Abstract
The purpose of this study was to examine the profound effects of the amino acid linkers on the melanoma targeting and pharmacokinetic properties of novel 111In-labeled lactam bridge-cyclized DOTA-[X]-CycMSHhex {1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid-[X]-c[Asp-His-dPhe-Arg-Trp-Lys]-CONH2, X=GlyGlyNle, GlyGluNle or NleGlyGlu} peptides.
Methods
Three novel DOTA-GGNle-CycMSHhex, DOTA-GENle-CycMSHhex and DOTA-NleGE-CycMSHhex peptides were designed and synthesized. The melanocortin-1 (MC1) receptor binding affinities of the peptides were determined in B16/F1 melanoma cells. The melanoma targeting and pharmacokinetic properties of 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex were determined in B16/F1 melanoma-bearing C57 mice.
Results
DOTA-GGNle-CycMSHhex and DOTA-GENle-CycMSHhex displayed 2.1 and 11.5 nM MC1 receptor binding affinities, whereas DOTA-NleGE-CycMSHhex showed 873.4 nM MC1 receptor binding affinity. The introduction of the -GlyGly- linker maintained high melanoma uptake while decreased the renal and liver uptakes of 111In-DOTA-GlyGlyNle-CycMSHhex. The tumor uptake values of 111In-DOTA-GGNle-CycMSHhex were 19.05 ± 5.04 and 18.6 ± 3.56 % injected dose/gram (%ID/g) at 2 and 4 h post-injection. 111In-DOTA-GGNle-CycMSHhex exhibited 28, 32 and 42% less renal uptake values than 111In-DOTA-Nle-CycMSHhex we reported previously, and 61, 65 and 68% less liver uptake values than 111In-DOTA-Nle-CycMSHhex at 2, 4 and 24 h post-injection, respectively.
Conclusion
The amino acid linkers exhibited the profound effects on the melanoma targeting and pharmacokinetic properties of the 111In-labeled lactam bridge-cyclized α-MSH peptides. Introduction of the -GlyGly- linker maintained high melanoma uptake while reducing the renal and liver uptakes of 111In-DOTA-GlyGlyNle-CycMSHhex, highlighting its potential as an effective imaging probe for melanoma detection, as well as a therapeutic peptide for melanoma treatment when labeled with a therapeutic radionuclide.
Keywords: Alpha-melanocyte stimulating hormone, Radiolabeled cyclic peptide, Melanoma imaging
INTRODUCTION
Over the last decade, both radiolabeled linear and cyclized alpha-melanocyte stimulating hormone (α-MSH) peptides have been designed to target G protein-coupled melanocortin-1 (MC1) receptors (1–5) for melanoma radioimaging and radiotherapy (6–20). Due to the stabilization of secondary structures (i.e. beta turns), the cyclic peptides possess less conformational freedom and higher stabilities than the linear peptides. Furthermore, the stabilization of secondary structures makes the cyclic peptides better fit the receptor binding pocket, thus enhancing their receptor binding affinities. At the present time, disulfide bond, metal and lactam bridge have been successfully utilized to cyclize the radiolabeled α-MSH peptides (9–13,15–20). Among these cyclization strategies, metal and lactam bridge cyclization resulted in greater tumor uptake and lower renal uptake values of the radiolabeled α-MSH peptides than the disulfide bridge cyclization (12,13,15–20).
We have successfully developed a novel class of 111In-labeled lactam bridge-cyclized DOTA-conjugated α-MSH peptides for primary and metastatic melanoma detection (15–19). Initially, a Lys-Asp lactam bridge was used to cyclize the MC1 receptor binding motif (His-dPhe-Arg-Trp) to yield a 12-amino acid cyclic α-MSH peptide {CycMSH: c[Lys-Nle-Glu-His-dPhe-Arg-Trp-Gly-Arg-Pro-Val-Asp]}. 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) was conjugated to the N-terminus of the CycMSH with or without an amino acid linker (-GlyGlu-) for radiolabeling. 111In-DOTA-GlyGlu-CycMSH displayed high melanoma uptake (10.40 ± 1.40 % ID/g at 2 h post-injection) in B16/F1 melanoma-bearing C57 mice (15). Using 111In-DOTA-GlyGlu-CycMSH as an imaging probe, both flank primary and pulmonary metastatic melanoma lesions could be clearly visualized by small animal single photon emission computed tomography (SPECT)/CT (15,16).
Recently, we have identified another novel DOTA-conjugated lactam bridge-cyclized α-MSH peptide with a 6-amino acid peptide ring {DOTA-Nle-CycMSHhex: DOTA-Nle-c[Asp-His-dPhe-Arg-Trp-Lys]-CONH2} for melanoma targeting. The receptor binding motif of His-dPhe-Arg-Trp was directly cyclized by an Asp-Lys lactam bridge. Interestingly, the reduction of the ring size dramatically enhanced the melanoma uptake (19.39 ± 1.65 % ID/g at 2 h post-injection) and reduced the renal uptake (9.52 ± 0.44 % ID/g at 2 h post-injection) of 111In-DOTA-Nle-CycMSHhex compared to 111In-DOTA-GlyGlu-CycMSH in B16/F1 melanoma-bearing C57 mice (15,19).
Hydrocarbon, amino acid and polyethylene glycol (PEG) linkers displayed profound favorable effects in the receptor binding affinities and pharmacokinetics of radiolabeled bombesin (21–25), RGD (26–29) and α-MSH peptides (15,16). To examine the effects of the amino acid linkers on melanoma targeting and pharmacokinetic properties, we designed three novel DOTA-conjugated lactam bridge-cyclized CycMSHhex peptides with different amino acid linkers in this study based on the unique structure of DOTA-Nle-CycMSHhex we previously reported (19). A neutral -Gly-Gly- (GG) linker and a negatively-charged -Gly-Glu- (GE) linker were inserted between the DOTA and Nle to generate DOTA-GGNle-CycMSHhex and DOTA-GENle-CycMSHhex. Furthermore, the negatively-charged -Gly-Glu- linker was introduced between Nle and CycMSHhex to yield DOTA-NleGE-CycMSHhex. The MC1 receptor binding affinities of these three peptides were determined in B16/F1 melanoma cells. Only DOTA-GGNle-CycMSHhex and DOTA-GENle-CycMSHhex displayed low nanomolar MC1 receptor binding affinities. Hence, we further determined the melanoma targeting and pharmacokinetic properties of 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex in B16/F1 melanoma-bearing C57 mice.
MATERIALS AND METHODS
Chemicals and Reagents
Amino acids and resin were purchased from Advanced ChemTech Inc. (Louisville, KY) and Novabiochem (San Diego, CA). DOTA-tri-t-butyl ester was purchased from Macrocyclics Inc. (Richardson, TX) for peptide synthesis. 125I-Tyr2-[Nle4, d-Phe7]-α-MSH {125I-(Tyr2)-NDP-MSH} was obtained from PerkinElmer, Inc. (Waltham, MA) for in vitro receptor binding assay. 111InCl3 was purchased from MDS Nordion, Inc. (Ottawa, ON, Canada) for radiolabeling. All other chemicals used in this study were purchased from Thermo Fischer Scientific (Waltham, MA) and used without further purification. B16/F1 murine melanoma cells were obtained from American Type Culture Collection (Manassas, VA).
