Abstract
The present study addressed the role of switch detection in cognitive flexibility by testing the effect of transition cues (i.e., cues that directly signal the need to switch or maintain a given task goal) in a cued set-shifting paradigm at age 5. Children performed better, especially on switch trials, when transition cues were combined with traditional task cues (i.e., cues that directly signal the relevant task on a given trial), relative to conditions without transition cues. This effect was not influenced by explicit knowledge of transition cues or transition cue transparency, suggesting transition cues did not need to be semantically processed to be beneficial. These findings reveal that young children’s difficulties in set-shifting situations partially stem from failures to monitor for the need to switch.
Keywords: executive control, set-shifting, flexibility, goal management, preschool children
Human volitional behavior is largely driven by goals. Daily activities are organized as a function of the short- and long-term goals that one desires to reach, and the achievement of these goals, or failure thereof, strongly influences future behaviors. Goals refer to “intention[s] to accomplish a task, achieve some specific state of the world, or take some mental or physical actions” (Altmann & Trafton, 2002, p. 39). Goal attainment is supported by executive control, that is, the set of processes that regulate thoughts and actions by monitoring (e.g., activating, maintaining, inhibiting) the information that one attends to and how this information is processed and by flexibly adjusting responses, especially in situations where concurrent goal-irrelevant information interferes. Understanding the relation between goals and children’s executive control is essential because executive control is largely involved in cognitive development (Deák, 2003; Carlson & Moses, 2001), academic achievement (Bull, Espy, Wiebe, Sheffield, & Nelson, in press), and problem behaviors (Espy, Wiebe, Sheffield, Clark, & Moehr, 2011).
One prominent facet of executive control where goals are paramount is cognitive flexibility, that is, the ability to switch between multiple tasks as a function of changing environmental demands. Cognitive flexibility undergoes marked improvement over the preschool period and beyond (e.g., Carlson, 2005; Cragg & Nation, 2009; Cepeda, Kramer, & Gonzalez de Sather, 2001; Espy, Bull, Martin, & Stroup, 2006; Espy & Cwik, 2004; Jacques & Zelazo, 2001; Smidts, Jacobs, & Anderson, 2004; Zelazo, Müller, Frye, & Marcovitch, 2003). Although flexibility is widely acknowledged to involve multiple components (Cragg & Chevalier, in press; Diamond, 2006; Garon, Bryson, & Smith, 2008), there is substantial debate about their nature and interaction. A set of recent studies, conducted with a variety of paradigms, suggests that flexibility development in preschoolers is driven partly by the increasing ability to select and maintain relevant task goals, both in situations where these goals are stated explicitly to children (Marcovitch, Boseovski, & Knapp, 2007; Marcovitch, Boseovski, Knapp, & Kane, in press; Morton & Munakata, 2002) and where goals must be inferred indirectly from task cues (Chevalier & Blaye, 2009; Chevalier, Sheffield, Nelson, Clark, Wiebe, & Espy, 2010; Towse, Lewis, & Knapp, 2007), response feedback (Chevalier, Dauvier, & Blaye, 2009) or stimulus features (Snyder & Munakata, 2010). However, little is known about the processes that contribute to the representation, selection, and maintenance of task goals, which we term goal management throughout the rest of the paper. In the present study, we examined the contribution of switch detection, that is, the monitoring for the necessity to switch task goal, to preschooler’s goal management. To this end, we manipulated the amount of information that directly signals whether children must maintain or switch away from a given task goal in a paradigm putatively tapping cognitive flexibility.
Managing goals is especially challenging where children have to decide on their own when to switch and what task goal is relevant on the basis of environmental information such as task cues. Such a requirement is a common feature of cognitive flexibility measures at preschool age (Shape School—Espy, 1997; Espy et al., 2006; Advanced DCCS—Zelazo, 2006) and at school age and beyond (the cued task-switching paradigm—see Kiesel, Steinhauser, Wendt, Falkenstein, Jost, Philipp, & Koch, 2010; Vandierendonck, Liefooghe, & Verbruggen, 2010). For instance, the Shape School requires children to switch unpredictably between shape and color-matching tasks as a function of whether stimuli wear a hat (shape is relevant) or not (color is relevant). Like other task-switching paradigms, the Shape School contains simple blocks where the same task is relevant on all trials (simple-block trials) and mixed blocks in which children either have to repeat the task from the previous trial (no-switch trials) or switch to the other task (switch trials). These three trial types allow for the computation of two distinct switch costs, mixing and local costs (Koch, Prinz, & Allport, 2005). The distinction between these costs is heuristic insofar as it attempts to tease apart the difficulties associated with multiple tasks being mixed from those with switching to a new task per se, and thus offers the potential to delineate the multiple processes that comprise cognitive flexibility. More specifically, mixing costs correspond to the drop in performance from simple-block trials to no-switch trials within mixed blocks. As none of these trials require switching, no-switch trials mainly differ from simple-block trials in the necessity to hold two task-sets active in working memory. Most importantly for the present study, on no-switch trials, the child needs to determine which task is relevant on both switch and no-switch trials by forming representations of the relevant task goals on the basis of task cues (Miyake, Emerson, Padilla, & Ahn, 2004; see also Baddeley, Chincotta, & Adlam, 2001; Gruber & Goschke, 2004; Reimers & Maylor, 2005; Rubinstein, Meyers, & Evans, 2001). As such, mixing costs are considered primarily sensitive to the selection of the relevant task goal (Kray & Lindenberger, 2000; Rubin & Meiran, 2005).
Local costs correspond to the additional drop in performance from no-switch trials to switch trials (both within mixed blocks). On both of these trial types, once the relevant task goal is identified (e.g., color task), the corresponding task set, that is, the set of parameters related to task rules, attention orientation and response selection, must be (re)activated in order to achieve the goal (Vandierendonck et al., 2010). Task-set activation is more demanding on switch trials than no-switch trials, because of additional interference and/or additional processes (e.g., inhibitory process) to implement on switch trials. As both switch and no-switch trials require determining the relevant task goals whereas only switch trials require actually switching, local costs are thought to reflect primarily the additional processes and/or higher difficulty specifically associated with switching per se (Chevalier & Blaye, 2009; Rubin & Meiran, 2005). Both mixing and local costs decrease over childhood, pointing to age-related improvement in both goal management and task-set switching (Cepeda et al., 2001; Chevalier & Blaye, 2009; Cragg & Nation, 2009; Ellefson, Shapiro, & Chater, 2006; Reimers & Maylor, 2005).
The difficulty of goal management varies as a function of task cue transparency, that is, the degree of association between task cues and task goals, which has differential impacts on mixing and local costs with age. When asked to switch between color and shape-matching rules, 5- to 6-year-olds show reduced mixing costs with transparent cues (e.g., strings of multiple colors for color and black square for shape) relative to arbitrary cues (e.g., gray background for one dimension and black background for the other) (Blaye & Chevalier, in press; Chevalier & Blaye, 2009, see also Towse et al., 2007). Transparent cues are thought to more directly activate the corresponding task goal representation whereas less transparent or arbitrary cues require further processing to enable the corresponding task goals (Logan & Schneider, 2006; Miyake et al., 2004). Interestingly, the effect of task cue transparency changes with age. First, its magnitude decreases over childhood but remains significant even in adults, suggesting that goal management increases in efficiency with age (Chevalier & Blaye, 2009). Second, whereas task cue transparency affects mixing costs but not local costs on both accuracy and reaction times in preschoolers (i.e., similar effect on no-switch and switch trials), it modulates both types of cost on reaction times in adults (i.e., greater effect on switch than no-switch trials) (Arbuthnott & Woodward, 2002; Logan & Bundesen, 2003; Logan & Schneider, 2006; Miyake et al., 2004). These findings suggest that the processes or strategies underlying goal management may differ somewhat in preschoolers and adults.
In addition to the likely stronger conceptual representations of task goals with age (which is not the topic of the present study)—, age-related changes in goal management may relate to differences in (a) switch detection, that is, decision about the necessity to switch or not, and/or (b) task selection (or identification), that is, decision about what to switch to (see Jamadar, Mitchie, & Karayanidis, 2010). Generally, task cues provide direct information on the relevant task identity. The effect of task cue transparency on switching performance suggests that task selection is a main contributor to goal management and an essential source of difficulty for preschoolers. Task cues provide only indirect information on whether a switch is needed as this information may be available only after task identity has been accessed. Further, in task-switching paradigms, switch and no-switch trials are equally associated with each task cue, potentially making switch detection even more challenging.
