Abstract
The lymph gland is the major site of hematopoiesis in Drosophila. During late larval stages three types of hemocytes are produced, plasmatocytes, crystal cells, and lamellocytes, and their differentiation is tightly controlled by conserved factors and signaling pathways. JAK/STAT is one of these pathways which have essential roles in vertebrate and fly hematopoiesis. We show that Stat has opposing cell-autonomous and non-autonomous functions in hemocyte differentiation. Using a clonal approach we established that loss of Stat in a set of prohemocytes in the cortical zone induces plasmatocyte maturation in adjacent hemocytes. Hemocytes lacking Stat fail to differentiate into plasmatocytes, indicating that Stat positively and cell-autonomously controls plasmatocyte differentiation. We also identified the GATA factor pannier (pnr) as a downstream target of Stat. By analyzing the phenotypes resulting from clonal loss and over-expression of pnr in lymph glands, we find that Pnr is positively regulated by Stat and specifically required for the differentiation of plasmatocytes. Stat and Pnr represent two essential factors controlling blood cell maturation in the developing lymph gland and exert their functions both in a cell-autonomous and non-cell-autonomous manner.
Keywords: Drosophila, hematopoiesis, JAK/STAT, GATA factor, Pnr
Introduction
In Drosophila, as in vertebrates, hematopoiesis occurs in two phases. In the first, the “primitive” phase, hemocytes develop in the early embryo from head mesoderm and supply the pool of circulating blood cells. During the second, the “definitive” phase, hemocytes are produced in a small organ, the lymph gland. At late embryonic stages, the lymph gland can be recognized as a cluster of ~20 cells on either side at the anterior of the dorsal vessel. The gland grows during larval development into a multilobal organ alongside the heart tube. The anterior, primary lobe, is the largest. It consists of at least three compartments; the medullary zone (MZ), the cortical zone (CZ), and the Posterior Signaling Center (PSC) (Crozatier and Meister, 2007; Jung et al., 2005; Meister, 2004; Minakhina and Steward, 2010; Sinenko et al., 2009).
The PSC functions as the hematopoietic niche, controlling overall homeostasis within the lymph gland and may also regulate the maintenance and division program of the hematopoietic stem cells (HSCs) (Krzemien et al., 2007; Mandal et al., 2007). The existence of stem cells in the Drosophila lymph gland was shown by the analysis of mitotic clones induced in embryos and first instar larvae (Minakhina and Steward, 2010). Both persistent and transient clones were observed, indicating the presence of hematopoietic stem cells and daughter prohemocytes.
During development, prohemocytes differentiate into plasmatocytes, crystal cells, and lamellocytes. Plasmatocytes, the predominant type, have phagocytic functions and secrete extracellular matrix components and immune peptides similar to human white blood cells. Lamellocytes are rare under normal conditions, but upon immune challenge increase in numbers and function in encapsulation of foreign bodies or parasites. Crystal cells are non-adhesive hemocytes responsible for melanization during wound healing and encapsulation of parasites. During the onset of metamorphosis or as a part of an immune reaction the lymph gland bursts, and the differentiated hemocytes are released into the hemolymph (for review see Jung et al., 2005; Lanot et al., 2001; Lemaitre and Hoffmann, 2007; Markus et al., 2009; Sorrentino et al., 2002). Hemocyte development in the lymph gland is tightly controlled not only by signals from the PSC, but also by signals from the fat body and from circulating hemocytes.
A number of genes and pathways regulate hematopoiesis in Drosophila. Mutations in proteins such as GATA-factors, the early B-cell factor (EBF)/Collier, Ras, NF-κB, Notch, JAK/STAT, and Wingless can result in over-proliferation of hemocytes or a lack of differentiation of specific cell types (Agaisse and Perrimon, 2004; Gao et al., 2009; Krzemien et al., 2007; Lebestky et al., 2003; Sinenko et al., 2004). The homologs of these genes function in human hematopoiesis and are implicated in leukemias and lymphomas. We investigated the role of two transcriptional regulators; STAT (Signal Transducer and Activator of Transcription) and the GATA factor Pannier (Pnr) in Drosophila hemocyte differentiation.
The evolutionarily conserved Janus kinase (JAK)/STAT signaling cascade plays a key role in a variety of biological processes, such as the immune response, cell fate determination, cell migration, planar cell polarity, and stem cell maintenance. In Drosophila there is only one STAT protein, Stat92E, and it is activated by Hopscotch (Hop), the only JAK family kinase. Hyperactive hop alleles, hopTum-l and hopT42, and over-expression of UAS-hopTum-l or UAS-hop transgenes cause blood cell over-proliferation and formation of melanotic masses in larvae and adults (Hanratty and Dearolf, 1993; Harrison et al., 1995). This phenotype can be suppressed by loss of function alleles of Stat92e (Hou et al., 2002; Hou et al., 1996). Genetic studies have identified several components of the pathway, including the cytokine-like molecules Unpaired (Upd, Upd2, and Upd3), the receptor Domeless (Dome), and Socs36E, a negative regulator of the JAK/STAT pathway (Brown et al., 2001; Harrison et al., 1998; Karsten et al., 2002). Recent studies identified an additional receptor Latran (Lat) that regulates JAK/STAT signaling during the immune response to parasite infection (Makki et al., 2010). Multiple in vitro and in vivo studies have identified the consensus binding site of Stat92e and more than a dozen target genes, including dome, socs36e, even-skipped (eve), chronologically inappropriate morphogenesis (chinmo), and u-shaped (ush) that appear to be positively regulated by JAK/STAT signaling (Agaisse et al., 2003; Flaherty et al., 2010; Flaherty et al., 2009; Gao et al., 2009; Ghiglione et al., 2002; Karsten et al., 2002; Yan et al., 1996).