Peptide Synthesis
New DOTA-GGNle-CycMSHhex, DOTA-GENle-CycMSHhex and DOTA-NleGE-CycMSHhex peptides were synthesized using standard fluorenylmethyloxycarbonyl (Fmoc) chemistry according to our published procedure (19) with modifications. Briefly, linear peptide backbones of (tBu)3DOTA-Gly-Gly-Nle-Asp(O-2-PhiPr)-His(Trt)-dPhe-Arg(Pbf)-Trp(Boc)-Lys(Dde), (tBu)3DOTA-Gly-Glu(OtBu)-Nle-Asp(O-2-PhiPr)-His(Trt)-dPhe-Arg(Pbf)-Trp(Boc)-Lys(Dde) and (tBu)3DOTA-Nle-Gly-Glu(OtBu)-Asp(O-2-PhiPr)-His(Trt)-dPhe-Arg(Pbf)-Trp(Boc)-Lys(Dde) were synthesized on Sieber Amide resin by an Advanced ChemTech multiple-peptide synthesizer (Louisville, KY). Seventy micromoles of resin, 210 µmol of each Fmoc-protected amino acid and 210 µmol of (tBu)3DOTA were used for the synthesis. The protecting group of Dde was removed by 2% hydrazine for peptide cyclization. The protecting group of 2-phenylisopropyl was removed and the protected peptide was cleaved from the resin treating with a mixture of 2.5% of trifluoroacetic acid (TFA) and 5% of triisopropylsilane. After the precipitation with ice-cold ether and characterization by liquid chromatography-mass spectroscopy (LC-MS), each protected peptide was dissolved in H2O/CH3CN (50:50) and lyophilized to remove the reagents. Then, each protected peptide was further cyclized by coupling the carboxylic group from the Asp with the epsilon amino group from the Lys. The cyclization reaction was achieved by an overnight reaction in dimethylformamide (DMF) using benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium-hexafluorophosphate (PyBOP) as a coupling agent in the presence of N,N-diisopropylethylamine (DIEA). After the characterization by LC-MS, each cyclized protected peptide was dissolved in H2O/CH3CN (50:50) and lyophilized to remove the reagents. The protecting groups were totally removed by treating with a mixture of trifluoroacetic acid (TFA), thioanisole, phenol, water, ethanedithiol and triisopropylsilane (87.5:2.5:2.5:2.5:2.5:2.5) for 2 h at room temperature (25 °C). Each peptide was precipitated and washed with ice-cold ether four times, purified by reverse phase-high performance liquid chromatography (RP-HPLC) and characterized by LC-MS.
In vitro Receptor Binding Assay
The receptor binding affinities (IC50 values) of DOTA-GGNle-CycMSHhex, DOTA-GENle-CycMSHhex and DOTA-NleGE-CycMSHhex were determined by in vitro competitive binding assay according to our published procedure (19) with modifications. B16/F1 cells in 24-well cell culture plates (5×105 cells/well) were incubated at room temperature (25°C) for 2 h with approximately 60,000 cpm of 125I-Tyr2-NDP-MSH in the presence of 10−12 to 10−5 M of each peptide in 0.3 mL of binding medium {Dulbecco’s Modified Eagle’s Medium with 25 mM N-(2-hydroxyethyl)-piperazine-N’-(2-ethanesulfonic acid), pH 7.4, 0.2% bovine serum albumin (BSA), 0.3 mM 1,10-phenathroline}. The medium was aspirated after the incubation. The cells were rinsed twice with 0.5 mL of ice-cold pH 7.4, 0.2% BSA / 0.01 M phosphate buffered saline (PBS) and lysed in 0.5 mL of 1 N NaOH for 5 minutes. The activities associated with cells were measured in a Wallac 1480 automated gamma counter (PerkinElmer, Waltham, MA). The IC50 value of each peptide was calculated using Prism software (GraphPad Software, La Jolla, CA).
Peptide Radiolabeling with 111In
Since DOTA-NleGE-CycMSHhex exhibited at least 78-fold lower receptor binding affinity than DOTA-GGNle-CycMSHhex and DOTA-GENle-CycMSHhex, we only further evaluated DOTA-GGNle-CycMSHhex and DOTA-GENle-CycMSHhex. 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex were prepared in a 0.5 M NH4OAc-buffered solution at pH 4.5 according to our published procedure (19). Briefly, 50 µL of 111InCl3 (37–74 MBq in 0.05 M HCl aqueous solution), 10 µL of 1 mg/mL peptide aqueous solution and 400 µL of 0.5 M NH4OAc (pH 4.5) were added into a reaction vial and incubated at 75°C for 45 mins. After the incubation, 10 µL of 0.5% EDTA (ethylenediaminetetraacetic acid) aqueous solution was added into the reaction vial to scavenge potential unbound 111In3+ ions. The radiolabeled complexes were purified to single species by Waters RP-HPLC (Milford, MA) on a Grace Vydac C-18 reverse phase analytical column (Deerfield, IL) using the following gradient at a 1 mL/min flowrate. The mobile phase consisted of solvent A (20 mM HCl aqueous solution) and solvent B (100% CH3CN). The gradient was initiated and kept at 82:18 A/B for 3 min followed by a linear gradient of 82:18 A/B to 72:28 A/B over 20 mins. Then, the gradient was changed from 72:28 A/B to 10:90 A/B over 3 min followed by an additional 5 min at 10:90 A/B. Thereafter, the gradient was changed from 10:90 A/B to 82:18 A/B over 3 mins. Each purified peptide sample was purged with N2 gas for 20 min to remove the acetonitrile. The pH of the final solution was adjusted to 7.4 with 0.1 N NaOH and sterile saline for animal studies. In vitro serum stability of 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex were determined by incubation in mouse serum at 37°C for 24 h and monitored for degradation by RP-HPLC.
Biodistribution Studies
All animal studies were conducted in compliance with Institutional Animal Care and Use Committee approval. The pharmacokinetics of 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex were determined in B16/F1 melanoma-bearing C57 female mice (Harlan, Indianapolis, IN). The C57 mice were subcutaneously inoculated with 1×106 B16/F1 cells on the right flank for each mouse to generate B16/F1 melanomas. Ten days post inoculation, the tumor weights reached approximately 0.2 g. Each melanoma-bearing mouse was injected with 0.037 MBq of 111In-DOTA-GGNle-CycMSHhex or 111In-DOTA-GENle-CycMSHhex via the tail vein. Groups of 5 mice were sacrificed at 0.5, 2, 4 and 24 h post-injection, and tumors and organs of interest were harvested, weighed and counted. Blood values were taken as 6.5% of the whole-body weight. The specificities of the tumor uptake of 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex were determined by co-injecting 10 µg (6.07 nmol) of unlabeled NDP-MSH which is a linear α-MSH peptide analogue with picomolar MC1 receptor binding affinity.
Melanoma Imaging
Since 111In-DOTA-GGNle-CycMSHhex displayed more favorable tumor targeting and pharmacokinetic properties than 111In-DOTA-GENle-CycMSHhex, we only further evaluated the melanoma imaging property of 111In-DOTA-GGNle-CycMSHhex. One B16/F1 melanoma-bearing C57 mouse (10 days post the cell inoculation) was injected with 14.8 MBq of 111In-DOTA-GGNle-CycMSHhex via the tail vein. The mouse was sacrificed for small animal SPECT/CT (Nano-SPECT/CT®, Bioscan) imaging at 2 h post-injection. The CT imaging was immediately followed by the whole-body SPECT imaging. The SPECT scans of 24 projections were acquired. Reconstructed SPECT and CT data were visualized and co-registered using InVivoScope (Bioscan, Washington DC).
Metabolites of 111In-DOTA-GGNle-CycMSHhex in Melanoma and Urine
Both melanoma and urine were collected from the mouse used for SPECT/CT imaging to analyze the metabolites of 111In-DOTA-GGNle-CycMSHhex in melanoma and urine. The tumor was homogenized by a VWR homogenizer for 5 mins. Equal volume of ethanol was added into the tumor sample. The tumor sample was vortexed and then centrifuged at 16,000 g for 5 mins. The supernatant was transferred into a glass test tube and purged with N2 gas for 20 min to remove the ethanol. Aliquots of the supernatant were injected into HPLC. The urinary sample was directly centrifuged at 16,000 g for 5 min prior to the HPLC analysis. Thereafter, aliquots of the urine were injected into HPLC. The HPLC gradient described above was used for the analyses of metabolites.
Statistical Analysis
Statistical analysis was performed using the Student’s t-test for unpaired data. A 95% confidence level was chosen to determine the significant difference in tumor and renal uptakes between 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex, as well as the significant difference in tumor and renal uptakes between 111In-DOTA-GGNle-CycMSHhex or 111In-DOTA-GENle-CycMSHhex with/without NDP-MSH co-injection. The differences at the 95% confidence level (p<0.05) were considered significant.
