoepitope in the TMJ cartilage was significantly different between normal mice and those with acute PGIA, but the differences became statistically significant after the mice reached the chronic phase of PGIA. In the arthritic ankle joints, aggrecan degradation (diminished safranin O staining and generation of NITEGE neoepitope) was significantly greater than in the controls already at the acute phase of PGIA, and progressed further in chronic disease [Table I]. These results suggested that aggrecan degradation occurred in both the ankles and the TMJs in PGIA, but was more progressive in the ankles than in the TMJs.
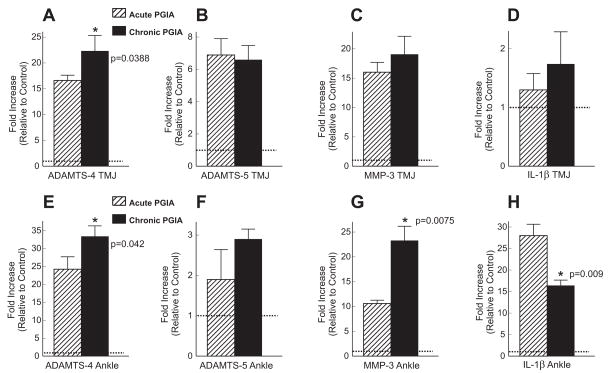
The presence of very little or no synovial inflammation coupled with massive loss of aggrecan is one of the typical features of OA22. Pro-inflammatory cytokines, such as IL-1β, TNFα, IL-6, or IL-17, have been implicated in the initiation of matrix breakdown in cartilage22,23. Among these cytokines, IL-1β, which can also be produced by the chondrocytes themselves in OA, is thought to be the main effector of aggrecan breakdown via induction of aggrecanase and MMP expression in the chondrocytes23. We found evidence of upregulated expression of the genes encoding ADAMTS-4, ADAMTS-5 and MMP-3 (~20-fold, ~7-fold, and ~18-fold increases, respectively, relative to normal controls) in the TMJs of animals with both acute and chronic PGIA [Figs 3(A-C)], but the IL-1β gene was not upregulated (only ~1.5-fold higher than control) in these TMJ samples [Fig. 3(D)]. The expression levels of aggrecanases and MMP-3 in the arthritic peripheral joints increased as inflammation progressed [Figs 3(E, F, G)], but the overall magnitude of gene expression was similar to the levels found in the TMJs, except for ADAMTS-5, which was expressed at slightly lower levels in the arthritic ankle joints. In contrast, the IL-1β gene was highly over-expressed in the arthritic limbs at both the acute and the chronic phases of PGIA relative to the normal control, although there was a statistically significant decrease in expression as arthritis progressed into the chronic phase [Fig. 3(H)]. This latter observation suggested that the inflamed limb joints were the major sources of the “catabolic” cytokine IL-1β.
Fig. 3.
Expression of genes encoding matrix-degrading enzymes and interleukin (IL)-1β in the TMJs and ankle joints of mice with PGIA. Quantitative real-time PCR (qRT-PCR) was employed to compare the expression levels of these genes between control and arthritic mice in the TMJs (A–D) and the ankle joints (E–H) in PGIA. The results are from three independent experiments (N=3), using RNA of cartilage and synovial tissue (pooled from the joints of 2–3 mice per group), and are expressed as fold change (increase) in expression level relative to the control (mean ± 95% confidence interval). For comparison of the magnitude of gene upregulation, the control levels are indicated by horizontal dotted lines. Statistically significant differences (p<0.05) between “acute” and “chronic” PGIA samples are depicted by asterisks and the p values are indicated. (A) The gene encoding the aggrecanase ADAMTS-4 was upregulated in the TMJs of mice with both acute (crossed bars) and chronic (black bars) arthritis compared to TMJs of non-arthritic mice (dashed line). (B) The aggrecanase ADAMTS-5 was also upregulated, but to a lesser extent. (C) Over-expression of MMP-3 (stromelysin) was also detected in the PGIA samples. (D) The expression level of IL-1β in the TMJs of arthritic mice was only slightly above the level observed in control mice. (E) ADAMTS-4 was highly upregulated in the arthritic joints at both the acute and chronic phases of PGIA. (F) ADAMTS-5 was only marginally upregulated in the same joint samples. (G) MMP-3 was moderately over-expressed in the joints of animals with acute PGIA and strongly over-expressed in animals with chronic disease. (H) Conversely, IL-1β was massively upregulated in ankle joints with acute arthritis and moderately in those with chronic inflammation. The most notable difference between the TMJs and ankle joints was the very high level of IL-1β gene expression in the arthritic ankles (graph H) compared to the nearly normal levels in the TMJs (graph D) of corresponding groups of mice.
