Abstract
Bridging of long peripheral nerve gaps remains a significant clinical challenge. Electrospun nanofibers have been used to direct and enhance neurite extension in vitro and in vivo. While it is well established that oriented fibers influence neurite outgrowth and Schwann cell migration, the mechanisms by which they influence these cells are still unclear. In this study, thin films consisting of aligned poly-acrylonitrile methyl acrylate (PAN-MA) fibers or solvent casted smooth, PAN-MA films were fabricated to investigate the potential role of differential protein adsorption on topography-dependent neural cell responses. Aligned nanofibers films promoted enhanced adsorption of fibronectin compared to smooth films. Studies employing function-blocking antibodies against cell adhesion motifs suggest that fibronectin plays an important role in modulating Schwann cell migration and neurite outgrowth from dorsal root ganglion (DRG) cultures. Atomic Force Microscopy demonstrated that aligned PAN-MA fibers influenced fibronectin distribution, and promoted aligned fibronectin network formation compared to smooth PAN-MA films. In the presence of topographical cues, Schwann cell-generated fibronectin matrix was also organized in a topographically sensitive manner. Together these results suggest that fibronectin adsorption mediated the ability of topographical cues to influence Schwann cell migration and neurite outgrowth. These insights are significant to the development of rational approaches to scaffold designs to bridge long peripheral nerve gaps.
Keywords: neural tissue engineering, fibronectin, Schwann cell migration, protein distribution, electrospun nanofibers, peripheral nerve regeneration
1. Introduction
Functional recovery after peripheral nerve injury is critically dependent on both the rate as well as degree of regeneration and reinnervation of target tissues [1]. Each year, approximately 100,000 patients undergo peripheral nerve surgeries in United States and Europe [2]. Even though microsurgery techniques are adequate for short nerve lesions, no satisfactory methods are available to bridge long peripheral nerve gaps, and the “gold standard” of using autografts has several drawbacks that limit its use. Besides falling short on the extent of regeneration, several studies have shown that delays in repair after injury contribute to poor functional recovery [3, 4]. Hence, there is a critical need to improve both the extent and rate of regeneration after peripheral nerve injury.
Synthetic biomaterial-based nerve conduits have been developed as alternatives to autografts [5-8]. Across short gaps (< 8 mm), these conduits support provisional fibrin cable formation which acts as a substrate for Schwann cell (SC) and fibroblast (FB) migration into the nerve gap from proximal and distal nerve stumps[9]. These cells help reorganize the extracellular matrix (ECM) and provide the trophic support to the regenerating axons enabling bridging of the nerve gap [10]. However, nerve conduits have not been effective in bridging critically sized nerve gaps that are typically (greater than 3 cm in humans and greater than 1.3-1.5 cm in rats), and functional recovery is rarely attained [4]. Therefore, strategies to augment nerve conduit effectiveness by including additional physical and biochemical elements within conduit lumens have been proposed.
Magnetically aligned collagen fibers [11], hydrogels [12, 13] and synthetic micro filaments [14, 15] have been tested both in vitro and in vivo and have shown promise as supporting substrates for peripheral nerve cells. Specifically, aligned electrospun fibers have been widely explored for enhancing nerve cell function. Their high surface area to volume ratio and their ability to provide contact guidance have made them an attractive scaffold for bridging peripheral nerve gaps [16, 17].
Previous studies from our laboratory have shown that poly(acrylonitrile-co-methylacrylate) (PAN-MA) based aligned fiber films stacked in a polysulfone conduit successfully bridge long peripheral nerve gaps in rats without the need of any exogenous factors by enabling efficient Schwann cell migration [8]. A subsequent study demonstrated that a single thin film of aligned PAN-MA fiber which occupied only 0.6% of the total volume of the conduit was able to bridge a 14 mm gap in rats [18].
Whereas these and other studies demonstrate the ability of electrospun films to enhance nerve regeneration, the mechanisms by which they influence regeneration and peripheral glial cells such as Schwann cells remain unclear. It is evident that surface topography significantly influences cell behavior in vitro and in vivo [19, 20]. Varying topography of electrospun fibers alters cell adhesion, spreading, proliferation, migration and differentiation in bone [21] and nerve regeneration [22] as well as in guiding stem cell fate [23]. Substrate curvature modulates neurite extension [24] and ECM may play a role in effecting this behavior of cells [25]. The present study explores the relationship between differential protein adsorption on electrospun PAN-MA films and smooth solvent cast PAN-MA films.
2. Materials and Methods
2.1 Fabrication of polymer films with aligned and smooth topographies
Polymer solutions (7%) were made by dissolving poly(acrylonitrile-co-methylacrylate) (PAN-MA) (Sigma, MW 8000) in N,N,-dimethylformamide (DMF) at 60 °C. For electrospinning, the solution was pumped through a syringe at a rate of 1mL/h at a voltage of 6-10 kV. The polymer stream was directed at an aluminum foil-covered metal drum rotating at 2400 rpm for 15 minutesin order to produce aligned fibers. A 2% solution of the same polymer prepared in DMF was cast on a glass coverslip to obtain smooth films with the same chemistry. A UV lamp was used to sterilize the samples. The diameter of the fibers was characterized using scanning electron microscopy (S-800 SEM, Hitachi) and quantified using Image-Pro software (Media Cybernetics). Strips of aligned and smooth films (2 cm × 1 cm) were glued to the bottom of a 35 mm petri dish for in vitro assessment of topography.
2.2 Harvesting of Schwann cells and dorsal root ganglia (DRG)
Schwann cells were purified from the sciatic nerves of postnatal day 1 (P1) rat pups (Harlan) using a protocol modified from Brockes et al[26]. Briefly, sciatic nerves were dissected into 1 mm segments and dissociated in 1.33% collagenase (Worthington Biochemical) solution for 30 min. The nerve segments were then treated with 0.25% Trypsin/EDTA (Invitrogen, Carlsbad, CA) for 30 min. Cells were then mechanically dissociated using a pipette and incubated in culture media (DMEM/F12 (Fisher, Hampton, NH))supplemented with 10% fetal bovine serum(Gemini, Sacramento, CA) and neuregulin 1 (NRG1) (R&D systems) (50 ng/mL). After 24 h, the culture media was replaced with similar media supplemented with arabinoside (Ara-C) (10-5) (Sigma) for 48 h to remove the faster proliferating fibroblasts. Purity of cells was assessed by immunostaining with S100 (DAKO). Cultures with purity of greater than 95% were used in in vitro assays.
DRGs were also harvested from P1 rat pups. The nerve roots were removed and the ganglia were seeded on aligned fiber based films. To encourage attachment to the films, the ganglia were first incubated for several hours with only a thin layer of medium. Afterwards, each experimental condition was fully covered with DMEM/F12 media with 10% FBS and 50 ng/mL nerve growth factor (NGF) (Roche). Effects of topography on Schwann cell migration and neurite outgrowth under different experimental conditions was characterized using these DRG cultures.
