Abstract
It remains unclear whether abdominal obesity increases cardiovascular disease (CVD) risk independent of the metabolic abnormalities which often accompany it. Therefore, the objective of the current study was to evaluate the independent effects of abdominal obesity versus metabolic syndrome and diabetes on the risk for incident coronary heart disease and stroke. The Framingham Offspring, Atherosclerosis Risk in Communities, and Cardiovascular Health studies were pooled to assess the independent effects of abdominal obesity (waist circumference >102 cm for men and >88 cm for women) versus metabolic syndrome (excluding the waist circumference criterion) and diabetes on risk for incident coronary heart disease and stroke in 20,298 men and women aged ≥45 years. The average follow-up was 8.3 (standard deviation 1.9) years. There were 1,766 CVD events. After adjustment for demographic factors, smoking, alcohol intake, number of metabolic syndrome components and diabetes, abdominal obesity was not significantly associated with an increased risk of CVD (hazard ratio [95% confidence interval] 1.09 [0.98, 1.20]). However, after adjustment for demographics, smoking, alcohol intake, and abdominal obesity, having 1–2 metabolic syndrome components, the metabolic syndrome, and diabetes were each associated with a significantly increased risk of CVD (2.12 [1.80, 2.50], 2.82 [1.92, 4.12] and 5.33 [3.37, 8.41], respectively). Although abdominal obesity is an important clinical tool for identification of individuals likely to possess metabolic abnormalities, these data suggest that the metabolic syndrome and diabetes are considerably more important prognostic indicators of CVD risk.
INTRODUCTION
Obesity is known to increase the risk of cardiovascular disease (CVD). However, we have recently shown that a substantial proportion (approximately 30%) of obese U.S. adults do not have the clustering of cardiometabolic abnormalities commonly associated with obesity including hypertension, dyslipidemia, and elevated levels of fasting glucose, insulin resistance, and systemic inflammation.(1) Two prior studies suggest that obesity may increase the risk for CVD only among persons with hypertension, dyslipidemia, or type 2 diabetes(2, 3). Additionally, new research suggests that the cardiovascular risk reduction of weight loss may differ in obese persons with versus without cardiometabolic disturbances(4, 5). Therefore, examination of the CVD risks associated with obesity independent of the cardiometabolic disturbances which often, but not always, accompany it is of considerable public health and clinical importance. Previous studies of the independent CVD risks associated with obesity have provided contradictory evidence (2, 3, 6–13), and have largely failed to directly examine whether CVD risk is elevated when obesity is unaccompanied by these cardiometabolic disturbances. Instead, most published studies used statistical adjustment to account for the effects of metabolic status. In addition, despite that abdominal obesity is known to confer greater risk of CVD than BMI-measured obesity, very few prior studies have addressed whether abdominal obesity is associated with increased risk of CVD even when it is unaccompanied by the cardiometabolic abnormalities thought to result from it. Therefore, the purpose of the current study was to evaluate the independent effects of abdominal obesity and cardiometabolic abnormalities on the risk for incident CVD. Three large population-based cohort studies of men and women were pooled to obtain sufficient sample size and numbers of incident CVD events to assess the risk of CVD associated with obesity in those both with and without cardiometabolic abnormalities, and to statistically evaluate whether metabolic status modifies the association between obesity and incident CVD.
METHODS and PROCEDURES
Study Population
The population for the current analyses is derived from the pooling of three large, limited-access public use databases from the following studies: the Framingham Offspring Study (FOS), the Atherosclerosis Risk in Communities Study (ARIC), and the Cardiovascular Health Study (CHS). Informed consent and appropriate institutional review board approval was obtained by each center for the three studies.
The FOS was initiated in 1971, and recruited 5,124 men and women aged 5 to 70 years who were children or spouses of participants in the Framingham Heart Study. As the FOS did not measure waist circumference until the 4th examination, this examination was used as the baseline visit for the current pooling of data. ARIC recruited 15,792 men and women aged 45 to 64 years in 1987 to 1989, and CHS initially recruited 5,201 participants aged ≥65 years in 1989–1990 using Medicare eligibility files. In 1992, an additional 687 black participants were recruited. Details regarding recruitment and study procedures for each of the three studies have been published previously.(14–16)
Pooling of the three longitudinal databases resulted in a dataset with 26,744 individuals. Exclusion criteria from the pooled dataset included missing age information or baseline age <45 years (total n=2,277; missing=1,198; <45 years=1,079); body mass index (BMI) values in the underweight range (<18.5 kg/m2; n=163); missing data on waist circumference (n=85); history of CVD (CHD, stroke, and peripheral vascular disease in all studies and heart failure for CHS and the FOS cohorts) at study baseline for ARIC and CHS, and at the original study baseline or at any time between baseline and the 4th follow-up visit for the FOS (n=3,158); missing data on the four-level metabolic obesity variable (n=273), and reported fasting fewer than 8 hours prior to the study visit (n=490), leaving data from 20,298 individuals for the current analyses.