Of these genes, only dome and socs36e have been shown by clonal analysis to be both positively and cell-autonomously regulated by Stat92E (Bach et al., 2007; Bach et al., 2003; Ghiglione et al., 2002). Stat92E has also been shown to negatively regulate several genes such as Serrate (Ser), Delta (Dl), wingless (wg) and pnr in imaginal discs, (Ekas et al., 2006; Flaherty et al., 2009; Tsai et al., 2007). Dl and Ser are repressed by activated Stat92E in a cell-autonomous manner (Flaherty et al., 2009). Despite progress in understanding of the JAK/STAT pathway, the effectors and mechanisms of its action in hematopoiesis remain largely unknown. One of the potential targets of Stat92E in hemocytes is a single Drosophila homolog of Friend of GATA (FOG) called U-shaped (Ush) (Fossett et al., 2001; Gao et al., 2009). Ush functions in hemocyte differentiation and has been shown to bind at least two Drosophila GATA factors, Srp and Pnr (Haenlin et al., 1997; Waltzer et al., 2002), but how Ush and these GATA factors function together in hematopoiesis is not clear.
In vertebrates, members of the GATA family of transcription factors are among the most important regulators of hematopoiesis and heart development. In Drosophila, there are five GATA factors: pannier, serpent (srp), grain (grn), and the less well characterized GATAd and GATAe. Srp functions primarily in blood, gut and fat body formation, and so far remains the only GATA factor with a defined role in Drosophila hematopoiesis (Fossett et al., 2003; Mandal et al., 2004; Rehorn et al., 1996; Sam et al., 1996). During embryogenesis Srp is required for hemocyte precursor formation and is essential for specifying plasmatocytes and crystal cells in larval development (Fossett et al., 2003; Lebestky et al., 2000; Mandal et al., 2004; Rehorn et al., 1996). Pnr is known to function in Drosophila eye and wing formation. In embryos, pnr is required for the establishment of the hemangioblast, a common progenitor of cardiac and blood cells, and dosage of pnr is critical for proper development of the adult heart (Gajewski et al., 1999; Mandal et al., 2004; Qian and Bodmer, 2009).
We studied the function of Stat92e and pnr specifically in hematopoiesis and found that both factors have non-cell-autonomous and autonomous functions in hemocyte development. Stat non-cell-autonomously negatively regulates the temporal and spatial maturation program of hemocytes in the lymph gland, and is also required cell-autonomously for plasmatocyte differentiation. We show that the GATA factor Pnr, not known previously to function in hematopoiesis, is a downstream target of STAT, and we demonstrate that it also is cell-autonomously essential for plasmatocyte differentiation.
Material and methods
Drosophila strains and genetics
The following Drosophila strains were used for clonal analysis: FRT82B stat92e397/TM6 (Silver and Montell, 2001); FRT82B pnrVX6/TM6 (Heitzler et al., 1996); pnrMD237 /TM3 (Calleja et al., 1996) from Bloomington Stock Center (Bloomington); UAS-STAT92e RNAi and UAS-Pnr RNAi (VDRC); UAS-Hop/TM3 (Harrison et al., 1995). Stocks used to generate flip-out clones y w hsFLP Ay-GAL4 (Ito et al., 1997) and MARCM clones y w hsFLP Tub-GAL4 UAS-GFP:myc-nls, tubP-GAL80 (Lee and Luo, 2001) were obtained from Ken Irvine (Rutgers University). GFP stocks; UAS-GFP:myc-nls and UAS-mCD8-GFP/CyO (Bloomington). The pnr-LacZ reporter line controlled by regulatory elements of pnr-α and pnr-β was obtained from Philippe Ramain (IGBMC, France). The wild-type UAS-pnr transgene encoding Pnr-α and capable of partially rescueing the pnrVX6 phenotypes (Qian and Bodmer, 2009) and pxn-GAL4, 2X UAS-GFP were obtained from Utpal Banerjee (UCLA).
Clonal analysis and RNAi knock down experiments
Clones were generated using the Flip-out (Ito et al., 1997) or MARCM techniques (Lee and Luo, 2001; Lee et al., 2000) and labeled with GFP:myc-nls or mCD8-GFP. The wild type Flip-out clones were used as controls for RNAi and overexpression experiments. Drosophila progeny were maintained at 25°C prior and subsequent to heat-shock. Clones were induced by heat-shock at 38°C for 1 hour at embryonic stages (4-20 hrs after egg lay) or of third instar larvae (5th day after egg lay). Lymph glands were dissected from second and third instar larvae, fixed and stained. Larvae were staged according to age (time after first and second molt), gut color, mouth hook, and spiracle morphology. Upon dissection the size and morphology of the brain and discs served as additional controls. Wasp infection of wild-type and pnrMD237 homozygous larvae was done with the help of Chiyedza Small as described in Sorrentino et al. (2002).
Immunochemistry and imaging
Larval lymph glands were dissected, fixed, immunostained in glass dissecting dishes, and analyzed as described in Jung et al. (2005) and Minakhina and Steward (2010). Antibodies specific for plasmatocytes (P1) were obtained from Dr. I. Ando (Biological Research Center, Szeged) and used at 1:300 dilution. Rabbit anti-PPO2 antibody obtained from George Christophides (Imperial College, London, 1:2000), rabbit anti-Pxn antibody from John Fessler and Sergey Sinenko (UCLA, 1:700), and anti-Antp antibody from the Developmental Studies Hybridoma Bank (Glicksman and Brower, DSHB, 1:20) were used as crystal cell, CZ and PSC markers, respectively. Antibodies specific for lamellocytes (PS4) were provided by Delphine Pennetier and Alain Vincent (Université Toulouse, Toulouse 1:200). Goat anti-LacZ (Abcam) antibodies were used at 1:1000 dilution.
Samples were examined with a Zeiss Axioplan-2 microscope. Images were captured using a Leica DM IRBE laser scanning confocal microscope (objectives 40× and 63× oil), analyzed with Leica Microsystems software and further processed using Adobe PhotoShop.
Results
The JAK/STAT pathway in hemocyte differentiation
During the third instar larval stage lymph glands undergo considerable growth of the cortical zone and an increase in differentiated hemocytes (Fig. 1A-D). The primary lobe of the gland is 4-6 cells deep, and flattens towards the distal side of the cortex. Partially differentiated prohemocytes and differentiated blood cells forming the CZ are marked by the expression of Peroxidasin (Pxn) (Jung et al., 2005; Nelson et al., 1994; Stramer et al., 2005). Terminally differentiated plasmatocytes are recognized by staining with P1 antibody (Asha et al., 2003; Kurucz et al., 2007) and are seen in the surface cell layer of the CZ during the late third instar stage (z-section in Fig. 1B-D). Their appearance largely coincides with the final expansion of the CZ before the lymph gland bursts (Fig. 1C-C’). Crystal cells can be identified by the expression of Lz or Pro-phenoloxidase (PPO) (Christophides et al., 2002; Gajewski et al., 2007; Lebestky et al., 2000) and are found scattered throughout the CZ (z-section in Fig. 1 B-D).
