Abstract
Understanding the basis of normal heart remodeling can provide insight into the plasticity of the cardiac state, and into the potential for treating diseased tissue. In Drosophila, the adult heart arises during metamorphosis from a series of events, that include the remodeling of an existing cardiac tube, the elaboration of new inflow tracts, and the addition of a layer of longitudinal muscle fibers. We have identified genes active in all these three processes, and studied their expression in order to characterize in greater detail normal cardiac remodeling. Using a Transglutaminase-lacZ transgenic line, that is expressed in the inflow tracts of the larval and adult heart, we confirm the existence of five inflow tracts in the adult structure. In addition, expression of the Actin87E actin gene is initiated in the remodeling cardiac tube, but not in the longitudinal fibers, and we have identified an Act87E promoter fragment that recapitulates this switch in expression. We also establish that the longitudinal fibers are multinucleated, characterizing these cells as specialized skeletal muscles. Furthermore, we have defined the origin of the longitudinal fibers, as a subset of lymph gland cells associated with the larval dorsal vessel. These studies underline the myriad contributors to the formation of the adult Drosophila heart, and provide new molecular insights into the development of this complex organ.
Keywords: Drosophila, heart, dorsal vessel, inflow tract, metamorphosis, lymph gland
1. Introduction
Understanding the molecular basis of heart development in animals is critical to our appreciation of congenital cardiac diseases, and to understanding how cardiac tissue might be remodeled in response to injury or disease. These studies are hampered by the complexity of the mammalian cardiac organ, which comprises tissues arising from a number of distinct cellular origins. Nevertheless, significant insights into cardiac disease mechanisms have been uncovered over the last several years, and a striking finding is that many of the identified disease genes are those for which a requirement in cardiac specification and differentiation had previously been reported (see Srivastava, 2006; Garg, 2006 for reviews).
It is now broadly established and accepted that the molecular mechanisms of cardiac specification and differentiation in animals are based upon evolutionarily-conserved transcriptional and signaling networks (Cripps and Olson, 2002; Brand, 2003). Importantly, since a number of human disease genes were first identified in the Drosophila system as regulators of embryonic heart specification (Medioni et al., 2009), model organisms, including Drosophila, have provided significant material for the identification of cardiogenic genes in vertebrates.
The Drosophila cardiac tissue arises in the embryo from bilateral rows of dorsal mesodermal cells, which migrate towards each other during the morphogenetic process known as dorsal closure. The result of this event is the generation of the dorsal vessel, a linear cardiac tube, located at the dorsal midline of the animal. This vessel comprises an inner double row of contractile cardiomyocytes, surrounded by a number of pericardial cells, and by a small number of paired lymph glands near the anterior of the vessel (Bodmer and Frasch, 1999).
While the dorsal vessel is required for circulation of hemolymph in Drosophila, it is essentially the sole blood vessel in the animal, since flies have an open circulatory system. During late embryogenesis and throughout larval development, hemolymph enters the cardiac tube in the posterior part of the vessel, a region termed the heart, located in abdominal segments A5-A7. Hemolymph enters through three pairs of specialized valves, termed ostia, which open during diastole and are forced closed during systole. As the heart contracts, hemolymh passes anteriorly through a region of the dorsal vessel termed the aorta, and is dispersed into the body cavity near the brain. Hemolymph then percolates back through the body, to re-enter the dorsal vessel through the ostia (Rizki, 1978). The pericardial cells have long been thought to have a role in detoxification (Rizki, 1978), and the lymph glands are known to be hematopoietic organs for larval and adult life (see for example Jung et al., 2005).
This detailed functional understanding of the Drosophila cardiac tube with its associated tissues has recently been examined at the molecular and genetic levels. The predominant myocardial cells express the homeobox gene tinman (tin), which is required for specification of the cardiac field (Bodmer, 1993; Azpiazu and Frasch, 1993), whereas the contractile ostia arise from a subset of myocardial cells that express the orphan nuclear receptor gene seven-up (svp) (Molina and Cripps, 2001; Ponzielli et al., 2002).