2.3 Neurite outgrowth and Schwann cell migration assay
To evaluate the effects of the underlying topography on neurite outgrowth and Schwann cell migration, DRGs were cultured for 7 days on electrospun aligned PAN-MA and solvent cast smooth PAN-MA films, fixed with Histochoice (Fisher) for 20 min and washed three times with 1× PBS. Cells were tagged overnight at 4°C with the primary antibody solutions: neurofilament 160 kDa (NF160, 1:500, mouse IgG1, Sigma) to stain for neurons and S-100 (1:250, rabbit, IgG, DakoCytomation) to stain for Schwann cells. The following secondary antibodies were used: goat anti-rabbit IgG Alexa 488/594, goat anti-mouse IgG1 Alexa 488/594. Fifteen of the longest NF160+ axons and 15 furthest S100+ Schwann cells were measured from the edge of the DRGs as shown in Figure 2. Image Pro was used to quantify the migration distance of Schwann cells and the extent of neurite extension under the effects of various conditions used.
Figure 2.
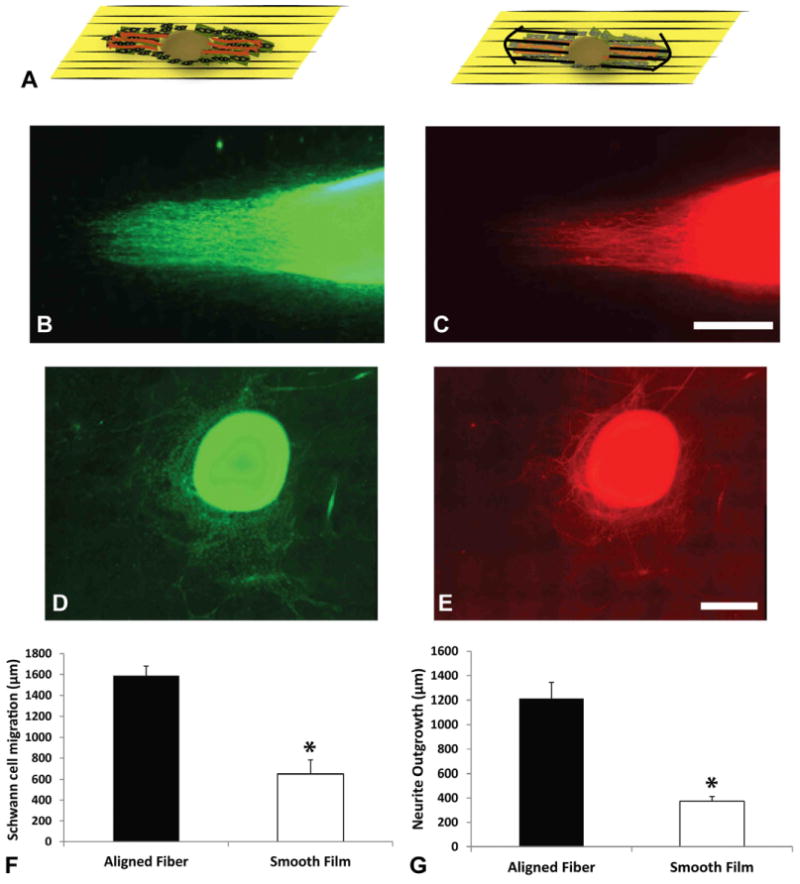
Schematic diagram illustrating how Schwann cells and neurons extend from the DRG body (A). Images of Schwann cell migration (using S100 staining, green) and neurite outgrowth (using NF160, red) on aligned fibers (B, C) and smooth film (D, E) from rat DRG in vitro. (F, G): Show the quantitative comparison of both fiber topographies in Schwann cell migration and neurite outgrowth. Aligned fibers show significantly higher migration distances and growth. *p < 0.05. Error Bars = Std. Dev.
2.4 Fibronectin adsorption assay
Fibronectin adsorption on electrospun PAN-MA and solvent-cast PAN-MA film topographies was analyzed using a modified enzyme linked immunosorbent assay (ELISA). Circular film segments 5mm in diameter were generated using a sharp metal mask and the aligned fibers and smooth films were glued to the bottom of a 96 well tissue culture plate which was pre-coated with Protein Block (Pierce) solution to prevent protein adsorption on the dish. Fetal bovine serum (100%) was added to the films overnight. PAN-MA films were then washed thoroughly to remove unbound proteins three times with 0.1 % Tween 20 in 1× PBS. Protein block solution was applied to the substrates followed by an overnight incubation withanti-fibronectin antibody (1:3000) (Millipore). Next day; substrates were washed extensively followed by incubation with HRP conjugated secondary antibody (1:3000) for 1 hr. Substrates were washed again and incubated with TMB substrate solution and the peroxidase/TMB reaction was terminated using stop solution (Cell Signaling). A standard curve was obtained by incubating FN (0.5 ng – 10 ng, r2 = 0.85) in a 96 well plate by performing the above mentioned ELISA concurrently. Substrates incubated in 1× PBS were used as controls. Absorbance at 450 nm was read by using a spectrophotometer to quantify the amount of antibody bound to fibronectin adsorbed onto the different topographies. Surface area for both aligned and smooth films were characterized by Atomic Force Microscopy. 3D surface area were quantified using SPIP (nanoScience Instruments) software and used to normalize the adsorption data.
2.4 Fibronectin depletion and competition assays
To investigate whether fibronectin (FN) plays a role in affecting the migration of Schwann cells on aligned fibers, experiments were performed with FN-depleted serum. Briefly, Gelatin Sepharose media (GE healthcare) was incubated with fetal bovine serum (5 ml of Sepharose for 50 ml of Serum)overnight at 4°C. This solution was centrifuged to remove the beads and western blot analysis was performed to evaluate the percent depletion of FN. We obtained serum which was greater than 95% depleted of FN (data not shown). Fibers were incubated in FN-depleted serum before DRGs were seeded. Serum without FN depletion was used as a control. DRGs were cultured for one week in respective media and Schwann cell migration was measured as discussed previously in section 2.3.
To evaluate if FN adhesion motifs play a role in mediating the effects of fibers on behavior of neuronal cells, DRGs were seeded and allowed to attach to aligned fiber films overnight. Cyclo-GRGDSP(AnaSpec) was added to media (0.5 mg/mL) every two days and the DRGs were cultured for 6 days. Control cultures with no peptide were also maintained for the same amount of time. Schwann cell migration and neurite outgrowth were measured as discussed above (Section 2.3) to assess the effects of competitively binding to cell integrin.
Finally, to investigate whether fibronectin-specific adhesion motifs mediate fiber influenced cell migration, an antibody inhibition assay using the HFN7.1 anti-FN clone was performed. HFN7.1 binds to the central cell binding domain on human fibronectin with no detectable cross reactivity to rat fibronectin. DRGs were allowed to attach overnight in media containing human serum. HFN7.1 (DSHB, Iowa) and isotype control (M18, DSHB, Iowa) antibodies were added to the cultures and maintained for 3 days. Additionally, cultures without any antibody treatment were used to determine if human serum had any negative effects on cell growth. Schwann cell migration and neurite outgrowth were quantified using methods described above (Section 2.3).
2.5 Atomic force microscopy
AFM experiments were performed using a Multimode AFM equipped with a NanoScope IIIa controller from Veeco (Manchester, U.K.) operating in tapping mode in air; the Nanoscope software was used. Si-cantilevers from Veeco (Manchester, U.K.) were used with a force constant of 2.8 N/m and resonance frequency of 89 kHz. The phase signal was set to zero at a frequency of 5% lower than the resonance one. The drive amplitude was 750 mV, and the amplitude set point Asp was 1.6 V. Fibronectin from human plasma (Sigma) was adsorbed on the different substrates by immersing the material sheets in 20 μg/mL FN in PBS for 1hr. After protein adsorption, samples were rinsed 3 times with 1× PBS to eliminate the non-adsorbed protein. Remaining drops on the surface were dried by exposing the sample to a nitrogen flow for 2-3 min. AFM was performed in the tapping mode in air immediately after sample preparation. Height, phase, and amplitude magnitudes were recorded for each image.