In order to act on the TMJ cartilage at an area that is distant from the sites of production, IL-1β has to be present in the circulation. Indeed, while IL-1β was not detectable in the sera of control animals, it was present in the sera of mice with either acute or chronic PGIA [Table II]. Moreover, additional pro-inflammatory cytokines, such as TNFα, IL-6, and IL-17, all of which could also contribute to cartilage matrix breakdown22, were detected in the sera of arthritic animals at both the acute and chronic phases of PGIA [Table II].
Table II.
Serum levels of pro-inflammatory cytokines in mice with acute or chronic PGIA and in non-arthritic controls
| Cytokine (pg/ml) | Acute PGIA (N = 10) | Chronic PGIA (N = 12) | Control (N = 8) |
|---|---|---|---|
| IL-1β | |||
| Mean (CI 95%) | 9.5 (7.48 – 11.52) | 9.31 (7.88 – 10.74) | 0 (0) |
| TNFα | |||
| Mean (CI 95%) | 9.27 (8.01 – 10.53) | 8.34 (6.63 – 10.05) | 0 (0) |
| IL-6 | |||
| Mean (CI 95%) | 17.5 (14.92 – 20.08) | 14.3 (12.75 – 15.31) | 0 (0) |
| IL-17 | |||
| Mean (CI 95%) | 5.27 (4.06 – 6.48) | 5.35 (4.64 – 6.06) | 0 (0) |
Serum levels of interleukin (IL)-1β, tumor necrosis factor (TNF)α, IL-6, and IL-17 are expressed in pg/ml. Values of the means and 95% confidence levels (CI 95%) are shown. None of these cytokines were detectable in serum samples of control (DDA adjuvant-injected, non-arthritic) mice (last column). The differences between mice with acute and chronic PGIA were not statistically significant in the serum levels of any of these cytokines (p>0.05). N: number of mice.
Discussion
This is the first study to demonstrate that degenerative changes develop in the mandibular cartilage of the TMJs of mice during the progression of autoimmune inflammatory joint disease in PGIA, an animal model of RA. The lack of previous investigations in RA models is surprising because the TMJ is a synovial joint, which may become a target of autoimmune reactions in both RA and animal models of the human disease. A significant proportion of RA patients experience pain in their TMJs, and analyses of synovial fluid samples clearly indicates the involvement of this joint not only in OA, but also in RA11,24,25. In a recent study, up to 72 % of newly diagnosed RA patients were found to have TMJ involvement24. This percentage is close to those found in studies of RA patients with longer disease duration21,25–27 or in pediatric patients with juvenile idiopathic arthritis28,29, a disease with RA-like features.
We found evidence of substantial loss of PG (aggrecan) in the mandibular cartilage of TMJs, with minimal or no local synovial inflammation, in mice that developed severe inflammatory arthritis in their limb joints. This finding was consistent in animals that entered the chronic phase of arthritis (disease duration: 10 days or longer), and aggrecan loss was occasionally associated with structural damage (fibrillation or fissures) in the TMJ cartilage. In the ankle joints, severe synovitis was accompanied by chondrocyte loss from the superficial zone (pitting). Although pitting was mainly restricted to sites where the cartilage was in contact with inflammatory cells, GAG-containing aggrecan fragments were lost from the entire cartilage. Whereas the TMJ of mice with severe PGIA showed evidence of structural impairment, aggrecan degradation in mandibular cartilage was not greater, and appeared to be less progressive, than in the inflamed ankle joints. This suggests that the insults that triggered aggrecan loss and/or the response of the joints to those stimuli could be different at these anatomically distinct sites. Histopathologic abnormalities were not found in the joints of age- and sex-matched control (PBS/DDA-injected) mice, which allowed us to exclude the possibility of any age- or gender-related association30.