It has also been established that anteroposterior (AP) patterning in the cardiac tube is essential to its normal function in the embryo and larva (reviewed in Lo and Frasch, 2003). The posterior heart is larger and more muscular than the more anterior aorta (Figure 1A, B). In addition, the ostia arise from Svp cells located in the posterior heart, while Svp cells located in the aorta do not, during the larval stage, develop into inflow tracts (Molina and Cripps, 2001). Moreover, lymph glands arise only in the anterior segments, apparently at the expense of pericardial cells in that anterior location (Rodriguez et al., 1996). AP patterning decisions in the muscular cardiac tube are controlled by members of the Antennapedia and Bithorax Complexes of homeotic selector genes, where abdominal-A function is required to specify the posterior heart, and the portion of the aorta that contains Svp cells is specified by the actions of Ultrabithorax and Antennapedia genes (Lovato et al., 2002; Ryan et al., 2005; Ponzielli et al., 2002; Lo et al., 2002; Perrin et al 2004). Similarly, the choice between pericardial cell fate and lymph gland fate is regulated in part by the activity of Ultrabithorax (Rodriguez et al., 1996). Clearly, the larval cardiac tissue represents a complex organ, comprising a number of distinct cell types, with diverse and unique functions.
Figure 1. Structure and metamorphosis of the Drosophila dorsal vessel.
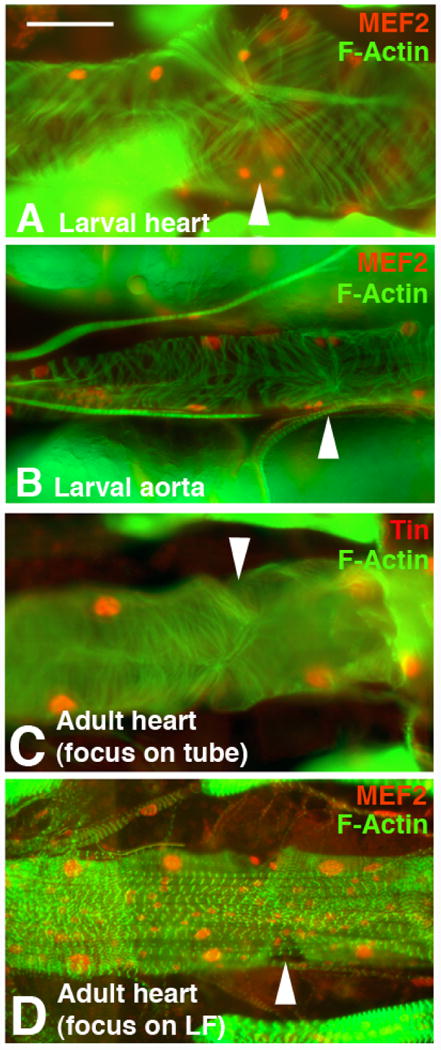
The larval dorsal vessel comprises a posterior heart (A), and a more anterior aorta (B). The heart is larger and has a more extensively elaborated myofibrillar structure, as visualized with phalloidin (green). In the heart, Svp cells (smaller paired nuclei) mark the locations of each ostium (arrowhead). In the aorta, the Svp cells do not form perforations nor invaginations (arrowhead in B). MEF2 (red) accumulates in all cardial cell nuclei. C: In the adult heart, the cardiac tube is extensively elaborated compared to the larva, and ostia are present (arrowhead). D: The ventral surface of the adult heart develops a layer of longitudinally-oriented fibers, contributed by new cells laid down during metamorphosis. These new cells, as well as the cardiac tube cells, accumulate MEF2 (red in D). Images modified from Molina and Cripps (2001). Bar, 25μm.
