Abstract
Death Receptor 5 (DR5) induces apoptosis in various types of cells and is a potential therapeutic target. We have investigated whether targeting DR5 could be used to eliminate pathogenic B lymphocytes from systemic lupus erythematosus (SLE) patients. We examined DR5 expression and function on B lymphocytes from healthy controls subjects, SLE patients, and human tonsil. DR5 was expressed similarly on all B cell subpopulations, including resting and activated B cells. Expression of DR5 was equivalent on B cells from SLE patients and healthy subjects. Additionally, DR5 expression was unchanged after B lymphocyte stimulation. However, B cells were resistant to DR5-induced apoptosis, including after in vitro activation. No changes in subsets of B cells were observed in subjects of a trial of CS-1008, an agonist anti-DR5. While DR5 shows promise as a way to selectively eliminate tumor cells and activated synoviocytes, these data suggest DR5 alone cannot be used as a target to remove pathogenic SLE B cells.
Keywords: Death Receptor 5, B lymphocyte, Systemic lupus erythematosus, Cancer
INTRODUCTION
TNF-related apoptosis-inducing ligand (TRAIL) is a pro-apoptotic member of the TNF ligand family that is expressed in a variety of tissues [1; 2]. A soluble form of the ligand is produced after protease-induced cleavage [3]. TRAIL has five known receptors. TRAIL receptor 1 (also known as Death receptor 4/DR4) and TRAIL receptor 2 (also called Death receptor 5/DR5, KILLER and TRICK2) are capable of inducing cell death [4; 5; 6]. Conversely, TRAIL receptor 3 (also known as Decoy receptor 1/DcR1, LIT, and TRID) and TRAIL receptor 4 (also called Decoy receptor 2/DcR2, TRUNDD) cannot cause apoptosis due to lack of or truncated death domains and serve as decoy receptors blocking TRAIL-mediated apoptosis [7; 8]. TRAIL receptor 5, osteoprotegerin, is a soluble TRAIL decoy receptor that aids in the development and activation of osteoclasts in bone remodeling [9]. Early data showed sensitivity to TRAIL-mediated apoptosis in a variety of malignant cell lines, making this protein a promising target to eradicate cancer cells [10; 11]. However, later studies showed TRAIL was able to induce cell death in non-cancerous cells including thymocytes, neural cells and human hepatocytes [12; 13; 14].
The toxicity of TRAIL itself has shifted the focus to individual TRAIL receptors as a possible treatment for cancer. Ligation of DR5 induced cell death in many DR5-expressing cancer cell lines [15; 16]. DR5 mediates apoptosis through the organization of the intracellular death inducing signaling complex (DISC), which includes DR5, caspase 8 and Fas-associated death domain (FADD). After receptor engagement and trimerization, DR5 recruits FADD to its intracellular death domain. FADD interacts with caspase 8, which leads to oligomerization and autoactivation of caspase 8 and activation of downstream effector caspases [17; 18; 19; 20].
TRA-8 is an agonist antibody to DR5. It induces cell death in cell lines and in vivo human tumor explant models. Previous reports show that TRA-8 causes apoptosis, without additional cross-linking, in many DR5 expressing cancer cell lines as well as synovial fibroblasts isolated from patients with rheumatoid arthritis [16; 21]. Unlike TRAIL, TRA-8 does not cause apoptosis in normal hepatocytes [16]. CS-1008, a humanized form of TRA-8, is undergoing initial testing in cancer patients. A Phase I study (ClinicalTrials.gov NCT00320827) has been completed and Phase II trials are underway.
DR5 is also expressed in a wide range of non-malignant tissues [4; 6; 22]. Mice deficient in TRAIL are hypersensitive to collagen-induced arthritis and streptozotocin-induced diabetes, suggesting a role for TRAIL receptors in control of autoimmunity [23]. Ligation of DR5, including with TRA-8, has been shown to cause apoptosis in synovial fibroblasts isolated from rheumatoid arthritis patients [21; 24], and thus could potentially be used to treat this disease. Alternatively, DR5 is also expressed on some lymphocytes. If TRA-8 were capable of inducing apoptosis in lymphocytes, then this could potentially be used to treat human autoimmune diseases, particularly if the mechanism involved a role for DR5 in activation-induced cell death. Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by B cell hyperactivity and autoantibody production. These self-reactive antibodies lead to tissue damage and play a major role in the pathogenesis of SLE [25]. Selective elimination of pathogenic autoreactive B cells would likely have therapeutic benefits. An effective strategy to target these cells is needed. Human IL-6 differentiated plasma B cells and murine plasma B cells generated from a T-dependent immune response were susceptible to TRAIL-mediated apoptosis [26]. DR5 expression and function among primary B cell subsets, both resting and activated, is unknown, and sensitivity to DR5-mediated apoptosis in cells associated with pathogenesis of lupus has yet to be studied.
Our group investigated whether targeting DR5 with TRA-8 could eliminate activated B cells in SLE. We compared DR5 expression on human tonsil B lymphocytes and between different B cell populations in healthy controls and in SLE patients. The ability of DR5 to induce apoptosis was assessed using TRA-8. We show that resting and activated primary B cell subsets isolated from tonsil, normal and SLE whole blood express DR5. There was no increase in DR5 expression of B cells from patients with SLE compared to healthy controls. Stimulation of primary B cells did not increase DR5 protein levels. We also compared lymphocyte populations in subjects in the phase I trial of CS-1008 before and after treatment. In all studies, although primary B cell subsets expressed DR5, these populations were resistant to TRA-8-mediated apoptosis. CD40 and IL-4 stimulated primary B cells were also insensitive to TRA-8-induced cell death. We report that primary B cell sensitivity to DR5-induced apoptosis requires more than DR5 protein expression alone. These data suggest an intracellular regulation of DR5-mediated apoptosis in non-cancerous B lymphocytes that differs from transformed cells. On the other hand, these data suggest that potential treatment approaches targeting DR5 on certain cells, such as rheumatoid synovial cells, will not deplete DR5-expressing lymphocytes.
METHODS
Tissue Samples, Antibodies and Reagents
Juvenile human tonsils were obtained from the UAB Tissue Procurement Facility. Whole blood was acquired from lupus patients with established active disease (Table 1), from healthy controls, or from patients with cancer receiving CS-1008, in heparin collection tubes. Samples were obtained in accordance with institutional policies and after Institutional Review Board approval. FITC anti-CD27, PE-Cy5 anti-CD38, and APC anti-CD19 were purchased from BD Pharmingen. PE anti-CD27 was purchased from eBiosciences. PE mouse IgG1 was purchased from Caltag. Unlabeled mIgG1 was purchased from Southern Biotechnology Associates. 2B4 and TRA-8 anti-human DR5 antibodies were kindly provided by Tong Zhou [16; 27]. Recombinant human IL-4 was purchased from R&D Systems. Monoclonal anti-human CD40 was purchased from Ancell. DiOC6 was purchased from Molecular Probes. Caspase 8 was purchased from Cell Signaling. p38 was purchased from Santa Cruz. HRP-conjugated secondary antibodies were purchased from Jackson ImmunoResearch.