Loss of PG from the TMJ cartilage of arthritic animals was reminiscent of the changes described for cartilage in joints affected with OA in humans or in animal models of cartilage degeneration22,31. Aggrecan fragmentation could be attributed to upregulation of catabolic enzymes, and we found that mRNA levels of aggrecanases ADAMTS-4 and ADAMTS-5, and stromelysin (MMP-3) were upregulated, and the neoepitopes generated by these enzymes (-NITEGE and -VDIPEN) accumulated in the TMJs in animals with PGIA. These results were similar to those in the inflamed ankle joints of the same animals.
Pro-inflammatory cytokines have a central role in aggrecanase and MMP upregulation, and the key pro-inflammatory cytokine appears to be IL-1β, although TNFα and IL-6, either directly or via the activation of the IL-1β pathway, may also be involved in the upregulation of aggrecanases and MMPs22,23. Chondrocytes have been shown to produce IL-1β in response to various stimuli, and this cytokine can activate the catabolic pathway in cartilage22, which ultimately leads to local matrix breakdown. In support of this, induction of chondrocyte-specific expression of the IL-1β gene resulted in OA-like histopathological changes and dysfunction in the TMJs of transgenic mice32. Moreover, a positive correlation was found between the incidence of TMJ dysfunction and serum levels of the IL-1 receptor (as a proxy for IL-1β) in RA patients33. We were surprised to find that the chondrocytes of the damaged mandibular cartilage did not upregulate IL-1β expression in mice with PGIA. However, this cytokine was markedly over-expressed in the joint tissues of arthritic limbs of the same animals, suggesting that exogenous IL-1β (released into the circulation from inflamed joints) could be responsible for the catabolic activity of mandibular cartilage chondrocytes and/or surrounding synovial cells. Indeed, serum samples from arthritic mice contained high amounts of IL-1β and other pro-inflammatory cytokines, including TNFα, IL-6, and IL-17. Notably, the serum levels of these cytokines in our arthritic mice were comparable to concentrations found in TMJ synovial fluid samples from patients with RA or OA25,34,35.
We conclude that unlike the peripheral joints, the TMJ is not a target of the autoimmune attack in PGIA. The OA-like cartilage damage in the TMJs of arthritic mice is likely due to the effects of systemic factors, such as circulating cytokines, released from inflamed joints. Sustained production of these pro-inflammatory cytokines (i.e., chronic disease) appeared to be necessary to induce TMJ cartilage degradation in a consistent manner. Serum concentrations of pro-inflammatory cytokines decline at a late phase of chronic PGIA (several weeks after disease onset)3,4. Therefore, it is possible that the process of TMJ cartilage degradation slows down after the “burn-out” of limb joint inflammation. Finally, as these animals experience pain in their arthritic limbs, it remains to be determined if there is pain-related overuse of the TMJ (e.g., increased gnawing) that can contribute to cartilage damage in that joint.
In summary, we show evidence of cartilage matrix degradation in the TMJs of mice having autoimmune inflammatory arthritis in their limb joints, and propose that systemic pro-inflammatory cytokines could be involved in TMJ pathology. Our results suggest that early treatment of RA, especially with biologics, such as IL-1 or TNF antagonists25,36–38, holds promise for the prevention of irreversible tissue damage in the TMJs of RA patients.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH/NIAMS R01 AR040310 and R21 AR052679).