2.6 Fibronectin organization by Schwann cells
Purified Schwann cells were seeded at a density of 2 × 105 cells on aligned fiber and smooth films in a 35-mm tissue culture plate and cultured for one week. Fibronectin laid down by glial cells was observed by staining with anti-rat fibronectin antibody (1:100) (Millipore). Goat anti-rabbit IgG Alexa 488 was used to stain for the matrix laid down by the glial cells.
2.7 Statistical analysis
ANOVA, combined with Tukey post hoc tests, was used to calculate the significance of differences between mean values. A p value less than 0.05 was considered statistically significant.
3. Results
3.1 Schwann cell migration and neurite outgrowth from DRG
Aligned PAN-MA fibers were generated using an electrospinning process. Smooth PAN/MA smooth films were obtained by solvent casting the solution of the same polymer. Under SEM, aligned fibers had an average diameter of 800 ± 96 nm while the smooth films' largest feature size was approximately 4 nm (Fig.1). Therefore, we were successful in generating a substrate with anisotropic topographical features, and another with a relatively ‘smooth’ topography with the same underlying substrate chemistry, PAN-MA.
Figure 1.
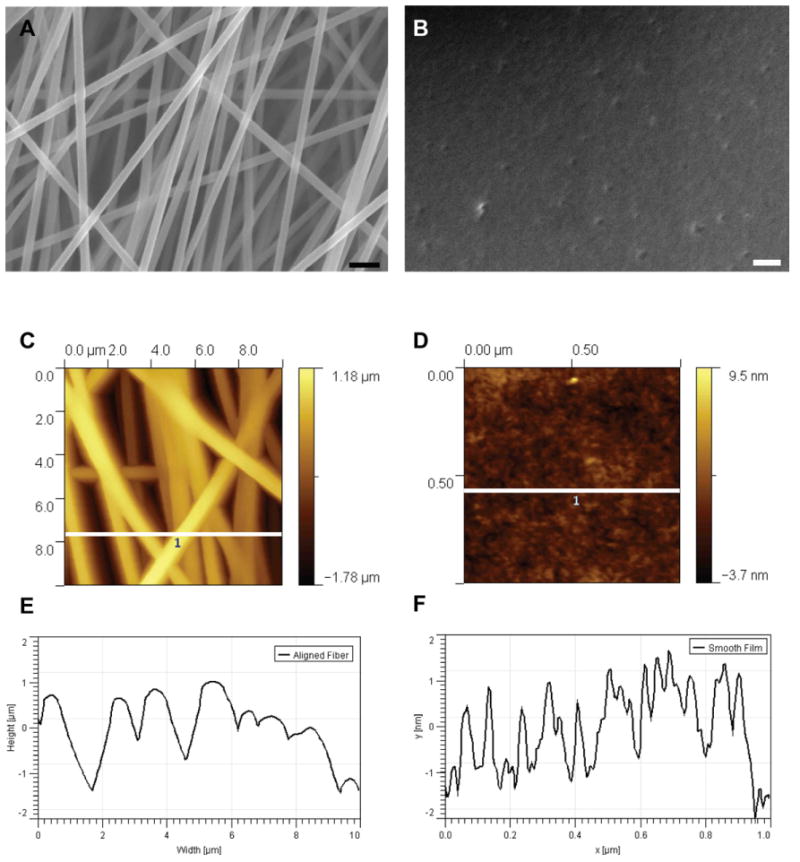
Scanning electron microscopy images of PAN-MA aligned fibers (A) and smooth film (B). Scale bar represents 2 μm in (A) and 0.3 μm in (B). Atomic force microscope analysis of fiber topographies (C, D). (E, F) Height profiles (white lines in (C) and (D)). Scan area for (C): 10 × 10 μm2 and (D): 1 × 1 μm2.
DRGs were seeded on the different topographies to quantify Schwann cell migration and neurite outgrowth. Schwann cells migrated parallel to the direction of the fibers. The migration distance of S100 positive Schwann cells was significantly greater, 1588 ± 93μm, on aligned fibers compared to the smooth films, 371 ± 59μm, where Schwann cell migration was less and not directed (p < 0.05) (Fig 2). Similar results were observed for neurite outgrowth. NF160 positive axons extended along the fibers to significantly greater extent (1212 ± 131μm, p < 0.05) compared to smooth films(373± 39μm). These results demonstrate that guidance cues from aligned fibers enhanced the migration of cells as well as directed neuronal outgrowth.
3.2 FN Adsorption from serum
We investigated the role of protein adsorption in mediating DRG and Schwann cell interactions with the underlying topography. Since fibronectin has been implicated in influencing Schwann cell migration, we characterized fibronectin adsorption from serum on aligned electrospun films relative to adsorption on smooth films. We quantified the amount of fibronectin adsorbed on aligned fibers and smooth films using a modified ELISA based assay. Membranes of aligned PAN-MA fibers exhibited significantly (p < 0.05) higher FN adsorption, 4.81 ± 0.32 ng/cm2 compared to 3.08 ± 0.19 ng/cm2 for smooth films.
3.3 Effects of FN depletion from serum on DRG behavior
Since differential fibronectin adsorption was observed on between fibers and films, the role of FN on the migration of Schwann cell on aligned fiber films was studied to determine whether FN adsorption from serum plays an important role in stimulating Schwann cell migration. Fibronectin depleted media was used to test the effects of fibronectin on Schwann cell migration.
DRGs attached evenly on both experimental substrates but Schwann cell migration was significantly diminished on substrates incubated in fibronectin-depleted media with a migration distance of 1144 ± 508 μm, as compared to control conditions, 2146 ± 315 μm. DRGs showed directed growth on both conditions. However, S100 positive cells migrated faster (p < 0.05) on substrates exposed to control serum (Fig 3).
Figure 3.