Blood Pressure, Anthropometrics, and Questionnaire Data
For each of the three studies, blood pressure was measured in the seated position after a short rest period and averaged across two readings. BMI was calculated as the weight in kilograms divided by height in meters squared, and categorized as normal weight (BMI<25 kg/m2), overweight (BMI 25.0–29.9 kg/m2), or obese (BMI≥30 kg/m2). Waist circumference was measured at the level of the umbilicus while the participant was standing, among all three studies. Abdominal obesity was defined as waist circumference >102 cm for men and >88 cm for women. Smoking and alcohol intake were assessed by questionnaire in each study. Since the amount of alcohol consumed was not assessed identically across studies, participants were coded as current drinkers or not.
Laboratory Measurements
Blood samples were obtained after an 8 hour or longer fast in each study. Laboratory methods have been previously reported for all three studies.(17–22) Of relevance to the current analyses, glucose was measured in serum with a Kodak Ektachem E-700 among CHS participants and by a hexokinase/glucose-6-phosphate dehydrogenase method among ARIC participants, and in plasma with a hexokinase reagent kit (A-gent glucose test; Abbott, South Pasadena, CA) among FOS participants. In all three studies, plasma triglycerides and HDL-cholesterol were measured enzymatically (HDL-cholesterol after precipitation of low and very low density lipoproteins with dextran sulfate-magnesium ions sulfate).
Metabolic Syndrome and Diabetes Definitions
Metabolic syndrome components were defined according to the National Cholesterol Education Program’s Adult Treatment Panel III (ATP III) revised recommendations(23): 1.) fasting serum or plasma glucose ≥100 mg/dL, 2.) fasting serum triglycerides ≥150 mg/dL, 3.) fasting serum HDL-cholesterol <40 mg/dL for men and <50 mg/dL for women, and 4.) systolic/diastolic blood pressure ≥130/85 mmHg or self-reported use of antihypertensive medications. The waist circumference criterion was not included as a metabolic syndrome component as it was used to define obesity. Diabetes was defined as a fasting serum or plasma glucose ≥126 mg/dL or self-reported use of anti-diabetes medications. Participants were categorized into one of four metabolic abnormality groups: 1.) no metabolic syndrome components or diabetes (“normal” metabolism), 2.) 1–2 metabolic syndrome components and no diabetes (1–2 components), 3.) ≥3 metabolic syndrome components and no diabetes (metabolic syndrome), and 4.) diabetes (diabetes).
Cardiovascular Disease Event Ascertainment and Follow-up Time Determination
To ensure consistency of event reporting across the three studies, the current analyses are limited to probable and definite fatal and non-fatal CHD and stroke. In all three studies, CHD was defined as myocardial infarction (MI), silent MI, or CHD death and stroke included both hemorrhagic and ischemic subtypes. Event ascertainment has been previously reported for all studies.(24–27) Briefly, each study obtained medical record data through chart abstraction for use in event classification, and utilized an adjudication committee to determine final event classification. Among all three studies, data abstracted for CHD determination included cardiac pain, ECG findings, and cardiac enzymes, and for stroke determination neurological evaluations, imaging studies, and pathology reports. Specific algorithms for event determination were similar, though not identical,l for all three studies, with the exception of MI determination, for which CHS adopted the ARIC protocol identically.
As each of the three studies varied in the length of available follow-up, these analyses are limited to nine years of follow-up to ensure adequate sample size throughout. Follow-up time for analyses of CVD (CHD and stroke considered together) was calculated as the time between the baseline visit (visit 4 for the FOS) and the first CHD or stroke event, or between the baseline visit and the last known contact with the participant for those without events.
Statistical Methods
The distributions of demographic, anthropometric, and laboratory variables were compared across both abdominal obesity and metabolic status groupings using chi-square tests for categorical variables and Kruskal Wallis or Mann Whitney U tests for continuous variables. Failure curves were generated using the Kaplan Meier method for the eight metabolic categories (i.e., abdominal obesity cross-classified by metabolic abnormality grouping).
After initial adjustment for demographic factors, smoking status, and alcohol intake, the independent effects of abdominal obesity and metabolic status on the risks of CVD, CHD, and stroke were examined by further adjustment of the Cox proportional hazard ratios associated with abdominal obesity for metabolic status grouping; and further adjustment of the Cox proportional hazard ratios associated with metabolic status grouping for abdominal obesity. A two-stage approach was used for all Cox proportional hazards regression modeling. First, study-specific hazard ratios and 95% confidence intervals of each outcome were calculated. Pooled hazard ratios were then calculated by combining the study-specific hazard ratios, weighted by the inverse of their variance, using a random effects model. For CHD event analyses, individuals whose first event was a stroke were censored at the time of their stroke, while for stroke analyses, individuals whose first event was a CHD event were censored at the time of their CHD event. There were two cases where a CHD event and stroke event occurred on the same day, and these individuals were counted only once in total CVD analyses. Statistical interactions between abdominal obesity and metabolic status on the risk of CVD were tested via multiplicative interaction terms (i.e. abdominal obesity group × metabolic status and waist circumference, modeled as a continuous variable, × metabolic status). To ensure that covariability between abdominal obesity and metabolism was not affecting resulting estimates, analyses of abdominal obesity were stratified by metabolic status and vice versa. The proportional hazards assumption was evaluated by Schoenfeld residuals in all Cox models, and was not violated.