Since the Drosophila larva undergoes metamorphosis to the adult body plan, and since the adult heart is predominantly located in abdominal segments A1-A5 (Miller, 1950), we and others have investigated the cellular and molecular changes that occur in the dorsal vessel during this time (Molina and Cripps, 2001; Monier et al., 2005; Zeitouni et al., 2007). The larval heart proper is largely histolyzed during pupal development, and the adult heart arises from the larval aorta, which shows a significant increase in size and myofibril density during pupal remodeling (compare Figure 1B to Figure 1C). Most of the larval inflow tracts are lost during this process, as they were located in the larval heart, and new ostia develop from Svp cells located in the larval aorta, such that there are four sets of ostia located in the adult abdomen. In addition, longitudinal muscle fibers are added to the ventral surface of the adult heart (Figure 1D). The metamorphosis of the cardiac tube is accomplished by a broad reprogramming of gene expression in the dorsal vessel (Zeitouni et al., 2007), a significant portion of which arises from ecdysone-induced changes in the expression of homeotic selector genes (Monier et al., 2005).
Although these studies have uncovered significant new insight into cardiac developmental processes, there remains much to learn concerning the cellular events that occur during development of the adult heart. These details are particularly important to understand given the recent application of the adult Drosophila heart as a model for human cardiac disease. It is now clear that the Drosophila heart undergoes cardiac aging, with many of the same characteristics of aging mammalian hearts (Ocorr et al., 2007). In addition, the adult heart can be used to screen for new genes whose products are essential to normal cardiac function (Neely et al., 2010). Wasserthal (2007) also recently reported that a fifth pair of ostia perforate the cardiac tube, these ostia being located in the thorax, close to its junction with the abdomen. However, relatively little is known of the origin of these two anterior ostia. In addition, the nature and origin of the longitudinal fibers has not been assessed, despite these fibers representing a novel mechanism of cardiac remodeling in this system.
In this paper, we report on a series of investigations into the development and pattering of the adult heart. Firstly, we confirm the findings of Wasserthal (2007), demonstrating the existence of five sets of ostia in the adult heart, and we demonstrate that all these sets of ostia originate from Svp cardial cells. Secondly, we assess actin gene expression in the adult cardiac tube, and demonstrate the differential expression of two actin gene family members in the heart tube. One of these genes, Actin87E, is a marker of remodeling in the tube, but is not expressed in the longitudinal fibers. Thirdly, we assess the identity and origin of the longitudinal fibers, demonstrating that they arise from a subset of lymph gland cells, that become skeletal muscle precursors, and populate the ventral surface of the adult heart. These important observations add new complexity to the structure of the Drosophila adult heart, and define a new and potentially novel process of cardiac development through the transdifferentiation of non-muscular cells into myocytes.
2. Results
2.1 The adult heart comprises five sets of inflow tracts arising from Svp cells
We previously reported that the Svp cells in the aorta of the larva are remodeled into inflow tracts for use in the adult, and that larval inflow tracts in the heart region are histolyzed (Molina and Cripps, 2001). These findings were extended by Monier et al (2005), who demonstrated that the most anterior pair of larval ostia, located in the A5 abdominal segment, are retained in the adult structure. More recently, Wasserthal (2007) demonstrated that the posterior of the adult thorax contains a further pair of ostia. These findings suggested that there are five pairs of inflow tracts in the adult, spanning from the posterior of the thorax to the persistent larval ostia in segment A5.
To confirm these findings, and to determine if the thoracic ostia have the same Svp cell origin as other ostia, we studied heart structure in adult animals carrying a Transglutaminase (Tg)-lacZ transgene. Tg is expressed in all Svp cells of the embryonic dorsal vessel (Iklé et al., 2008), and we found that this pattern of expression persisted until the adult stage in Tg-lacZ animals. In fillets of young transgenic adults stained for ß-galactosidase (ß-gal) activity, we observed five sets of cardial cells stained, spanning from the posterior of the thorax, to abdominal segment A5 (Figure 2A). Note that in this preparation, the pericardial cells also stained for ß-gal activity. This pericardial cell stain is not a reflection of transgene activity, but instead represents endogenous enzyme function in those cells.
Figure 2. Expression of a Tg-lacZ reporter in the adult identifies five sets of ostia.