Table 1.
Patient Demographics
| Subject number | Gender | Race | Age | SLE meds | organs |
|---|---|---|---|---|---|
| 1 | F | AA | 31 | HCQ* 400 mg/d Pred 30 mg/d |
Pleuritis nephritis |
| 2 | F | AA | 22 | CTX × 1 Mycophenolate 500 mg/d HCQ 400 mg/d Pred 40 mg/d |
Nephritis Arthritis rash |
| 3 | F | AA | 24 | CTX q mo Pred 60 mg/d HCQ 400 mg/d |
Pleuritis, Nephritis |
| 4 | F | AA | 21 | HCQ 400 mg/d CTX q 3m Pred 10 mg qod |
Nephritis Rash arthritis |
| 5 | F | AA | 31 | Azathioprine 150 mg/d Pred 20 mg/d |
Nephritis Neuropathy |
| 6 | F | C | 50 | HCQ 400 mg/d Mycophenolate 2.0 gm/d Pred 10 mg qod |
Nephritis |
| 7 | M | AA | 38 | Pred 20 mg/d | Nephritis |
| 8 | F | AA | 36 | HCQ 200 mg/d Mycophenolic acid 360mg bid Prednsione 5 mg/d |
Nephritis Pneumonitis Thrombocytopenia |
HCQ – hydroxychloroquine; CTX – cyclophosphamide; Pred-prednisone
Flow Cytometric Analysis of DR5
For analysis of DR5 expression, whole blood or tonsil mononuclear cells, prepared by density centrifugation on ficoll, were stained with 5μg/mL 2B4-PE or control mouse IgG1-PE in combination with fluorochrome-conjugated antibodies to CD27, CD38 and CD19. Fluorescence was detected with a BD FACS Calibur and the data analyzed with FlowJo (TreeStar). For analysis of apoptosis, cells were incubated for 15 min with 0.03μg/mL DiOC6 at 37°C, washed with PBS and stained with antibodies against CD27, CD38, and CD19.
Cell Cultures
Jurkat T leukemia cells or mononuclear cells derived from whole blood or from tonsils were cultured at 1 × 106 cells/mL in 24 well plates in IMDM supplemented with 10% FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin. In stimulation assays, cells were left untreated or stimulated with anti-CD40 (1μg/mL), 5ug/mL Biotinylated DA4.4 Fab’ followed by 5ug/mL Streptavidin, and/or IL-4 (100ng/mL). For tests of apoptosis, cells were cultured with 50ng/mL TRA-8 or control mouse IgG1.
Immunoprecipitation and Whole Cell Lysate
Unstimulated or TRA-8 treated Jurkat T leukemia cells or tonsil mononuclear cells, either unseparated or enriched for B cells by negative selection using a B cell isolation kit (Miltenyi Biotec) according to manufacturer specifications, were lysed at intervals. Whole cell lysates or immunoprecipitates formed using 2B4 crosslinked sepharose beads [27] were analyzed by SDS-PAGE as previously described [28].
Changes in Lymphocytes in Subjects Receiving CS-1008
Blood samples were obtained from 11 consenting participants in a phase I trial of CS-1008 [29] in patients, ages 38–88, of whom 58% were female, with different forms of malignancy, including carcinomas or lymphoma (1 subject) (ClinicalTrials.gov NCT00320827, described in J Clin Oncol 26: 2008 (May 20 suppl; abstr 3537)). Mononuclear cells were stained with panels of antibodies to B (anti-IgD/FITC, -CD27/PE, -CD38/PacBlue, and -CD19/APC) or T cells (anti-CD4/FITC, -CD27/PE, -CD28/PerCP-Cy5.5, -CCR7/PE-Cy7, -CD45RA/APC, and -CD8/APC-Cy7). Data were acquired on a BD LSRII and analyzed for percentages of different cell types with FlowJo (TreeStar). The fold change in a subset was calculated as the percentage of those cells at a post-treatment time point divided by the percentage of those cells in the pre-treatment sample from the same subject.
RESULTS
Primary human B cells express DR5
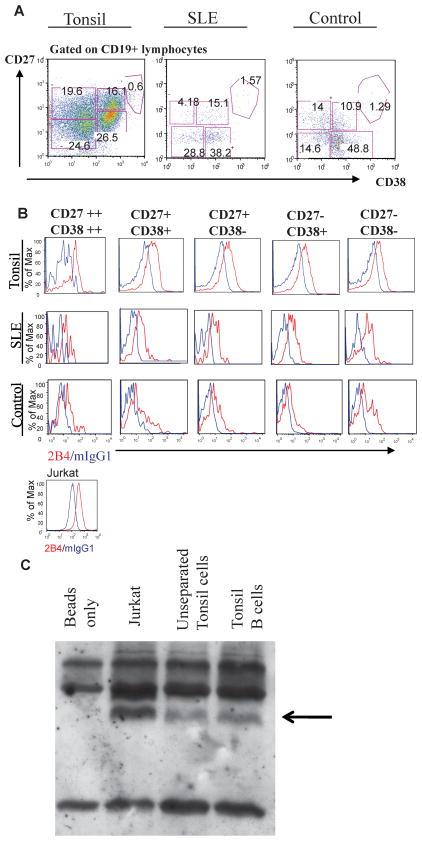
To determine whether primary B subsets express DR5, we performed flow cytometry analysis on B cells isolated from tonsils or from whole blood from healthy controls or patients with active SLE. CD19+ B cell subsets were identified by expression of CD27 and CD38. Using these parameters, B cells can be separated into five subpopulations: CD27− CD38− (resting naïve), CD27+ CD38− (memory), CD27− CD38+ (early germinal center in tonsil; transitional in whole blood), CD27+ CD38+ (germinal center in tonsil; activated/plasmablast precursors in whole blood), and CD27++ CD38++ (plasmablasts and plasma cells). DR5 expression was analyzed on each subset by co-staining using the 2B4 anti-human DR5 monoclonal antibody. Figure 1 illustrates subset distribution (Fig. 1A) and DR5 expression (Fig. 1B) on each subset in representative samples of a tonsil, whole blood from a healthy control and from a SLE patient. As expected, percentages of B cell subsets were altered in the SLE subject compared to healthy control, including a decrease in memory B cells and an increase in plasma cells (Fig. 1A). DR5 staining was seen in all B cell subsets, as well as Jurkat T cell leukemia cells used as a positive control (Fig. 1B). DR5 expression was also analyzed by western blot. A band of similar molecular weight to that immunoprecipitated from Jurkat cells was observed in preparations of unseparated human tonsil mononuclear cells, which remained after enrichment of B cells (Fig. 1C).