Footnotes
Authors’ Contributions
All authors significantly participated and were involved in the (1) conception and design (2) drafting the manuscript or revising it critically for intellectual content, and (3) all authors approved the final version of the paper before submission. Dr. Glant and Dr. Mikecz have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests
The authors declare that they have no competing interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albani S, Carson DA, Roudier J. Genetic and environmental factors in the immune pathogenesis of rheumatoid arthritis. Rheum Dis Clin North Am. 1992;18:729–40. [PubMed] [Google Scholar]
- 2.Goronzy JJ, Weyand CM. Developments in the scientific understanding of rheumatoid arthritis. Arthritis Res Ther. 2009:249. doi: 10.1186/ar2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glant TT, Finnegan A, Mikecz K. Proteoglycan-induced arthritis: immune regulation, cellular mechanisms and genetics. Crit Rev Immunol. 2003;23:199–250. doi: 10.1615/critrevimmunol.v23.i3.20. [DOI] [PubMed] [Google Scholar]
- 4.Glant TT, Mikecz K. Proteoglycan aggrecan-induced arthritis. A murine autoimmune model of rheumatoid arthritis. Methods Mol Med. 2004;102:313–38. doi: 10.1385/1-59259-805-6:313. [DOI] [PubMed] [Google Scholar]
- 5.Haskin CL, Milam SB, Cameron IL. Pathogenesis of degenerative joint disease in the human temporomandibular joint. Crit Rev Oral Biol Med. 1995;6:248–77. doi: 10.1177/10454411950060030601. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87:296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- 7.Franks AS. Temporomandibular joint in adult rheumatoid arthritis. A comparative evaluation of 100 cases. Ann Rheum Dis. 1969;28:139–45. doi: 10.1136/ard.28.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gleissner C, Kaesser U, Dehne F, Bolten WW, Willershausen B. Temporomandibular joint function in patients with longstanding rheumatoid arthritis - I. Role of periodontal status and prosthetic care - a clinical study. Eur J Med Res. 2003;8:98–108. [PubMed] [Google Scholar]
- 9.Bessa-Nogueira RV, Vasconcelos BC, Duarte AP, Goes PS, Bezerra TP. Targeted assessment of the temporomandibular joint in patients with rheumatoid arthritis. J Oral Maxillofac Surg. 2008;66:1804–11. doi: 10.1016/j.joms.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 10.Ueno T, Kagawa T, Kanou M, Ishida N, Fujii T, Fukunaga J, et al. Pathology of the temporomandibular joint of patients with rheumatoid arthritis--case reports of secondary amyloidosis and macrophage populations. J Craniomaxillofac Surg. 2003;31:252–56. doi: 10.1016/s1010-5182(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 11.Gynther GW, Holmlund AB, Reinholt FP, Lindblad S. Temporomandibular joint involvement in generalized osteoarthritis and rheumatoid arthritis: a clinical, arthroscopic, histologic, and immunohistochemical study. Int J Oral Maxillofac Surg. 1997;26:10–16. doi: 10.1016/s0901-5027(97)80838-7. [DOI] [PubMed] [Google Scholar]
- 12.Harper RP, Kerins CA, McIntosh JE, Spears R, Bellinger LL. Modulation of the inflammatory response in the rat TMJ with increasing doses of complete Freund’s adjuvant. Osteoarthritis Cartilage. 2001;9:619–24. doi: 10.1053/joca.2001.0461. [DOI] [PubMed] [Google Scholar]
- 13.Habu M, Tominaga K, Sukedai M, Alstergren P, Ohkawara S, Kopp S, et al. Immunohistochemical study of interleukin-1beta and interleukin-1 receptor antagonist in an antigen-induced arthritis of the rabbit temporomandibular joint. J Oral Pathol Med. 2002;31:45–54. doi: 10.1046/j.0904-2512.2001.10057.x. [DOI] [PubMed] [Google Scholar]
- 14.Glant TT, Mikecz K, Arzoumanian A, Poole AR. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 1987;30:201–12. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg L. Chemical basis for the histological use of safranin-O in the study of articular cartilage. J Bone Joint Surg. 1971;53-A:69–82. [PubMed] [Google Scholar]
- 16.Bardos T, Kamath RV, Mikecz K, Glant TT. Anti-inflammatory and chondroprotective effect of TSG-6 (tumor necrosis factor-α-stimulated gene-6) in murine models of experimental arthritis. Am J Pathol. 2001;159:1711–21. doi: 10.1016/s0002-9440(10)63018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westling J, Fosang AJ, Last K, Thompson VP, Tomkinson KN, Hebert T, et al. ADAMTS4 cleaves at the aggrecanase site (Glu373-Ala374) and secondarily at the matrix metalloproteinase site (Asn341-Phe342) in the aggrecan interglobular domain. J Biol Chem. 2002;277:16059–66. doi: 10.1074/jbc.M108607200. [DOI] [PubMed] [Google Scholar]