An efficient strategy to decide about the necessity to switch consists in detecting any perceptual change between the task cue presented on the previous trial (and activated in working memory) and the new task cue (Monsell & Mizon, 2006). Any task cue match signals that the same goal must be maintained whereas the need to switch can be inferred from any task cue perceptual mismatch. Such a perceptual mismatch strategy is especially efficient in that it allows switch detection before any semantic processing of the task cue, which facilitates semantic processing on no-switch trials (or makes it even unnecessary) because individuals can quickly reactivate or keep active the task goal from the previous trial. However, this strategy is efficient as long as most changes in task cues actually concur with task switches. When two task cues are associated with each task (e.g., the cues “odd-even” and “parity” for a parity task, and the cues “high-low” and “magnitude” for a magnitude task), a change in task cue can concur with a task switch (e.g., from “high-low” [magnitude task] on trial N-1 to “parity” [parity task] on trial N) but can also occur when the same task repeats (e.g., from “high-low” [magnitude task] on trial N-1 to “magnitude” on trial N [magnitude task]). Consistent with the perceptual mismatch hypothesis, task cue changes incur local costs in adults even when no task switch is required (Logan & Bundesen, 2003; Monsell & Mizon, 2006; Logan & Schneider, 2006). The magnitude of the cue-change cost (without task switch) is all the more important when switch trials are frequent, making it highly probable that a change in task cues does signal switch trials (Monsell & Mizon, 2006). Thus, adults likely use switch probability in combination with task cue changes to infer switches.
Although the perceptual mismatch strategy considerably decreases the amount of task cue processing on no-switch trials, it requires maintaining the information on the task cue of the previous trial in working memory. Therefore, its cognitive cost/benefit ratio is probably less advantageous for young children who have lower working memory capacity on average in comparison to adults (e.g., Gathercole, Pickering, Ambridge, & Wearing, 2004). In addition, such a strategy implies substantial metacognitive skills (to estimate the probability of switches, spot the association between switches and cue changes, and use this information strategically) that preschoolers lack. Indeed preschoolers semantically process task cues more independently on each trial than do adults (or to completely fail at times to process task cues—regardless of the type of trial—when they use the same dimension across all mixed-block trials; Chevalier et al., 2010). Preschoolers’ similar semantic processing of task cues on all trials of the mixed block would explain why task cue transparency affects mixing costs and not local costs (i.e., same effect on switch and no-switch trials). In contrast, the perceptual mismatch strategy in adults likely leads to deeper or more frequent semantic processing of task cues on switch trials than no-switch trials, hence accounting for the effect of task cue transparency on local costs in adults (i.e., stronger effect on switch than no-switch trials). Most importantly, if, as we hypothesize, preschoolers do not use perceptual change in task cues to infer switches, then they must encounter switch detection difficulty and thus they should benefit from environmental information that directly signals the necessity to switch task.
Unlike task cues, transition cues (e.g., “same” for no-switch trials and “different” for switch trials) directly signal the type of transition required on each trial and thus provide direct information regarding switch detection. Transition cues have never been used with children, but adults show poorer overall performance and larger local costs with transition cues than with traditional task cues (Forstmann, Brass, & Koch, 2005; Van Loy, Liefooghe, & Vandierendonck, 2010; Saeki & Saito, 2009; Schneider & Logan, 2006). Any switch detection advantage conferred by transition cues likely is overridden by the necessity of combining transition cue information with information about the previous task goal in working memory in order to infer the newly relevant task goal, which probably requires additional endogenous control as suggested by stronger frontolateral and frontomedial activation with transition cues than task cues (Forstmann, Brass, Koch, & Cramon, 2005). Based on findings in adults, children likely would not behave more flexibly if task cues were replaced with transition cues because any advantage in terms of improved switch detection would be overridden by additional demands on endogenous control to activate the specific goal. However, as preschoolers probably encounter acute difficulty in switch detection, they may benefit from transition cues when such cues are combined with task cues so that preschool children may benefit from both transition cues to directly detect switches and task cues to directly infer task identity.
In the present study, we investigated the role of switch detection in cognitive flexibility in 5- to 6-year-old children, a developmental period when children are expected to select and maintain goals with increasing efficiency in task-switching paradigms. Children were tested on versions of the Shape School that required switching between color- and shape-matching tasks as a function of transition and task cues. All versions contained visual task cues. Critically, we contrasted conditions with only task cues and conditions with both visual task cues and auditory transition cues. If, as we hypothesized, children encounter switch detection difficulty because they do not use the perceptual mismatch strategy, they should perform better in conditions with transition cues than in conditions without such cues. As detecting switches is critical switching per se, transition cues should yield benefits on switch trials especially, hence reducing local costs.
In addition, the perceptual mismatch strategy in adulthood suggests that switch detection can be based at least partially on perceptual processes. The question arises as whether transition cues need to be semantically processed to help children detect switches or if the perceptual change in transition cues, which occurs infrequently since there are fewer switch trials, will be sufficient to enhance switch detection. To answer this question, we manipulated the degree of transition cue transparency, that is, the degree with which these cues were semantically associated with trial type (switch vs. no-switch). Further, children’s explicit knowledge of transition cue meanings was checked after completion of the Shape School. If semantic processing is required to benefit from transition cues, children should perform better with transparent transition cues (“same” for no-switch trials and “different” for switch trials) than arbitrary transition cues (the pseudo-words “kopo” and “jada”) because semantic processing is easier for transparent cues. Similarly, children showing explicit knowledge of cue meanings, reflecting deeper processing, should outperform those who fail to recall cue meanings. In contrast, if semantic processing is not necessary to detect switches, transparent transition cues should not help any further than arbitrary ones, and explicit knowledge of transition cue meanings should not influence performance.
Finally, we also varied the degree of transparency of task cues in order to explore the extent to which the difficulty of switch detection was dependent on the difficulty of task selection. Given that this study was the first to combine task cues and transition cues of which we are aware, we had no specific prediction regarding the interaction of task cue transparency and transition cue transparency. Based on previous studies in children, we expected task cue transparency to affect mixing costs but not local costs (Blaye & Chevalier, in press; Chevalier & Blaye, 2009).
Method
Participants
Study participants included fifty-seven 5- to 6-year old children from a small Midwestern city (M = 67.39 months, SD = 3.70 months, range = 59–73 months). They were randomly split in two experimental conditions: 29 children were assigned to the Arbitrary Task Cue condition and 28 children to the Transparent Task Cue condition. Information about age, sex, race, gross household monthly income, levels of maternal education, and verbal abilities (as assessed by the Verbal Comprehension section of the abbreviated form of the Woodcock-Johnson III; Brief Intellectual Ability assessment [BIA]; Woodcock, McGrew, & Mather, 2001) is provided in Table 1 (income, maternal education and race information was missing for one child in the Arbitrary Task Cue condition). None of these variables significantly differed across conditions. Before enrollment in the study, parents completed a telephone screening to ensure that children were not diagnosed with developmental or language delays or behavioral disorders. At screening, all parents reported their children were monolingual. Parental informed consent was obtained for all children prior to participation.
Table 1.
Demographic Information and Verbal Abilities for Participants in the Arbitrary Task Cue
| Variables | Task Cue Condition | Condition Comparison | |
|---|---|---|---|
| Arbitrary Task Cue | Transparent Task Cue | ||
| N | 29 | 28 | |
| Age (years) |
M = 5.56 SD = .33 Range = 59–73 months |
M = 5.69 SD = .30 Range = 60–73 months |
t(55) = −1.60, p = .11 |
| Sex | Girls: 41% Boys: 59% |
Girls: 43% Boys: 57% |
χ2(1, N = 57) = .01, p = .91 |
| Race | White Non-Hispanic: 93% Other: 7% |
White Non–Hispanic: 79% Other: 21% |
Fisher’s exact test (N = 56), p = .10 |
| Household monthly income(US $) |
M = 5,209 SD = 2,737 Range = 1,020–12,000 |
M = 4,811 SD = 2,374 Range = 674–11,250 |
t(54) = .58, p = .56 |
| Maternal education(years) |
M = 15.79 SD = 2.33 Range = 12–20 |
M = 16.32 SD = 3.22 Range = 12–26 |
t(54) = −.71, p = .47 |
| Verbal abilitiesa |
M = 105.72 SD = 16.92 Range = 75–138 |
M = 104.39 SD = 19.73 Range = 70–140 |
t(55) = .27, p = .78 |
Group and Transparent Task Cue Group
Note. Information about race, household monthly income, and maternal education was missing for one participant in the Arbitrary Task Cue condition.
Standard score for the Verbal Comprehension section of the BIA.
Materials
The Shape School (adapted from Espy, 1997; Espy et al., 2006) was run on a 14-in. monitor Latitude EA300 DELL laptop using Eprime 1.2 (Psychology Software Tools, Pittsburgh, PA) and responses were entered by pressing one of four horizontally arranged buttons on a response pad connected to the laptop. Children were presented with bidimensional stimuli and had to switch between a shape-matching task and a color-matching task by pressing one of four response buttons as quickly and accurately as possible. The instructions were conveyed in a story about school activities. Stimuli were 12 cm × 9 cm cartoon characters whose main body part was a square or a circle colored in blue or red. All stimuli were bivalent (i.e., they afforded responses on both color and shape). Each possible response option (red, blue, square, circle) corresponded to one of the response buttons. Two different response arrangements were created with the constraint that shape and color responses were interweaved. Each participant was assigned one response arrangement that remained the same throughout the session. Response arrangements were counterbalanced across participants. Response pictures (a red patch, a blue patch, a black square and a black circle) were affixed to the response buttons to decrease working memory demand related to retention of response button meanings. Participants responded with the same index finger on all trials and had to go back to a spot equidistant from all buttons after responding. This procedure was adopted because pilot data showed children had difficulty keeping their four fingers on response buttons simultaneously.