Sensitivity analyses were conducted by assessing the outcomes of fatal and non-fatal events, separately, by assessing outcomes in subgroups defined by age (45 to 64 years and ≥65 years) and sex, and using BMI groups (<25 kg/m2, 25–29.9 kg/m2, and ≥30 kg/m2) in place of abdominal obesity. Sensitivity analyses were also performed excluding events within the first two years in an attempt to remove the influence of subclinical disease at baseline on the results, and incorporating cholesterol-lowering medication use into the HDL-cholesterol criterion of the metabolic syndrome definition (n=637). This lead to an additional 65 people re-categorized as having 1–2 components, and 93 people re-categorized as having metabolic syndrome. All analyses were conducted using STATA version 10.
RESULTS
The average follow-up was 8.3 (SD 1.9) years, and over this time there were a total of 1 766 fatal or non-fatal incident CVD events (1 118 CHD events and 648 stroke events). Since two individuals had a CHD event and a stroke simultaneously, the number of events used for the combined CVD analyses was 1 764. Compared to non-obese individuals, those with abdominal obesity were more likely to be 45 to 64 years of age, women, African American, less educated, never-smokers, never or former drinkers, to have metabolic syndrome or diabetes, and to have worse levels of metabolic syndrome components (Table 1). Compared to individuals without any metabolic syndrome components or diabetes, individuals with the metabolic syndrome or diabetes were older, and more likely to be men, African American, never smokers, less educated, never or former drinkers, obese, and to have worse levels of CVD risk factors (Table 2).
Table 1.
Baseline Characteristics of Study Participants by Abdominal Obesity Groups
| Non-obese (n=10,357) |
Abdominally Obese (n=9,941) |
p-value | |
|---|---|---|---|
| Age Group, % | |||
| 45 to 64.9 years | 7,858 (75.9%) | 7,740 (77.9%) | 0.001 |
| ≥65 years | 2,499 (24.1%) | 2,201 (22.1%) | |
| Sex, % | < 0.001 | ||
| Women | 4,590 (44.3%) | 6,826 (68.7%) | |
| Men | 5,767 (55.7%) | 3,115 (31.3%) | |
| Race, % | < 0.001 | ||
| Caucasian | 8,744 (84.4%) | 7,536 (75.8%) | |
| African American | 1,613 (15.6%) | 2,405 (24.2%) | |
| High School Education, % | < 0.001 | ||
| ≤ High School | 5,229 (50.5%) | 5,966 (60.0%) | |
| > High School | 5,128 (49.5%) | 3,975 (40.0%) | |
| Cigarette Smoking, % | |||
| Never | 3,931 (38.6%) | 4,570 (46.4%) | < 0.001 |
| Current | 2,491 (24.5%) | 1,994 (20.3%) | |
| Former | 3,766 (37.0%) | 3,283 (33.3%) | |
| Alcohol Consumption, % | < 0.001 | ||
| Never or Former | 3,801 (36.8%) | 4,868 (49.2%) | |
| Current | 6,541 (63.3%) | 5,032 (50.8%) | |
| Cardiometabolic Status, % | |||
| Normal | 2,752 (26.6%) | 1,159 (11.7%) | < 0.001 |
| 1–2 Components | 5,773 (55.7%) | 4,941 (49.7%) | |
| Metabolic Syndrome | 1,249 (12.1%) | 2,322 (23.4%) | |
| Diabetes | 583 (5.6%) | 1,519 (15.3%) | |
| Systolic BP, mmHg | 123.4 (20.4) | 127.6 (19.8) | < 0.001 |
| Diastolic BP, mmHg | 73.2 (11.4) | 74.9 (11.1) | < 0.001 |
| HDL Cholesterol, mmol/L | 1.41 (0.45) | 1.30 (0.40) | < 0.001 |
| Triglycerides, mmol/La | 1.12 (0.82–1.57) | 1.40 (1.02–1.98) | < 0.001 |
| Glucose, mmol/La | 5.34 (5.02–5.72) | 5.61 (5.22–6.20) | < 0.001 |
| Waist Circumference, cm | 86.6 (9.0) | 104.4 (10.7) | < 0.001 |
Values in table are n (%) or mean (standard deviation) unless otherwise indicated.
Values are median (interquartile range)
Table 2.