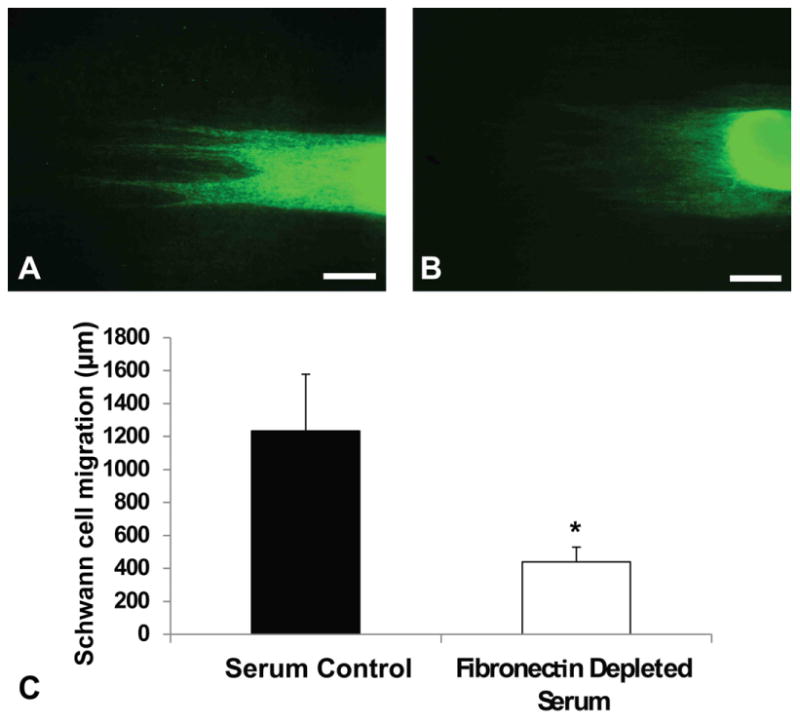
Quantitative analysis of Schwann cell migration in fibronectin depleted serum condition. Schwann cell migration from DRG in normal serum (A) and fibronectin depleted serum (B). (C): Migration in serum is significantly higher than migration in depleted serum. *p < 0.05. Error bar = Std. dev. Scale bar = 400 μm
3.4 Influence of competitive binding of Cyclo-GRDGS on FN mediated response
To further investigate the cellular interaction with adsorbed proteins on aligned fiber topographies, a Cyclo-GRGDS competitive assay was used. Cyclo-GRGDS was used to compete with the cell binding of adsorbed proteins on aligned fibers. As shown in Fig 4, at six days, Schwann cells migrated 371 ± 59 μm on substrates treated with soluble Cyclo-GRGDS compared to 1217 ± 73 μm on normal culture conditions. Moreover, neurite growth was significantly reduced (p<0.05) with GRGDS, 269 ± 84 μm, in comparison to normal media conditions, 965 ± 70 μm. These results show Schwann cell migration and neurite outgrowth on aligned nanofibers involves adhesion to an RGD-containing molecule adsorbed from serum or deposited by cells.
Figure 4.
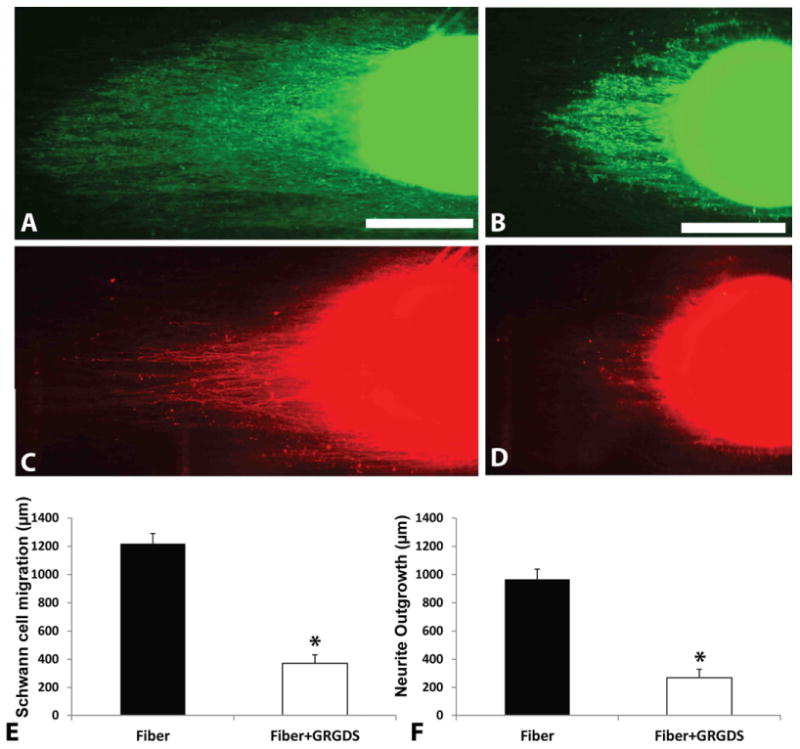
Schwann cell migration and neurite outgrowth analysis in GRGDSP inhibited media. Schwann cell migration from DRG in normal media (A) and GRGDSP media (B). Images of neurite outgrowth in respective conditions (C, D). Scale bars in (A) and (B) indicate 400 μm. Fibers without peptide inhibition displayed significantly higher migration (E) and neurite outgrowth (F). *p < 0.05. Error bar = Std. dev.
3.5 Inhibition of integrin binding motif of fibronectin
Next, we investigated the role of fibronectin by using antibodies specific to the central cell binding motifs of fibronectin molecule. HFN7.1 antibody was used to inhibit the effects of fibronectin adsorbed from human serum. Compared to the controls (M18 isotype control and normal human serum), HFN7.1 significantly (p < 0.05) inhibited Schwann cell migration and neurite outgrowth (565 ± 118 μm on M18, 521 ± 65 μm on untreated control and 318 ± 87 μm on HFN7.1) from DRGs. It was observed that Schwann cells migrated 585 ± 109 μm on substrates incubated with M18, 572 ± 148 μm on normal serum condition, while only 355 ± 80 μm on HFN7.1 incubated substrates. There was no significant difference between cell behavior on isotype treated and control condition suggesting that the antibody does not influence regular Schwann cell migration and axonal sprouting (Fig 5). Thus, integrin engagement of the cell binding domain of adsorbed FN appears essential for efficient Schwann cell migration and neurite outgrowth.
Figure 5.
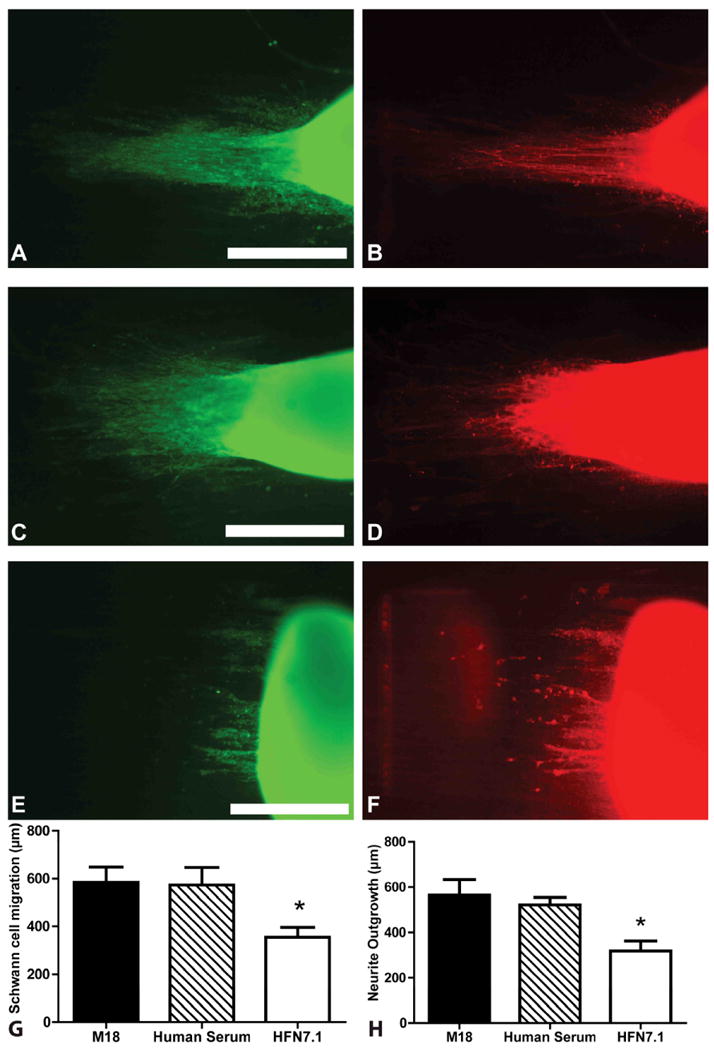
DRG Schwann cell migration and neurite outgrowth in HFN7.1 antibody inhibited fibers. Migration and outgrowth in M18 isotype control (A, B), normal human serum (C, D), and HFN7.1 antibody incubated fibers (E, F). Left panel is stained for Schwann cell (S100, green) and right panel is stained for neurites (NF160, red) Scale bars = 400 μm. Both controls demonstrated significantly higher Schwann migration and neurite outgrowth when compared to HFN7.1 incubated DRGS (G, H) respectively. *p<0.05. Error bar = Std. dev.