Two types of cues were used in the Shape School (Figure 1). All task cues (i.e., cues associated with the relevant dimension/matching task) were visual and integrated spatially with stimuli. Arbitrary task cues were associated arbitrarily with the dimensions (matching tasks) they signaled. For half of the participants, a top hat on the top of the stimulus signaled that color was relevant and a baseball cap signaled that shape was relevant, whereas the reverse was true for the other half. Transparent task cues were more strongly associated with dimensions: a baseball cap with a rainbow pattern signaled the color task whereas a baseball cap with geometrical shape patterns signaled the shape task. In contrast to task cues, transition cues (i.e., cues associated with trial types) were auditory. Transition cues were auditory to limit the complexity of visual information that children had to process and minimize encoding competition with task cues. Arbitrary transition cues consisted of pseudo-words. Half of the children heard “kopo” on simple-block and no-switch trials, and “jada” on switch trials, whereas the other half heard “jada” for simple-block and no-switch trials, and “kopo” on switch trials. Using pseudo-words ensured that these cues were arbitrarily associated with trial types. Transparent transition cues were highly associated with trial types: the word “same” on simple-block and no-switch trials, and “different” on switch trials. Finally, when no transition cues were used (i.e., children had no meaningful information on trial transition), children heard the same pseudo-word, “diyo”, on every trial in order to equate the amount of perceptual information to encode across conditions. All auditory cues had the same duration (600 ms) and were recorded by the same female voice. To ensure comparability with traditional versions of the Shape School, task cues and transition cues had the exact same onset as the stimuli (i.e., cues-stimulus interval = 0 ms). Each trial started with a 500-ms fixation point followed by the simultaneous onset of the stimulus, the task cue, and the transition cue. The stimulus and task cue remained on screen until the response was entered. The response triggered the next fixation point.
Figure 1.
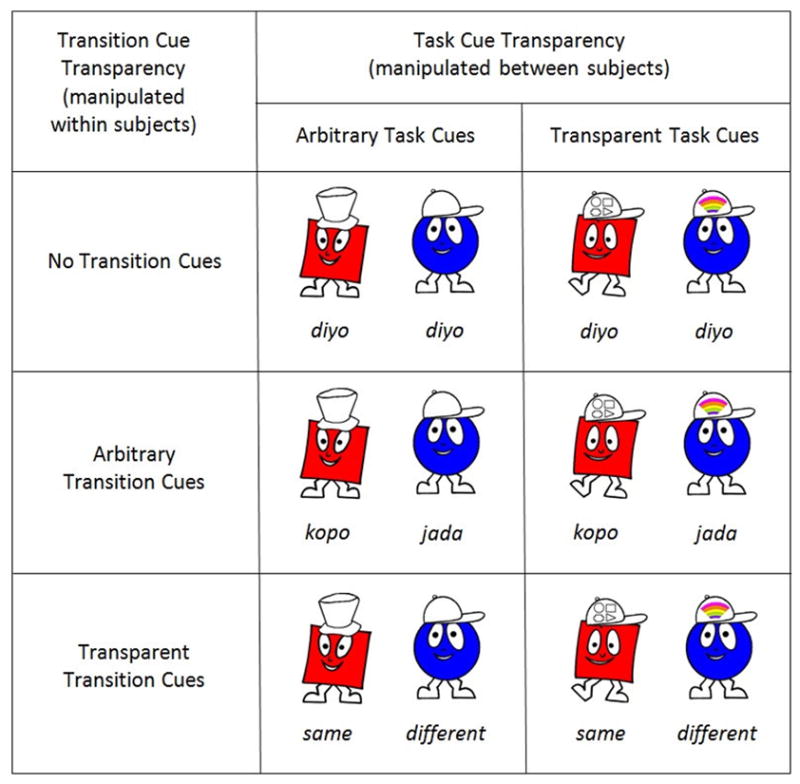
Illustration of task cues, transition cues and a subset of the stimuli used for each level of task cue transparency and transition cue transparency. In these examples, the character on the left appears on a no-switch trial and the one on the right on a switch trial. The contingency between arbitrary transition cues and trial type was counterbalanced. Task cues consist of hats on top of the stimuli and transition cues consist of auditory messages. In the No Transition Cue versions, the same auditory message was played on all trials (except for start trials).
Procedure
Each participant was tested individually by a trained examiner at the laboratory in a single session of approximately one hour. The session started with one version of the Shape School, followed by the Verbal Comprehension section of the BIA to assess verbal abilities and a second version of the Shape School. The child then was invited to take a 10-minute break during which (s)he received a snack. After the break, the child was administered the third version of the Shape School. Meanwhile, the accompanying parent filled out a short background questionnaire. The child received a developmentally appropriate toy after session completion.
Transition cue transparency (no transition cues, arbitrary transition cues, transparent transition cues) was manipulated within subjects, whereas task cue transparency (arbitrary task cues, transparent task cues) was manipulated between subjects. Half of the participants were assigned to the Arbitrary Task Cue condition and completed three versions of the Shape School, one for each modality of transition cue transparency (order counterbalanced across participants), all of which contained arbitrary task cues. The other half of participants were assigned to the Transparent Task Cue condition and took the three versions of the Shape School, corresponding to the three modalities of transition cue transparency (order counterbalanced across participants), all with transparent task cues. Cue combinations are illustrated in Figure 1.
Children were told that they would see cartoon characters and they would have to press the button corresponding to each character’s name. Some characters were from Mr. Color’s class and their names were their color whereas other characters were in Ms. Shape’s class and their names were their shapes. To know which class each character belonged, they would have to look at the character’s hat and listen to the auditory message. Then, it was stressed that all characters from Mr. Color’s class wore the same hat [color task cue] and all characters from Ms. Shape’s class had the other hat [shape task cue]. To help them decide about the characters’ class, they would also hear [no-switch transition cue] when the character belonged to the same class as the previous character, and [switch transition cue] when the character belonged to the other class.
Each version started with two simple blocks where children were told that all the characters that they would see were in the same class and they were explicitly told the name of the class. There were 4 warm-up trials and 15 subsequent test trials in each simple block. In such blocks, the same dimension-matching task was relevant constantly across all trials, but it changed when the participant moved from the first to the second simple block (e.g. color was relevant in the first simple block and shape in the second one). Dimension order was counterbalanced across conditions and participants. If one dimension came first for the first version a participant completed, then the second version started with the other dimension. At the beginning of each simple block, the relevant dimension and corresponding task cue were emphasized and, for the relevant conditions, the examiner also pointed out that the children would hear the non-switch transition cue because all stimuli belonged to the same class. After completion of the two simple blocks, children were told that they would see characters from both classes at the same time and proceeded to the mixed block. Color and shape task cues and, in the relevant conditions, switch and non-switch transitions cues were emphasized along with dimensions being mixed on subsequent trials. The mixed block contained 6 warm-up trials and 44 test trials and was split in two parts separated by a short break. Mixed-block test trials fell into 14 switch trials (i.e. the relevant task changes), 28 no-switch trials (i.e. the relevant task repeats), and 2 start trials (the very first trial of the block plus the first trial after the break). Start trials for each block contained no transition cues because these trials marked no transitions. The mixed block in each version contained equal numbers of each stimulus and each correct response, as well as equal numbers of color-matching and shape-matching trials. The color- and shape-matching tasks switched unpredictably. In all blocks, the examiner provided guidance and feedback on warm-up trials (e.g., “What did you hear? So what does it mean? Is he in the same class or in the other class? Look! Which class is it? Yes, that’s why he wears a [task cue]. So what’s his name? Where do you press?”). Children did not receive any help or feedback on any test trials. At the end of each version, children were asked to recall the meaning of each task cue and transition cue (except for the No Transition Cue conditions where they were queried about task cue meanings only) to check their knowledge of cue meanings (e.g., “What class, color or shape [order counterbalanced], did [cue] mean?”, “Did [cue] mean the same or a different class [order counterbalanced]?”).
Data Analysis
All analyses were run after discarding the start trial of each block and the start trial following the mixed-block break, because these were neither switch nor no-switch trials and contained no transition cues. Outliers (> 10,000 ms, < 200 ms) also were excluded (1.2% of trials). In addition, RT analyses were performed including only successful trials immediately preceded by a successful trial. Children with missing data for some cells of the experimental design (e.g., switch trials for shape in the Arbitrary Task Cue – No Transition Cue condition) were excluded from RT analyses (N = 7). Data were analyzed using mixed ANOVAs and planned contrasts. When appropriate (as evidenced by Mauchly’s [1940] tests), the Greenhouse-Geisser correction (Greenhouse & Geisser, 1959) was applied for violation of the assumption of sphericity. Mixing costs were examined, contrasting simple-block trials1 and no-switch trials of the mixed block. Local costs were examined, contrasting switch and no-switch trials within the mixed block.