Figure 1. Differentiation of hemocytes within the cortical zone (CZ).

During the third instar larval stage the cortical prohemocytes (pxn∷GFP, green) proliferate and differentiate into crystal cells (expressing PPO, cytoplasmic marker, pink) and plasmatocytes (P1, membrane marker, red). Confocal projections, z-sections (on the top of each projection, A-C’), and the schematic representation of a mature lymph gland (D) show that the gradual increase in plasmatocyte numbers on the surface of the CZ coincides with its expansion. During the same time the number of crystal cells also increases, but they are distributed throughout all layers of the CZ. Cells in the PSC are stained with anti-Antp (nuclear, red). (E, F) Projections of lymph glands expressing pxn∷Stat92e RNAi show drastic increase of plasmatocytes and crystal cells in the CZ during the mid-third instar (10-18 hours before pupariation, E, E’) and late third instar larval stages (2-6 hours before pupariation, F, F’) compared to wild type (A-C’). Plasmatocyte maturation is induced in the inner layers of the CZ (F, z-section). (G, G’) Projection of a lymph gland expressing pxn∷pnr RNAi; few fully differentiated plasmatocyte are detected, while crystal cells are increase in 30% of cases. DNA is shown in blue. Scale bars are 10 μm.
A function of the JAK/STAT pathway in hemocyte differentiation is suggested by the dominant gain-of-function phenotype of hopscotch (hopTum). The mutation causes over-proliferation of hemocytes and an increase in lamellocytes, which contribute to the formation of melanotic masses in larvae and adult flies (Agaisse et al., 2003; Harrison et al., 1995; Luo et al., 2002). JAK/STAT was also postulated to be necessary in the MZ to maintain a pool of undifferentiated hemocytes and to regulate the maturation of plasmatocyte and crystal cells (Gao et al., 2009; Krzemien et al., 2007; Sorrentino et al., 2007). To investigate the requirement for the pathway in the lymph gland, we knocked down Stat92e by RNAi, specifically in the lymph gland cortical zone, using the pxn-GAL4 driver, and found elevated plasmatocyte and crystal cell differentiation (Fig. 1E-F’). However, knocking down Stat92e in the MZ (dome-GAL4; UAS-Stat92eRNAi, not shown) caused no significant change in hemocyte differentiation, suggesting that Stat92e functions in committed cortical cells rather then medullary prohemocytes.
To further define Stat function in hematopoiesis we generated Stat92e loss-of-function clones in lymph glands. Stat92e397 is a strong recessive allele with a change of a highly conserved tryptophan 594 within the SH2 domain to a stop codon, resulting in a nonfunctional protein (Silver and Montell, 2001). We found that unlike the commonly used Stat92e85c9, this allele does not produce any dominant phenotype (Gao et al., 2009). We have previously used the MARCM technique to induce clones in the embryonic and first instar lymph gland and characterized four types of wild type clones (Minakhina and Steward, 2010). Here we used the same approach to induce Stat92E397 MARCM clones and found that they can also be divided into the same four types. The clones appear similar to wild type clones except for cohesive clones located in the CZ. When the clone was located in the inner layers of the CZ, close to the MZ, the hemocytes surrounding the clone on the distal side underwent premature plasmatocyte differentiation not observed in other cells in the inner layer (Fig. 2A, B). When Stat92E397 clones are located at the surface of the CZ, the Stat cells within the clone are retarded in their development and show at best low levels of the P1 marker but fail to undergo final differentiation into plasmatocytes (Fig. 2C). Clones located in the PSC (not shown), in the MZ (Fig. 2D arrows), and single hemocytes resulting from cell mixing (Fig. 2B, arrow) do not visibly affect differentiation within or around the clone.
Figure 2. Cell-autonomous and non-autonomous functions of Stat92e.

(A-C) Confocal crossections trough lymph glands with Stat92e397 MARCM clones and (D-F) Stat92eRNAi flip-out clones. (A, B, and E). Loss of Stat in the clones (green) located in the inner part of the CZ enhances the maturation of adjacent hemocytes into plasmatocytes (membrane marker, red) and sometimes crystal cells (cytoplasmic, pink). Loss of Stat in the clones located at the surface of the CZ (C and F) causes delay in plasmatocyte maturation within the clone, but does not affect crystal cell differentiation (PS4, read in G). (H-L) Over-expression of UAS-hop in flip out clones induces lamellocytes differentiation in a non-cell-autonomous manner. Confocal cross-sections of lymph gland lobe show that differentiated lamellocytes (red) appear near the clones (H) and on the surface of the lobe (I). 4-5 days after induction of clones, the lymph glands burst, the number of circulating hemocytes increases, and lamellocytes form aggregates (K and L). Lamellocytes are elongated cells and have larger nuclei than plasmatocytes (visualized with F-actin, red). Both wild type blood cells (non-green) and hemocytes that express hop (labeled with GFP, green) are increased in numbers and differentiate into lamellocytes. (J) Control blood smear shows low number of blood cells; rare wild type flip-out clones are labeled with GFP (green). DNA is shown in blue. Scale bars are 10 μm.
Similar phenotypes were observed in Stat92e-RNAi knock-down clones (see Materials and Methods, Fig. 2D-F). All 27 clones found in the inner part of the CZ are bordered by areas with increased P1 staining (Fig. 2D, inset E), 12 of those areas also contain crystal cells. The clones located at the surface of the lobe also showed a severe reduction in plasmatocyte differentiation within the clones (Fig.2D, inset F); the level of P1 in 20 of 23 clones was below detection, and the other 3 clones showed P1 staining that was significantly weaker than that in surrounding cells. Crystal cells were observed in the clones with the same frequencies as in wild type tissues (Fig. 2G). Stat92e-RNAi knock-down clones located in the medulla (13 clones) and PSC (8 clones) showed no visible changes in size or differentiation.
These results show that Stat is not required in the PSC or the MZ, but has a cell-autonomous function that positively controls maturation of plasmatocytes in the CZ. Further, Stat has a non-cell-autonomous function. We find that the local signaling emitted from Stat CZ clones stimulate the maturation of surrounding hemocytes, indicating that Stat normally inhibits this signal and keeps adjacent cells from differentiating.