3.6 Influence of topography on fibronectin organization and production
To investigate the role of topography and its effect on protein adsorption, AFM was used to understand the distribution and organization of FN on aligned fibers and smooth films. Height, amplitude and phase profiles of human plasma fibronectin adsorbed on different topographies were evaluated in tapping-mode AFM. Aligned fibers exhibited the presence of FN fibrils, mostly aligned along the length of the fiber. On smooth films, fibronectin did not form any supramolecular structure and had a more globular morphology (Fig 6). Together with the previous results, this phenomenon observed on aligned PANMA fibers suggests that this organized fibronectin matrix could play a vital role in aligning cells to the underlying substrates thereby affecting their migration.
Figure 6.
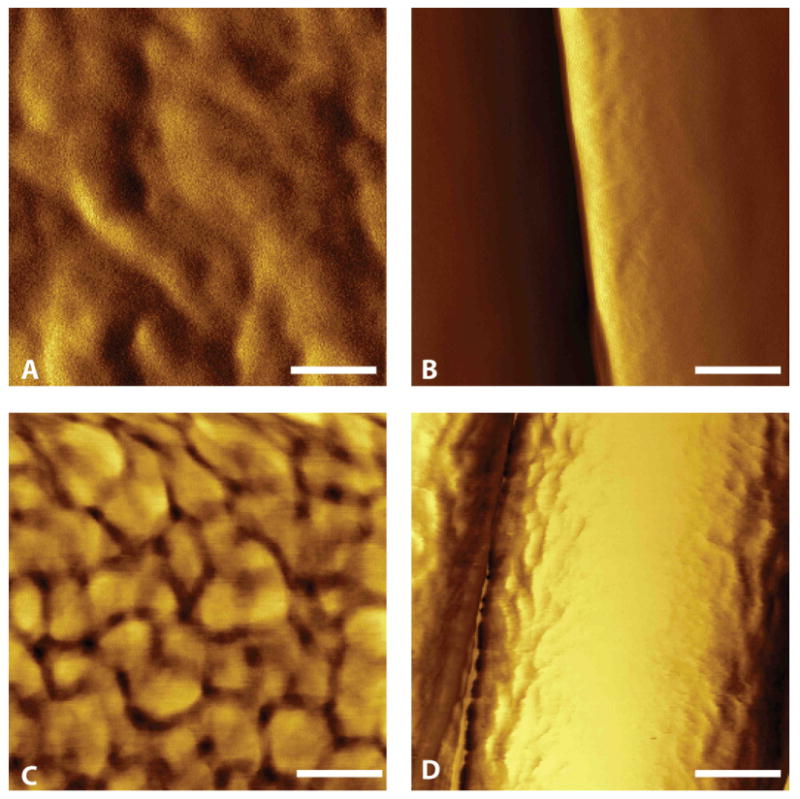
Atomic force microscopy images of PAN-MA polymer topography. (A): Smooth film. (B): Aligned PAN-MA fiber scaffold. (C) Smooth film with fibronectin. (D) Aligned fibers with fibronectin. Scale bar = 100 nm.
The ability of the aligned fibers to organize the matrix laid down by these cells was also examined. Purified P3 rat Schwann cells were cultured on aligned fibers and smooth films. FN antibody was used to observe the organization of FN matrix laid down by these cells. Aligned fibers promoted the formation of oriented network of fibronectin in comparison to smooth films which had a more disorganized matrix (Fig 7). This data demonstrates that the fibers not only enhance the adsorption of proteins but also influence the production and organization of ECM produced by the cells.
Figure 7.
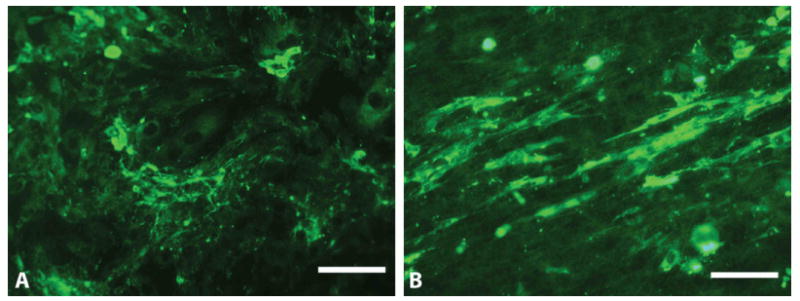
ECM organization on smooth film (A) and aligned fibers (B). Scale bars = 100 μm.
4. Discussion
Previous work from our laboratory as well as others has demonstrated that luminal fillers in nerve guidance channels can enhance peripheral nerve regeneration. Presumably, these luminal fillers interact with the normal regeneration process and influence the early stages of regeneration [27]. Luminal fillers may aid the formation of Bands of Bunger (longitudinal arrangement of Schwann cells and Laminin-1) by influencing the migration of Schwann cells [8, 18]. Schwann cells play an important role in the regeneration process by not only myelinating axons, but also producing growth factors such as NGF, BDNF, NT-3 and CNTF to help peripheral nerve regeneration [28]. Schwann cells also secrete and organize extra cellular matrix molecules such as vitronectin, laminin and fibronectin to enhance neurite outgrowth [29, 30]. Thus, Schwann cells have an important role in the PNS repair, and therefore, contact guidance-based strategies have been developed to enhance neurite growth have also served to enhance Schwann cell migration.
Several studies demonstrate that electrospun nanofibers align and direct Schwann cell migration as well as to guide neurite outgrowth. Electrospun PLLA fibers were used to bridge a 10 mm nerve gap in rats and showed functional recovery close to that of an autograft [15]. In another study, PCL/gelatin based aligned electrospun fibers improved Schwann cell attachment and proliferation [31]. Wang et. al fabricated aligned chitosan based nanofibers to specifically align Schwann cells for PNS repair [32]. They observed that aligned electrospun fibers promoted cell growth as well as support high axon count compared to a scaffold of non-oriented fibers in a 10 mm gap in rats. Whereas these studies show the benefits of using aligned electrospun scaffolds to repair nerve defects, it is difficult to realize their potential since a majority of these studies are performed in non-critical gaps where spontaneous regeneration occurs in empty conduits with no fillers.