Results
Cue Questions
About half of the children demonstrated explicit knowledge of transition cues by correctly recalling their meanings (i.e., they answered all 4 questions correctly): 15 children in the Arbitrary Task Cue condition and 14 children in the Transparent Task Cue condition. To investigate the extent to which explicit knowledge of transition cue meanings moderated performance, we categorized children into two groups: successful recall and failed recall (one or more errors). Unlike transition cues, the vast majority of children correctly answered questions about task cues. Only five children in the Arbitrary Task Cue condition and four children in the Transparent Task Cue condition failed to recall task cue meanings (two of them in each condition also failed questions about transition cues). As the main focus of the present experiment was on transition cues, children who failed to recall task cue meanings were dropped from subsequent analyses in order to ensure that any potential effect of transition cue knowledge was not driven by task cue knowledge. Therefore, the final sample included 24 children in each condition. Out of these children, 12 passed and 12 failed transition cue questions in each condition. The children who correctly recalled transition cue meanings came from households whose gross monthly income was significantly higher than children who failed to recall transition cue meanings (M = $5,067, SD = 2,428, and M = $4,048, SD = 2,072 respectively, t(45) = 2.67, p = .01), and demonstrated marginally higher verbal abilities (M successful recall group = 111, SD = 17 vs. M failed recall group = 102, SD = 18), t(46) = 1.74, p = .08. The two groups did not significantly differ in mean age, sex, race/ethnicity, or maternal education (all ps > .10).
Accuracy and Latency
The performance of children who correctly answered task cue questions is reported in in Table 2. Their performance was analyzed with 2 (Task Cue) × 2 (Recall Group) × 3 (Transition Cue) × 3 (Trial Type) × 2 (Dimension) mixed ANOVAs with Task Cue and Recall Group as between-subjects variables and Transition Cue, Trial Type, and Dimension as within-subjects variables. Dimension was included in these analyses because prior studies have shown asymmetrical local and mixing costs between color and shape tasks (e.g., Ellefson et al., 2006). One ANOVA was run on accuracy rates and the other on latency. They yielded significant main effects of trial type on both accuracy, F(2, 88) = 37.55, MSE = .03, p < .0001, partial η2 = .46, and latency, F(2, 74) = 138, MSE = 1082462, p < .0001, partial η2 = .79. Accuracy decreased across simple-block trials (M = 91%), no-switch trials (M = 86%) and switch trials (M = 79%), leading to significant mixing cost (M = 5.4%), F(1,44) = 12.02, MSE = .41, p = .001, partial η2 = .22, and local costs (M = 6.8%), F(1,44) = 44.68, MSE = .18, p < .0001, partial η2 = .50. Reaction times increased across simple-block trials (M = 1420 ms), no-switch trials (M = 2298 ms), and switch trials (M = 2980 ms), yielding significant mixing cost (M = 877 ms), F(1, 37) = 143.48, MSE = 7865265, p < .0001, partial η2 = .79, and local cost (M = 682 ms), F(1, 37) = 49.71, MSE = 13731947, p < .0001, partial η2 = .57. Significant mixing costs show that repeating a given task was more difficult when tasks changed unpredictably than when the same task was relevant on the entire block. Significant local costs show that switching to the other task was more difficult than repeating the same task in the mixed block.
Table 2.
Mean Accuracy in Percentage and Latency in Milliseconds (and Standard Errors) as a Function of Transition Cues, Task Cues, Trial Type, Dimension, and Recall Group.
| Transition Cues Trial Type | Dimension | Accuracy (%) |
Latency (ms) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Arbitrary Task Cues | Transparent Task Cues | Arbitrary Task Cues | Transparent Task Cues | ||||||
| Successful Recall | Failed Recall | Successful Recall | Failed Recall | Successful Recall | Failed Recall | Successful Recall | Failed Recall | ||
| No Transition Cues | |||||||||
| Simple-block trials | Color | 86.9 (5.7) | 92.8 (5.7) | 83.6 (5,7) | 92.3 (5.7) | 1366 (201) | 1495 (201) | 1281 (210) | 1325 (222) |
| Shape | 97 (3.8) | 92.9 (3.8) | 91.4 (3.8) | 84.5 (3.8) | 1759 (139) | 1325 (139) | 1415 (145) | 1396 (153) | |
| No-Switch trials | Color | 91 (4.6) | 87.9 (4.6) | 86.9 (4.6) | 81.6 (4.6) | 2425 (213) | 2050 (213) | 2294 (223) | 1854 (235) |
| Shape | 95.8 (6.5) | 76.1 (6.6) | 80.2 (6.5) | 88 (6.5) | 2275 (212) | 2553 (212) | 2404 (222) | 2103 (234) | |
| Switch trials | Color | 76.2 (6.4) | 75 (6.4) | 77.4 (6.4) | 70.4 (6.4) | 2917 (338) | 3431 (338) | 2960 (355) | 2238 (374) |
| Shape | 80.9 (8.1) | 64.3 (8.1) | 75 (8.1) | 76 (8.1) | 3035 (245) | 3543 (245) | 3151 (257) | 2355 (271) | |
| Arbitrary Transition Cues | |||||||||
| Simple-block trials | Color | 96.4 (3.7) | 88.5 (3.7) | 97.6 (3.7) | 90.5 (3.7) | 1321 (142) | 1337 (142) | 1305 (149) | 1509 (157) |
| Shape | 91.1 (4.9) | 95.8 (4.9) | 82.4 (4.9) | 94.6 (4.9) | 1533 (194) | 1627 (194) | 1719 (204) | 1440 (215) | |
| No-Switch trials | Color | 83.7 (5.1) | 73.6 (5.1) | 89.3 (5.1) | 90.9 (5.1) | 2430 (246) | 2405 (246) | 2072 (258) | 1901 (272) |
| Shape | 83.9 (5.8) | 68.3 (5.8) | 84.8 (5.8) | 87.5 (5.8) | 2795 (303) | 2925 (303) | 2307 (318) | 2450 (335) | |
| Switch trials | Color | 84.3 (6.3) | 65.1 (6.3) | 85.7 (6.3) | 76.2 (6.3) | 3005 (305) | 3500 (305) | 2667 (320) | 2589 (337) |
| Shape | 85.2 (5.3) | 71.2 (5.3) | 85.5 (5.3) | 83.3 (5.3) | 3434 (413) | 3785 (413) | 3209 (433) | 2605 (457) | |
| Transparent Transition Cues | |||||||||
| Simple-block trials | Color | 96.4 (4.1) | 91.1 (4.1) | 88.7 (4.1) | 88.1 (4.1) | 1284 (93) | 1239 (93) | 1220 (97) | 1283 (103) |
| Shape | 95.8 (4.8) | 93.5 (4.8) | 91.4 (4.8) | 81.5 (4.8) | 1471 (181) | 1565 (181) | 1476 (190) | 1397 (200) | |
| No-Switch trials | Color | 93.4 (5.7) | 83.5 (5.7) | 88.5 (5.7) | 82.7 (5.7) | 2189 (212) | 2327 (212) | 1790 (223) | 2315 (235) |
| Shape | 89.3 (4.5) | 85.1 (4.5) | 92.1 (4.5) | 91.9 (4.5) | 2433 (253) | 2548 (253) | 2053 (265) | 2248 (279) | |
| Switch trials | Color | 86.9 (5.8) | 74.4 (5.8) | 86 (5.8) | 86.9 (5.8) | 2560 (365) | 3314 (364) | 2514 (382) | 2724 (402) |
| Shape | 83.1 (5.6) | 76.8 (5.6) | 84.3 (5.6) | 82.9 (5.6) | 3025 (308) | 3345 (308) | 2741 (323) | 2876 (341) | |
The effects of transition cues and task cue transparency on mixing costs and local costs are organized below as a function of the three questions addressed in this study. In addition to the effects reported below, there was a significant main effect of dimension on latency, F(1, 37) = 30.02, MSE = 292713, p < .0001, partial η2 = .45. Reaction time were longer for the shape-matching task (M = 2342 ms) than the color-matching task (M = 2123 ms). However, dimension did not interact with any other variables (ps > .09), hence failing to show any asymmetrical switch costs between color and shape. All other effects were not significant (all ps > .07).
Do children benefit from direct information signaling the necessity to switch?
To answer this question, we examined whether children benefited from the presence of transition cues, looking specifically at the main effect of transition cues and its interaction with trial types. These effects, if significant, were further explored with planned comparisons between each of the Arbitrary and Transparent Transition Cue conditions and the No Transition Cue conditions (the comparisons between transparent and arbitrary transition cues are presented in the next subsection). For accuracy, the main effect of transition cue was not significant (p > .12), but transition cue significantly interacted with trial type, F(4,176) = 3.89, MSE = .02, p = .01, partial η2 = .08. This interaction is illustrated in Figure 2. Independent of task cues, local costs were significantly lower in the Arbitrary and Transparent Transition Cue conditions (M = 3.2% and M = 5%, respectively) than in the No Transition Cue Conditions (M = 11%), F(1,44) = 5.33, MSE = .17, p = .02, partial η2 = .46, and F(1,44) = 9.58, MSE = .07, p = .003, partial η2 = .18, respectively (in the “local costs” section of the bottom panel of Figure 2, the gray and white bars are shorter than the black bar). In contrast, the mixing cost in the No Transition Cue conditions (M = 4.2%) did not differ from those in the Transparent and Arbitrary Transition Cue conditions (M = 2.5% and M = 9.3%, respectively), ps > .10 (see the “mixing costs” section of the bottom panel of Figure 2). Thus, relative to “traditional” situations without any transition cues, the presence of transition cues, irrespective of their transparency, helped children switch accurately between tasks, as suggested by the specific effect of transition cues on local costs (which isolate the difficulty of switching) and not mixing costs (which reflect the difficulty of deciding between two potential tasks).