RESULTS
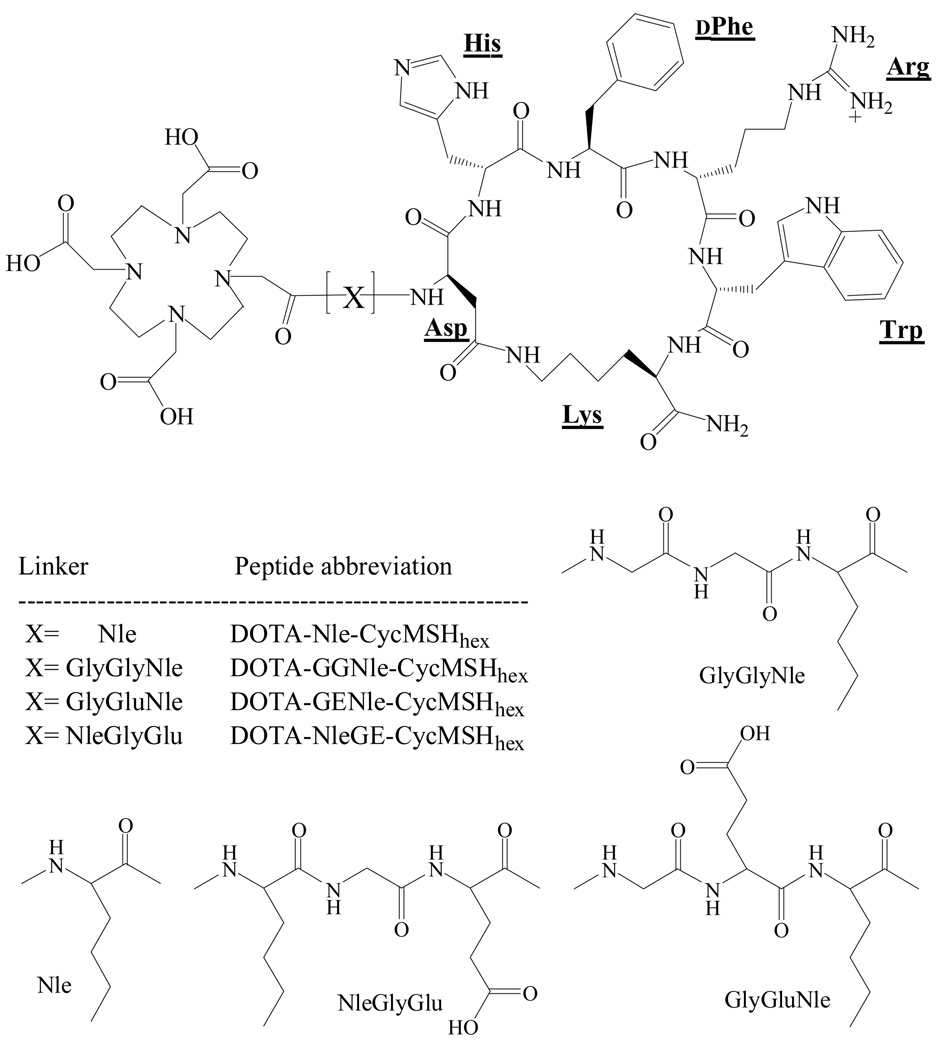
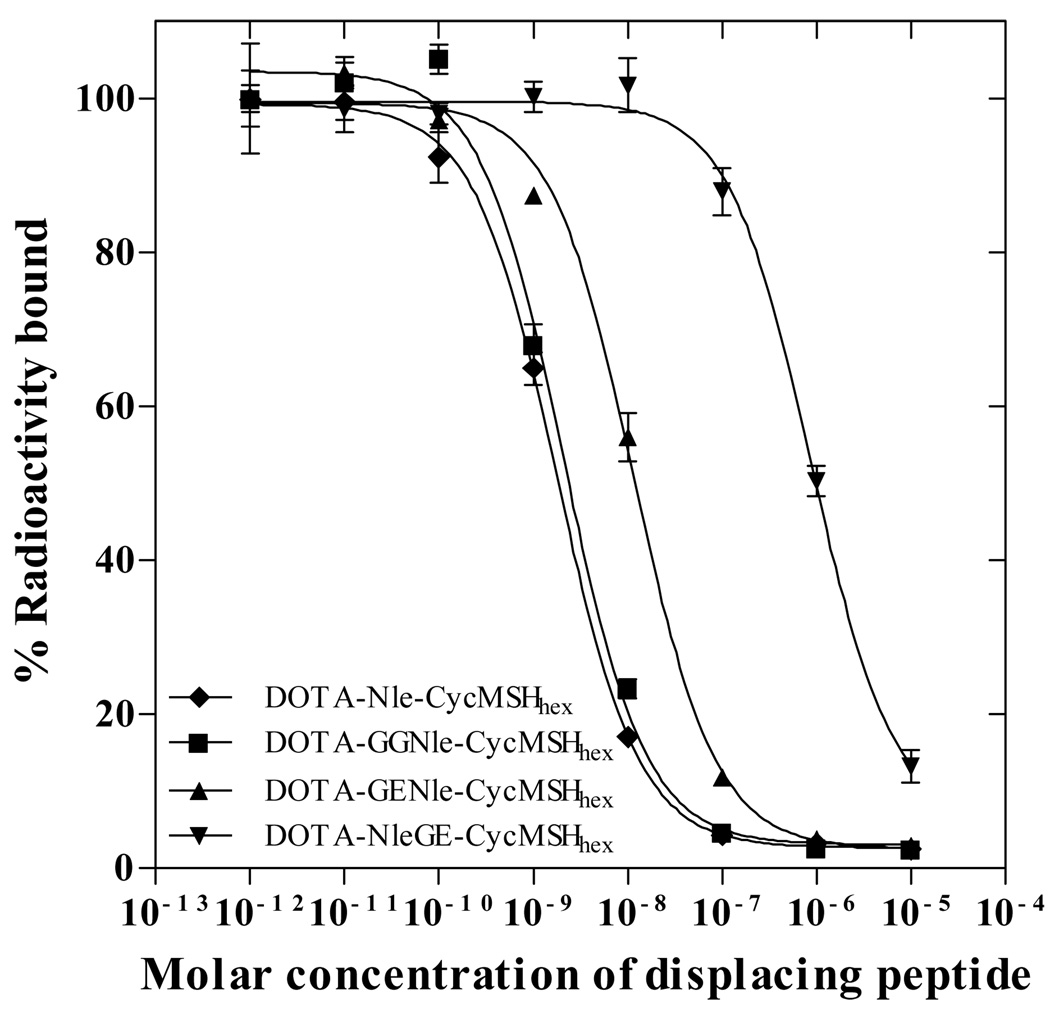
Three novel α-MSH peptides, DOTA-GGNle-CycMSHhex, DOTA-GENle-CycMSHhex and DOTA-NleGE-CycMSHhex were synthesized and purified by HPLC. All three peptides displayed greater than 95% purity after HPLC purification. The schematic structures of the peptides are shown in Figure 1. The identities of the peptides were confirmed by electrospray ionization mass spectrometry. The calculated and found molecular weights of the peptides are presented in Table 1. The receptor binding affinities of the peptides were determined in B16/F1 melanoma cells. The IC50 values of DOTA-GGNle-CycMSHhex, DOTA-GENle-CycMSHhex and DOTA-NleGE-CycMSHhex were 2.1, 11.5 and 873.4 nM in B16/F1 cells, respectively (Table 1 and Fig. 2).
Figure 1.
Structures of DOTA-Nle-CycMSHhex, DOTA-GGNle-CycMSHhex, DOTA-GENle-CycMSHhex and DOTA-NleGE-CycMSHhex. The structure of DOTA-Nle-CycMSHhex was cited from the reference 19 for comparison.
Table 1.
DOTA-conjugated lactam bridge-cyclized alpha-MSH peptides.
| DOTA-Nle- CycMSHhex |
DOTA-GGNle- CycMSHhex |
DOTA-GENle- CycMSHhex |
DOTA-NleGE- CycMSHhex |
|
|---|---|---|---|---|
| Amino acid linker between DOTA and the cyclic peptide moiety | -Nle- | -Gly-Gly-Nle- | -Gly-Glu-Nle- | -Nle-Gly-Glu- |
| Calculated molecular weight (Da) | 1368.5 | 1482.6 | 1554.6 | 1554.6 |
| Found molecular weight (Da) | 1368.2 | 1482.0 | 1554.0 | 1554.0 |
| Molecular Formula | C64H93N19O15 | C68H99N21O17 | C71H103N21O19 | C71H103N21O19 |
| MC1R binding affinity (nM) | 1.8 | 2.1 | 11.5 | 873.4 |
| HPLC retention time (min) | 14.3 | 14.8 | 15.4 | 9.6 |
| HPLC retention time for 111In-conjugate (min) | 10.7 | 17.7 | 21.7 | N/A |
The data of DOTA-Nle-CycMSHhex was cited from the reference 19 for comparison.