We further tested if JAK-STAT pathway hyper-activation also has a non-cell-autonomous effect. This hyper activation can be triggered by ectopic expression of UAS-hopTum or of the wild type UAS-hop transgene (Bach et al., 2003; Harrison et al., 1995). General and hemocyte specific expression of both transgenes result in similar hematopoietic phenotypes including hemocyte proliferation, premature burst of the lymph gland, lamellocyte differentiation, and formation of melanotic masses (Asha et al., 2003; Hanratty and Ryerse, 1981; Harrison et al., 1995; Luo et al., 2002; Zettervall et al., 2004 and data not shown). To avoid systemic effects on hematopoiesis and to monitor the immediate effect of pathway activation on hemocyte differentiation, we have generated flip-out clones (see Materials and Methods) expressing UAS-hop in 3rd instar larvae, and analyzed these clones 24 and 48 hours after their induction. 48 hours after induction lymph glands had burst, wild type and Hop expressing hemocytes had mixed and differentiated so that the clones could not be studied. 24 hours after induction about 50% of larvae still carried intact lymph glands. These were dissected and stained for hemocyte markers. 26 of 36 lymph gland lobes (>70%) with clones showed maturation of lamellocytes in close proximity to the clone (Fig. 2H-I), compared to 10% in controls. Only in 3 lobes lamellocytes were detected within the clone (1-2 lamellocytes). Thus upregulation of JAK-STAT activates the ‘lamellocyte program’ non-cell-autonomously. Changes in plasmatocyte or crystal cell differentiation were not observed within 24 hours after clone induction.
To test if non-cell-autonomous function of JAK/STAT may be responsible for the increase in circulating hemocytes and lamellocytes we generated flip-out clones expressing UAS-hop in first instar larvae and analyzed circulating blood cells at the third instar larval stage. Blood smears of control larvae contain small cells, presumably plasmatocytes, occasionally a cell derived from a wild type clone is present (arrow; Fig. 2J). The blood smears from larvae with UAS-hop clones had increased numbers of hemocytes and aggregates of lamellocytes (Fig. 2K, L). Importantly, both hemocytes expressing Hop (green) and non-Hop-expressing cells proliferated and differentiated into lamellocytes, supporting the observation that Stat hyper activation regulates a non-cell-autonomous signal that mimics the response to parasitic infection.
Pnris essential for plasmatocyte differentiation
We found that the normal differentiation program within the lymph gland can be disrupted by loss of function of the GATA factor pnr. Pxn-GAL4 driven expression of pnr-RNAi in the CZ causes drastic reduction in plasmatocyte differentiation and sometimes an increase in crystal cells (Fig. 1G-G’), indicating that pnr also functions in the CZ.
We used the clonal approach to further study Pnr in hemocyte development. pnr loss of function (pnrVX6) MARCM clones were generated and scored as described previously (Minakhina and Steward, 2010). All four types of clones were observed as in wild type. To determine the fates of the cells within and around the clones, the lymph glands were stained for hemocyte differentiation markers (Fig. 3A-D’). Clonal loss of pnr did not cause visible non-autonomous effects, but abolished plasmatocyte maturation within all clones in CZ (16 clones). Figure 3A and 3B shows a surface section of a lymph gland with large pnrVX6 clones in the CZ where hemocytes failed to differentiate into mature plasmatocytes. pnrVX6 cells were able to partially differentiate,visualized by presence of Pxn, (Fig. 3C) and produce crystal cells, 5 of 16 clones contained crystal cells (Fig. 3D, D’). Reduction of plasmatocytes was also observed when Pnr levels were reduced in pnr-RNAi flip-out clones (result not shown), suggesting that Pnr functions cell-autonomously in cortical prohemocytes and is specifically required for plasmatocyte differentiation.
Figure 3. Cell-autonomous function of pnr in lymph gland development and terminal differentiation of plasmatocytes.
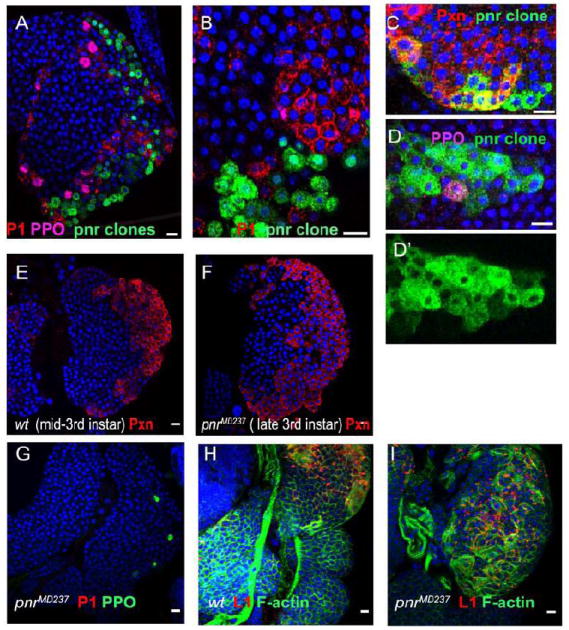
(A-D’) Confocal cross-sections through the surface layers of lymph glands with pnrVX6 MARCM clones. (A, B) pnrVX6 cells (green) fail to differentiate into plasmatocytes (P1, red). (C) Clonal loss of Pnr does not affect early stages of hemocyte differentiation, illustrated by Pxn (red) expression within the clone (green), nor does it affect crystal cell maturation (D, D’, PPO, purple). Confocal cross-sections of lymph glands from mid-third instar (10-18 hours before pupariation) wild type (E) and late third instar (2-4 hours before pupariation) pnrMD237 (F, G) lymph glands. In pnrMD237 the expression of the early differentiation marker Pxn (compare E and F, red) in the CZ is not altered, but further expansion of the CZ and plasmatocyte maturation are inhibited (no P1 staining in G). (H, I) pnr does not affect lamellocyte differentiation upon wasp infection. Flat, elongated lamellocytes are visualized by staining with L1 antibody (patchy red) and Phalloidin (green). DNA is shown in blue. DNA is shown in blue. Scale bars are 10 μm.