Figure 2.
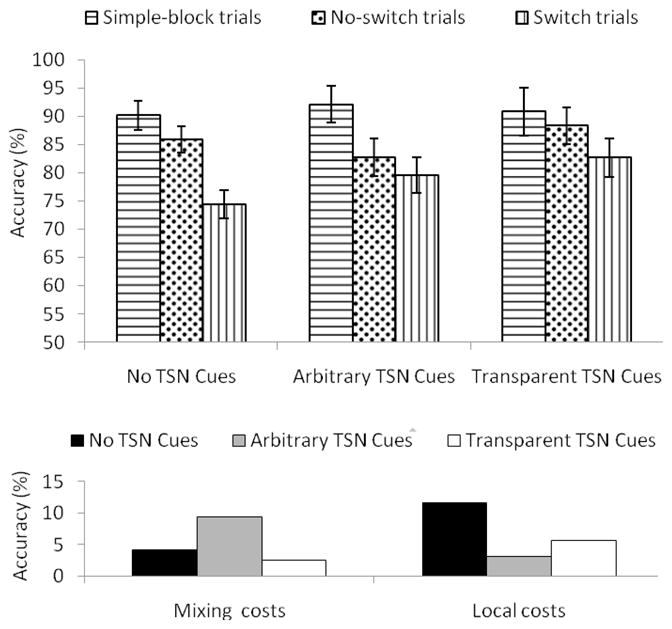
Mean accuracy rates in percentage as a function of transition cues and trial types (top panel) and corresponding mixing costs and local costs (bottom panel). TSN = transition. Mixing costs correspond to the drop in performance between simple-block trials and no-switch trials. Local costs correspond to the drop in performance between no-switch trials and switch trials. Error bars indicate standard errors of the means.
For latency, transition cues had a significant main effect, F(2, 74) = 3.42, MSE = 518353, p = .03, partial η2 = .09, but did not interact with trial type (p > .88). Reaction times were marginally longer with arbitrary transition cues (M = 2328 ms) than without any transition cues (M = 2206 ms), F(1,37) = 3.10, MSE = 7009258, p = .08, partial η2 = .08 (in the “combined” section of Figure 3, the gray bar is the highest). Transparent Transition Cue (M = 2328 ms) did not differ from No Transition Cue conditions, p = .48 (white vs. black bars in the “combined” section of Figure 3). This finding suggests that, in contrast to their beneficial effect on accuracy, transition cues did not facilitate children’s speed of performance overall. In fact, responses tended to be even longer when transition cues were arbitrary.
Figure 3.
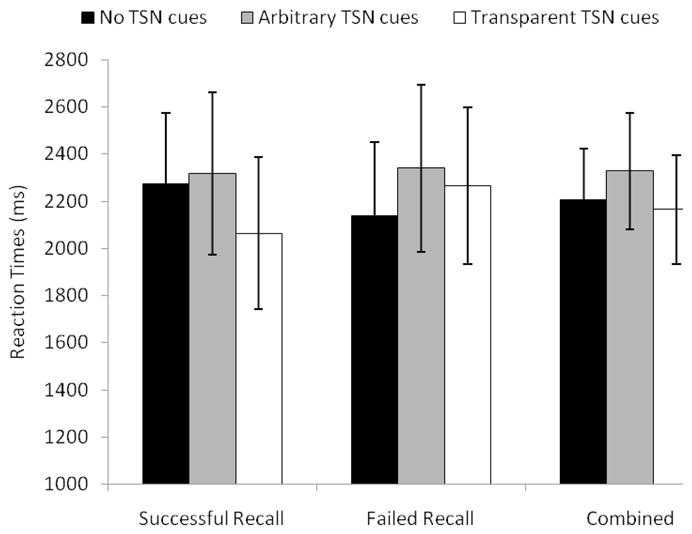
Mean reaction times in milliseconds as a function of transition cues and recall groups. TSN = transition. Error bars indicate standard errors of the means.
What is the role of semantic processing of transition cues in improving switch detection?
The first method of examining semantic processing of transition cues was to look at the significant interaction between transition cues and trial types for accuracy and the significant main effect of transition cues for latency reported in the previous subsection, but this time, to compare between Transparent Transition Cue conditions and Arbitrary Transition Cue conditions. For accuracy, Arbitrary and Transparent Transition Cue conditions did not differ in local cost (Arbitrary Transition Cues: M = 3.2%; Transparent Transition Cues: M = 5.7%), p > .11 (gray vs. white bars in the “local section” of the bottom panel of Figure 2). However, transparent transition cues yielded smaller mixing costs than arbitrary transition cues, F(1,44) = 5.33, MSE = .17, p = .02, partial η2 = .11 (white vs. gray bars in the “mixing costs” section of the bottom panel of Figure 2). In addition, reaction times were overall (i.e., irrespective of the trial type) longer with arbitrary transition cues (M = 2328 ms) than with transparent transition cues (M = 2164 ms), F(1,37) = 6.23, MSE = 6322436, p = .01, partial η2 = .23 (gray vs. white bars in the “combined” section of Figure 3). These findings suggest that transparent transition cues may not help detect switches any more efficiently than arbitrary transition cues. However, transparent transition cues were more quickly processed overall in comparison to arbitrary transition cues.
A second way of examining the issue of semantic processing was to determine if the effects of transition cues differed whether children correctly recalled their meanings or failed to do so. The main effect of the recall group and all the related interactions were all non significant for accuracy (ps > .09). For latency, there was a significant Transition Cues × Recall Group interaction, F(2, 74) = 3.35, MSE = 518353, p = .04, partial η2 = .08 (Figure 3). For the successful recall group, latency was shorter with transparent transition cues (M = 2063 ms) than with arbitrary transition cues (M = 2317 ms) and without any transition cues (M = 2273 ms), F(1, 37) = 7.67, MSE = 526870, p = .008, partial η2 = .17, and F(1, 37) = 6.27, MSE = 444085, p = .01, partial η2 = .15, respectively (in the “successful recall” section of Figure 3, the white bar is the shortest). In contrast, for the failed recall group, latency marginally increased from the No Transition Cue conditions (M = 2139 ms) to the Arbitrary Transition Cue conditions (M = 2339 ms), F(1, 37) = 4.09, MSE = 584105, p = .05, partial η2 = .10 (in the “failed recall” section of Figure 3, the black bar is the shortest). These results suggest that the children who recalled transition cue meanings successfully, and thus were the most likely to process their meanings, performed the fastest when transition cues were transparent, whereas arbitrary transition cues tended to slow down responses of the children who failed to recall transition cue meanings.
Is the difficulty of switch detection dependent on the difficulty of task selection?
To determine the extent to which switch detection and task selection are related, we examined the interactions involving both task cue transparency and transition cues. None of these interactions were significant (all ps > .11), suggesting that the effect of transition cues was not moderated by task cue transparency.
Independent of transition cues, task cue transparency significantly interacted with trial type on both accuracy, F(2,88) = 4.92, MSE = .03, p = .01, partial η2 = .10, and reaction times, F(2, 74) = 3.20, MSE = 1082462, p = .04, partial η2 = .08. Relative to arbitrary task cues, transparent task cues led to significantly smaller accuracy mixing costs (Transparent Task Cues: M = 1.9%; Arbitrary Task Cues: M = 8.9%), F(1,44) = 5.12, MSE = .41, p = .02, partial η2 = .10, and marginally smaller latency mixing costs (Transparent Task Cues: M = 752 ms; Arbitrary Task Cues: M = 1003 ms), F(1, 37) = 2.94, MSE = 7865265, p = .09, partial η2 = .07. In contrast, task cue transparency did not affect local costs for either accuracy (Transparent Task Cues: M = 6.2%; Arbitrary Task Cues: M = 7.3%), or latency (Transparent Task Cues: M = 570 ms; Arbitrary Task Cues: M = 795 ms), ps > .25. Consistent with prior studies, task cue transparency reduced mixing costs only, suggesting that transparent task cues help children select the relevant task goal (on both switch and no-switch trials), but did not affect the processes specifically related to switching per se.