- 18.Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5:94–103. doi: 10.1186/ar630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahm A, Mrosek E, Spank H, Erggelet C, Kasch R, Esser J, et al. Changes in content and synthesis of collagen types and proteoglycans in osteoarthritis of the knee joint and comparison of quantitative analysis with Photoshop-based image analysis. Arch Orthop Trauma Surg. 2010;130:557–64. doi: 10.1007/s00402-009-0981-y. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Tiilikainen P, Pirttiniemi P, Kainulainen T, Pernu H, Raustia A. MMP-3 and -8 expression is found in the condylar surface of temporomandibular joints with internal derangement. J Oral Pathol Med. 2005;34:39–45. doi: 10.1111/j.1600-0714.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–46. [PubMed] [Google Scholar]
- 23.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–35. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 24.Hajati AK, Alstergren P, Nasstrom K, Bratt J, Kopp S. Endogenous glutamate in association with inflammatory and hormonal factors modulates bone tissue resorption of the temporomandibular joint in patients with early rheumatoid arthritis. J Oral Maxillofac Surg. 2009;67:1895–903. doi: 10.1016/j.joms.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alstergren P, Benavente C, Kopp S. Interleukin-1beta, interleukin-1 receptor antagonist, and interleukin-1 soluble receptor II in temporomandibular joint synovial fluid from patients with chronic polyarthritides. J Oral Maxillofac Surg. 2003;61:1171–78. doi: 10.1016/s0278-2391(03)00678-5. [DOI] [PubMed] [Google Scholar]
- 26.Srinivas R, Sorsa T, Tjaderhane L, Niemi E, Raustia A, Pernu H, et al. Matrix metalloproteinases in mild and severe temporomandibular joint internal derangement synovial fluid. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:517–25. doi: 10.1067/moe.2001.115136. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida K, Takatsuka S, Hatada E, Nakamura H, Tanaka A, Ueki K, et al. Expression of matrix metalloproteinases and aggrecanase in the synovial fluids of patients with symptomatic temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:22–27. doi: 10.1016/j.tripleo.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Walton AG, Welbury RR, Thomason JM, Foster HE. Oral health and juvenile idiopathic arthritis: a review. Rheumatology (Oxford) 2000;39:550–555. doi: 10.1093/rheumatology/39.5.550. [DOI] [PubMed] [Google Scholar]
- 29.Arabshahi B, Cron RQ. Temporomandibular joint arthritis in juvenile idiopathic arthritis: the forgotten joint. Curr Opin Rheumatol. 2006;18:490–495. doi: 10.1097/01.bor.0000240360.24465.4c. [DOI] [PubMed] [Google Scholar]
- 30.Silbermann M, Livne E. Age-related degenerative changes in the mouse mandibular joint. J Anat. 1979;129:507–20. [PMC free article] [PubMed] [Google Scholar]
- 31.Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthr Cart. 1998;6:374–76. doi: 10.1053/joca.1998.0140. [DOI] [PubMed] [Google Scholar]
- 32.Lai YC, Shaftel SS, Miller JN, Tallents RH, Chang Y, Pinkert CA, et al. Intraarticular induction of interleukin-1beta expression in the adult mouse, with resultant temporomandibular joint pathologic changes, dysfunction, and pain. Arthritis Rheum. 2006;54:1184–97. doi: 10.1002/art.21771. [DOI] [PubMed] [Google Scholar]
- 33.Voog U, Alstergren P, Eliasson S, Leibur E, Kallikorm R, Kopp S. Progression of radiographic changes in the temporomandibular joints of patients with rheumatoid arthritis in relation to inflammatory markers and mediators in the blood. Acta Odontol Scand. 2004;62:7–13. doi: 10.1080/00016350310007860. [DOI] [PubMed] [Google Scholar]
- 34.Fu K, Ma X, Zhang Z, Pang X, Chen W. Interleukin-6 in synovial fluid and HLA-DR expression in synovium from patients with temporomandibular disorders. J Orofac Pain. 1995;9:131–37. [PubMed] [Google Scholar]
- 35.Vernal R, Velasquez E, Gamonal J, Garcia-Sanz JA, Silva A, Sanz M. Expression of proinflammatory cytokines in osteoarthritis of the temporomandibular joint. Arch Oral Biol. 2008;53:910–915. doi: 10.1016/j.archoralbio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Abramson SB, Amin A. Blocking the effects of IL-1 in rheumatoid arthritis protects bone and cartilage. Rheumatology (Oxford) 2002;41:972–80. doi: 10.1093/rheumatology/41.9.972. [DOI] [PubMed] [Google Scholar]
- 37.Stoustrup P, Kristensen KD, Kuseler A, Pedersen TK, Gelineck J, Herlin T. Intra-articular vs. systemic administration of etanercept in antigen-induced arthritis in the temporomandibular joint. Part II: mandibular growth. Pediatr Rheumatol Online J. 2009;7:6. doi: 10.1186/1546-0096-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland LW, et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:614–24. doi: 10.1002/art.10141. [DOI] [PubMed] [Google Scholar]