pnr encodes two protein isoforms, Pnr-α and Pnr-β The pnr-β start of transcription lies about 8.3 kb upstream of the pnr-α start but as a result of alternative splicing, pnr-β encodes a shorter protein, lacking the non-conserved N-terminus. In embryos and wing discs the two isoforms are expressed in overlapping, but not identical patterns and they are able to regulate gene expression in both a synergetic and antagonistic manner (Fromental-Ramain et al., 2008). The pnrMD237 allele carries a P{GawB} insertion in the 5’UTR of pnr-β that strongly impairs pnr-β expression but enhances pnr-α (Fromental-Ramain et al., 2007). pnrMD237 homozygous larvae are viable, slightly delayed in development, and die as pharate adults. Homozygous pnrMD237 lymph glands develop a normal MZ-CZ organization, cortical cells express Pxn and contain crystal cells (Fig. 3H). However, the final CZ expansion and plasmatocyte differentiation does not occur. The morphology of the lymph gland of pnrMD237 mutant larvae about 2-4 hours before pupariation is similar to that in mid-third instar wild type larvae (Fig. 3E, F compare to Fig. 1A-C’). Like wild type, pnr lymph glands contain very few lamellocytes, but the reaction to wasp infection is normal, in that most prohemocytes differentiate into lamellocytes (Fig. 3J). Thus, the loss of Pnr-β or upregulation of Pnr-α blocks the late wave of hemocyte proliferation and differentiation of plasmatocytes.
To test if the upregulation of pnr-α is responsible for the lymph gland phenotypes, we used a “wild type” UAS-pnr transgene that encodes Pnr-α (Fromental-Ramain et al., 2008; Qian and Bodmer, 2009). We found that pnr-α expression within random clones leads to a reduction in lymph gland size, lack of plasmatocyte differentiation, and causes a delay in larval development (Fig. 4A, B). The most severe phenotypes were observed when pnr-α expressing clones were induced during embryogenesis: clone sizes within the MZ and CZ varied between 5 to 30 cells, significantly smaller than the 50-200 cell wild type clone size, and the size of the lymph glands was less than half of normal size (Fig. 4A, B). All 18 analyzed lymph gland clones showed the phenotype.
Figure 4. Pnr-α overexpression in the lymph glands.

(A-C) Ectopic expression of UAS-pnr-α in random flip-out clones (A, B), and in the CZ (C; pxn-GAL4, UAS-GFP, UAS-pnr-α, green) causes a small organ size, and lack of plasmatocytes in lymph glands of larvae 2-4 hours before pupariation.
(D-F) Plasmatocyte maturation (P1, membrane, red) in CZ is restored by pnr-α expression in Stat92e knock down clones. Expression of pnr-α in Stat92e knock down clonesdoes not modify the non-cell–autonomous phenotype of Stat (D,E; elevated differentiation in adjacent cells), and suppress Stat92e cell autonomous phenotype. Note high level of plasmatocyte marker (membrane, red) in the clone cells (green) on the CZ surface (F, compare to Fig. 2F). DNA is shown in blue. Scale bars are 10 μm.
To rule out that signaling from non-hematopoietic tissues controlled lymph gland size and development, we over-expressed pnr-α specifically in differentiating hemocytes using the pxn-GAL4 driver This over-expression led to similar phenotypes as were obtained when pnr-α was expressed in random clones in the larva, including slow development, smaller lymph gland size, substantial failure of CZ growth, and lack of plasmatocyte differentiation (Fig. 4C). Thus pnr-α over-expression in few hemocytes affects hemocyte proliferation in the whole gland in a non-cell-autonomous manner, and also generates a signal that suppresses cell proliferation in other tissues. Furthermore, higher levels of pnr-α expression, or perhaps the imbalance between the two pnr isoforms, are responsible for the pleiotropic mutant phenotype of pnrMD237.
Pnr expression is regulated by Stat92E
Analysis of pnr and Stat92e clones showed that both genes have similar cell autonomous functions; both are required for plasmatocyte maturation and do not affect crystal cell development. To test if Stat92E positively regulates pnr expression we generated UAS-Stat92e RNAi, UAS-pnr-α flip-out clones. Within these clones pnr-α over-expression suppressed the delay of plasmatocyte maturation that was observed in clones with reduced Stat92e levels. Within all (11) UAS-Stat92e RNAi, UAS-pnr-α flip-out clones located on the surface of the CZ, plasmatocytes express high levels of the P1 marker like the adjacent hemocytes (compare Fig. 2C, F and Fig. 4F).
In the same larvae we looked at how the Stat92e non-cell-autonomous effects are influenced by expression of pnr-α and found that the two effects were additive. These larvae showed similar phenotypes to larvae with pnr-α ectopic expression only; most of the larvae exhibited slow development, small clone size, and lymph gland undergrowth (Fig. 4 A, D). However, in these underdeveloped glands the differentiation of plasmatocytes was increased. This premature differentiation of plasmatocytes is never seen when pnr-α is over-expressed in a wild type background (Fig. 4 compare A, B, C, and D). These results confirm again that in the cortical zone of the developing lymph gland Stat-dependent paracrine signaling restricts premature plasmatocyte differentiation and occurs independently of pnr.
The Pnr locus contains several putative Stat binding sites; a cluster of 3 STAT consensus sites is present about 4kb upstream of the pnr-β transcriptional start site and 4 single sites are distributed between introns and shared regulatory regions of both pnr isoforms. Because the Stat92e cell-autonomous phenotype, the failure of plasmatocyte maturation,can be rescued by pnr-α overexpression we hypothesized that pnr expression may be directly regulated by Stat92E. To test this possibility we used a pnr-LacZ reporter that spans regulatory regions of both pnr-α and pnr-β and closely mimics the expression pattern of endogenous pnr (Fromental-Ramain et al., 2008). The reporter is expressed in larval discs and all lymph glands sells (Fig. 5) and can be detected from the first instar larval stage till pupariation. Analysis of pnr-LacZ expression in Stat92e-RNAi knock-down clones showed that reduction of Stat causes reduction of pnr expression in lymph gland cells during the second and early third instar stages (Fig. 5). However at late third instar stage the expression of the reporter within the clone was indistinguishable from adjacent tissues (data not shown). Therefore, Stat92E regulates the expression of pnr during early lymph gland development, but it is not required for pnr activation during third instar larvae stage.