Figure 1. Individual Primary B Cell Subsets Express Death Receptor 5.
(A) CD19+ lymphocytes were resolved into subsets based on expression levels of CD27 and CD38. (B) anti-human DR5 2B4 (red) and mIgG1 (blue) staining of B cell subsets from tonsil, healthy control and SLE samples. Representative data from one sample per group tested are shown. Lower panel: 2B4 (red) and mIgG1 (blue) staining of Jurkats. (C) DR5 immunoprecipitation using 2B4 (human anti-DR5 antibody) cross-linked sepharose beads. Immunoprecipitates were collected from Jurkat (lane 2), unseparated (lane 3) and B cell enriched tonsil mononuclear cells (lane 4). The arrow denotes the specific band not seen using 2B4 sepharose beads free of lysate (lane 1).
SLE and healthy control B cells express similar levels of DR5
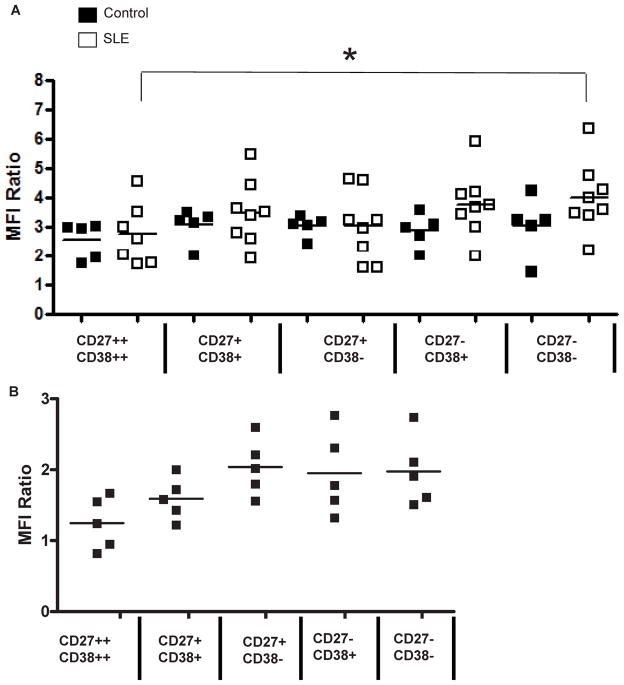
Figure 2 presents aggregate data on DR5 expression in each of the B cell subsets, in whole blood stains from five healthy control subjects and eight lupus patients. Demographic data for the SLE subjects is provided in Table 1. The controls were all healthy African-American females, ages 23–33. DR5 expression was measured as the ratio of mean fluorescent intensity (MFI) of the 2B4 anti-human DR5 antibody to the MFI of control mouse IgG1 (a ratio of one would denote no increase in staining over background). Expression of DR5 was detected on B cells from all individuals tested (Fig. 2A). No difference was seen between resting naïve (CD27− CD38−) and activated (CD27+ CD38+ and CD27++ CD38++) B cell subsets, although in SLE subjects there was a statistically significant decrease in 2B4 binding on CD27++ CD38++ plasmablasts, compared to CD27− CD38− naïve B cells. Surface expression of DR5 was equivalent in subsets of B cells from SLE and healthy subjects. Tonsil B cell subsets also had similar MFI ratios (Fig. 2B).
Figure 2. SLE and Healthy Control B Cell Subpopulations Have Comparable Amounts of DR5.
The ratio of the mean fluorescent intensity (MFI) of 2B4 anti-DR5 binding to the MFI of control mIgG1 antibody binding on the indicated subsets of circulating B cells in the blood is shown for five healthy control subjects and eight SLE subjects (A). The same subsets were analyzed in mononuclear cells from tonsils (B).
DR5 expression is not increased after stimulation in vitro
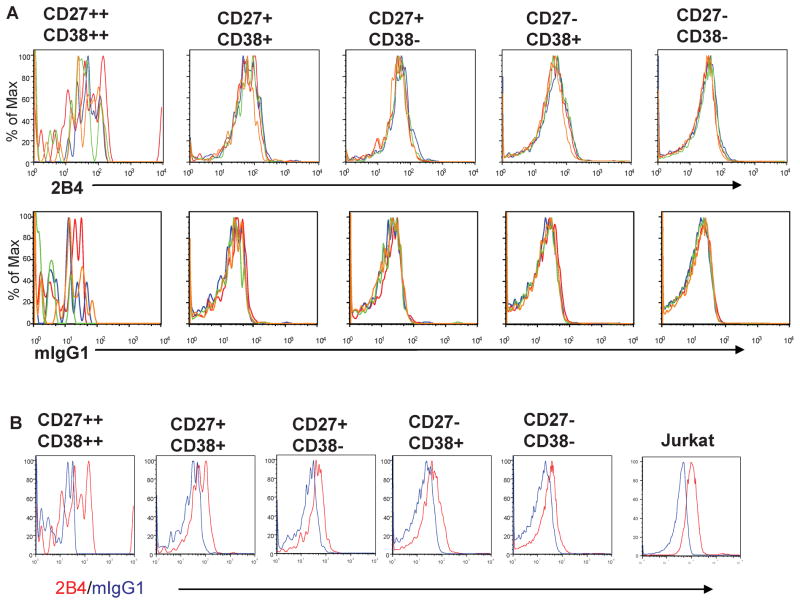
Protein expression of other TNF receptor family members, FAS and TNF-R, increase on B cells after stimulation. We investigated whether DR5 protein levels on B lymphocytes would similarly increase after stimulation with CD40 and/or IL-4. No stimulation-induced increase in DR5 was observed in any tonsil B cell subset (Fig. 3A top row). Retention of DR5 expression after overnight culture was confirmed (Fig. 3B). Other combinations of stimuli, including the B cell antigen receptor and IL-4, alone or in combination with CD40, were also tested in similar experiments, and also did not alter DR5 expression (data not shown, but equivalent results to the experiments shown in Fig. 3).
Figure 3. B Cell Stimulation Does Not Increase DR5 Surface Expression.
(A) Tonsil mononuclear cells were incubated overnight in medium alone (red) or with 100ng/mL IL-4 (blue) or 1 μg/mL CD40 (green) or IL-4 plus CD40 (yellow). Cells were then stained with 2B4 anti-human DR5 or mIgG1 control antibody in combination with antibodies to CD19, CD27 and CD38. In (A), overlay plots are shown of staining results with either 2B4 (top row) or mIgG1 control (bottom row) of B cell subsets from each stimulation condition. In (B), overlay plots are shown of staining results with 2B4 (red) and mIgG1 control (blue) for each B cell subset cultured overnight in medium alone.