Our previous work has shown that aligned thin films fabricated from electrospun PAN-MA supported enhanced Schwann cell infiltration, axonal growth as well as improved functional recovery in a critical length (17mm) nerve gap in rat [8]. Furthermore, a single thin film of oriented fibers which occupied only 0.6% of the total conduit volume was sufficient to enhance Schwann cell migration and bridge a 14 mm nerve gap [18]. These studies clearly show that topographical cues from aligned electrospun fibers can augment Schwann cell migration and lead to axonal regeneration. But the mechanisms by which aligned fiber based scaffolds promote neural cell or Schwann cell function are still unknown. In order to fabricate scaffolds that exceed autograph performance, an understanding of how aligned topographical cues enhance Schwann cell migration and direct neuronal growth is useful. Therefore, in the current study, we investigated how the effects of aligned fiber-based scaffolds mediate enhanced neurite extension and Schwann cell migration.
To discern the role of topography, we fabricated both aligned PAN-MA fiber-based films using an electrospinning process, and smooth PAN-MA films using solvent casting with minimal topographical cues. The topography of aligned fibers and smooth films were apparent both quantitatively and qualitatively as shown in Fig 1 by SEM and AFM analysis. Schwann cell migration and axonal growth are critical events for successful peripheral nerve regeneration after injury [33, 34]. We measured the extent of Schwann cell migration and DRG neurite outgrowth using cell-specific immunostaining on aligned fiber films and smooth films. Presence of aligned topography significantly enhanced the migration of Schwann cells and directed the growth of neural cell along the long axis of the fibers (Fig 2). It has been speculated that nanoscale topographies alter cellular responses by controlling the elongation and alignment of the nucleus.[35]
In order to evaluate the mechanistic interplay between the topographical features of the underlying film and cellular behavior, we examined at how topography alters adsorption of fibronectin, a critical protein mediating Schwann cells behavior and neurite extension [36, 37]. During regeneration, cell interaction with ECM proteins play a vital role in controlling cell attachment, migration, proliferation and differentiation [38]. These interactions also lead to cytokine activity, cell apoptosis and are responsible for activating intracellular signaling [39]. Earlier studies have shown that nanoporous scaffolds have led to higher adsorption of serum ECM proteins such as fibronectin, laminin and vitronectin compared to solid scaffolds [40]. Protein adsorption and organization by the underlying matrix has shown to affect the alignment of cells as well as control their phenotype [41]. Specifically, in the current study we explored how fibronectin adsorption from serum is regulated by the underlying topography. Fibronectin coated tissue culture plastic increased the migration of Schwann cell in vitro [42]. Also, neuronal growth from DRG was influenced by fibronectin composition in another study [43]. Fibronectin fibers promoted the alignment of Schwann cell focal contacts and organized the F-actin structure in parallel to the fibers [44]. Furthermore, oriented mats from the same fibers were used to bridge a 10 mm nerve defect in rats and showed faster rate of growth compared to muscle graft.[45] Therefore, FN represents an important ECM protein in mediating both Schwann cell and axonal response during the course of nerve regeneration.
When we quantified FN adsorption on aligned PAN-MA films compared to smooth PAN-MA films, more fibronectin adsorbed on aligned topographical films compared to smooth films. However, aligned fibers also demonstrated a higher surface area compared to smooth PAN-MA films as determined using atomic force microscopy (approximately 1.14 times higher on the aligned topography compared to smooth films, data not shown). After normalization surface area, we observed significant differences in the amount of fibronectin adsorbed per unit surface remained higher on aligned fibers compared to smooth films. At this time there are some caveats worth discussing. One of the limitations of AFM is that it has a scan depth of 5 μm while the fiber based films are 10 μm. Thus we may be underestimating the surface area of the fiber based films thereby exaggerating the calculation of adsorption of FN per unit surface area. Based on our calculations, even if the surface area of the fiber based films is 35% higher than that of the smooth films, the amount of FN adsorbed onto the fibers would still be statistically higher compared to the smooth films. Further the FN antibodies used in the ELISA might also interact with the adsorbed protein differently. As the dimensions of an individual Schwann cell and DRG exceed that of individual fibers, the cellular effects observed by simply be either due to greater concentration of FN being presented to cells, or the FN distribution along fibers is anisotropic compared to smooth films, causing anisotropic cellular behavior.
To investigate whether fibronectin was necessary for the observed anisotropic cell response on aligned fibers, we incubated fibers with fibronectin-depleted media and quantified that the migration of Schwann cells from DRGs. Schwann cell migration was significantly lower on FN-depleted serum substrates (Fig 3). There was some migration of cell on FN-depleted serum substrates and this phenomenon is due to the cell producing their own ECM molecules that is responsible for their migration. DRG glial cells have previously been shown to synthesize their own ECM [42]. This data strongly suggests that FN plays a critical role in Schwann cell migration and neurite extension on aligned polymeric films.
To further evaluate the functional role of FN mediated enhanced Schwann cell migration and neurite extension on aligned fiber based films compared to smooth films, Cyclo-GRDSP peptide was used to competitively bind to integrins on cells. Cyclo-GRDSP exposure to Schwann cells significantly inhibited Schwann cell migration and neurite outgrowth on aligned films compared to control substrates (Fig 4). This suggests that cell behavior on aligned fiber topographies is mediated by integrin-based adhesion. We recognize of course that while Cyclo-GRGDSP blocks integrin binding, it is not specific to FN - RGD binding sites are found on several ECM proteins such as fibronectin, vitronectin, osteopointin, fibrinogen and laminin [46]. Receptors on cells that detect the specific RGD sequence are often structurally similar and thus the binding motifs of specific adsorbed proteins that are responsible for mediating downstream cellular activities are not easily detected with RGD based peptides. Therefore, our study demonstrates that ability of the fibers to present the cell binding motifs of fibronectin is essential to trigger downstream effects that lead to more efficient and enhanced Schwann cell migration and neuronal regeneration. Since we were interested in evaluating if the presentation of adsorbed fibronectin by aligned fibers is responsible for modulation cell activity, we used the monoclonal antibody HFN7.1 to specifically inhibit the RGD binding site of adsorbed fibronectin from human serum. HFN7.1 inhibited Schwann cell migration from DRGs on aligned fibers suggesting that binding of fibronectin-specific RGD motifs on aligned fiber based topography are responsible for cell phenotype (Fig 5). Since αv integrins on Schwann cells have shown to mediate their migration on tissue culture plastic [43], we can speculate that adsorbed fibronectin on fibers also enhances Schwann cell migration via αv receptors on migrating Schwann cells. These blocking assays, when considered together, give new insights into how the effects of aligned fibers are translated to the neuronal cells.