Baseline Characteristics of Study Participants by Metabolic Syndrome and Diabetes Status
| Normal (n=3,911) |
1–2 Components (n=10,714) |
Metabolic Syndrome (n=3,571) |
Diabetes (n=2,102) |
p-value | |
|---|---|---|---|---|---|
| Age Group, % | |||||
| 45 to 64.9 years | 3,354 (85.8%) | 8,119 (75.8%) | 2,670 (74.8%) | 1,455 (69.2%) | <0.001 |
| ≥65 years | 557 (14.2%) | 2,595 (24.2%) | 901 (25.2%) | 647 (30.8%) | |
| Sex, % | |||||
| Women | 2,589 (66.2%) | 5,941 (55.5%) | 1,740 (48.7%) | 1,146 (54.5%) | <0.001 |
| Men | 1,322 (33.8%) | 4,773 (44.6%) | 1,831 (51.3%) | 956 (45.5%) | |
| Race, % | |||||
| Caucasian | 3,362 (86.0%) | 8,479 (79.1%) | 3,019 (84.5%) | 1,420 (67.6%) | <0.001 |
| African American | 549 (14.0%) | 2,235 (20.9%) | 552 (15.5%) | 682 (32.5%) | |
| ≥ High School Education, % | |||||
| < High School | 1,813 (46.4%) | 5,828 (54.4%) | 2,161 (60.5%) | 1,393 (66.3%) | <0.001 |
| ≥ High School | 2,098 (53.6%) | 4,886 (45.6%) | 1,410 (39.5%) | 709 (33.7%) | |
| Cigarette Smoking, % | |||||
| Never | 1,769 (45.9%) | 4,432 (42.0%) | 1,357 (38.4%) | 943 (45.3%) | 0.001 |
| Current | 821 (21.3%) | 2,412 (22.8%) | 850 (24.1%) | 402 (19.3%) | |
| Former | 1,268 (32.9%) | 3,720 (35.2%) | 1,324 (37.5%) | 737 (35.4%) | |
| Alcohol Consumption, % | |||||
| Never or Former | 1,391 (35.7%) | 4,455 (41.7%) | 1,557 (43.7%) | 1,266 (60.5%) | <0.001 |
| Current | 2,511 (64.4%) | 6,230 (58.3%) | 2,004 (56.3%) | 828 (39.5%) | |
| Abdominal Obesity, % | |||||
| Non-Obese | 2,752 (70.4%) | 5,773 (53.9%) | 1,249 (35.0%) | 583 (27.7%) | <0.001 |
| Obese | 1,159 (29.6%) | 4,941 (46.1%) | 2,322 (65.0%) | 1,519 (72.3%) | |
| BMI Group, % | |||||
| Normal Weight | 2,239 (57.3%) | 3,850 (36.0%) | 615 (17.2%) | 338 (16.1%) | <0.001 |
| Overweight | 1,318 (33.7%) | 4,560 (42.6%) | 1,610 (45.1%) | 807 (38.5%) | |
| Obese | 353 (9.0%) | 2,299 (21.5%) | 1,343 (37.6%) | 954 (45.5%) | |
| Systolic BP, mmHg | 110.8 (10.4) | 126.6 (20.3) | 132.9 (19.0) | 134.2 (21.5) | <0.001 |
| Diastolic BP, mmHg | 68.5 (8.0) | 74.7 (11.5) | 77.3 (11.1) | 75.4 (11.9) | <0.001 |
| HDL Cholesterol, mmol/L | 1.63 (0.39) | 1.40 (0.42) | 1.03 (0.26) | 1.18 (0.36) | <0.001 |
| Triglycerides, mmol/La | 0.90 (0.70–1.15) | 1.19 (0.89–1.54) | 2.06 (1.73–2.60) | 1.68 (1.18–2.46) | <0.001 |
| Glucose, mmol/La | 5.11 (4.86–5.29) | 5.40 (5.08–5.77) | 5.83 (5.56–6.16) | 8.38 (7.22–11.38) | <0.001 |
| Waist Circumference, cm | 87.7 (11.7) | 94.5 (12.6) | 101.2 (11.8) | 103.9 (13.0) | <0.001 |
| BMI, kg/m2 | 24.9 (3.8) | 27.0 (4.6) | 29.2 (4.8) | 30.1 (5.4) | < 0.001 |
Values in table are n (%) or mean (standard deviation) unless otherwise indicated.
Values are median (interquartile range)
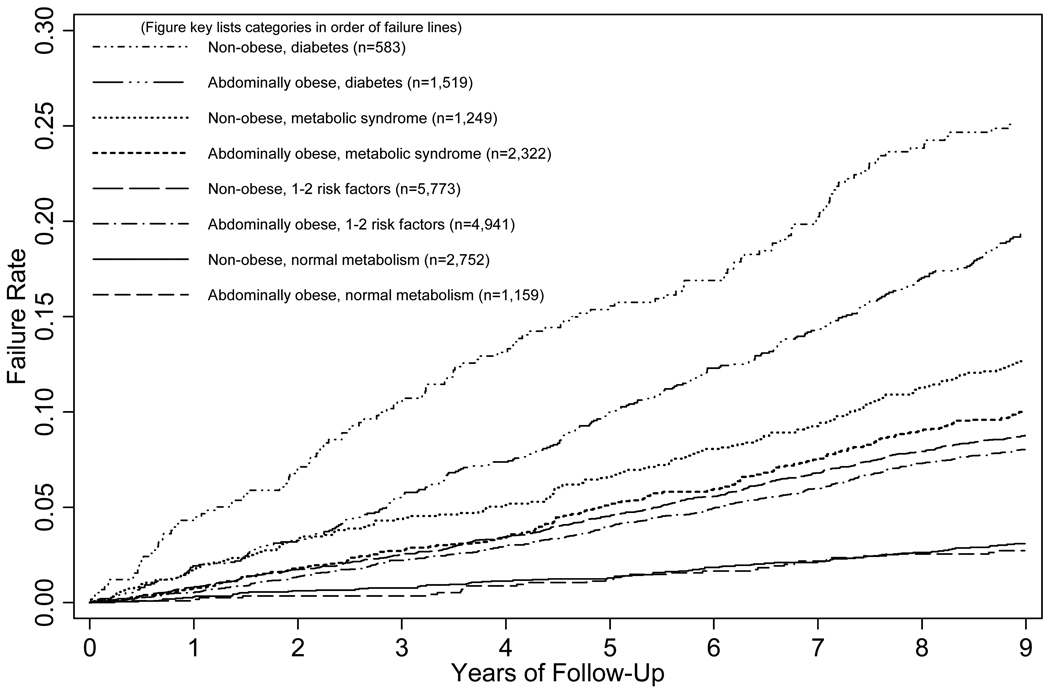
When abdominal obesity and metabolic categories were considered in combination, three groups of failure curves emerged corresponding to the metabolic categories (0 risk factors, 1–2 components or metabolic syndrome, and diabetes) (Figure 1).