Primary B cells are not sensitive to DR5-mediated apoptosis
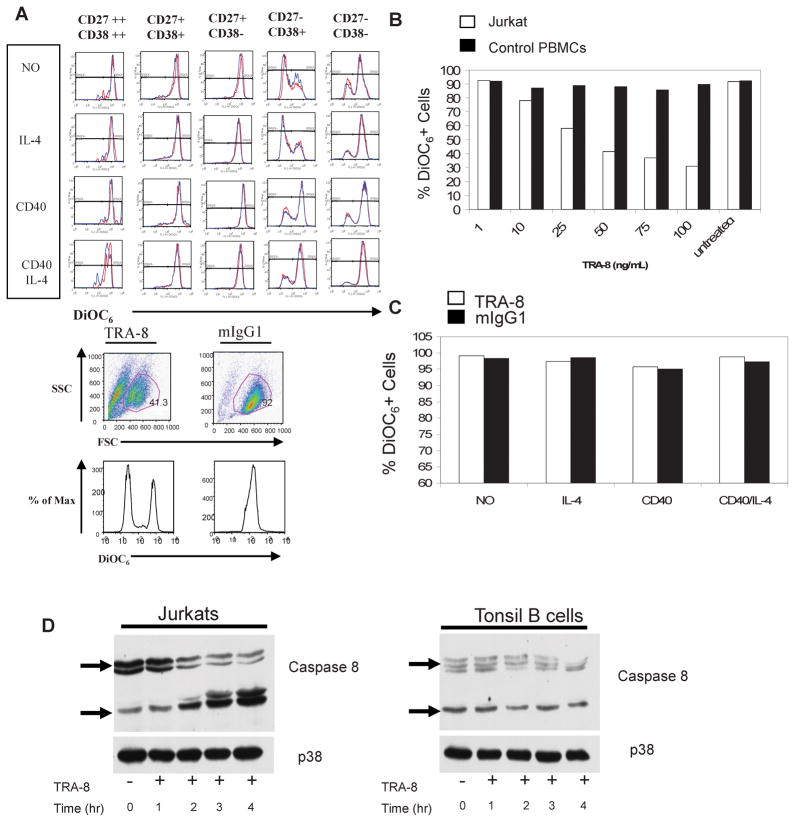
To determine if the DR5 present on primary B cells was functionally active, we incubated tonsil lymphocytes with the agonistic monoclonal anti-human DR5 antibody, TRA-8, using a range of antibody concentrations and durations of culture. Jurkat T cells were used as a positive control. The mitochondrial fluorescent dye DiOC6 was used to reveal cells undergoing apoptosis. Apoptotic cells, with defective mitochondrial permeability, leak the dye and lose DiOC6 fluorescence. Tonsil B cell subsets were not sensitive to TRA-8 induced apoptosis, whether resting or stimulated in vitro with CD40 and/or IL-4 (Fig. 4A, top panels). Germinal center B cells (CD27− CD38+) died quickly in culture medium alone but were partially rescued by CD40, demonstrating the sensitivity of the DiOC6 and the biologic effect of the CD40. In contrast, Jurkat cells were susceptible to TRA-8-induced cell death (Fig. 4A, bottom panel).
Figure 4. Primary B Cells are Resistant to DR5-Mediated Apoptosis.
(A, top) Tonsil mononuclear cells were incubated for 24 hours in the presence of 50ng/mL TRA-8 anti-human DR5 (red) or 50ng/mL control mIgG1 (blue). The cells were stimulated with medium only or with CD40 (1 μg/mL) or IL-4 (100ng/mL) or both. Cells were then stained with 0.3μg/mL DiOC6 and antibodies to CD19, CD27 and CD38 and analyzed by flow cytometry. (A, bottom) Jurkat cells were incubated for 24 hours with TRA-8 or mIgG1 and stained with DiOC6. (B) 1 million Jurkats (open bars) or healthy control peripheral blood mononuclear cells (closed bars) were incubated overnight in the presence of increasing amounts of TRA-8 or left untreated and then stained with DiOC6. (C) 1 million SLE peripheral blood mononuclear cells were incubated overnight in the presence of 50ng/mL TRA-8 (open bars) or 50ng/mL mIgG1 (closed bars) along with CD40 (1μg/mL) and/or IL-4 (100ng/mL) stimulation. Cells were then stained with DiOC6. (D) Jurkat (left panel) and B cell enriched tonsil mononuclear cells (right panel) were left untreated or stimulated with 100ng/mL TRA-8 for 1–4 hours. Whole cell extracts were collected and analyzed for cleaved caspase 8. p38 was used as a loading control.
CD19+ peripheral blood mononuclear cells (PBMCs) isolated from healthy controls were also found to be insensitive to TRA-8 induced apoptosis (Fig. 4B). A 100ng/mL dose of TRA-8 was unable to induce apoptosis in healthy control B cells, in contrast to the dose-dependent increase in apoptosis in Jurkat cells (Fig. 4B). CD19+ PBMCs from SLE subjects also were not susceptible to DR5-mediated apoptosis (Fig. 4C).
Whole cell extracts from Jurkat and tonsil B cells were analyzed for TRA-8-induced caspase activation. TRA-8 treatment caused caspase 8 cleavage in a time-dependent manner in Jurkat cells but not in tonsil B cells (Fig. 4D)
DR5 agonist does not alter subsets of circulating lymphocytes in vivo
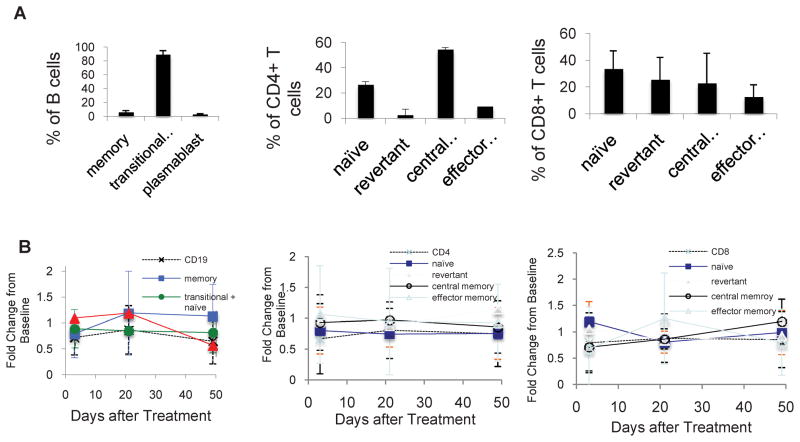
Blood samples were obtained before and at days 2, 21 and 49 after treatment from subjects with different malignancies enrolled in a dose-escalation trial of CS-1008. B cells were identified as CD19+ cells, and subsets identified on the basis of expression of IgD, CD27 and CD38, including transitional and naïve (CD27− CD38+), memory (CD27+ CD38−) and plasmablasts (CD27++ CD38++). CD4+ and CD8+ T cells were identified on the basis of CD45RA and CCR7 expression as naïve (CD45RA+ CCR7+), central memory (CD45RA− CCR7+), effector memory (CD45RA− CCR7−) and revertant memory (CD45RA+ CCR7−). There was considerable variability in the percentages of B or T cells and their subsets between subjects at baseline, presumably due to effects of the malignancy and its treatment (Fig. 5A). For each individual, we measured the fold change in the percentage of each subset at each time point after treatment, relative to the baseline percentage for each subset in that individual. The average and standard deviation in the fold change were then calculated for each subset at each time point for all subjects. The standard deviations in the fold change from baseline were relatively large for subsets that represented only a small percentage of cells (e.g., plasmablasts, revertant CD4+ T cells). However, the change after treatment in the average percentage of the circulating subsets of B or T cells was minimal (Fig. 5B) and in no case reached statistical significance by paired student’s t test. Similarly, no significant changes in the proportion of IgD+ memory B cells or of CD27− or CD28− T cells were observed (data not shown).