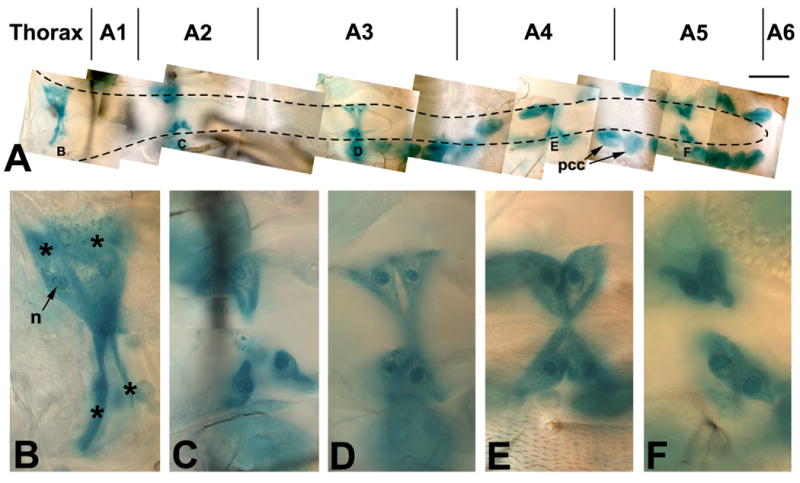
A: Photomontage of the adult dorsal vessel from the thorax (left) to A6 (right). Tg-lacZ expression is revealed by X-gal staining (blue-green), and identifies the five sets of Svp cells that form ostia (B-F). Note that the pericardial cells (pcc) have endogenous ß-gal activity. B-F: Photomontages of each of the sets of Svp cells identified in panel A. Each Svp cell set comprises four cells, denoted by asterisks in B. In each cell, the location of the nucleus can be determined based upon enriched accumulation of ß-gal activity. Bar, 25μm for A; 5μm for B-F.
Closer observation of the Tg-lacZ positive cardial cells (Figure 2, B-F) revealed that each set comprised two pairs of elongated cells with one nucleus per cell. In many instances, the cells showed invaginations or perforations into the cardiac tube, identifying them as ostia.
These findings confirmed that there are five pairs of ostia in the adult, and further demonstrated that all these ostia arise from cells of the Svp lineage.
2.2 Metamorphosis of the cardiac tube: analysis of actin gene expression
In order to define changes in the patterns of gene expression in the heart during metamorphosis, we analyzed expression of members of the actin gene family in the cardiac tube. There is temporal and spatial regulation of actin gene expression in skeletal muscles in Drosophila and other animals (reviewed in Bernstein et al., 1993). Since the cardiac tube undergoes metamorphosis, and since the adult heart comprises distinct muscle cells forming the heart versus the longitudinal fibers, we reasoned that there might be unique patterns of actin expression in this organ. Previous studies (Fyrberg et al., 1983; Tobin et al., 1990; Baker et al., 2005) indicated that the only myofibrillar actin genes expressed in the adult abdomen are Act57B and Act87E. We therefore analyzed the expression of each of these genes in the dorsal vessel, by in situ hybridization of antisense probes to transverse sections of third instar larvae, and to transverse sections of adult abdomens. To ensure that we were observing signals specific to each actin gene, the probes were generated from unique 3′UTR sequences (Fyrberg et al., 1983; Kelly et al., 2002). We also carried out hybridizations using control sense probes, for which representative stains are presented.
While the signal detected for each probe was relatively low, resulting from the thin layer of muscle tissue that comprises the dorsal vessel, we observed two interesting findings. Firstly, Act57B, the major embryonic and larval myofibrillar actin gene, was expressed in the cardiac tube at both the larval stage (Figure 3A) and at the adult stage (Figure 3B). In addition, Act57B was expressed in the larval skeletal body wall muscles, and in the body wall muscles of the adult. This signal in the heart and skeletal muscles was specific, since no muscle-specific signal was observed when an Act57B sense probe was hybridized to sections of larvae (data not shown) and adults (Figure 3C). This result was further confirmed by the activity of an Act57B-lacZ transgene (Kelly et al., 2002). In transverse cryosections of these transgenic animals, there was strong ß-gal activity in the adult heart and in abdominal body wall muscles (Figure 3D).
Figure 3. Expression of actin genes in the dorsal vessel during development.