Discussion
The present study examined the role of switch detection in 5-year-olds’ flexibility by testing combinations of transition cues and task cues in the Shape School and varying the degree of transparency of both cue types. The first question of interest was whether children can benefit from transition cues that directly signal the necessity to switch tasks. The results showed that both arbitrary and transparent transition cues reduced local costs but not mixing costs on accuracy, suggesting that transition cues are especially beneficial on trials where children actually have to implement a task switch. Second, we investigated whether the semantic transparency of transition cue contributes to improving switch detection. Arbitrary transition cues led to greater accuracy mixing costs than transparent transitions and to longer reaction times overall. In addition, whereas arbitrary transition cues tended to increase reaction times for children who failed to recall transition cue meanings, reaction times were the fastest with transparent transition cues for children who correctly recalled transition cue meanings. These findings suggest that perceptual processing of transition cues is sufficient for transition cues to improve switch detection, but semantic processing may help children process cue information faster. Third, we investigated whether the difficulty of switch detection is moderated by the difficulty of task selection. The effect of transition cues did not interact with task cue transparency, suggesting that transition cues and task cues are relatively independent of each other in their effects on mixing and local costs.
The beneficial effect of transition cues shows that preschoolers gain from information that directly helps them detect switches when such information is presented in conjunction with task cues that directly signal the relevant task identity. This finding suggests that part of preschoolers’ goal management difficulty relates to detection of the necessity to switch to a new task goal and that switch detection can be improved experimentally. To our knowledge, this study is the first to disentangle the difficulty of switch detection from task selection across all ages and, as such, it extends a growing body of research pointing to goal management as a key component of preschoolers’ cognitive flexibility (e.g., Blaye & Chevalier, in press; Chevalier & Blaye, 2009; Chevalier, Blaye, Dufau, & Lucenet, 2010; Chevalier et al., 2010; Marcovitch et al., 2007; Morton & Munakata, 2002; Snyder & Munakata, 2010; Towse et al., 2007).
As expected, the presence of transition cues reduced local costs but not mixing costs, suggesting that transition cues were especially helpful when a switch was actually needed. Although children probably need to actively monitor for the necessity to switch or to maintain a task on all trials of the mixed block, the decision to switch or maintain may vary in difficulty between switch and no-switch trials. In the present study, no-switch trials outnumbered switch trials (2/3 versus 1/3 of trials, respectively), which may have led children to assume by default that the same task would repeat on the next trial, hence facilitating maintenance detection but making it more difficult to detect switches. Alternatively, independent of trial type contingency, switch detecting a switch may always be more difficult than maintenance detection because of some cognitive bias to least cognitively demanding activity, that is, repeating the same task. Either way, transition cues are expected to affect switch trials to a greater extent than no-switch trials with such a trial contingency. However, varying the ratio of switch and no-switch trials in future studies should clarify whether maintenance detection can be made easier than switch detection when no-switch trials outnumber switch trials so that children would expect by default a switch on the next trial. Such a finding would speak to switch detection processes occurring on all trials but with variable difficulty and would also inform on preschoolers’ sensitivity to trial contingency.
In contrast to the clearly beneficial effect of the presence of transition cues relative to task cues alone, the difference between arbitrary and transparent transition cues was not as evident. On the one hand, transparent transition cues did not lead to any greater substantive decrease in local costs than did arbitrary transition cues, showing that the difficulty of semantic processing does not moderate the benefit of transition cues. Further, successful recall of transition cue meanings had no effect on accuracy, suggesting that explicit knowledge about these cues and semantic processing of transition cues may not be necessary to facilitate switch detection. The relative infrequency of transition cues associated with a switch may sharply contrast with the frequent cues marking no-switch trials. This break in the flow of perceptual information may help children realize the switch status of those trials and increase their processing of task cues to select the relevant task goal, hence explaining the unique facilitating effect of transition cues on switch trials.
On the other hand, arbitrary transition cues yielded bigger mixing costs on accuracy than transparent transition cues, and also resulted in longer reaction times overall. These results suggest that arbitrary transition cues, but not transparent ones, have an overall detrimental effect (not related to switch detection) that interferes with performance on all trials. However, this interference is offset by the beneficial effect of arbitrary transition cues on switch detection in switch trials. Prior research in young children has shown that (a) auditory information is more salient than visual information, (b) the simultaneous presentation of both types of information results in auditory information overshadowing visual information (i.e., slowing down its processing), and (c) the overshadowing effect is stronger when auditory information is unfamiliar (Robinson & Sloutsky, 2007; 2008; 2010). Consistent with the overshadowing hypothesis, both the perceptual modality and the transparency of task cues affect preschoolers’ flexibility. More specifically, relative to visual task cues, auditory presentation of task cues increases the beneficial effect of transparent task cues on performance but also magnifies the detrimental effect of arbitrary task cues (Chevalier & Blaye, 2009). In the present study, the perceptual processing of auditory transition cues, especially arbitrary cues that are unfamiliar (pseudo-words), may have slowed down performance because of an overshadowing effect. Furthermore, the children who failed to recall correctly transition cue meanings were marginally slowest to respond with transition cues, whereas the children who recalled correctly responded fastest with transparent transition cues relative to arbitrary or no transition cues. The children who demonstrated explicit knowledge of transition cues probably processed these cues both perceptually and semantically, which helped them better activate their representations and remember their meanings. The easier semantic processing of transparent transition cues may have facilitated the speed at which these children processed these cues, hence reducing the overshadowing effect. In brief, perceptual processing is probably sufficient for transition cues to help children detect switches. Semantic processing of transparent transition cues may help further, in a more general fashion, to process auditory information faster and reduce the overshadowing effect on visual information. Using visual transition cues in future research should clarify this issue.
Interestingly, children who demonstrated explicit knowledge of transition cue meanings came from households with significantly higher gross monthly income, suggesting that children’s processing of transition cues was influenced by individual differences in sociodemographic background, consistent with a growing literature on broader executive control (e.g., Ardila, Rosselli, Matute, & Guajardo, 2005; Noble, McCandliss, & Farah, 2007; Noble, Norman, & Farah, 2005; Stevens, Lauinger, & Neville, 2009; Wiebe, Sheffield, Nelson, Clark, Chevalier, & Espy, in press). Children from higher sociodemographic backgrounds may draw upon higher working memory capacity to maintain transition cues active in memory and semantically process these cues.
Contrary to transition cues which mainly affected local costs, task cue transparency only affected mixing costs, suggesting that task cues similarly influence preschoolers’ performance on all trial types in the mixed block. Our current findings replicate those from previous investigations (Blaye & Chevalier, in press; Chevalier & Blaye, 2009), despite the additional presence of auditory information in the present study. More importantly for the present study, task cue transparency did not interact with the effect of transition cues. These findings suggest that switch detection and task selection component processes may be relatively independent of each other in preschoolers, although further research is needed to capture the dynamics of goal management and its development with age.
Preschoolers’ difficulty of switch detection on the Shape School may seem at odds with successful performance by age 4 on the Dimensional Change Card Sort (DCCS), a widespread paradigm where children need to sort bidimensional cards by one dimension (e.g. color) for a series of trials before switching to the other dimension (e.g., shape) for a second series of trials. However, this discrepancy likely results because on the DCCS, the switch is explicitly announced and the next relevant dimension is repeated by the experimenter before each trial, hence posing very minimal switch detection and task selection demands. In contrast on the Shape School, children have to monitor for the necessity to switch and select the relevant task on the basis of task cues, which probably explains why performance keeps improving on such tasks after 5 years of age (Cepeda et al., 2001; Chevalier & Blaye, 2009). Besides goal management demands, the requirement to switch back and forth between the same tasks may pose greater inhibitory demands than on the DCCS where children never have to return to a previously inhibited task (Diamond, 2009).
As many theoretical accounts of flexibility development have been designed to explain performance on the DCCS, they often overlook goal management and mainly focus on difficulties of attention reorientation to relevant stimulus information related to problems in inhibition (Kirkham, Cruess, & Diamond, 2003; Bialystok & Martin, 2004) or selective attention (Brooks, Hanauer, Padowska, & Rosman, 2003; Hanania & Smith, 2010). Accounts that address the role of goal management implicitly assume that children clearly identify the need to switch and the newly relevant goal but then fail to maintain it in working (or active) memory (Marcovitch et al., 2007; Morton & Munakata, 2002). The only account that directly considers task selection is perhaps the Cognitive Complexity and Control (CCC) theory (Zelazo et al., 2003), which stresses the role of higher-order rules that enable selection of the relevant task. However, this theory posits that children successfully use such higher-order rules by age 4 and thus fails to account for task selection difficulties arising after age 4 in paradigms such as the Shape School. Most importantly, the CCC theory does not capture switch detection. The difficulty of switch detection shown in the present study is perhaps closer to the claim that preschoolers do not understand the necessity to switch on the DCCS (Deák, 2003; Kloo & Perner, 2005). However, conceptual limitations (i.e., lack of understanding that a stimulus can be multi-represented by several dimensions; Kloo & Perner, 2005) that supposedly account for this phenomenon clearly do not apply to 5-year-olds who, unlike 3-year-olds in the DCCS, do not systematically perseverate in switching paradigms such as the Shape School.