Figure 5. Stat positively regulates pnr expression in the lymph glands.

(A-B’) Confocal crossections trough second instar and early third instar stage lymph glands expressing pnr-LacZ (red). Loss of Stat in Stat92eRNAi flip-out clones (green) causes the reduction of pnr-driven LacZ expression. The reduction is seen in second instar lymph glands (A,A’) and in secondary lobes of early third instar larvae (B, B’).
Discussion
Cell-autonomous functions of Pnr and Stat
In vertebrates several GATA family members are prominently expressed in hematopoietic cells and are responsible for an array of blood cell fate decisions (for review see Cantor and Orkin, 2002). In Drosophila the GATA factor interactor Ush and the GATA protein Srp are required for the specification of hemocyte precursors and for the proper maturation of plasmatocytes and crystal cells (Fossett et al., 2003; Waltzer et al., 2003). We find that the GATA factor Pnr has a more specific role and is required in late stages of hematopoiesis. The phenotype of pnr clones in lymph glands suggests that it is specifically and cell-autonomously necessary to promote plasmatocyte development from committed (Pxn-positive) prohemocytes. The functional requirement of pnr in prohemocytes overlaps with the expression pattern of pnr-LacZ detected in the entire lymph gland.
Knock down of Stat92e in flip-out clones reduces pnr-LacZ expression within the clones. The Stat92e and pnr clones both show failure of terminal plasmatocyte maturation and no effect on crystal cell development. Importantly, the Stat92e cell-autonomous phenotype can be rescued by over-expression of pnr-α in the same cells. The pnr locus contains several putative Stat binding sites in the regulatory regions of pnr-α and pnr-β, therefore Stat92E may well directly activate transcription of both pnr isoforms in prohemocytes (Fig. 6). The late third instar larval lymph glands the expression of pnr (pnr-LacZ) does not require Stat92E indicating that pnr expression in the lymph glands is also regulated by additional factors (Haenlin et al., 1997; Fromental-Ramain et al., 2008). This may also explain the observation that loss of Stat92E has somewhat weaker effects on plasmatocyte maturation than loss of Pnr.
Figure 6. Lymph gland cortical zone signaling.

Together these results confirm the role Pnr in plasmatocyte differentiation and a function of Stat92E as a positive and cell-autonomous regulator of pnr expression. They also explain why cells within Stat92e clones do not mature into plasmatocytes even though they receive the same differentiation cues as hemocytes surrounding the clone (Fig. 2C, E, F).
Non-cell-autonomous functions of Pnr and Stat92e
The terminal maturation of hemocytes within the CZ is temporally and spatially regulated. Plasmatocyte maturation is suppressed until the late third instar larval stage, and plasmatocytes differentiate only on the surface of the CZ (Fig. 1 A, C). Immune challenge and genetic manipulations of hematopoietic factors can accelerate plasmatocyte differentiation. There are two reciprocal forces regulating hemocyte differentiation in the lymph gland CZ, signals that keep cells from differentiation, and signals that control hemocyte maturation positively. One of the repressing signaling pathways is JAK/STAT. Stat92E is required in the inner layers of the CZ to prevent terminal differentiation prior to late third instar stage. Loss of Stat92E causes an increase in differentiation in neighboring cells even at earlier developmental stages (Figs. 1E-E’) and in the inner layers of the CZ that normally does not contain mature plasmatocytes (Fig. 2). These results suggest that Stat92E regulates a paracrine signal that keeps hemocyte from maturation or represses a cascade of differentiation signals.
In the absence of immune challenge the developing lymph gland maintains a low to moderate level of JAK/STAT activity. The pathway is strongly stimulated during the immune response or can be genetically over-activated, resulting in a boost of hemocyte proliferation and a vast increase in lamellocytes (Agaisse and Perrimon, 2004; Luo et al., 2002). Over-expression of Hop in a small number of cells (flip out clones) also causes hemocyte proliferation and lamellocytes differentiation in the surrounding wild type cells. This non-cell-autonomous effect is observed both within lymph glands and in circulating hemocytes (Fig. 2). Hop expression in the lymph gland causes an immediate response, the maturation of committed lamellocyte precursors. Over a longer period activation of the JAK/STAT pathway induces a systemic response, similar to the response to parasitic infection. Both these responses are non-cell-autonomous. Hanratty and colleagues showed that transplantation of HopTum hemocytes into wild type hosts caused over-proliferation of blood cells and melanotic masses formation (Hanratty and Dearolf, 1993; Hanratty and Ryerse, 1981; Harrison et al., 1995). This may represent another example of the non-cell-autonomous function of the JAK/STAT pathway.
Similar to Stat, Pnr regulates a set of signals that can affect lymph gland development in a non-cell-autonomous manner. The ectopic over-expression of pnr-α in hemocytes and in random flip-out clones has a systemic inhibitory effect on tissue growth, lymph gland development, and particularly on CZ expansion, and, indirectly on plasmatocytes development. Change of the ratio of pnr-α and pnr-β isoforms in pnrMD237 homozygous mutants causes a similar phenotype. Further, when pnr-α is expressed in Stat92e clones plasmatocyte differentiation in the CZ is restored. This suggests that the over-expression of pnr-α by itself does not prevent plasmatocyte differentiation but rather causes a delay in lymph gland development. As a consequence of this delay the lymph gland misses an essential set of signals that promote plasmatocyte maturation simultaneously with CZ expansion. We propose that the correct balance between pnr-α and pnr-β is essential for non-cell autonomous function of Pnr in regulating the expansion of the CZ.
Our data underline the complexity of cell interactions during the maturation and differentiation of hemocytes and provide evidence that the transcription factors Stat and Pnr regulate cell-cell communications and homeostasis within the lymph gland. We were able to separate the cell-autonomous and non-autonomous functions of Stat92E in hemocyte maturation and showed that Pnr is a downstream target of the cell-autonomous Stat92E function, essential for plasmatocyte maturation (Fig. 6). What signaling pathway transduces the Stat paracrine inhibitory effect on plasmatocyte differentiation is still unclear, but it is possible that the JAK/STAT pathway itself is involved.