Figure 5. Agonist DR5 antibody does not alter lymphocyte subsets in vivo.
(A) Percentages of CD19+ B cell (left), CD4+ T cell (middle) and CD8+ T cell (left) subpopulations, resolved by flow cytometry, at baseline in patients with various malignancies enrolled in the phase 1 study of CS-1008. (B) Fold change in percentages of subsets of CD19+ B cell (left), CD4+ T cell (middle) and CD8+ T cell (left) in patients at days 2, 21, and 49 after CS-1008 treatment.
DISCUSSION
DR5 has been reported by many to be increased on malignant cells and tissues. Engagement of this receptor causes apoptosis in many cancer cells [15; 16]. Elimination of malignantly transformed cells using this molecule is being currently explored as a possible cancer therapy. Synovial fibroblasts isolated from autoimmune rheumatoid arthritis patients, which exhibit a semi-transformed phenotype, also exhibit DR5 up-regulation and sensitivity to DR5-induced apoptosis [16; 21; 24]. SLE B cells possess characteristics of malignantly transformed cells, such as hyperproliferation and hyperactivity. Here we investigated DR5 expression and function in primary B cells, focusing on SLE B lymphocytes to see if DR5 could be used to eradicate pathogenic activated B cells. Investigation of DR5 expression on non-cancer cells has just begun and remains controversial. We show that primary B cells do express DR5 but are insensitive to DR5-mediated apoptosis.
A previous study reported little DR5 expression on CD19+ PBMCs isolated from healthy control volunteers. The noted ratio of anti-human DR5 MFI to isotype control MFI was 1.2 [30]. In contrast, we found that CD19+ B cell mean MFI ratios from control and SLE subjects were 2.92 and 3.68, respectively (data not shown). Tonsil mononuclear B cells had a mean MFI of 1.92 (data not shown), and expression was confirmed by western blotting. Human B cell subsets have been heavily characterized in blood and tonsil [31; 32; 33; 34]. B lymphocyte gating strategies using CD27 (memory cell marker) and CD38 (activation marker) allow the separation of five distinct B cell subpopulations (Fig. 1A). All primary B cell subsets examined expressed DR5 (Figs. 1B, 2A, and 2B). Interestingly, DR5 expression was comparable among most B cell subsets. In SLE patients, CD27++ CD38++ plasmablasts expressed less DR5 than CD27− CD38− naïve B cells, but otherwise resting and activated B cell populations expressed similar levels of DR5. This observation is consistent with previous reports that resting and activated murine B cells had similar DR5 mRNA levels [26]. An autoimmune-disease related increase in B lymphocyte DR5 expression compared to healthy controls was not seen.
The relevance of TRAIL-mediated apoptosis in SLE continues to be examined. Upregulation of TRAIL mRNA was found in PBMCs isolated from SLE patients with active disease [35]. Previous reports showed that low neutrophil counts negatively correlated with serum TRAIL in SLE patients [36]. Elevated serum TRAIL concentrations positively associated with anti-SSA/SSB antibodies in SLE patients [37]. Increased expression of TRAIL on T cells was found in SLE patients with active disease [38]. Moreover, increased T cell TRAIL expression has been shown to cause autologous cell death of monocytes in SLE patients [39]. Auto-antigen specific T cells were also found resistant to TRAIL-mediated apoptosis [40].
CD40, BCR and/or IL-4 stimulation of B cells did not increase DR5 in any primary B cell subset (Fig. 3). The Fas death receptor is also a member of the TNF receptor family and is involved in B cell activation-induced cell death. Fas expression and function in B cells have been extensively studied. Unlike DR5, expression of this pro-apoptotic death receptor varies between B cells subsets. Naïve tonsil B cells do not express Fas, while memory and germinal center B cells express moderate to high levels suggesting antigen stimulation is required for Fas expression [41; 42; 43]. Studies show CD40 stimulation causes a substantial increase in Fas in all three populations. Susceptibility to Fas-mediated apoptosis is only acquired after CD40 co-stimulation [42]. Our data implies that DR5 is not involved in activation-induced apoptosis and that antigen exposure is unnecessary for DR5 expression.
DR5 function in primary B cell subsets was examined by using an agonistic monoclonal anti-human DR5 antibody, TRA-8. TRA-8 induces apoptosis without additional cross-linking and competes with the natural DR5 ligand TRAIL for binding [16]. While B cells isolated from tonsil, healthy control, and SLE subjects express DR5, all B cell populations were found to be resistant to TRA-8 induced apoptosis after overnight stimulation (Figs. 4A, 4B, and 4C). Co-stimulation with CD40 and/or IL-4 did not increase susceptibility to TRA-8 induced apoptosis. Stimulation through the BCR alone or with combinations of CD40 and/or IL-4 were also ineffective in increasing sensitivity to TRA-8 induced cell death. No cell death was detected in any treatment condition tested (data not shown). A 24 hour incubation with 50ng/mL TRA-8 was unable to produce primary B cell death and excludes the notion that primary B cells have a delayed effect to TRA-8 induced apoptosis (Fig. 4A). Increased concentrations of TRA-8 (75- and 100 ng/mL) were unable to induce apoptosis in primary B lymphocytes. Additionally, primary B cells and Jurkat T cells were incubated with 20ng/mL of DR5 ligand TRAIL and 1ug/mL cross-linker overnight and assessed for cell death. Primary B cells were insensitive to TRAIL-induced cell death while Jurkat T cells were susceptible to TRAIL-mediated apoptosis (data not shown).