FIGURE 1.
Kaplan-Meier Plot of CVD Event Rates by the Eight Joint Obesity/Metabolic Abnormality Categories
After adjustment for age, sex, race, education, smoking status, and alcohol intake, abdominal obesity was associated with a 33% increased risk of total CVD (CHD and stroke) in pooled analyses (Table 3). However, after further adjustment for metabolic status, abdominal obesity was no longer significantly associated with increased risk of CVD. Metabolic syndrome and diabetes were associated with 100% to 400% increased risk of incident CVD events after initial adjustment for age, sex, race, education, smoking status, and alcohol intake, as well as after further adjustment for abdominal obesity. Hazards ratios for CHD and stroke showed similar patterns and effect magnitudes.
Table 3.
Pooled Hazards Ratios (95% Confidence Intervals) for Incident CVD Events Associated with Abdominal Obesity and Metabolic Status
| CHD |
Stroke |
Total CVD |
|
|---|---|---|---|
| Adjusted HR (95% CI) |
Adjusted HR (95% CI) |
Adjusted HR (95% CI) |
|
| Abdominal Obesity | |||
| Model 1a | |||
| Non-obese (reference) | 1.00 | 1.00 | 1.00 |
| Obese | 1.40 (1.10 -1.79) | 1.18 (1.00 – 1.39) | 1.33 (1.09 – 1.63) |
| P-value | <0.01 | 0.05 | <0.01 |
| Model 1a + Adjustment for Metabolic Status grouping | |||
| Non-obese (reference) | 1.00 | 1.00 | 1.00 |
| Obese | 1.13 (0.99 – 1.29) | 1.00 (0.85 – 1.19) | 1.09 (0.98 – 1.20) |
| P-value | 0.06 | 0.97 | 0.12 |
| Metabolic Status | |||
| Model 1a | |||
| Normal (reference) | 1.00 | 1.00 | 1.00 |
| 1–2 Components | 2.20 (1.70 – 2.84) | 1.84 (1.34 – 2.52) | 2.09 (1.71 – 2.54) |
| Metabolic Syndrome | 2.92 (1.89 – 4.53) | 1.86 (1.29 – 2.64) | 2.67 (1.79 – 3.98) |
| Diabetes | 5.19 (3.22 – 8.36) | 4.20 (2.38 – 7.40) | 5.14 (3.13 – 7.76) |
| P-value | <0.001 | <0.001 | <0.001 |
| Model 1a + Adjustment for Abdominal Obesity | |||
| Normal (reference) | 1.00 | 1.00 | 1.00 |
| 1–2 Components | 2.15 (1.66 – 2.78) | 1.86 (1.31 – 2.64) | 2.05 (1.68 – 2.50) |
| Metabolic Syndrome | 2.77 (1.90 – 4.03) | 1.86 (1.29 – 2.68) | 2.56 (1.78 – 3.69) |
| Diabetes | 4.90 (3.25 – 7.39) | 4.22 (2.35 – 7.59) | 4.91 (3.11 – 7.76) |
| P-value | <0.001 | <0.001 | <0.001 |
Adjusted for age, sex, race, education, smoking status, and alcohol intake.