Acknowledgments
We thank Girish Deshpande for reading the manuscript, Istvan Ando, Erika Bach, Utpal Banerjee, George K. Christophides, Douglas Harrison, Kenneth Irvine, Delphine Pennetier, Philippe Ramain, Sergey Sinenko, Alain Vincent for fly stocks and antibodies. We thank Chiyedza Small and Shubha Govind for help with the wasp experiment and Le Nguyen for help with stocks and fly food. This work was supported by grants from the New Jersey Commission on Cancer Research (NJCCR) and the National Institutes of Health NIHD018055, and by the Goldsmith Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev. 2004;198:72–82. doi: 10.1111/j.0105-2896.2004.0133.x. [DOI] [PubMed] [Google Scholar]
- Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell. 2003;5:441–450. doi: 10.1016/s1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- Asha H, Nagy I, Kovacs G, Stetson D, Ando I, Dearolf CR. Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics. 2003;163:203–215. doi: 10.1093/genetics/163.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. doi: 10.1016/j.modgep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Bach EA, Vincent S, Zeidler MP, Perrimon N. A sensitized genetic screen to identify novel regulators and components of the Drosophila janus kinase/signal transducer and activator of transcription pathway. Genetics. 2003;165:1149–1166. doi: 10.1093/genetics/165.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Hu N, Hombria JC. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr Biol. 2001;11:1700–1705. doi: 10.1016/s0960-9822(01)00524-3. [DOI] [PubMed] [Google Scholar]
- Calleja M, Moreno E, Pelaz S, Morata G. Visualization of gene expression in living adult Drosophila. Science. 1996;274:252–255. doi: 10.1126/science.274.5285.252. [DOI] [PubMed] [Google Scholar]
- Cantor AB, Orkin SH. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene. 2002;21:3368–3376. doi: 10.1038/sj.onc.1205326. [DOI] [PubMed] [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Muller HM, Osta MA, Paskewitz SM, Reichhart JM, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, von Mering C, Xu J, Zheng L, Bork P, Kafatos FC. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Crozatier M, Meister M. Drosophila haematopoiesis. Cell Microbiol. 2007;9:1117–1126. doi: 10.1111/j.1462-5822.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- Ilejll LA, Baeg GH, Flaherty MS, Ayala-Camargo A, Bach EA. JAK/STAT signaling promotes regional specification by negatively regulating wingless expression in Drosophila. Development. 2006;133:4721–4729. doi: 10.1242/dev.02675. [DOI] [PubMed] [Google Scholar]
- Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, Zavadil J, Banerjee U, Bach EA. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev Cell. 2010;18:556–568. doi: 10.1016/j.devcel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty MS, Zavadil J, Ekas LA, Bach EA. Genome-wide expression profiling in the Drosophila eye reveals unexpected repression of notch signaling by the JAK/STAT pathway. Dev Dyn. 2009;238:2235–2253. doi: 10.1002/dvdy.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossett N, Hyman K, Gajewski K, Orkin SH, Schulz RA. Combinatorial interactions of serpent, lozenge, and U-shaped regulate crystal cell lineage commitment during Drosophila hematopoiesis. Proc Natl Acad Sci U S A. 2003;100:11451–11456. doi: 10.1073/pnas.1635050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossett N, Tevosian SG, Gajewski K, Zhang Q, Orkin SH, Schulz RA. The Friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc Natl Acad Sci U S A. 2001;98:7342–7347. doi: 10.1073/pnas.131215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromental-Ramain C, Vanolst L, Delaporte C, Ramain P. pannier encodes two structurally related isoforms that are differentially expressed during Drosophila development and display distinct functions during thorax patterning. Mech Dev. 2008;125:43–57. doi: 10.1016/j.mod.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Gajewski K, Fossett N, Molkentin JD, Schulz RA. The zinc finger proteins Pannier and GATA4 function as cardiogenic factors in Drosophila. Development. 1999;126:5679–5688. doi: 10.1242/dev.126.24.5679. [DOI] [PubMed] [Google Scholar]
- Gajewski KM, Sorrentino RP, Lee JH, Zhang Q, Russell M, Schulz RA. Identification of a crystal cell-specific enhancer of the black cells prophenoloxidase gene in Drosophila. Genesis. 2007;45:200–207. doi: 10.1002/dvg.20285. [DOI] [PubMed] [Google Scholar]
- Gao H, Wu X, Fossett N. Upregulation of the Drosophila Friend of GATA gene U-shaped by JAK/STAT signaling maintains lymph gland prohemocyte potency. Mol Cell Biol. 2009;29:6086–6096. doi: 10.1128/MCB.00244-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiglione C, Devergne O, Georgenthum E, Carballes F, Medioni C, Cerezo D, Noselli S. The Drosophila cytokine receptor Domeless controls border cell migration and epithelial polarization during oogenesis. Development. 2002;129:5437–5447. doi: 10.1242/dev.00116. [DOI] [PubMed] [Google Scholar]
- Haenlin M, Cubadda Y, Blondeau F, Heitzler P, Lutz Y, Simpson P, Ramain P. Transcriptional activity of pannier is regulated negatively by heterodimerization of the GATA DNA-binding domain with a cofactor encoded by the u-shaped gene of Drosophila. Genes Dev. 1997;11:3096–3108. doi: 10.1101/gad.11.22.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanratty WP, Dearolf CR. The Drosophila Tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. Mol Gen Genet. 1993;238:33–37. doi: 10.1007/BF00279527. [DOI] [PubMed] [Google Scholar]
- Hanratty WP, Ryerse JS. A genetic melanotic neoplasm of Drosophila melanogaster. Dev Biol. 1981;83:238–249. doi: 10.1016/0012-1606(81)90470-x. [DOI] [PubMed] [Google Scholar]
- Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. Embo J. 1995;14:2857–2865. doi: 10.1002/j.1460-2075.1995.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DA, McCoon PE, Binari R, Gilman M, Perrimon N. Drosophila unpaired encodes a secreted protein that activates the JAK signaling pathway. Genes Dev. 1998;12:3252–3263. doi: 10.1101/gad.12.20.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzler P, Haenlin M, Ramain P, Calleja M, Simpson P. A genetic analysis of pannier, a gene necessary for viability of dorsal tissues and bristle positioning in Drosophila. Genetics. 1996;143:1271–1286. doi: 10.1093/genetics/143.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou SX, Zheng Z, Chen X, Perrimon N. The Jak/STAT pathway in model organisms: emerging roles in cell movement. Dev Cell. 2002;3:765–778. doi: 10.1016/s1534-5807(02)00376-3. [DOI] [PubMed] [Google Scholar]