Insensitivity to DR5-mediated apoptosis in DR5-bearing B cells is intriguing. We find that cell surface protein expression alone is insufficient to mediate cell death after DR5 ligation. An active DISC including FADD and caspase 8 is required for apoptotis signaling to occur. Alternatively, flice-like inhibitory protein (FLIP) can compete with caspase 8 for recruitment to FADD making the DR5-associated DISC nonfunctional. FLIP is a caspase 8 homolog that contains an inactive caspase domain and is unable to induce cell death. The role of FLIP in death receptor mediated apoptosis has been shown in the literature [44; 45; 46]. Over-expression of FLIP in cancer cells has been linked to resistance to TRAIL-mediated apoptosis [47; 48]. Conversely, after RNA interference of FLIP, cells that were found completely or partially resistant to TRAIL-mediated apoptosis developed increased sensitivity [49; 50; 51; 52; 53]. In preliminary studies, we examined FLIP expression in primary tonsil B lymphocytes by performing western blot analysis on whole cell lysates. Jurkat T cell FLIP protein levels decreased with increased stimulation with TRA-8. However, no reduction in FLIP, after increased TRA-8 stimulation, was observed in tonsil B lymphocytes (data not shown).
Additional mechanisms involved in susceptibility to TRAIL-induced cell death have been documented. TRAIL receptor post-translational modification has been linked to sensitivity in malignant cells. Wagner et. al noted that O-glycosylation of DR4 and DR5 initiated receptor clustering and caspase 8 activation [54]. Rossin and colleagues found that palmitoylation of DR4 was required for raft localization and receptor oligomerization in transfected HEK-293 cells [55]. Additionally, sensitivity to TRAIL-mediated apoptosis was found to correlate with expression of Bcl 2 family member, Mcl-1 in various cancer cell lines [56]. TRAIL susceptibility in Bax deficient colon cancer cells was noted after expression of c-myc [57]. Mechanisms linked with non-malignant normal cell resistance to TRAIL-mediated apoptosis remain unclear. Previously published reports found cells insensitive to TRAIL-mediated apoptosis were made sensitive after addition of a proteasome inhibitor [58; 59]. Likewise, we stimulated SLE peripheral blood mononuclear cells with TRA-8 in combination with the proteasome inhibitor lactacystin. SLE B lymphocytes remained resistant to TRA-8 induced cell death (data not shown). However, addition of lactacystin alone reduced the viability of naïve B cells (data not shown).
TRAIL has been shown to have various non-apoptotic functions including proliferation [60; 61; 62; 63] and cytokine production [64; 65]. The ability of TRAIL to reduce activation and proliferation in primary B cell subsets is an important question that should be further explored. In our studies, we did not perform experiments that directly measured cell proliferation. However, we can investigate TRA-8 induced cell activation indirectly, by examining CD38 expression, which was included in the flow cytometry panel to identify B cell subsets. Cell surface CD38 expression on B cells has been shown to increase after CD40 in vitro activation and the increase was observed on the cells that entered a proliferative phase [66; 67]. We did not observe an increase in B cell CD38 expression after TRA-8 stimulation and therefore do not believe that TRA-8 induces activation in primary B cells.
Our results suggest that targeting DR5 will not eliminate pathogenic and hazardous B cells in SLE. However, given the susceptibility of primary human synoviocytes to DR5-mediated apoptosis, the lack of effect on B (or T) cells documented here suggest that a treatment approach with CS-1008 will not result in depletion of DR5-expressing lymphocytes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–82. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 2.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–90. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 3.Wajant H, Moosmayer D, Wuest T, Bartke T, Gerlach E, Schonherr U, Peters N, Scheurich P, Pfizenmaier K. Differential activation of TRAIL-R1 and -2 by soluble and membrane TRAIL allows selective surface antigen-directed activation of TRAIL-R2 by a soluble TRAIL derivative. Oncogene. 2001;20:4101–6. doi: 10.1038/sj.onc.1204558. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity. 1997;7:821–30. doi: 10.1016/s1074-7613(00)80400-8. [DOI] [PubMed] [Google Scholar]
- 5.Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–3. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 6.Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG, Rauch CT. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–97. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–20. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 8.Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997;186:1165–70. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–7. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 10.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–63. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 12.Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, Strom SC. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–7. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- 13.Simon AK, Williams O, Mongkolsapaya J, Jin B, Xu XN, Walczak H, Screaton GR. Tumor necrosis factor-related apoptosis-inducing ligand in T cell development: sensitivity of human thymocytes. Proc Natl Acad Sci U S A. 2001;98:5158–63. doi: 10.1073/pnas.091100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nitsch R, Bechmann I, Deisz RA, Haas D, Lehmann TN, Wendling U, Zipp F. Human brain-cell death induced by tumour-necrosis-factor-related apoptosis-inducing ligand (TRAIL) Lancet. 2000;356:827–8. doi: 10.1016/S0140-6736(00)02659-3. [DOI] [PubMed] [Google Scholar]
- 15.Buchsbaum DJ, Zhou T, Grizzle WE, Oliver PG, Hammond CJ, Zhang S, Carpenter M, LoBuglio AF. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin Cancer Res. 2003;9:3731–41. [PubMed] [Google Scholar]
- 16.Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T, Zhang H, Mountz JD, Koopman WJ, Kimberly RP, Zhou T. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–60. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 17.Thomas LR, Henson A, Reed JC, Salsbury FR, Thorburn A. Direct binding of Fas-associated death domain (FADD) to the tumor necrosis factor-related apoptosis-inducing ligand receptor DR5 is regulated by the death effector domain of FADD. J Biol Chem. 2004;279:32780–5. doi: 10.1074/jbc.M401680200. [DOI] [PubMed] [Google Scholar]