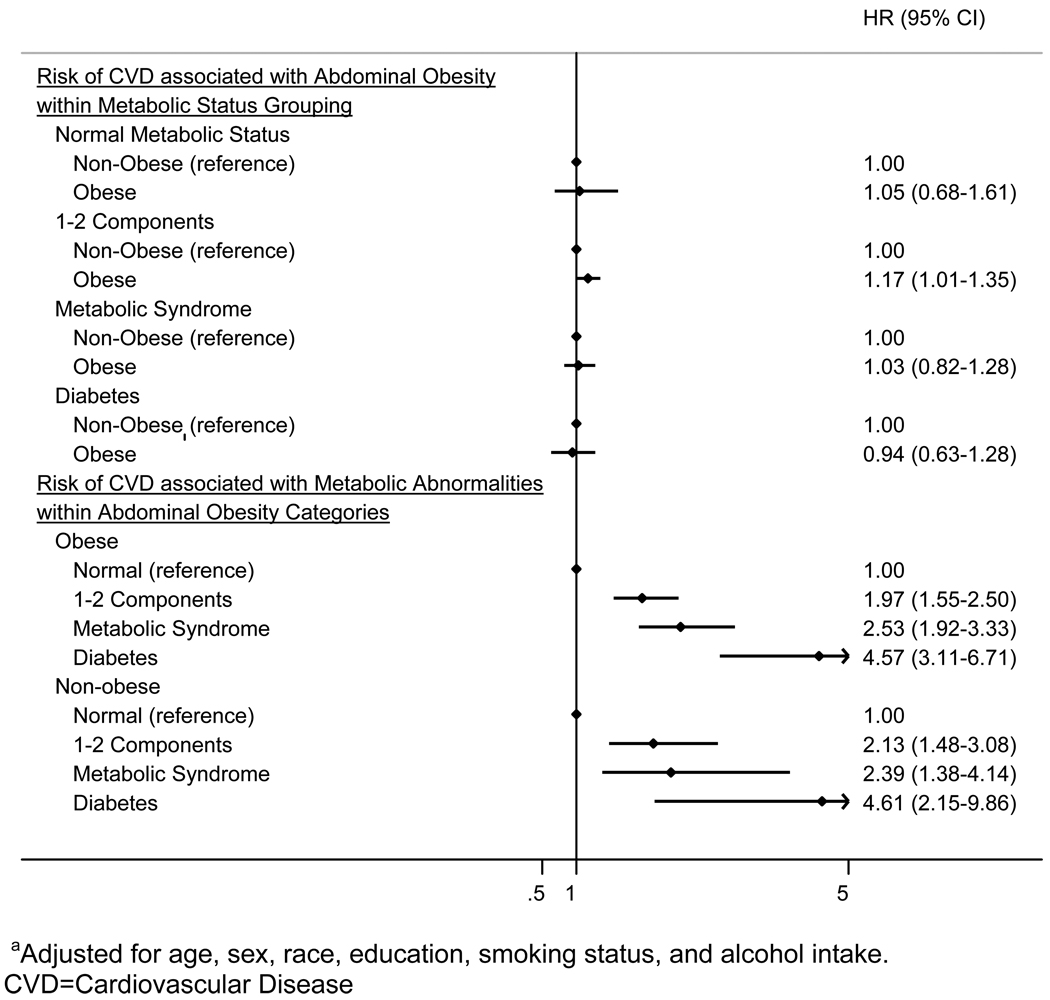
In stratified analyses, abdominal obesity was not significantly associated with an increased risk of incident CVD for participants with normal metabolism, metabolic syndrome or diabetes (Figure 2). However, an association was present between abdominal obesity and increased risk of incident CVD for those with 1–2 metabolic components. Among both non-obese and abdominally obese individuals, participants with 1–2 metabolic components, metabolic syndrome and diabetes had an increased risk of incident CVD compared to those with normal metabolism. Results were similar when waist circumference was modeled as a continuous variable (data not shown). There was no significant statistical interaction between metabolic status and either abdominal obesity or waist circumference expressed as a continuous variable on the risks of CVD (p=0.48 and p=0.49, respectively).
FIGURE 2.
Forest Plot of Pooled Adjusteda Hazards Ratios (95% Confidence Intervals) for Incident CVD Events Associated with Metabolic Status Stratified by Abdominal Obesity (Top) and Associated with Abdominal Obesity Stratified by Metabolic Status (Bottom)
Sensitivity Analyses
The results were markedly similar after excluding events that occurred within the first two years of follow-up (data not shown). Additionally, the associations were also similar when analyses were conducted stratified by sex, age group, and by fatal versus non-fatal event status, and considering cholesterol-lowering medication use in the metabolic syndrome definition (data not shown). Finally, all analyses were repeated using BMI categories (normal weight, overweight, obese) rather than waist circumference, and analyses were also similar (data not shown). When analyses were conducted for each of the three studies separately, a significant effect of abdominal obesity on risk for CVD (HR for total CVD events 1.17; 95% CI 1.01–1.37) was present after adjustment for metabolic status in the ARIC study, but not in the CHS or FOS (HR 1.01 [0.87–1.18] and 1.06 [0.73–1.54] respectively) (Supplementary On-Line Table 1). However, this difference between studies was not statistically significant, as indicated by a non-significant study × abdominal obesity interaction term (p=0.52). Similar to the pooled analysis, within each of the three studies, abdominal obesity was not statistically significantly associated with increased risk of CVD within metabolic status categories, but 1–2 metabolic components, metabolic syndrome, and diabetes were each associated with incident CVD events among both abdominally obese and non-obese participants (Supplementary On-Line Table 2).
DISCUSSION
The present analyses of data pooled from three large cohort studies showed that after accounting for metabolic status, abdominal obesity was not associated with a significantly increased risk for CHD or stroke in nearly every case, the one exception being among those with 1–2 metabolic components. However, the presence of metabolic syndrome components, metabolic syndrome, or diabetes were each associated with approximately 2 to 5 times increased risk for CHD or stroke over an average follow-up of 8 years, even after accounting for abdominal obesity.