Figure 2.
The in vitro competitive binding curves of DOTA-Nle-CycMSHhex, DOTA-GGNle-CycMSHhex, DOTA-GENle-CycMSHhex and DOTA-NleGE-CycMSHhex in B16/F1 melanoma cells. The IC50 values of DOTA-Nle-CycMSHhex, DOTA-GGNle-CycMSHhex, DOTA-GENle-CycMSHhex and DOTA-NleGE-CycMSHhex were 1.8, 2.1, 11.5 and 873.4 nM respectively. The data of DOTA-Nle-CycMSHhex was cited from the reference 19 for comparison.
We only further evaluated DOTA-GGNle-CycMSHhex and DOTA-GENle-CycMSHhex since both peptides displayed low nanomolar MC1 receptor binding affinities. . DOTA-GGNle-CycMSHhex and DOTA-GENle-CycMSHhex were readily labeled with 111In in 0.5 M ammonium acetate solution at pH 4.5 with greater than 95% radiolabeling yield. Each 111In-labeled peptide was completely separated from its excess non-labeled peptide by RP-HPLC. The retention times of the peptides and their 111In-labeled conjugates are showed in Table 1. The retention times of 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex were 17.7 and 21.7 min, respectively. 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex showed greater than 98% radiochemical purities after HPLC purification, and were stable in mouse serum at 37 °C for 24 h. Only intact 111In-labeled conjugates were detected by RP-HPLC after 24 h of incubation in mouse serum.
We further evaluated the melanoma targeting and pharmacokinetic properties of 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex in B16/F1 melanoma-bearing C57 mice. The biodistribution results of 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex are shown in Table 2. 111In-DOTA-GGNle-CycMSHhex exhibited rapid high melanoma uptake and prolonged tumor retention. The tumor uptake value of 111In-DOTA-GGNle-CycMSHhex was 18.39 ± 2.22 %ID/g at 0.5 h post-injection. The tumor uptake reached its peak value of 19.05 ± 5.04 % ID/g at 2 h post-injection. 111In-DOTA-GGNle-CycMSHhex displayed similar high tumor uptake (18.6 ± 3.56 %ID/g) at 4 h post-injection. Even at 24 h post-injection, there was 6.77 ± 0.84 %ID/g of 111In-DOTA-GGNle-CycMSHhex activity remained in the tumor. Approximately 98% of the tumor uptake of 111In-DOTA-GGNle-CycMSHhex was blocked by 10 µg (6.07 nmol) of non-radiolabeled NDP-MSH (p<0.05), demonstrating that the tumor uptake was specific and MC1 receptor-mediated. Whole-body clearance of 111In-DOTA-GGNle-CycMSHhex was rapid, with approximately 88.4% of the injected radioactivity cleared through the urinary system by 2 h post-injection. Normal organ uptakes of 111In-DOTA-GGNle-CycMSHhex were low (<1.31% ID/g) except for the kidneys at 2, 4 and 24 h post-injection. The liver uptake of 111In-DOTA-GGNle-CycMSHhex was less than 0.61 %ID/g at 2 h post-injection. The kidney uptake value was 15.19 ± 2.75 %ID/g at 0.5 h post-injection, and decreased to 6.84 ± 0.92 %ID/g at 2 h post-injection. Co-injection of NDP-MSH didn’t significantly reduce the renal uptake of the 111In-DOTA-GGNle-CycMSHhex activity at 2 h post-injection, indicating that the renal uptake was not MC1 receptor-mediated. High tumor uptake and prolonged tumor retention coupled with rapid whole-body clearance resulted in high tumor/blood and high tumor/normal organ uptake ratios that were achieved as early as 0.5 h post-injection. The tumor/liver uptake ratios of 111In-DOTA-GGNle-CycMSHhex were 33.42 and 31.0 at 2 and 4 h post-injection, whereas the tumor/kidney uptake ratios of 111In-DOTA-GGNle-CycMSHhex were 2.79 and 2.73 at 2 and 4 h post-injection.
Table 2.
Biodistribution of 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex in B16/F1 melanoma-bearing C57 mice. The data were presented as percent injected dose/gram or as percent injected dose (mean ± SD, n=5)
| 111In-DOTA-GGNle-CycMSHhex | 111In-DOTA-GENle-CycMSHhex | |||||||
|---|---|---|---|---|---|---|---|---|
| Tissues | 0.5 h | 2 h | 4 h | 24 h | 0.5 h | 2 h | 4 h | 24 h |
| Percent injected dose/gram (%ID/g) | ||||||||
| Tumor | 18.39±2.22 | 19.05±5.04 | 18.6±3.56 | 6.77±0.84 | 11.75±2.00* | 8.99±1.91* | 5.3±2.84* | 4.40±0.87* |
| Brain | 0.21±0.18 | 0.03±0.03 | 0.04±0.03 | 0.01±0.01 | 0.07±0.01 | 0.02±0.01 | 0.04±0.04 | 0.03±0.01 |
| Blood | 3.17±0.45 | 0.12±0.11 | 0.01±0.01 | 0.02±0.01 | 1.28±0.09 | 0.16±0.05 | 0.14±0.06 | 0.01±0.01 |
| Heart | 1.35±0.26 | 0.24±0.12 | 0.01±0.02 | 0.01±0.01 | 0.66±0.17 | 0.06±0.04 | 0.06±0.04 | 0.06±0.02 |
| Lung | 2.97±0.71 | 0.28±0.07 | 0.13±0.10 | 0.07±0.05 | 1.31±0.29 | 0.31±0.14 | 0.20±0.04 | 0.12±0.05 |
| Liver | 1.41±0.22 | 0.57±0.09 | 0.60±0.03 | 0.60±0.10 | 0.67±0.17 | 0.50±0.12 | 0.36±0.03 | 0.26±0.01 |
| Spleen | 0.93±0.37 | 0.17±0.06 | 0.15±0.10 | 0.12±0.13 | 0.54±0.13 | 0.24±0.11 | 0.19±0.10 | 0.14±0.01 |
| Stomach | 2.18±0.28 | 1.30±0.12 | 1.14±0.13 | 1.17±0.48 | 0.95±0.15 | 0.28±0.03 | 0.49±0.14 | 0.41±0.01 |
| Kidneys | 15.19±2.75 | 6.84±0.92 | 6.82±1.19 | 5.44±1.58 | 9.06±2.20* | 5.54±0.63* | 6.25±0.51 | 4.21±0.03 |
| Muscle | 0.37±0.26 | 0.01±0.01 | 0.02±0.02 | 0.02±0.01 | 0.32±0.09 | 0.06±0.03 | 0.11±0.05 | 0.09±0.01 |
| Pancreas | 0.99±0.27 | 0.23±0.12 | 0.14±0.06 | 0.10±0.01 | 0.40±0.08 | 0.12±0.10 | 0.13±0.08 | 0.15±0.04 |
| Bone | 0.59±0.39 | 0.10±0.09 | 0.10±0.08 | 0.04±0.04 | 0.13±0.10 | 0.08±0.05 | 0.02±0.01 | 0.06±0.01 |
| Skin | 2.16±1.28 | 0.27±0.12 | 0.27±0.28 | 0.26±0.08 | 1.63±0.43 | 0.37±0.11 | 0.12±0.10 | 0.16±0.13 |
| Percent injected dose (%ID) | ||||||||
| Intestines | 1.65±0.26 | 1.30±0.32 | 0.97±0.38 | 0.74±0.13 | 0.95±0.14 | 0.68±0.26 | 1.45±0.85 | 0.76±0.45 |
| Urine | 60.80±4.05 | 88.46±1.75 | 88.39±3.06 | 93.23±1.60 | 83.56±0.49 | 89.65±6.24 | 91.38±1.85 | 93.57±0.12 |
| Uptake ratio of tumor/normal tissue | ||||||||
| Tumor/Blood | 5.80 | 158.75 | 1860.00 | 338.50 | 9.18 | 56.19 | 37.86 | 440.00 |
| Tumor/Kidneys | 1.21 | 2.79 | 2.73 | 1.24 | 1.30 | 1.62 | 0.85 | 1.05 |
| Tumor/Lung | 6.19 | 68.04 | 143.08 | 96.71 | 8.97 | 29.00 | 26.50 | 36.67 |
| Tumor/Liver | 13.04 | 33.42 | 31.00 | 11.28 | 17.54 | 17.98 | 14.72 | 16.92 |
| Tumor/Muscle | 49.70 | 1905.00 | 930.00 | 338.50 | 36.72 | 149.83 | 48.18 | 48.89 |
| Tumor/Skin | 8.51 | 70.56 | 68.89 | 26.04 | 7.21 | 24.30 | 44.17 | 27.50 |
P<0.05, significance comparison in tumor and kidney uptakes between 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex.