As discussed earlier, FN's ability to mediate enhanced Schwann cell migration and neurite extension on fiber-based films could be explained either by increased FN contacting each cell, or by FN's distribution being different on fiber-based films compared to smooth PAN-MA films. Atomic force microscopy was utilized to further probe how topography influences the distribution and organization of adsorbed fibronectin. Surface chemistry, roughness and surface energy have all been shown to influence the protein adsorption process (Fig 6).[47] Surface roughness has shown to significantly affect fibronectin conformation which can lead to poor cell adhesion and actin reorganization.[47, 48] In our studies, aligned fibers topography promoted organized distribution of adsorbed fibronectin. Fibronectin fibrils also aligned parallel to the axis of the fibers. Since, previous studies have shown the dependence of focal contact formation to surface properties [49, 50]; it is likely that aligned PAN-MA fibers enhance the elongation of cellular processes by oriented fibronectin network formation. Smooth topography did not reveal any organization of fibronectin, suggesting that the enhancement of Schwann cell migration and neurite outgrowth on aligned fibers is mediated by the ability of the polymer fibers to influence the organization of adsorbed fibronectin at the material interface. Fibronectin assembly into fibrils upon adsorption on some family of materials has shown to enhance its biological activity, including cytoskeleton development, focal adhesion formation, matrix secretion and differentiation.[48, 51]
Organization of fibronectin secreted by glial cells cultured on aligned fibers is also enhanced by underlying topographical cues. Aligned fibers enabled formation of organized fibronectin network compared to smooth films (Fig 7). Matrix organization by glial cells is a vital step in the nerve regeneration process and the ability of the aligned fibers to affect that step can be beneficial during regeneration. Even though the major focus of the present study was to elucidate the role played by fibronectin in modulating cell behavior during neuronal regeneration, it is likely that other ECM components such as laminin may also play a pivotal role in cell interaction with topography. Further studies are warranted to understand the contribution of other ECM proteins mediating topographical enhancement of neurite extension and Schwann cell migration. In this study, we demonstrate that fibronectin plays a critical role, and that blocking its function even in the presence of other ECM proteins is sufficient to decrease Schwann cell migration and neurite extension. We also demonstrate that fibronectin presentation to neuronal cells due to the protein adsorption, conformation and organization on fiber-based films may all contribute to enhanced Schwann cell migration and neurite extension.
5. Conclusions
Data from our studies suggest that fibronectin presentation, conformation and organization contributes heavily to enhanced Schwann cell migration and neurite outgrowth on fiber-based films compared to smooth films of the same composition. In order to develop scaffolds that match or exceed the performance of autografts, a deeper understanding of the mechanisms by which scaffold properties affect the nerve regeneration is critical.
Acknowledgments
The authors would like to acknowledge support from the following grants: NIH R01NS065109, NIH R01NS044409 and NSF graduate research fellowship.HFN7.1 and M18 antibody was obtained from the Developmental Studies Hybridoma Bank, which was developed under the auspices of the National Institute of Child Health and Human Development and is maintained by the University of Iowa, Department of Biological Sciences. Manuel Salmerón-Sánchez was supported by the Spanish Government through PR2009-0351 to stay in Atlanta (Georgia Institute of Technology) for a sabbatical during 2010.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen MB, Zhang F, Lineaweaver WC. Luminal fillers in nerve conduits for peripheral nerve repair. Ann Plast Surg. 2006;57(4):462–71. doi: 10.1097/01.sap.0000237577.07219.b6. [DOI] [PubMed] [Google Scholar]
- 2.Schlosshauer B, Dreesmann L, Schaller HE, Sinis N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery. 2006;59(4):740–7. doi: 10.1227/01.NEU.0000235197.36789.42. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 3.Hoke A, Brushart T. Introduction to special issue: Challenges and opportunities for regeneration in the peripheral nervous system. Exp Neurol. 2010;223(1):1–4. doi: 10.1016/j.expneurol.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribeiro-Resende VT, Koenig B, Nichterwitz S, Oberhoffner S, Schlosshauer B. Strategies for inducing the formation of bands of Bungner in peripheral nerve regeneration. Biomaterials. 2009;30(29):5251–9. doi: 10.1016/j.biomaterials.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Shin RH, Friedrich PF, Crum BA, Bishop AT, Shin AY. Treatment of a segmental nerve defect in the rat with use of bioabsorbable synthetic nerve conduits: a comparison of commercially available conduits. J Bone Joint Surg Am. 2009;91(9):2194–204. doi: 10.2106/JBJS.H.01301. [DOI] [PubMed] [Google Scholar]
- 6.Evans GR. Challenges to nerve regeneration. Semin Surg Oncol. 2000;19(3):312–8. doi: 10.1002/1098-2388(200010/11)19:3<312::aid-ssu13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Evans GR, Brandt K, Widmer MS, Lu L, Meszlenyi RK, Gupta PK, et al. In vivo evaluation of poly(L-lactic acid) porous conduits for peripheral nerve regeneration. Biomaterials. 1999;20(12):1109–15. doi: 10.1016/s0142-9612(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 8.Kim YT, Haftel VK, Kumar S, Bellamkonda RV. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29(21):3117–27. doi: 10.1016/j.biomaterials.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams LR, Longo FM, Powell HC, Lundborg G, Varon S. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for a bioassay. J Comp Neurol. 1983;218(4):460–70. doi: 10.1002/cne.902180409. [DOI] [PubMed] [Google Scholar]
- 10.Williams LR. Exogenous fibrin matrix precursors stimulate the temporal progress of nerve regeneration within a silicone chamber. Neurochem Res. 1987;12(10):851–60. doi: 10.1007/BF00966306. [DOI] [PubMed] [Google Scholar]
- 11.Ceballos D, Navarro X, Dubey N, Wendelschafer-Crabb G, Kennedy WR, Tranquillo RT. Magnetically aligned collagen gel filling a collagen nerve guide improves peripheral nerve regeneration. Exp Neurol. 1999;158(2):290–300. doi: 10.1006/exnr.1999.7111. [DOI] [PubMed] [Google Scholar]
- 12.Dodla MC, Bellamkonda RV. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials. 2008;29(1):33–46. doi: 10.1016/j.biomaterials.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillon GP, Yu X, Sridharan A, Ranieri JP, Bellamkonda RV. The influence of physical structure and charge on neurite extension in a 3D hydrogel scaffold. J Biomater Sci Polym Ed. 1998;9(10):1049–69. doi: 10.1163/156856298x00325. [DOI] [PubMed] [Google Scholar]
- 14.Ngo TT, Waggoner PJ, Romero AA, Nelson KD, Eberhart RC, Smith GM. Poly(L-Lactide) microfilaments enhance peripheral nerve regeneration across extended nerve lesions. J Neurosci Res. 2003;72(2):227–38. doi: 10.1002/jnr.10570. [DOI] [PubMed] [Google Scholar]
- 15.Koh HS, Yong T, Teo WE, Chan CK, Puhaindran ME, Tan TC, et al. In vivo study of novel nanofibrous intra-luminal guidance channels to promote nerve regeneration. J Neural Eng. 2010;7(4):046003. doi: 10.1088/1741-2560/7/4/046003. [DOI] [PubMed] [Google Scholar]
- 16.Nisbet DR, Forsythe JS, Shen W, Finkelstein DI, Horne MK. Review paper: a review of the cellular response on electrospun nanofibers for tissue engineering. J Biomater Appl. 2009;24(1):7–29. doi: 10.1177/0885328208099086. [DOI] [PubMed] [Google Scholar]
