Bidirectional ventricular tachycardia (VT) is characterized by beat-to-beat alternation of the QRS morphology and axis on ECG. It classically occurs in patients with digitalis toxicity, but also has been noted in genetic disorders such as catecholaminergic polymorphic VT and Anderson-Tawil syndrome (ATS). Mutations in KCNJ2, which encodes the inward rectifier K+ channel Kir2.1 (current: IK1), were identified in patients with ATS. However, the mechanism of bidirectional VT remains unclear.
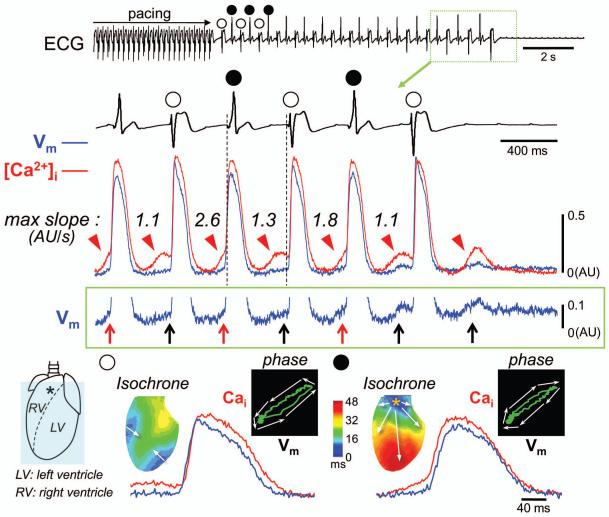
Here we present an image of bidirectional VT due to beat-to-beat alteration in diastolic level of [Ca2+]i and triggered activity in a rabbit model of ATS. The isolated heart was perfused with 37°C oxygenated Tyrode’s solution, and stained with Rhod-2 AM and RH237 to optically monitor changes in epicardial [Ca2+]i and membrane voltage (Vm), respectively (0.35 × 0.35 mm2 per pixel, 2 ms/flame). The ATS model was created by 5 mmol/L cesium chloride which mainly blocks IK1 at this concentration.1 Supraventricular origin of cardiac arrhythmias was excluded by creating AV block. An episode of bidirectional VT occurred after addition of 0.3 μmol/L isoproterenol and rapid ventricular pacing (Figure). The R-R interval during the VT was constant at 360 ms, except the period before spontaneous VT termination when R-R intervals were gradually prolonged. The odd VT beats (unfilled circles) arose from the outside of the mapped region. The even VT beats (filled circles) displayed a focal activation pattern originating from the right ventricular outflow tract (RVOT). The optical tracings shown in the Figure were obtained from the site marked by an asterisk. The even VT beats likely arose from that epicardial site because the local activation preceded the onset of QRS in ECG. However, it is also possible that these beats originated from Purkinje cells or deeper layers of myocardium and broke through the epicardium prior to QRS onset. During the diastole of the VT, spontaneous [Ca2+]i elevation (red arrowheads) and delayed afterdepolarizations (upward arrows) were observed at this site. Because the DAD amplitudes in intact hearts were smaller than that observed in single cell recordings due to electrotonic interactions between cells with and without DADs and signal averaging in optical mapping,2 we magnified the Vm tracing to make the DADs more visible. Note that the maximal slopes of the diastolic [Ca2+]i elevation alternate from beat to beat. The larger of the two slopes was associated with a DAD with a faster upstroke triggering an action potential (red upward arrows), forming the even beats. The diastolic [Ca2+]i elevation with gentle slopes was associated with a failure to trigger (black upward arrows), allowing an outside wavefront to activate the mapped region, forming the odd beats. Phase plots of [Ca2+]i versus Vm show a counterclockwise trajectory in the odd beat, but clockwise trajectory in the even beat, indicating that the RVOT site was passively activated by a propagated wavefront, followed by secondary increase in [Ca2+]i during the odd beat (i.e. Ca2+-induced Ca2+ release), while [Ca2+]i event occurred before Vm event during the even beat (i.e. spontaneous Ca2+ release from the SR). It is likely that two foci of triggered activities alternatingly trigger rather than compete with each other to generate bidirectional VT.
Figure.
Acknowledgments
Sources of Funding: NIH grants P01 HL78931, R01 HL78932, and 71140; a Nihon Kohden/St Jude Medical electrophysiology fellowship (Dr Maruyama); an AHA Established Investigator Award (Dr Lin); and a Medtronic-Zipes endowment (Dr Chen).
Abbreviations
- ATS
Andersen-Tawil Syndrome
- AU
arbitrary unit
- RVOT
right ventricular outflow tract
- VT
ventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests: None.
This manuscript was processed by a guest editor
Reference
- 1.Morita H, Zipes DP, Morita ST, Wu J. Mechanism of U wave and polymorphic ventricular tachycardia in a canine tissue model of Andersen-Tawil syndrome. Cardiovasc.Res. 2007;75:510–518. doi: 10.1016/j.cardiores.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 2.Maruyama M, Joung B, Tang L, et al. Diastolic intracellular calcium-membrane voltage coupling gain and postshock arrhythmias: role of Purkinje fibers and triggered activity. Circ Res. 2010;106:399–408. doi: 10.1161/CIRCRESAHA.109.211292. [DOI] [PMC free article] [PubMed] [Google Scholar]