- Hou XS, Melnick MB, Perrimon N. Marelle acts downstream of the Drosophila HOP/JAK kinase and encodes a protein similar to the mammalian STATs. Cell. 1996;84:411–419. doi: 10.1016/s0092-8674(00)81286-6. [DOI] [PubMed] [Google Scholar]
- Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Karsten P, Hader S, Zeidler MP. Cloning and expression of Drosophila SOCS36E and its potential regulation by the JAK/STAT pathway. Mech Dev. 2002;117:343–346. doi: 10.1016/s0925-4773(02)00216-2. [DOI] [PubMed] [Google Scholar]
- Krzemien J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–328. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- Kurucz E, Vaczi B, Markus R, Laurinyecz B, Vilmos P, Zsamboki J, Csorba K, Gateff E, Hultmark D, Ando I. Definition of Drosophila hemocyte subsets by cell-type specific antigens. Acta Biol Hung. 2007;58(Suppl):95–111. doi: 10.1556/ABiol.58.2007.Suppl.8. [DOI] [PubMed] [Google Scholar]
- Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila. Dev Biol. 2001;230:243–257. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- Lebestky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–149. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- Lebestky T, Jung SH, Banerjee U. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 2003;17:348–353. doi: 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25:307–316. doi: 10.1016/s0896-6273(00)80896-x. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Luo H, Rose PE, Roberts TM, Dearolf CR. The Hopscotch Jak kinase requires the Raf pathway to promote blood cell activation and differentiation in Drosophila. Mol Genet Genomics. 2002;267:57–63. doi: 10.1007/s00438-001-0632-7. [DOI] [PubMed] [Google Scholar]
- Makki R, Meister M, Pennetier D, Ubeda JM, Braun A, Daburon V, Krzemien J, Bourbon HM, Zhou R, Vincent A, Crozatier M. A short receptor downregulates JAK/STAT signalling to control the Drosophila cellular immune response. PLoS Biol. 2010;8:e1000441. doi: 10.1371/journal.pbio.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal L, Banerjee U, Hartenstein V. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat Genet. 2004;36:1019–1023. doi: 10.1038/ng1404. [DOI] [PubMed] [Google Scholar]
- Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog-and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus R, Laurinyecz B, Kurucz E, Honti V, Bajusz I, Sipos B, Somogyi K, Kronhamn J, Hultmark D, Ando I. Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2009;106:4805–4809. doi: 10.1073/pnas.0801766106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M. Blood cells of Drosophila: cell lineages and role in host defence. Curr Opin Immunol. 2004;16:10–15. doi: 10.1016/j.coi.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Minakhina S, Steward R. Hematopoietic stem cells in Drosophila. Development. 2010;137:27–31. doi: 10.1242/dev.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, Olson PF, Parker CG, Fessler JH. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. Embo J. 1994;13:3438–3447. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Bodmer R. Partial loss of GATA factor Pannier impairs adult heart function in Drosophila. Hum Mol Genet. 2009;18:3153–3163. doi: 10.1093/hmg/ddp254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehorn KP, Thelen H, Michelson AM, Reuter R. A molecular aspect of hematopoiesis and endoderm development common to vertebrates and Drosophila. Development. 1996;122:4023–4031. doi: 10.1242/dev.122.12.4023. [DOI] [PubMed] [Google Scholar]
- Sam S, Leise W, Hoshizaki DK. The serpent gene is necessary for progression through the early stages of fat-body development. Mech Dev. 1996;60:197–205. doi: 10.1016/s0925-4773(96)00615-6. [DOI] [PubMed] [Google Scholar]
- Silver DL, Montell DJ. Paracrine signaling through the JAK/STAT pathway activates invasive behavior of ovarian epithelial cells in Drosophila. Cell. 2001;107:831–841. doi: 10.1016/s0092-8674(01)00607-9. [DOI] [PubMed] [Google Scholar]
- Sinenko SA, Kim EK, Wynn R, Manfruelli P, Ando I, Wharton KA, Perrimon N, Mathey-Prevot B. Yantar, a conserved arginine-rich protein is involved in Drosophila hemocyte development. Dev Biol. 2004;273:48–62. doi: 10.1016/j.ydbio.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Sinenko SA, Mandal L, Martinez-Agosto JA, Banerjee U. Dual role of wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Dev Cell. 2009;16:756–763. doi: 10.1016/j.devcel.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino RP, Carton Y, Govind S. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev Biol. 2002;243:65–80. doi: 10.1006/dbio.2001.0542. [DOI] [PubMed] [Google Scholar]
- Sorrentino RP, Tokusumi T, Schulz RA. The Friend of GATA protein U-shaped functions as a hematopoietic tumor suppressor in Drosophila. Dev Biol. 2007;311:311–323. doi: 10.1016/j.ydbio.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Stramer B, Wood W, Galko MJ, Redd MJ, Jacinto A, Parkhurst SM, Martin P. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol. 2005;168:567–573. doi: 10.1083/jcb.200405120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YC, Yao JG, Chen PH, Posakony JW, Barolo S, Kim J, Sun YH. Upd/Jak/STAT signaling represses wg transcription to allow initiation of morphogenetic furrow in Drosophila eye development. Dev Biol. 2007;306:760–771. doi: 10.1016/j.ydbio.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Waltzer L, Bataille L, Peyrefitte S, Haenlin M. Two isoforms of Serpent containing either one or two GATA zinc fingers have different roles in Drosophila haematopoiesis. Embo J. 2002;21:5477–5486. doi: 10.1093/emboj/cdf545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltzer L, Ferjoux G, Bataille L, Haenlin M. Cooperation between the GATA and RUNX factors Serpent and Lozenge during Drosophila hematopoiesis. Embo J. 2003;22:6516–6525. doi: 10.1093/emboj/cdg622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Small S, Desplan C, Dearolf CR, Darnell JE., Jr Identification of a Stat gene that functions in Drosophila development. Cell. 1996;84:421–430. doi: 10.1016/s0092-8674(00)81287-8. [DOI] [PubMed] [Google Scholar]
- Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, Ando I, Hultmark D. A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci U S A. 2004;101:14192–14197. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]