- 17.Venugopal J, Prabhakaran MP, Low S, Choon AT, Zhang YZ, Deepika G, et al. Nanotechnology for nanomedicine and delivery of drugs. Curr Pharm Des. 2008;14(22):2184–200. doi: 10.2174/138161208785740180. [DOI] [PubMed] [Google Scholar]
- 18.Clements IP, Kim YT, English AW, Lu X, Chung A, Bellamkonda RV. Thin-film enhanced nerve guidance channels for peripheral nerve repair. Biomaterials. 2009;30(23-24):3834–46. doi: 10.1016/j.biomaterials.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman-Kim D, Mitchel JA, Bellamkonda RV. Topography, cell response, and nerve regeneration. Annu Rev Biomed Eng. 2010;12:203–31. doi: 10.1146/annurev-bioeng-070909-105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Leong KW. Nanoscale surfacing for regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(5):478–95. doi: 10.1002/wnan.74. [DOI] [PubMed] [Google Scholar]
- 21.Badami AS, Kreke MR, Thompson MS, Riffle JS, Goldstein AS. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27(4):596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 22.Chew SY, Mi R, Hoke A, Leong KW. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials. 2008;29(6):653–61. doi: 10.1016/j.biomaterials.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih YR, Chen CN, Tsai SW, Wang YJ, Lee OK. Growth of mesenchymal stem cells on electrospun type I collagen nanofibers. Stem Cells. 2006;24(11):2391–7. doi: 10.1634/stemcells.2006-0253. [DOI] [PubMed] [Google Scholar]
- 24.Smeal RM, Rabbitt R, Biran R, Tresco PA. Substrate curvature influences the direction of nerve outgrowth. Ann Biomed Eng. 2005;33(3):376–82. doi: 10.1007/s10439-005-1740-z. [DOI] [PubMed] [Google Scholar]
- 25.Manwaring ME, Walsh JF, Tresco PA. Contact guidance induced organization of extracellular matrix. Biomaterials. 2004;25(17):3631–8. doi: 10.1016/j.biomaterials.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 26.Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165(1):105–18. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- 27.Mukhatyar V, Karumbaiah L, Yeh J, Bellamkonda R. Tissue Engineering Strategies Designed to Realize the Endogenous Regenerative Potential of Peripheral Nerves. Adv Mater. 2009;21(46):4670–9. [Google Scholar]
- 28.Xu XM, Guenard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351(1):145–60. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- 29.Cornbrooks CJ, Carey DJ, McDonald JA, Timpl R, Bunge RP. In vivo and in vitro observations on laminin production by Schwann cells. Proc Natl Acad Sci U S A. 1983;80(12):3850–4. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGarvey ML, Baron-Van Evercooren A, Kleinman HK, Dubois-Dalcq M. Synthesis and effects of basement membrane components in cultured rat Schwann cells. Dev Biol. 1984;105(1):18–28. doi: 10.1016/0012-1606(84)90257-4. [DOI] [PubMed] [Google Scholar]
- 31.Gupta D, Venugopal J, Prabhakaran MP, Dev VR, Low S, Choon AT, et al. Aligned and random nanofibrous substrate for the in vitro culture of Schwann cells for neural tissue engineering. Acta Biomater. 2009;5(7):2560–9. doi: 10.1016/j.actbio.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Itoh S, Konno K, Kikkawa T, Ichinose S, Sakai K, et al. Effects of Schwann cell alignment along the oriented electrospun chitosan nanofibers on nerve regeneration. J Biomed Mater Res A. 2009;91(4):994–1005. doi: 10.1002/jbm.a.32329. [DOI] [PubMed] [Google Scholar]
- 33.Torigoe K, Hashimoto K, Lundborg G. A role of migratory Schwann cells in a conditioning effect of peripheral nerve regeneration. Exp Neurol. 1999;160(1):99–108. doi: 10.1006/exnr.1999.7202. [DOI] [PubMed] [Google Scholar]
- 34.Torigoe K. The role of migratory Schwann cells in nerve regeneration as studied by the film model. J Peripher Nerv Syst. 1997;2(3):227–31. [PubMed] [Google Scholar]
- 35.Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem Int Ed Engl. 2009;48(30):5406–15. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baron-Van Evercooren A, Kleinman HK, Seppa HE, Rentier B, Dubois-Dalcq M. Fibronectin promotes rat Schwann cell growth and motility. J Cell Biol. 1982;93(1):211–6. doi: 10.1083/jcb.93.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey SB, Eichler ME, Villadiego A, Rich KM. The influence of fibronectin and laminin during Schwann cell migration and peripheral nerve regeneration through silicon chambers. J Neurocytol. 1993;22(3):176–84. doi: 10.1007/BF01246356. [DOI] [PubMed] [Google Scholar]
- 38.Brown RA, Phillips JB. Cell responses to biomimetic protein scaffolds used in tissue repair and engineering. Int Rev Cytol. 2007;262:75–150. doi: 10.1016/S0074-7696(07)62002-6. [DOI] [PubMed] [Google Scholar]
- 39.Sell S, Barnes C, Smith M, McClure M, Madurantakam P, Grant J, et al. Extracellular matrix regenerated: tissue engineering via electrospun biomimetic nanofibers. Polym Int. 2007;56(11):1349–60. [Google Scholar]
- 40.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res A. 2003;67A(2):531–7. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 41.Tan JL, Liu W, Nelson CM, Raghavan S, Chen CS. Simple approach to micropattern cells on common culture substrates by tuning substrate wettability. Tissue Eng. 2004;10(5-6):865–72. doi: 10.1089/1076327041348365. [DOI] [PubMed] [Google Scholar]
- 42.Previtali SC, Malaguti MC, Riva N, Scarlato M, Dacci P, Dina G, et al. The extracellular matrix affects axonal regeneration in peripheral neuropathies. Neurology. 2008;71(5):322–31. doi: 10.1212/01.wnl.0000319736.43628.04. [DOI] [PubMed] [Google Scholar]
- 43.Milner R, Wilby M, Nishimura S, Boylen K, Edwards G, Fawcett J, et al. Division of labor of Schwann cell integrins during migration on peripheral nerve extracellular matrix ligands. Dev Biol. 1997;185(2):215–28. doi: 10.1006/dbio.1997.8547. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed Z, Brown RA. Adhesion, alignment, and migration of cultured Schwann cells on ultrathin fibronectin fibres. Cell Motil Cytoskeleton. 1999;42(4):331–43. doi: 10.1002/(SICI)1097-0169(1999)42:4<331::AID-CM6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed Z, Underwood S, Brown RA. Nerve guide material made from fibronectin: Assessment of in vitro properties. Tissue Eng. 2003;9(2):219–31. doi: 10.1089/107632703764664693. [DOI] [PubMed] [Google Scholar]
- 46.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238(4826):491–7. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez-Garcia C, Sousa SR, Moratal D, Rico P, Salmeron-Sanchez M. Effect of nanoscale topography on fibronectin adsorption, focal adhesion size and matrix organisation. Colloids Surf B Biointerfaces. 2010;77(2):181–90. doi: 10.1016/j.colsurfb.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 48.Rico P, Rodriguez Hernandez JC, Moratal D, Altankov G, Monleon Pradas M, Salmeron-Sanchez M. Substrate-induced assembly of fibronectin into networks: influence of surface chemistry and effect on osteoblast adhesion. Tissue Eng Part A. 2009;15(11):3271–81. doi: 10.1089/ten.TEA.2009.0141. [DOI] [PubMed] [Google Scholar]
- 49.Diener A, Nebe B, Luthen F, Becker P, Beck U, Neumann HG, et al. Control of focal adhesion dynamics by material surface characteristics. Biomaterials. 2005;26(4):383–92. doi: 10.1016/j.biomaterials.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 50.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004;25(28):5947–54. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 51.Gugutkov D, Gonzalez-Garcia C, Rodriguez Hernandez JC, Altankov G, Salmeron-Sanchez M. Biological activity of the substrate-induced fibronectin network: insight into the third dimension through electrospun fibers. Langmuir. 2009;25(18):10893–900. doi: 10.1021/la9012203. [DOI] [PubMed] [Google Scholar]