As a result, extant theoretical accounts of flexibility development remain incomplete and do not satisfactorily account for the multi-faceted nature of flexible behavior, nor for the changes over the entire preschool period and beyond. The present findings suggest that a more comprehensive account is needed that encompasses all aspects of goal management: switch detection, task goal selection and goal maintenance. The next major step in this endeavor will be to build on the demonstration of switch detection difficulty in preschoolers to delineate its developmental course throughout childhood and pinpoint the factors that promote switch detection processes and, more generally, goal management. Based on previous research, three factors seem especially promising in this respect. First, as stated earlier, the development of working memory may support the shift to a perceptual mismatch strategy that allows more efficient switch detection with age, which is all the more plausible as working memory has been shown already to support another element of goal management, goal maintenance (Marcovitch, Boseovski, Knapp, & Kane, 2010). Second, prior findings suggest that with age preschoolers are increasingly able to use experience on past trials to better anticipate the necessity to switch and which task will be relevant next, hence pointing to the potential contribution of metacognitive knowledge (Chevalier et al., 2009). Third, although empirical evidence is lacking in children, language may play a prominent role in the development of goal management in the light of research showing that adults use inner speech to select the relevant task goal when task cues are arbitrary (Miyake et al., 2004; Cragg & Nation, 2010).
To identify the factors promoting goal management, future research should examine the situational features that foster or hamper switch detection and task selection. For instance, the difficulty of switch detection may be high when cues that do not systematically signal a switch and the lack of clearly delineated phases in between switches, which are common features of some widely used flexibility paradigms (e.g., Creature Counting task from Test of Everyday Attention for Children; Manly, Anderson, Nimmo-Smith, Turner, Watson, & Robertson, 2001). In contrast, task selection may be heavily taxed by the requirement to infer the relevant task from environmental cues and the availability of more than two potential tasks, as it is the case in some paradigms (e.g., Flexible Induction of Meaning task; Deák, 2000; Flexible Item Selection Test; Jacques & Zelazo, 2001, see Snyder & Munakata, 2010, for consistent findings). The experimental manipulation of such features should bring new insights regarding the switch detection and task selection component processes, which in turn have the potential to further refine the understanding of children’s flexible switching behavior manifested by performance on a variety of flexibility paradigms. Finally, the identification of such features has the potential to refine the assessment and remediation of switching impairments in clinical populations. For instance, the use of transition cues could support the acquisition of switching abilities in children with cognitive impairments related to a number of neurodevelopmental disabilities.
In conclusion, the present study showed that preschoolers perform better on the Shape School when task cues are combined with transition cues. These findings suggest that preschoolers’ task switching difficulty partly originates in the detection of the necessity to change task goals. By highlighting for the very first time the role of switch detection, the present findings extend a growing body of research that points to the multifaceted nature of goal management and to its major role in flexibility and, more broadly, executive control.
Acknowledgments
This work was supported by NIH grants MH065668, DA023653, DA024769, HD038051, and HD050309. We are grateful to the participating families and the entire team at the Developmental Cognitive Neuroscience Lab of the University of Nebraska-Lincoln.
Footnotes
Preliminary analyses revealed no difference in accuracy between the first and second simple blocks (M = 90%, SD = 16, versus M = 90%, SD = 18, respectively), p > .94, but reaction times significantly decreased from the first simple block to the second (M = 1486 ms, SD = 593 ms, versus M = 1357 ms, SD = 457 ms, respectively), F(1, 51) = 6.66, MSE = 178500, p < .05, partial η2 = .12. Despite the significant decrease in reaction times, performance on the two simple blocks were collapsed in subsequent analyses to ensure comparability with studies using task-switching paradigms where simple blocks are virtually always collapsed (e.g., Cepeda et al., 2001; Ellefson et al., 2006).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nicolas Chevalier, Department of Psychology, University of Nebraska-Lincoln, Lincoln, Nebraska.
Sandra A. Wiebe, Department of Psychology, University of Alberta, Edmonton, Alberta, Canada
Kristina L. Huber, Department of Psychology & Office of Research, University of Nebraska-Lincoln, Lincoln, Nebraska
Kimberly Andrews Espy, Department of Psychology & Office of Research, University of Nebraska-Lincoln, Lincoln, Nebraska.
References
- Altmann EM, Trafton JG. Memory for goals: An activation-based model. Cognitive Science. 2002;26:39–83. [Google Scholar]
- Arbuthnott KD, Woodward TS. The influence of task-cue association and location on switch cost and alternating-switch cost. Canadian Journal of Experimental Psychology. 2002;56:18–29. doi: 10.1037/h0087382. [DOI] [PubMed] [Google Scholar]
- Ardila A, Rosselli M, Matute E, Guajardo S. The influence of the parents’ educational level on the development of executive functions. Developmental Neuropsychology. 2005;28:539–560. doi: 10.1207/s15326942dn2801_5. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Chincotta D, Adlam A. Working memory and the control of action: Evidence from task switching. Journal of experimental psychology: General. 2001;130:641–657. [PubMed] [Google Scholar]
- Bialystok E, Martin MM. Attention and inhibition in bilingual children: Evidence from the dimensional change card sort task. Developmental Science. 2004;7:325–339. doi: 10.1111/j.1467-7687.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- Blaye A, Chevalier N. The role of goal representation in inhibition and flexibility. Journal of Experimental Child Psychology. doi: 10.1016/j.jecp.2010.09.006. (in press) [DOI] [PubMed] [Google Scholar]
- Brooks PJ, Hanauer JB, Padowska B, Rosman H. The role of selective attention in preschoolers’ rule use in a novel dimensional card sort. Cognitive Development. 2003;18:195–215. [Google Scholar]
- Bull R, Espy KA, Wiebe SA, Sheffield TD, Nelson JM. Using confirmatory factor analysis to understand executive control in preschool children: Sources of variation in emergent mathematic skills. Developmental Science. doi: 10.1111/j.1467-7687.2010.01012.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM. Developmentally sensitive measures of executive function in preschool children. Developmental Neuropsychology. 2005;28:595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Moses LJ. Individual differences in inhibitory control and children’s theory of mind. Child Development. 2001;72:1032–1053. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JC. Changes in executive control across the life span: Examination of task-switching performance. Developmental Psychology. 2001;37:715–730. [PubMed] [Google Scholar]
- Chevalier N, Blaye A. Setting goals to switch between tasks: Effect of cue transparency on children’s cognitive flexibility. Developmental Psychology. 2009;45:782–797. doi: 10.1037/a0015409. [DOI] [PubMed] [Google Scholar]
- Chevalier N, Blaye A, Dufau S, Lucenet J. What visual information do children and adults consider while switching between tasks? Eye-tracking investigation of cognitive flexibility development. Developmental Psychology. 2010;46:955–972. doi: 10.1037/a0019674. [DOI] [PubMed] [Google Scholar]
- Chevalier N, Dauvier B, Blaye A. Preschoolers’ use of feedback for flexible behavior: Insights from a computational model. Journal of Experimental Child Psychology. 2009;103:251–267. doi: 10.1016/j.jecp.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Chevalier N, Sheffield TD, Nelson JM, Clark CAC, Wiebe SA, Espy KA. Underpinnings of the costs of flexibility in preschool children: The roles of inhibition and working memory. 2010. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg L, Chevalier N. The processes underlying cognitive flexibility in childhood. The Quarterly Journal of Experimental Psychology. doi: 10.1080/17470210903204618. (in press) [DOI] [PubMed] [Google Scholar]
- Cragg L, Nation K. Shifting development in mid-childhood: The influence of between-task interference. Developmental Psychology. 2009;45:1465–1479. doi: 10.1037/a0015360. [DOI] [PubMed] [Google Scholar]
- Cragg L, Nation K. Language and the development of cognitive control. Topics in Cognitive Science. 2010;2:631–642. doi: 10.1111/j.1756-8765.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- Deák GO. The growth of flexible problem solving: Preschool children use changing verbal cues to infer multiple word meanings. Journal of Cognition and Development. 2000;1:157–191. [Google Scholar]
- Deák GO. The development of cognitive flexibility and language abilities. In: Kail R, editor. Advances in Child Development and Behavior. Vol. 31. San Diego, CA: Academic Press; 2003. pp. 271–327. [DOI] [PubMed] [Google Scholar]
- Diamond A. The early development of executive functions. In: Bialystok E, Craik FIM, editors. Lifespan cognition mechanisms of change. Oxford: Oxford University Press; 2006. pp. 70–95. [Google Scholar]