As we anticipated, 111In-DOTA-GENle-CycMSHhex showed lower tumor uptake values than 111In-DOTA-GGNle-CycMSHhex at 0.5, 2 and 4 h post-injection. The tumor uptake values of 111In-DOTA-GGNle-CycMSHhex were 2, 2.5 and 3 times the tumor uptake values of 111In-DOTA-GENle-CycMSHhex at 0.5, 2 and 4 h post-injection, respectively (Table 2). Co-injection of non-radioactive NDP-MSH blocked 95.6% of the tumor uptake at 2 h post-injection (p<0.05), indicating that the tumor uptake of 111In-DOTA-GENle-CycMSHhex was MC1 receptor-specific. Despite the similar renal uptake of 111In-DOTA-GENle-CycMSHhex as 111In-DOTA-GGNle-CycMSHhex at 2, 4 and 24 h post-injection, 111In-DOTA-GENle-CycMSHhex showed 40% lower renal uptake than 111In-DOTA-GGNle-CycMSHhex at 0.5 h post-injection (p<0.05). The kidney uptake of 111In-DOTA-GENle-CycMSHhex was as low as 9.06 ± 2.20 %ID/g at 0.5 h post-injection and decreased to 5.54 ± 0.63 %ID/g at 2 h post-injection.
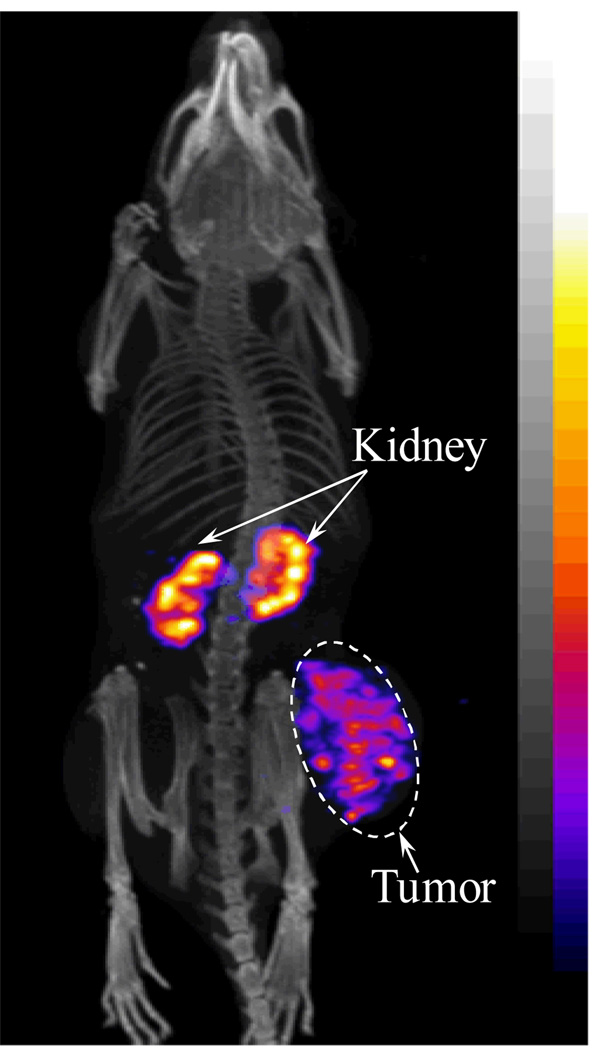
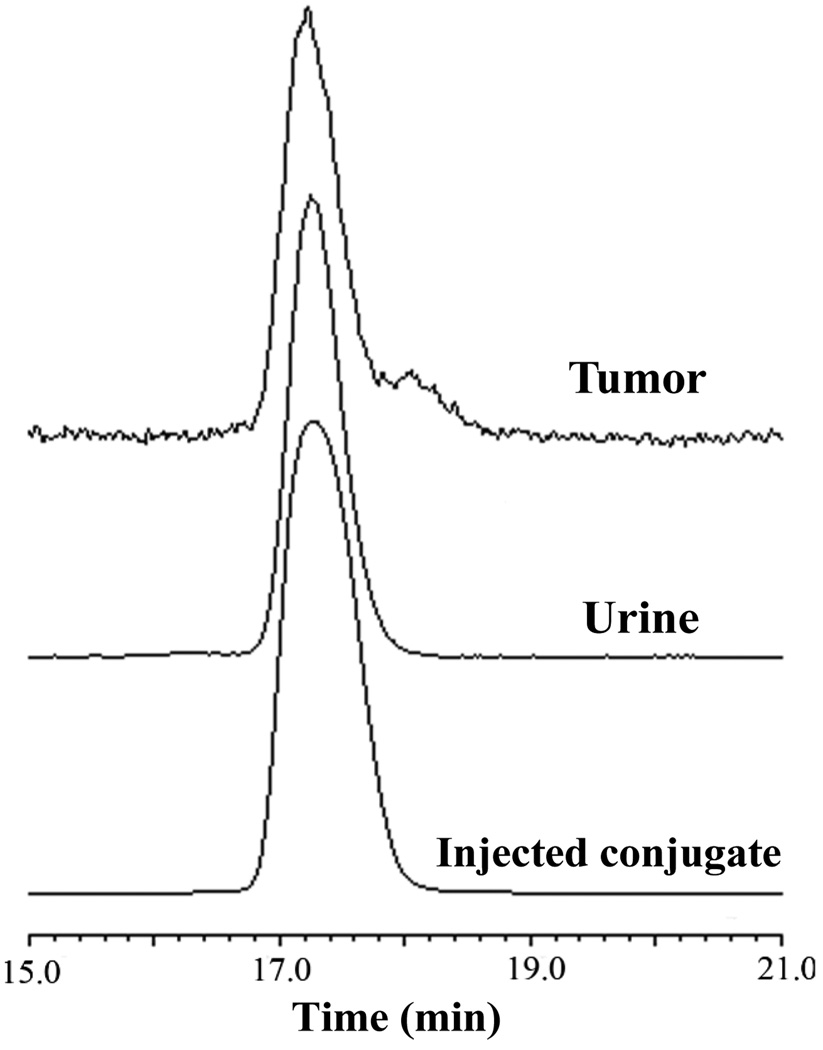
We further evaluated the melanoma imaging properties of 111In-DOTA-GGNle-CycMSHhex since 111In-DOTA-GGNle-CycMSHhex showed more favorable biodistribution properties than 111In-DOTA-GENle-CycMSHhex. The whole-body SPECT/CT images are presented in Figure 3. Flank melanoma tumors were clearly visualized by SPECT/CT using 111In-DOTA-GGNle-CycMSHhex as an imaging probe. The whole-body images showed high tumor to normal organ uptake ratios except for the kidneys, which was consistent with the biodistribution results. Melanoma and urinary metabolites of 111In-DOTA-GGNle-CycMSHhex were analyzed by RP-HPLC 2 h post-injection. Figure 4 illustrates both the HPLC profiles of melanoma and urine samples. 111In-DOTA-GGNle-CycMSHhex remained intact in the both tumor and urine 2 h post-injection (Fig. 4).
Figure 3.
Representative whole-body SPECT/CT images of a B16/F1 melanoma-bearing mouse (14 days post cell inoculation) at 2 h post-injection of 37.0 MBq of 111In-DOTA-GGNle-CycMSHhex.
Figure 4.
Radioactive HPLC profiles of 111In-DOTA-GGNle-CycMSHhex (injected conjugate) and its metabolites in urine and tumor at 2 h post-injection.