In the current study, we assessed the independent effects of abdominal obesity and metabolism by two different methods. After statistical adjustment for metabolic status, abdominal obesity was not associated with a statistically significantly increased risk for CVD. Similarly, stratified analyses of the association between abdominal obesity and CVD within metabolic status group showed abdominal obesity was not significantly associated with CVD among those with 0 components, metabolic syndrome, or diabetes. However, those with 1–2 metabolic syndrome components had a statistically significantly increased risk for CVD associated with abdominal obesity. While this latter result raises the possibility that abdominal obesity may increase the risk of CVD independent of metabolic abnormalities, this increased risk was modest (HR 1.17) compared with the increased risk associated with varying degrees of metabolic abnormalities (HRs ranging from 1.97 to 4.61), and was not found among those with 0 components, metabolic syndrome, or diabetes. Figure 2 presents a striking visual illustration of the strong risks of CVD associated with metabolic abnormalities compared with the lack of an association between abdominal obesity and CVD. Therefore, the current analyses support previous studies showing that the risk of CVD commonly associated with obesity is largely driven by concomitant metabolic abnormalities.(2, 3, 6–8)
The current results contradict some prior studies which have suggested the possibility that metabolic status may modify the obesity-CVD relationship.(2, 3) In the Aerobics Center Longitudinal Study (ACLS), abdominal obesity was associated with incident CVD events over 10 to 11 years of follow-up among middle-aged men who had hypertension, dyslipidemia, or diabetes but not among those without these factors.(2) Similar results were reported for obesity defined by BMI among middle-aged women in the Women’s Health Study.(3) However, in our stratified analyses, the HR for CVD events associated with abdominal obesity was 1.03 among those with metabolic syndrome and 0.94 among those with diabetes, suggesting that even in the presence of multiple metabolic abnormalities or diabetes, abdominal obesity was not significantly associated with elevated risk of CVD. The interaction term (abdominal obesity × metabolic status) was not statistically significant, further confirming the lack of effect modification in the current study.
BMI-defined obesity and abdominal obesity have been found to impart significantly increased risk of CVD independent of standard risk factors and diabetes in some studies. (9–11, 13) Notably, significant associations with obesity appear to be more frequently observed in studies of longer duration, raising the possibility that obesity increases long-term (20–25 years) risk of CVD, perhaps due to enhanced subclinical atherosclerosis in obese individuals. Obese, metabolically healthy individuals have been shown to have impaired endothelial dysfunction and greater carotid intima-media thickness.(28, 29) However, all but one of the incident CVD studies with longer-term follow-up used statistical adjustment to account for metabolic abnormalities, rather than stratification, and none accounted for the development of cardiometabolic abnormalities across follow-up. Therefore, it remains unclear whether obesity without concomitant metabolic abnormalities is associated with elevated long-term risk of CVD.
In stratified analyses, individuals who were abdominally obese but did not have any of the metabolic syndrome components or diabetes were not at significantly increased risk for CHD or stroke over an average follow-up of 8 years compared to similar individuals without obesity. However, stratified analyses also showed that individuals without abdominal obesity had a substantially increased risk for CHD or stroke over 8 years if they possessed metabolic syndrome components or diabetes compared to individuals without these factors. Similar results were found in sensitivity analyses among normal weight individuals, as defined by BMI. Therefore, in addition to highlighting the need for risk stratification via assessment of metabolic abnormalities among obese individuals, these data suggest that the metabolic syndrome may confer an approximate 3-fold increase in risk of incident CVD even in normal weight individuals.
The results of this study must be viewed within the context of its limitations. As indicated previously, measurement protocols were not identical for certain laboratory and questionnaire data across the three studies. However, we analyzed data in two stages and present both study-specific and pooled results. Results were similar for each of the three studies. There were insufficient numbers of African Americans to stratify results by race. The frequency and extent of assessment of body size and metabolic changes across follow-up were not uniform for all three studies and comparable data on physical activity was not available at baseline for each study, and therefore, these could not be taken into account in the statistical analyses. Additionally, in order to maintain sufficient numbers of events in all three studies across the follow-up period, we were limited to 9 years of follow-up. Longer follow-up is needed to examine whether the effect of obesity is more pronounced when risk of CVD is examined over an extended time frame.
However, this study also has a number of strengths. With the large sample size resulting from the pooling of longitudinal databases, substantial numbers of CHD and stroke events occurred, permitting the investigation of the independent risks of CVD associated with obesity and abnormal metabolism in important population subgroups. The large sample size also afforded us the power to assess independence of abdominal obesity from metabolic status via stratification, as well as to formally assess effect modification. Each of the three studies pooled in these analyses followed standardized data collection protocols for measurement of the variables included here-in, and each performed active follow-up for CVD events with formal adjudication of events.
In conclusion, the current analyses of individuals from three large, longitudinal population-based studies, suggests that the presence of metabolic abnormalities is a substantially stronger predictor than abdominal obesity of incident CHD and stroke over an average of 8 years. Although abdominal obesity and body size remain important clinical tools for identification of individuals likely to possess metabolic abnormalities, metabolic syndrome and diabetes are considerably more important prognostic indicators of CVD risk. These data underscore the need for close monitoring and treatment of adverse levels of blood pressure, lipids, and glucose even among normal weight or non-obese individuals.
Supplementary Material
ACKNOWLEDGEMENTS
The Cardiovascular Health Study (CHS), Atherosclerosis Risk in Communities Study (ARIC), and Framingham Offspring Study are conducted and supported by the NHLBI in collaboration with the CHS, ARIC, and Framingham Offspring study investigators, respectively. This manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the CHS, ARIC, or Framingham Offspring studies or the NHLBI.