- Diamond A. All or none hypothesis: A global-default mode that characterizes the brain and mind. Developmental Psychology. 2009;45:130–138. doi: 10.1037/a0014025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefson MR, Shapiro LR, Chater N. Asymmetrical switch costs in children. Cognitive Development. 2006;21:108–130. [Google Scholar]
- Espy KA. The Shape School: Assessing executive function inpreschool children. Developmental Neuropsychology. 1997;13:495–499. [Google Scholar]
- Espy KA, Bull R, Martin J, Stroup W. Measuring the development of executive control with the Shape School. Psychological Assessment. 2006;18:373–381. doi: 10.1037/1040-3590.18.4.373. [DOI] [PubMed] [Google Scholar]
- Espy KA, Cwik MF. The development of a Trail Making Test for young children: The TRAILS-P. The Clinical Neuropsychologist. 2004;18:1–12. doi: 10.1080/138540409052416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy KA, Wiebe SA, Sheffield TD, Clark CAC, Moehr MJ. Executive control and dimensions of problem behaviors in preschool children. Journal of Child Psychology and Psychiatry. 2011;52:33–46. doi: 10.1111/j.1469-7610.2010.02265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Brass M, Koch I. Methodological and empirical issues when dissociating cue-related from task-related processes in the explicit task-cuing procedure. Psychological Research. 2005;71:393–400. doi: 10.1007/s00426-005-0040-4. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Brass M, Koch I, von Cramon DY. Internally generated and directly cued task sets: An investigation with fMRI. Neuropsychologia. 2005;43:943–952. doi: 10.1016/j.neuropsychologia.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Developmental Psychology. 2004;40:177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Garon N, Bryson SE, Smith IM. Executive functions in preschoolers: A review using an integrative framework. Psychological Bulletin. 2008;134:31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Gruber O, Goschke T. Executive control emerging from dynamic interactions between brain systems mediating language, working memory and attentional processes. Acta Psychologica. 2004;115:105–121. doi: 10.1016/j.actpsy.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Hanania R, Smith LB. Selective attention and attention switching: towards a unified developmental approach. Developmental Science. 2010;13:622–635. doi: 10.1111/j.1467-7687.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques S, Zelazo PD. The Flexible Item Selection Task (FIST): A measure of Executive Function in preschoolers. Developmental Neuropsychology. 2001;20:573–591. doi: 10.1207/S15326942DN2003_2. [DOI] [PubMed] [Google Scholar]
- Jamadar S, Michie PT, Karayandis F. Sequence effects in cued task switching modulate response preparedness and repetition priming processes. Psychophysiology. 2010;47:365–386. doi: 10.1111/j.1469-8986.2009.00932.x. [DOI] [PubMed] [Google Scholar]
- Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, Koch I. Control and interference in task switching—A review. Psychological Bulletin. 2010;136:849–874. doi: 10.1037/a0019842. [DOI] [PubMed] [Google Scholar]
- Kirkham NZ, Cruess L, Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Developmental Science. 2003;6:449–467. [Google Scholar]
- Kloo D, Perner J. Disentangling dimensions in the dimensional change card-sorting task. Developmental Science. 2005;8:44–56. doi: 10.1111/j.1467-7687.2005.00392.x. [DOI] [PubMed] [Google Scholar]
- Koch I, Prinz W, Allport A. Involuntary retrieval in alphabetic-arithmetic tasks: Task-mixing and task-switching costs. Psychological Research. 2005;69:252–251. doi: 10.1007/s00426-004-0180-y. [DOI] [PubMed] [Google Scholar]
- Kray J, Lindenberger U. Adult age differences in task switching. Psychology and Aging. 2000;15:126–147. doi: 10.1037//0882-7974.15.1.126. [DOI] [PubMed] [Google Scholar]
- Logan GD, Bundesen C. Clever homunculus: Is there an endogenous act of control in the explicit task-cuing procedure? Journal of Experimental Psychology: Human Perception and Performance. 2003;29:575–599. doi: 10.1037/0096-1523.29.3.575. [DOI] [PubMed] [Google Scholar]
- Manly T, Anderson V, Nimmo-Smith I, Turner A, Watson P, Robertson IH. The differential assessment of children’s attention: The Test of Everyday Attention for Children (TEA-Ch), normative sample and ADHD performance. Journal of Psychology and Psychiatry. 2001;42:1065–1081. doi: 10.1111/1469-7610.00806. [DOI] [PubMed] [Google Scholar]
- Marcovitch S, Boseovski JJ, Knapp RJ. Use it or lose it: Examining preschoolers’ difficulty in maintaining and executing a goal. Developmental Science. 2007;10:559–564. doi: 10.1111/j.1467-7687.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- Marcovitch S, Boseovski JJ, Knapp RJ, Kane MJ. Goal Neglect and Working Memory Capacity in 4- to 6-Year-Old Children. Child Development. doi: 10.1111/j.1467-8624.2010.01503.x. (in press) [DOI] [PubMed] [Google Scholar]
- Mauchly JW. Significance test for sphericity of a normal n-variate distribution. Annals of Mathematical Statistics. 1940;11:204–209. [Google Scholar]
- Miyake A, Emerson MJ, Padilla F, Ahn JC. Inner speech as a retrieval aid for task goals: The effect of cue type and articulatory suppression in the random task cuing paradigm. Acta Psychologica. 2004;115:123–142. doi: 10.1016/j.actpsy.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Monsell S, Mizon GA. Can the task-cuing paradigm measure an endogenous task set reconfiguration process? Journal of Experimental Psychology: Human Perception and Performance. 2006;32:493–516. doi: 10.1037/0096-1523.32.3.493. [DOI] [PubMed] [Google Scholar]
- Morton JB, Munakata Y. Active versus latent representations: A neural network model of perseveration, dissociation, and decalage. Developmental Psychobiology. 2002;40:255–265. doi: 10.1002/dev.10033. [DOI] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science. 2005;8:74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Reimers S, Maylor EA. Task switching across the lifespan: Effects of age on general and specific switch costs. Developmental Psychology. 2005;41:661–671. doi: 10.1037/0012-1649.41.4.661. [DOI] [PubMed] [Google Scholar]
- Robinson CW, Sloutsky VM. Visual processing speed: effects of auditory input on visual processing. Developmental Science. 2007;10:734–740. doi: 10.1111/j.1467-7687.2007.00627.x. [DOI] [PubMed] [Google Scholar]
- Robinson CW, Sloutsky VM. Effect of auditory input on individuation tasks. Developmental Science. 2008;11:869–881. doi: 10.1111/j.1467-7687.2008.00751.x. [DOI] [PubMed] [Google Scholar]
- Robinson CW, Sloutsky VM. Effects of multimodal presentation and stimulus familiarity on auditory and visual processing. Journal of Experimental Child Psychology. 2010;107:351–358. doi: 10.1016/j.jecp.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin O, Meiran N. On the Origins of the Task Mixing Cost in the CuingTask-Switching Paradigm. Journal of Experimental Psychology : Learning, Memory and cognition. 2005;31:1477–1491. doi: 10.1037/0278-7393.31.6.1477. [DOI] [PubMed] [Google Scholar]
- Rubinstein JS, Meyer DE, Evans JE. Executive Control of Cognitive Processes in Task Switching. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:763–797. doi: 10.1037//0096-1523.27.4.763. [DOI] [PubMed] [Google Scholar]
- Saeki E, Saito S. Verbal representation in task order control: An examination with transition and task cues in random task switching. Memory & Cognition. 2009;37:1040–1050. doi: 10.3758/MC.37.7.1040. [DOI] [PubMed] [Google Scholar]
- Smidts DP, Jacobs R, Anderson V. The Object Classification Task for Children (OCTC): A measure of concept generation and mental flexibility in early childhood. Developmental Neuropsychology. 2004;26:385–401. doi: 10.1207/s15326942dn2601_2. [DOI] [PubMed] [Google Scholar]
- Schneider DW, Logan GD. Task switching versus cue switching: Using transition cuing to disentangle sequential effects in task-switching performance. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33:370–378. doi: 10.1037/0278-7393.33.2.370. [DOI] [PubMed] [Google Scholar]
- Snyder HR, Munakata Y. Becoming self-directed: Abstract representations support endogenous flexibility in children. Cognition. 2010;116:155–167. doi: 10.1016/j.cognition.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C, Lauinger B, Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: An event-related brain potential study. Developmental Science. 2009;12:634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towse JN, Lewis C, Knowles M. When knowledge is not enough: The phenomenon of goal neglect in preschool children. Journal of Experimental Child Psychology. 2007;96:320–332. doi: 10.1016/j.jecp.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Vandierendock A, Liefooghe B, Verbruggen F. Task switching: Interplay of reconfiguration and interference control. Psychological Bulletin. 2010;136:601–626. doi: 10.1037/a0019791. [DOI] [PubMed] [Google Scholar]
- Van Loy B, Liefooghe B, Vendierendonck A. Cognitive control in cued task switching with transition cues: Cue processing, task processing, and cue-task transition congruency. The Quarterly Journal of Experimental Psychology. 2010;63:1916–1935. doi: 10.1080/17470211003779160. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Sheffield TD, Nelson JM, Clark CAC, Chevalier N, Espy KA. The structure of executive control in 3-year-old children. Journal of Experimental Child Psychology. doi: 10.1016/j.jecp.2010.08.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Cognitive Abilities. Itasca, IL: Riverside; 2001. [Google Scholar]
- Zelazo PD. The Dimensional Change Card Sort (DCCS): A method of assessing executive function in children. Nature Protocols. 2006;1:297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Müller U, Frye D, Marcovitch S. The development of executive function in early childhood. Monographs of the Society for Research in Child Development. 2003;68(3) doi: 10.1111/j.0037-976x.2003.00260.x. Serial number 274. [DOI] [PubMed] [Google Scholar]