DISCUSSION
We have been interested in developing lactam bridge-cyclized α-MSH peptides to target the MC1 receptors for melanoma detection (15–19). Unique lactam bridge-cyclization makes the cyclic α-MSH peptides resistant to proteolytic degradations in vivo, as well as provides the flexibility for fine structural modification (15,17,19). Recently, we have identified 111In-DOTA-Nle-CycMSHhex with a 6-amino acid ring targeting the MC1 receptors for melanoma imaging (19). Among these reported 111In-labeled lactam bridge-cyclized α-MSH peptides (15,17,19), 111In-DOTA-Nle-CycMSHhex displayed the highest melanoma uptake values (24.94 ± 4.58 %ID/g at 0.5 h post-injection and 19.39 ± 1.65 %ID/g at 2 h post-injection) in B16/F1 melanoma-bearing mice (19). The reduction of the ring size improved the tumor uptake and reduced the renal uptake of 111In-DOTA-Nle-CycMSHhex, providing a new insight into the design of novel lactam bridge-cyclized α-MSH peptides for melanoma targeting.
Hydrocarbon, amino acid and PEG linkers have been used to optimize the receptor binding affinities, as well as modifying the pharmacokinetic properties of radiolabeled bombesin (21–25), RGD (26–29) and α-MSH peptides (15,16). For instance, Volkert and colleagues reported that the hydrocarbon linkers ranging from 5-carbon to 8-carbon between the DOTA and bombesin peptide resulted in 0.6–1.7 nM receptor binding affinities for the DOTA-conjugated bombesin peptides. Either shorter or longer hydrocarbon linkers dramatically reduce the receptor binding affinity by 100-fold (21). Rogers and colleagues reported the profound effects of amino acid linkers (-GlyGlyGly-, -GlySerGly-, -GlySerSer- and -GlyGluGly-) between the DOTA and bombesin peptide on tumor and normal organ uptakes of the radiolabeled peptides (25). 64Cu-labeled DOTA-conjugated bombesin peptide with the -GlyGlyGly- linker displayed the higher PC-3 tumor uptake, whereas the -GlySerGly- linker resulted in lower renal uptake (25). Recently, Liu and colleagues reported the improvement in tumor uptakes and pharmacokinetics of 64Cu- and 99mTc-labeled cyclic RGD peptides using the -GlyGlyGly- and PEG4 linkers (26–29). We also demonstrated that the introduction of a negatively-charged -GlyGlu- linker enhanced the melanoma uptake and reduced the renal uptake of 111In-DOTA-GlyGlu-CycMSH compared to 111In-DOTA-CycMSH (15). Hence, we evaluated the effects of -GlyGly- and -GlyGlu- linkers on melanoma targeting and pharmacokinetic properties of 111In-DOTA-[X]-CycMSHhex peptide constructs in this study.
DOTA-Nle-CycMSHhex displayed 1.8 nM MC1 receptor binding affinity in B16/F1 melanoma cells in our previous report (19). The MC1 receptor binding sequence of His-dPhe-Arg-Trp was directly cyclized by an Asp-Lys lactam bridge to generate the CycMSHhex moiety. The radiometal chelator DOTA was conjugated to the CycMSHhex moiety via a Nle to form DOTA-Nle-CycMSHhex peptide. Based on the unique structure of DOTA-Nle-CycMSHhex, we initially introduced the amino acid linker (-GlyGlu-) between the DOTA and Nle or between the Nle and CycMSHhex moiety to determine which position was suitable for an amino acid linker. We found that the moiety of Nle-CycMSHhex was critical for maintaining the low nanomolar MC1 receptor binding affinity of the peptide. The introduction of the -GlyGlu- linker between the Nle and CycMSHhex moiety dramatically reduced the MC1 receptor binding affinity to 873.4 nM, whereas the introduction of the -GlyGlu- linker between the DOTA and Nle only decreased the MC1 receptor binding affinity to 11.5 nM. Interestingly, the -GlyGly- linker between the DOTA and Nle maintained the MC1 receptor binding affinity as 2.1 nM, further indicating that the moiety of Nle-CycMSHhex played a crucial role in maintaining the low nanomolar MC1 receptor binding affinity of the peptide. Furthermore, the amino acid between the DOTA and the moiety of Nle-CycMSHhex also showed a significant impact on the MC1 receptor binding affinity of the peptide. The neutral -GlyGly- linker was better than negatively-charged -GlyGlu- linker in terms of maintaining the low nanomolar MC1 receptor binding affinity of the peptide. The IC50 value of DOTA-GENle-CycMSHhex was 5.5 times the IC50 value of DOTA-GGNle-CycMSHhex. It was likely that the electrostatic interaction between the negatively-charged Glu in the –GlyGlu- linker and the positively-charged Arg in the moiety of Nle-CycMSHhex affected the configuration of the MC1 receptor binding region (His- d-Phe-Arg-Trp). The difference in MC1 receptor binding affinity between DOTA-GGNle-CycMSHhex and DOTA-GENle-CycMSHhex (2.1 nM vs. 11.5 nM) was also observed in the difference in melanoma uptake between 111In-DOTA-GGNle-CycMSHhex and 111In-DOTA-GENle-CycMSHhex in B16/F1 melanoma-bearing C57 mice. The tumor uptake values of 111In-DOTA-GGNle-CycMSHhex were 2, 2.5 and 3 times the tumor uptake values of 111In-DOTA-GENle-CycMSHhex at 0.5, 2 and 4 h post-injection, respectively (Table 2). In our previous report, the introduction of a negatively-charged -GlyGlu- linker resulted in 44% lower renal uptake of 111In-DOTA-GlyGlu-CycMSH at 4 h post-injection compared to 111In-DOTA-CycMSH (15). In this study, 111In-DOTA-GENle-CycMSHhex showed 40% lower renal uptake (p<0.05) than 111In-DOTA-GGNle-CycMSHhex at 0.5 h post-injection (Table 2).