- 18.Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J, Krammer PH, Walczak H. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599–609. doi: 10.1016/s1074-7613(00)80211-3. [DOI] [PubMed] [Google Scholar]
- 19.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–20. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 20.Bodmer JL, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, Blenis J, Tschopp J. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol. 2000;2:241–3. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- 21.Ichikawa K, Liu W, Fleck M, Zhang H, Zhao L, Ohtsuka T, Wang Z, Liu D, Mountz JD, Ohtsuki M, Koopman WJ, Kimberly R, Zhou T. TRAIL-R2 (DR5) mediates apoptosis of synovial fibroblasts in rheumatoid arthritis. J Immunol. 2003;171:1061–9. doi: 10.4049/jimmunol.171.2.1061. [DOI] [PubMed] [Google Scholar]
- 22.MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem. 1997;272:25417–20. doi: 10.1074/jbc.272.41.25417. [DOI] [PubMed] [Google Scholar]
- 23.Lamhamedi-Cherradi SE, Zheng SJ, Maguschak KA, Peschon J, Chen YH. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL-/- mice. Nat Immunol. 2003;4:255–60. doi: 10.1038/ni894. [DOI] [PubMed] [Google Scholar]
- 24.Miranda-Carus ME, Balsa A, Benito-Miguel M, De Ayala CP, Martin-Mola E. Rheumatoid arthritis synovial fluid fibroblasts express TRAIL-R2 (DR5) that is functionally active. Arthritis Rheum. 2004;50:2786–93. doi: 10.1002/art.20501. [DOI] [PubMed] [Google Scholar]
- 25.Mitchison NA, Wedderburn LR. B cells in autoimmunity. Proc Natl Acad Sci U S A. 2000;97:8750–1. doi: 10.1073/pnas.97.16.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ursini-Siegel J, Zhang W, Altmeyer A, Hatada EN, Do RK, Yagita H, Chen-Kiang S. TRAIL/Apo-2 ligand induces primary plasma cell apoptosis. J Immunol. 2002;169:5505–13. doi: 10.4049/jimmunol.169.10.5505. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Wang H, Wang Z, Makhija S, Buchsbaum D, LoBuglio A, Kimberly R, Zhou T. Inducible resistance of tumor cells to tumor necrosis factor-related apoptosis-inducing ligand receptor 2-mediated apoptosis by generation of a blockade at the death domain function. Cancer Res. 2006;66:8520–8. doi: 10.1158/0008-5472.CAN-05-4364. [DOI] [PubMed] [Google Scholar]
- 28.Sun M, Song L, Li Y, Zhou T, Jope RS. Identification of an antiapoptotic protein complex at death receptors. Cell Death Differ. 2008;15:1887–900. doi: 10.1038/cdd.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yada A, Yazawa M, Ishida S, Yoshida H, Ichikawa K, Kurakata S, Fujiwara K. A novel humanized anti-human death receptor 5 antibody CS-1008 induces apoptosis in tumor cells without toxicity in hepatocytes. Ann Oncol. 2008;19:1060–7. doi: 10.1093/annonc/mdn015. [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa H, Yamada Y, Harasawa H, Tsuji T, Murata K, Sugahara K, Tsuruda K, Masuda M, Takasu N, Kamihira S. Restricted expression of tumor necrosis factor-related apoptosis-inducing ligand receptor 4 in human peripheral blood lymphocytes. Cell Immunol. 2004;231:1–7. doi: 10.1016/j.cellimm.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Liu YJ, Arpin C, de Bouteiller O, Guret C, Banchereau J, Martinez-Valdez H, Lebecque S. Sequential triggering of apoptosis, somatic mutation and isotype switch during germinal center development. Semin Immunol. 1996;8:169–77. doi: 10.1006/smim.1996.0021. [DOI] [PubMed] [Google Scholar]
- 32.Gagro A, Toellner KM, Grafton G, Servis D, Branica S, Radojcic V, Kosor E, Hrabak M, Gordon J. Naive and memory B cells respond differentially to T-dependent signaling but display an equal potential for differentiation toward the centroblast-restricted CD77/globotriaosylceramide phenotype. Eur J Immunol. 2003;33:1889–98. doi: 10.1002/eji.200323357. [DOI] [PubMed] [Google Scholar]
- 33.Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V. Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J Immunol. 2001;167:2361–9. doi: 10.4049/jimmunol.167.4.2361. [DOI] [PubMed] [Google Scholar]
- 34.Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR, Lipsky PE, Radbruch A, Dorner T. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. 2000;165:5970–9. doi: 10.4049/jimmunol.165.10.5970. [DOI] [PubMed] [Google Scholar]
- 35.Komatsuda A, Wakui H, Iwamoto K, Togashi M, Maki N, Masai R, Hatakeyama T, Sawada K. Up-regulation of TRAIL mRNA expression in peripheral blood mononuclear cells from patients with active systemic lupus erythematosus. Clin Immunol. 2007;125:26–9. doi: 10.1016/j.clim.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 36.Matsuyama W, Yamamoto M, Higashimoto I, Oonakahara K, Watanabe M, Machida K, Yoshimura T, Eiraku N, Kawabata M, Osame M, Arimura K. TNF-related apoptosis-inducing ligand is involved in neutropenia of systemic lupus erythematosus. Blood. 2004;104:184–91. doi: 10.1182/blood-2003-12-4274. [DOI] [PubMed] [Google Scholar]
- 37.Castellino G, Corallini F, Trotta F, Secchiero P. Elevated levels of TRAIL in systemic lupus erythematosus are associated to the presence of anti-SSA/SSB antibodies. Lupus. 2007;16:479–82. doi: 10.1177/0961203307079455. [DOI] [PubMed] [Google Scholar]
- 38.Rus V, Nguyen V, Puliaev R, Puliaeva I, Zernetkina V, Luzina I, Papadimitriou JC, Via CS. T cell TRAIL promotes murine lupus by sustaining effector CD4 Th cell numbers and by inhibiting CD8 CTL activity. J Immunol. 2007;178:3962–72. doi: 10.4049/jimmunol.178.6.3962. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan MJ, Lewis EE, Shelden EA, Somers E, Pavlic R, McCune WJ, Richardson BC. The apoptotic ligands TRAIL, TWEAK, and Fas ligand mediate monocyte death induced by autologous lupus T cells. J Immunol. 2002;169:6020–9. doi: 10.4049/jimmunol.169.10.6020. [DOI] [PubMed] [Google Scholar]
- 40.Lunemann JD, Waiczies S, Ehrlich S, Wendling U, Seeger B, Kamradt T, Zipp F. Death ligand TRAIL induces no apoptosis but inhibits activation of human (auto)antigen-specific T cells. J Immunol. 2002;168:4881–8. doi: 10.4049/jimmunol.168.10.4881. [DOI] [PubMed] [Google Scholar]
- 41.Lagresle C, Bella C, Daniel PT, Krammer PH, Defrance T. Regulation of germinal center B cell differentiation. Role of the human APO-1/Fas (CD95) molecule. J Immunol. 1995;154:5746–56. [PubMed] [Google Scholar]