This study was supported by the following grant from the National Heart, Lung, and Blood Institute of the NIH: R21-HL089625 (Dr. Wildman). The sponsor had no role in the design, conduct or reporting of the study.
Footnotes
DISCLOSURES
The authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168(15):1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 2.Katzmarzyk PT, Janssen I, Ross R, Church TS, Blair SN. The importance of waist circumference in the definition of metabolic syndrome: prospective analyses of mortality in men. Diabetes Care. 2006;29(2):404–409. doi: 10.2337/diacare.29.02.06.dc05-1636. [DOI] [PubMed] [Google Scholar]
- 3.Song Y, Manson JE, Meigs JB, Ridker PM, Buring JE, Liu S. Comparison of usefulness of body mass index versus metabolic risk factors in predicting 10-year risk of cardiovascular events in women. Am J Cardiol. 2007;100(11):1654–1658. doi: 10.1016/j.amjcard.2007.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes (Lond) 2006;30(10):1529–1534. doi: 10.1038/sj.ijo.0803304. [DOI] [PubMed] [Google Scholar]
- 5.Karelis AD, Messier V, Brochu M, Rabasa-Lhoret R. Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia. 2008;51(9):1752–1754. doi: 10.1007/s00125-008-1038-4. [DOI] [PubMed] [Google Scholar]
- 6.Kip KE, KE OC, Kelley DE, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women's Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109(6):706–713. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 7.Schulte H, Cullen P, Assmann G. Obesity, mortality and cardiovascular disease in the Munster Heart Study (PROCAM) Atherosclerosis. 1999;144(1):199–209. doi: 10.1016/s0021-9150(99)00055-6. [DOI] [PubMed] [Google Scholar]
- 8.Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91(8):2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 9.Hu G, Tuomilehto J, Silventoinen K, Sarti C, Mannisto S, Jousilahti P. Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Arch Intern Med. 2007;167(13):1420–1427. doi: 10.1001/archinte.167.13.1420. [DOI] [PubMed] [Google Scholar]
- 10.Rosengren A, Wilhelmsen L, Lappas G, Johansson S. Body mass index, coronary heart disease and stroke in Swedish women. A prospective 19-year follow-up in the BEDA study. Eur J Cardiovasc Prev Rehabil. 2003;10(6):443–450. doi: 10.1097/01.hjr.0000085253.65733.ef. [DOI] [PubMed] [Google Scholar]
- 11.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 12.St-Pierre AC, Cantin B, Mauriege P, et al. Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. CMAJ. 2005;172(10):1301–1305. doi: 10.1503/cmaj.1040834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flint AJ, Hu FB, Glynn RJ, et al. Excess weight and the risk of incident coronary heart disease among men and women. Obesity (Silver Spring) 2010;18(2):377–383. doi: 10.1038/oby.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 15.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 17.Ettinger WH, Wahl PW, Kuller LH, et al. Lipoprotein lipids in older people. Results from the Cardiovascular Health Study. The CHS Collaborative Research Group. Circulation. 1992;86(3):858–869. doi: 10.1161/01.cir.86.3.858. [DOI] [PubMed] [Google Scholar]
- 18.Shahar E, Chambless LE, Rosamond WD, et al. Plasma lipid profile and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2003;34(3):623–631. doi: 10.1161/01.STR.0000057812.51734.FF. [DOI] [PubMed] [Google Scholar]
- 19.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000;49(12):2201–2207. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 20.Kannel WB, Vasan RS, Keyes MJ, Sullivan LM, Robins SJ. Usefulness of the triglyceride-high-density lipoprotein versus the cholesterol-high-density lipoprotein ratio for predicting insulin resistance and cardiometabolic risk (from the Framingham Offspring Cohort) Am J Cardiol. 2008;101(4):497–501. doi: 10.1016/j.amjcard.2007.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atherosclerosis Risk in Communities Limited Access Data. 2008 6-16-2008. Ref Type: Internet Communication. [Google Scholar]
- 22.Meigs JB, Nathan DM, Wilson PW, Cupples LA, Singer DE. Metabolic risk factors worsen continuously across the spectrum of nondiabetic glucose tolerance. The Framingham Offspring Study. Ann Intern Med. 1998;128(7):524–533. doi: 10.7326/0003-4819-128-7-199804010-00002. [DOI] [PubMed] [Google Scholar]
- 23.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 24.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 25.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 26.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 27.Stokes J, III, Kannel WB, Wolf PA, Cupples LA, D'Agostino RB. The relative importance of selected risk factors for various manifestations of cardiovascular disease among men and women from 35 to 64 years old: 30 years of follow-up in the Framingham Study. Circulation. 1987;75(6 Pt 2):V65–V73. [PubMed] [Google Scholar]
- 28.Oflaz H, Ozbey N, Mantar F, et al. Determination of endothelial function and early atherosclerotic changes in healthy obese women. Diabetes Nutr Metab. 2003;16(3):176–181. [PubMed] [Google Scholar]
- 29.Khan U, Wang D, Thurston R, et al. Burden of subclinical cardiovascular disease in "metabolically benign" and "at-risk" overweight and obese women: The Study of Women's Health Across the Nation (SWAN) Submitted. 2010 doi: 10.1016/j.atherosclerosis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.