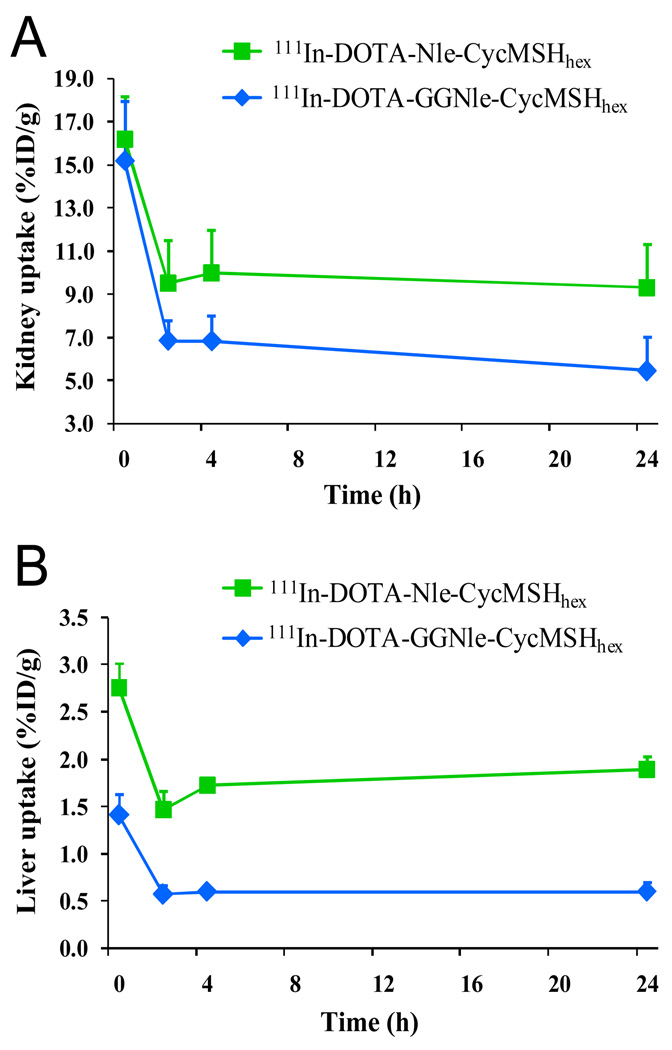
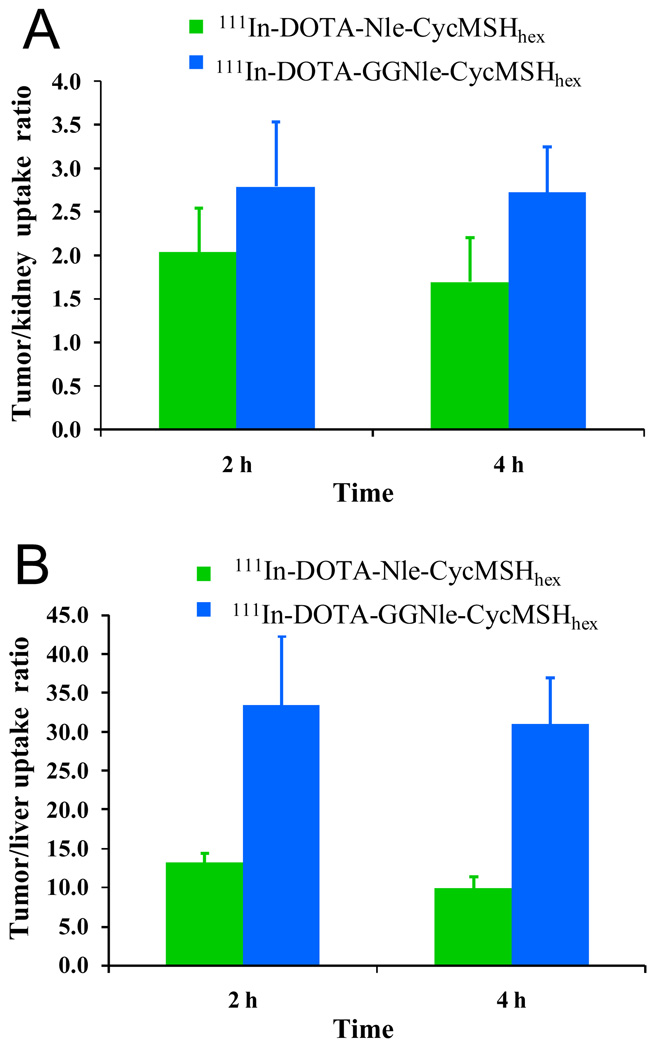
At the present time, the lactam bridge-cyclized 111In-DOTA-Nle-CycMSHhex and the metal-cyclized 111In-DOTA-Re(Arg11)CCMSH displayed the highest comparable melanoma uptakes among all reported 111In-labeled linear and cyclic α-MSH peptides (13,19). The melanoma uptake values were 17.29 ± 2.49 and 17.41 ± 5.63 %ID/g at 2 and 4 h post-injection for 111In-DOTA-Re(Arg11)CCMSH (13), whereas the melanoma uptake values were 19.39 ± 1.65 and 17.01 ± 2.54 %ID/g at 2 and 4 h post-injection for 111In-DOTA-Nle-CycMSHhex (19). Meanwhile, 111In-DOTA-Nle-CycMSHhex showed similar tumor/kidney uptake ratios as 111In-DOTA-Re(Arg11)CCMSH at 2 and 24 h post-injection (19). In this study, the introduction of the -GlyGly- linker maintained high melanoma uptakes of 111In-DOTA-GGNle-CycMSHhex (19.05 ± 5.04 and 18.6 ± 3.56 % ID/g at 2 and 4 h post-injection, respectively) compared to 111In-DOTANle-CycMSHhex, which was consistent with their similar MC1 receptor binding affinities (2.1 nM vs. 1.8 nM). Interestingly, the introduction of -GlyGly- linker reduced the liver and renal uptakes of 111In-DOTA-GGNle-CycMSHhex compared to 111In-DOTA-Nle-CycMSHhex (19). The reduction in liver and kidney uptakes might be attributed to the relatively faster whole-body clearance of 111In-DOTA-GGNle-CycMSHhex. Approximately 88% of 111In-DOTA-GGNle-CycMSHhex activity cleared out of the body via urinary system at 2 h post-injection, whereas 82% of 111In-DOTA-Nle-CycMSHhex activity washed out of the body via urinary tract at 2 h post-injection (19). 111In-DOTA-GGNle-CycMSHhex exhibited 61, 65 and 68% less liver uptake values than 111In-DOTA-Nle-CycMSHhex (Fig. 5), and 28, 32 and 42% less renal uptake values than 111In-DOTA-Nle-CycMSHhex at 2, 4 and 24 h post-injection (Fig. 5), respectively. The maintained high melanoma uptakes coupled with the decreased liver and renal uptakes resulted in enhanced tumor/liver and tumor/kidney uptake ratios for 111In-DOTA-GGNle-CycMSHhex compared to 111In-DOTA-Nle-CycMSHhex at 2 and 4 h post-injection (Fig. 6). The tumor/liver uptake ratios of 111In-DOTA-GGNle-CycMSHhex were 2.5 and 3.1 times the tumor/liver uptake ratios of 111In-DOTA-Nle-CycMSHhex at 2 and 4 h post-injection, whereas the tumor/kidney uptake ratios of 111In-DOTA-GGNle-CycMSHhex were 1.4 and 1.6 times the tumor/kidney uptake ratios of 111In-DOTA-Nle-CycMSHhex at 2 and 4 h post-injection.
Figure 5.
The kidney (A) and liver (B) uptake values of 111In-DOTA-Nle-CycMSHhex ( )and 111In-DOTA-GGNle-CycMSHhex (
)and 111In-DOTA-GGNle-CycMSHhex ( ). The data of 111In-DOTA-Nle-CycMSHhex was cited from the reference 19 for comparison.
). The data of 111In-DOTA-Nle-CycMSHhex was cited from the reference 19 for comparison.
Figure 6.
The tumor/kidney (A) and tumor/liver (B) ratios of 111In-DOTA-Nle-CycMSHhex ( ) and 111In-DOTA-GGNle-CycMSHhex (
) and 111In-DOTA-GGNle-CycMSHhex ( ) at 2 and 4 h post-injection. The data of 111In-DOTA-Nle-CycMSHhex was cited from the reference 19 for comparison.
) at 2 and 4 h post-injection. The data of 111In-DOTA-Nle-CycMSHhex was cited from the reference 19 for comparison.
As showed in Fig. 3, the enhanced tumor/liver and tumor/kidney uptake ratios of 111In-DOTA-GGNle-CycMSHhex generated high tumor imaging contrast to the background. The flank melanoma lesions were clearly visualized by SPECT/CT using 111In-DOTA-GGNle-CycMSHhex as an imaging probe, highlighting its potential as an effective imaging agent for melanoma detection. 111In-DOTA-GGNle-CycMSHhex maintained intact in melanoma and urine at 2 h post-injection (Fig. 4). From the therapeutic point of view, the enhanced tumor/liver and tumor/kidney uptake ratios of 111In-DOTA-GGNle-CycMSHhex would decrease the absorbed doses to the liver and kidneys when using the therapeutic radionuclide-labeled DOTA-GGNle-CycMSHhex for melanoma treatment. In other words, the improvement of tumor/liver and tumor/kidney uptake ratios would potentially increase the absorbed dose to the tumor while keeping the liver and kidneys safe when treating the melanoma with the therapeutic radionuclide-labeled DOTA-GGNle-CycMSHhex.
CONCLUSIONS
The amino acid linkers exhibited the profound effects on the melanoma targeting and pharmacokinetic properties of the 111In-labeled lactam bridge-cyclized α-MSH peptides. Introduction of the -GlyGly- linker maintained high melanoma uptake while reducing the renal and liver uptakes of 111In-DOTA-GlyGlyNle-CycMSHhex, highlighting its potential as an effective imaging probe for melanoma detection, as well as a therapeutic peptide for melanoma treatment when labeled with a therapeutic radionuclide.
ACKNOWLEDGMENTS
This work was supported by the NIH grant NM-INBRE P20RR016480. The image in this article was generated by the Keck-UNM Small Animal Imaging Resource established with funding from the W.M. Keck Foundation and the University of New Mexico Cancer Research and Treatment Center (NIH P30 CA118100).