- 42.Lagresle C, Mondiere P, Bella C, Krammer PH, Defrance T. Concurrent engagement of CD40 and the antigen receptor protects naive and memory human B cells from APO-1/Fas-mediated apoptosis. J Exp Med. 1996;183:1377–88. doi: 10.1084/jem.183.4.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrone P, Neidhardt EM, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–73. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–5. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 45.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer JL, Schroter M, Scaffidi C, Krammer PH, Peter ME, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–21. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 46.Jin TG, Kurakin A, Benhaga N, Abe K, Mohseni M, Sandra F, Song K, Kay BK, Khosravi-Far R. Fas-associated protein with death domain (FADD)-independent recruitment of c-FLIPL to death receptor 5. J Biol Chem. 2004;279:55594–601. doi: 10.1074/jbc.M401056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rippo MR, Moretti S, Vescovi S, Tomasetti M, Orecchia S, Amici G, Catalano A, Procopio A. FLIP overexpression inhibits death receptor-induced apoptosis in malignant mesothelial cells. Oncogene. 2004;23:7753–60. doi: 10.1038/sj.onc.1208051. [DOI] [PubMed] [Google Scholar]
- 48.Galligan L, Longley DB, McEwan M, Wilson TR, McLaughlin K, Johnston PG. Chemotherapy and TRAIL-mediated colon cancer cell death: the roles of p53, TRAIL receptors, and c-FLIP. Mol Cancer Ther. 2005;4:2026–36. doi: 10.1158/1535-7163.MCT-05-0262. [DOI] [PubMed] [Google Scholar]
- 49.Sharp DA, Lawrence DA, Ashkenazi A. Selective knockdown of the long variant of cellular FLICE inhibitory protein augments death receptor-mediated caspase-8 activation and apoptosis. J Biol Chem. 2005;280:19401–9. doi: 10.1074/jbc.M413962200. [DOI] [PubMed] [Google Scholar]
- 50.Siegmund D, Hadwiger P, Pfizenmaier K, Vornlocher HP, Wajant H. Selective inhibition of FLICE-like inhibitory protein expression with small interfering RNA oligonucleotides is sufficient to sensitize tumor cells for TRAIL-induced apoptosis. Mol Med. 2002;8:725–32. [PMC free article] [PubMed] [Google Scholar]
- 51.Lane D, Cartier A, L’Esperance S, Cote M, Rancourt C, Piche A. Differential induction of apoptosis by tumor necrosis factor-related apoptosis-inducing ligand in human ovarian carcinoma cells. Gynecol Oncol. 2004;93:594–604. doi: 10.1016/j.ygyno.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 52.Dolcet X, Llobet D, Pallares J, Rue M, Comella JX, Matias-Guiu X. FLIP is frequently expressed in endometrial carcinoma and has a role in resistance to TRAIL-induced apoptosis. Lab Invest. 2005;85:885–94. doi: 10.1038/labinvest.3700286. [DOI] [PubMed] [Google Scholar]
- 53.Perez LE, Parquet N, Shain K, Nimmanapalli R, Alsina M, Anasetti C, Dalton W. Bone marrow stroma confers resistance to Apo2 ligand/TRAIL in multiple myeloma in part by regulating c-FLIP. J Immunol. 2008;180:1545–55. doi: 10.4049/jimmunol.180.3.1545. [DOI] [PubMed] [Google Scholar]
- 54.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K, Huw L, Katta V, Cavet G, Hymowitz SG, Amler L, Ashkenazi A. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–7. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 55.Rossin A, Derouet M, Abdel-Sater F, Hueber AO. Palmitoylation of the TRAIL receptor DR4 confers an efficient TRAIL-induced cell death signalling. Biochem J. 2009;419:185–92. doi: 10.1042/BJ20081212. 2 p following 192. [DOI] [PubMed] [Google Scholar]
- 56.Ricci MS, Jin Z, Dews M, Yu D, Thomas-Tikhonenko A, Dicker DT, El-Deiry WS. Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol. 2004;24:8541–55. doi: 10.1128/MCB.24.19.8541-8555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ricci MS, Kim SH, Ogi K, Plastaras JP, Ling J, Wang W, Jin Z, Liu YY, Dicker DT, Chiao PJ, Flaherty KT, Smith CD, El-Deiry WS. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12:66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Sayers TJ, Brooks AD, Koh CY, Ma W, Seki N, Raziuddin A, Blazar BR, Zhang X, Elliott PJ, Murphy WJ. The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood. 2003;102:303–10. doi: 10.1182/blood-2002-09-2975. [DOI] [PubMed] [Google Scholar]
- 59.Yang X, Wang J, Liu C, Grizzle WE, Yu S, Zhang S, Barnes S, Koopman WJ, Mountz JD, Kimberly RP, Zhang HG. Cleavage of p53-vimentin complex enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis of rheumatoid arthritis synovial fibroblasts. Am J Pathol. 2005;167:705–19. doi: 10.1016/S0002-9440(10)62045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morel J, Audo R, Hahne M, Combe B. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces rheumatoid arthritis synovial fibroblast proliferation through mitogen-activated protein kinases and phosphatidylinositol 3-kinase/Akt. J Biol Chem. 2005;280:15709–18. doi: 10.1074/jbc.M414469200. [DOI] [PubMed] [Google Scholar]
- 61.Tsai HF, Lai JJ, Chou AH, Wang TF, Wu CS, Hsu PN. Induction of costimulation of human CD4 T cells by tumor necrosis factor-related apoptosis-inducing ligand: possible role in T cell activation in systemic lupus erythematosus. Arthritis Rheum. 2004;50:629–39. doi: 10.1002/art.20038. [DOI] [PubMed] [Google Scholar]
- 62.Secchiero P, Zerbinati C, Rimondi E, Corallini F, Milani D, Grill V, Forti G, Capitani S, Zauli G. TRAIL promotes the survival, migration and proliferation of vascular smooth muscle cells. Cell Mol Life Sci. 2004;61:1965–74. doi: 10.1007/s00018-004-4197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ehrlich S, Infante-Duarte C, Seeger B, Zipp F. Regulation of soluble and surface-bound TRAIL in human T cells, B cells, and monocytes. Cytokine. 2003;24:244–53. doi: 10.1016/s1043-4666(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 64.Li JH, Kirkiles-Smith NC, McNiff JM, Pober JS. TRAIL induces apoptosis and inflammatory gene expression in human endothelial cells. J Immunol. 2003;171:1526–33. doi: 10.4049/jimmunol.171.3.1526. [DOI] [PubMed] [Google Scholar]
- 65.Abdollahi T, Robertson NM, Abdollahi A, Litwack G. Identification of interleukin 8 as an inhibitor of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in the ovarian carcinoma cell line OVCAR3. Cancer Res. 2003;63:4521–6. [PubMed] [Google Scholar]
- 66.Tangye SG, Avery DT, Deenick EK, Hodgkin PD. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J Immunol. 2003;170:686–94. doi: 10.4049/jimmunol.170.2.686. [DOI] [PubMed] [Google Scholar]
- 67.Tangye SG, Avery DT, Hodgkin PD. A division-linked mechanism for the rapid generation of Ig-secreting cells from human memory B cells. J Immunol. 2003;170:261–9. doi: 10.4049/jimmunol.170.1.261. [DOI] [PubMed] [Google Scholar]