REFERENCES
- 1.Siegrist W, Solca F, Stutz S, et al. Characterization of receptors for alpha-melanocyte-stimulating hormone on human melanoma cells. Cancer Res. 1989;49:6352–6358. [PubMed] [Google Scholar]
- 2.Tatro JB, Reichlin S. Specific receptors for alpha-melanocyte-stimulating hormone are widely distributed in tissues of rodents. Endocrinology. 1987;121:1900–1907. doi: 10.1210/endo-121-5-1900. [DOI] [PubMed] [Google Scholar]
- 3.Miao Y, Whitener D, Feng W, Owen NK, Chen J, Quinn TP. Evaluation of the human melanoma targeting properties of radiolabeled alpha-melanocyte stimulating hormone peptide analogues. Bioconjug Chem. 2003;14:1177–1184. doi: 10.1021/bc034069i. [DOI] [PubMed] [Google Scholar]
- 4.Miao Y, Owen NK, Whitener D, Gallazzi F, Hoffman TJ, Quinn TP. In vivo evaluation of 188Re-labeled alpha-melanocyte stimulating hormone peptide analogs for melanoma therapy. Int J Cancer. 2002;101:480–487. doi: 10.1002/ijc.10640. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Cheng Z, Hoffman TJ, Jurisson SS, Quinn TP. Melanoma-targeting properties of 99mtechnetium-labeled cyclic alpha-melanocyte-stimulating hormone peptide analogues. Cancer Res. 2000;60:5649–5658. [PubMed] [Google Scholar]
- 6.Froidevaux S, Calame-Christe M, Tanner H, Eberle AN. Melanoma targeting with DOTA-alpha-melanocyte-stimulating hormone analogs: structural parameters affecting tumor uptake and kidney uptake. J Nucl Med. 2005;46:887–895. [PubMed] [Google Scholar]
- 7.Froidevaux S, Calame-Christe M, Schuhmacher J, et al. A gallium-labeled DOTA-alpha-melanocyte- stimulating hormone analog for PET imaging of melanoma metastases. J Nucl Med. 2004;45:116–123. [PubMed] [Google Scholar]
- 8.Froidevaux S, Calame-Christe M, Tanner H, Sumanovski L, Eberle AN. A novel DOTA-alpha-melanocyte-stimulating hormone analog for metastatic melanoma diagnosis. J Nucl Med. 2002;43:1699–1706. [PubMed] [Google Scholar]
- 9.Miao Y, Owen NK, Fisher DR, Hoffman TJ, Quinn TP. Therapeutic efficacy of a 188Re-labeled alpha-melanocyte-stimulating hormone peptide analog in murine and human melanoma-bearing mouse models. J Nucl Med. 2005;46:121–129. [PubMed] [Google Scholar]
- 10.Miao Y, Hylarides M, Fisher DR, et al. Melanoma therapy via peptide-targeted alpha-radiation. Clin Cancer Res. 2005;11:5616–5621. doi: 10.1158/1078-0432.CCR-05-0619. [DOI] [PubMed] [Google Scholar]
- 11.Wei L, Butcher C, Miao Y, et al. Synthesis and biologic evaluation of 64Cu-labeled rhenium-cyclized alpha-MSH peptide analog using a cross-bridged cyclam chelator. J Nucl Med. 2007;48:64–72. [PubMed] [Google Scholar]
- 12.Miao Y, Benwell K, Quinn TP. 99mTc- and 111In-labeled alpha-melanocyte-stimulating hormone peptides as imaging probes for primary and pulmonary metastatic melanoma detection. J Nucl Med. 2007;48:73–80. [PubMed] [Google Scholar]
- 13.Cheng Z, Chen J, Miao Y, Owen NK, Quinn TP, Jurisson SS. Modification of the structure of a metallopeptide: synthesis and biological evaluation of 111In-labeled DOTA-conjugated rhenium-cyclized alpha-MSH analogues. J Med Chem. 2002;45:3048–3056. doi: 10.1021/jm010408m. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Z, Xiong Z, Subbarayan M, Chen X, Gambhir SS. 64Cu-labeled alpha-melanocyte-stimulating hormone analog for MicroPET imaging of melanocortin 1 receptor expression. Bioconjug Chem. 2007;18:765–772. doi: 10.1021/bc060306g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao Y, Gallazzi F, Guo H, Quinn TP. 111In-labeled lactam bridge-cyclized alpha-melanocyte stimulating hormone peptide analogues for melanoma imaging. Bioconjug Chem. 2008;19:539–547. doi: 10.1021/bc700317w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo H, Shenoy N, Gershman BM, Yang J, Sklar LA, Miao Y. Metastatic melanoma imaging with an 111In-labeled lactam bridge-cyclized alpha-melanocyte-stimulating hormone peptide. Nucl Med Biol. 2009;36:267–276. doi: 10.1016/j.nucmedbio.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo H, Yang J, Gallazzi F, Prossnitz ER, Sklar LA, Miao Y. Effect of DOTA position on melanoma targeting and pharmacokinetic properties of 111In-labeled lactam bridge-cyclized α-melanocyte stimulating hormone peptide. Bioconjug Chem. 2009;20:2162–2168. doi: 10.1021/bc9003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo H, Yang J, Shenoy N, Miao Y. Gallium-67-labeled lactam bridge-cyclized alpha-melanocyte stimulating hormone peptide for primary and metastatic melanoma imaging. Bioconjug Chem. 2009;20:2356–2363. doi: 10.1021/bc900428x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo H, Yang J, Gallazzi F, Miao Y. Reduction of the ring size of radiolabeled lactam bridge-cyclized alpha-MSH peptide resulting in enhanced melanoma uptake. J Nucl Med. 2010;51:418–426. doi: 10.2967/jnumed.109.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Cheng Z, Owen NK, Hoffman TJ, Miao Y, Jurisson SS, Quinn TP. Evaluation of an 111In-DOTA-rhenium cyclized α-MSH analog: a novel cyclic-peptide analog with improved tumor-targeting properties. J Nucl Med. 2001;42:1847–1855. [PubMed] [Google Scholar]
- 21.Hoffman TJ, Gali H, Smith CJ, Sieckman GL, Hayes DL, Owen NK, Volkert WA. Novel series of 111In-labeled bombesin analogs as potential radiopharmaceuticals for specific targeting of gastrin-releasing peptide receptors expressed on human prostate cancer cells. J Nucl Med. 2003;44:823–831. [PubMed] [Google Scholar]
- 22.Garayoa EG, Schweinsberg C, Maes V, Brans L, Blauenstein P, Tourwe DA, Schibli R, Schbiger PA. Influence of the molecular charge on the biodistribution of bombesin analogues labeled with the [99mTc(CO)3]-core. Bioconjug Chem. 2008;19:2409–2416. doi: 10.1021/bc800262m. [DOI] [PubMed] [Google Scholar]
- 23.Fragogeorgi EA, Zikos C, Gourni E, Bouziotis P, Paravatou-Petsotas M, Loudos G, Mitsokapas N, Xanthopoulos S, Mavri-Vavayanni M, Livaniou E, Varvarigou AD, Archimandritis SC. Spacer site modifications for the improvement of the in vitro and in vivo binding properties of 99mTc-N3S-X-Bombesin[2–14] derivatives. Bioconjug Chem. 2009;20:856–867. doi: 10.1021/bc800475k. [DOI] [PubMed] [Google Scholar]
- 24.Garrison JC, Rold TL, Sieckman GL, Naz F, Sublett SV, Figueroa SD, Volkert WA, Hoffman TJ. Evaluation of the pharmacokinetic effects of various linking group using the 111In-DOTA-X-BBN(7–14)NH2 structural paradigm in a prostate cancer model. Bioconjug Chem. 2008;19:1803–1812. doi: 10.1021/bc8001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parry JJ, Kelly TS, Andrews R, Rogers BE. In vitro and in vivo evaluation of 64Cu-labeled DOTA-Linker-Bombesin(7–14) analogues containing different amino acid linker moieties. Bioconjug Chem. 2007;18:1110–1117. doi: 10.1021/bc0603788. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, He Z, Hsieh WY, Kim YS, Jiang Y. Impact of PKM linkers on biodistribution charateristics of the 99mTc-labeled cyclic RGDfK dimer. Bioconjug Chem. 2006;17:1499–1507. doi: 10.1021/bc060235l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi J, Wang L, Kim YS, Zhai S, Liu Z, Chen X, Liu S. Improving tumor uptake and excretion kinetics of 99mTc-labeled cyclic arginine-glycine-aspartic (RGD) dimers with triglycine linkers. J Med Chem. 2008;51:7980–7990. doi: 10.1021/jm801134k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Shi J, Kim YS, Zhai S, Jia B, Zhao H, Liu Z, Wang F, Chen X, Liu S. Improving tumor-targeting capability and pharmacokinetics of 99mTc-labeled cyclic RGD dimers with PEG4 linkers. Mol Pharm. 2009;6:231–245. doi: 10.1021/mp800150r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi J, Kim YS, Zhai S, Liu Z, Chen X, Liu S. Improving tumor uptake and pharmacokinetics of 64Cu-labeled cyclic RGD peptide dimers with Gly3 and PEG4 linkers. Bioconjug Chem. 2009;20:750–759. doi: 10.1021/bc800455p. [DOI] [PMC free article] [PubMed] [Google Scholar]