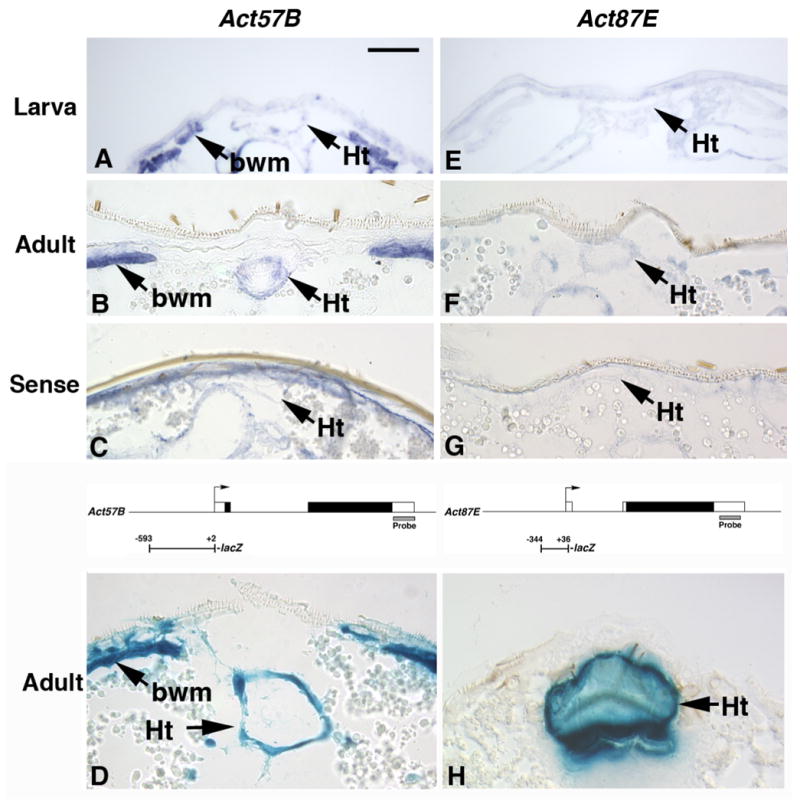
A, B: Act57B transcripts are present in the heart (Ht) and skeletal body wall muscles (bwm) at both larval (A) and adult (B) stages. C: An Act57B sense probe shows some non-specific reaction with the adult cuticle, but does not hybridize to transcripts in the dorsal vessel (arrow). D: An Act57B promoter-lacZ fusion is active in the adult dorsal vessel and skeletal muscles in transgenic animals. E, F: Act87E transcripts are not detected in the larval dorsal vessel, but are present in the adult dorsal vessel. G: An Act87E sense probe does not hybridize to transcripts in the adult dorsal vessel. H: An Act87E promoter-lacZ fusion is active in the adult dorsal vessel in transgenic animals. A-C, E-G, paraffin sections subjected to in situ hybridization; D, H, cryosections stained with X-gal. Diagrams above panels D and H show gene structures for Act57B and Act87E, respectively. Diagrams indicate the promoter fragments used for transgenic studies, plus the regions used as probes for in situ hybridization. Boxes represent exons, and open boxes represent un-translated parts of exons. Bar, 25μm.
Secondly, we found that Act87E expression was temporally regulated in the dorsal vessel. No specific signal was observed in larval sections (Figure 3E), but a signal was observed reproducibly in adult cardiac tissue (Figure 3F). Again, a sense probe for Act87E did not show cardiac-specific hybridization. We did not detect accumulation of Act87E transcripts above background levels in the larval or adult skeletal muscles, indicating either that Act87E is not expressed in these tissues, or that its transcript abundance is below the level of detection for our studies.
We also sought to confirm the Act87E expression pattern, via analysis of animals carrying an Act87E-lacZ fusion. This was generated by fusing a 381-bp promoter fragment of the Act87E gene to a minimal promoter-lacZ reporter, and creating transgenic animals carrying this construct. In these animals, we observed that there was significant Act87E-lacZ expression in the adult heart (Figure 3H).
Together, our results showed that the larval cardiac tube expresses a single myofibrillar actin isoform, whereas the adult cardiac tissue expresses two different actin genes. Our results are based upon combination of a direct assay (in situ hybridization) with an indirect assay (analysis of promoter-lacZ fusions in transgenic animals). Together, these approaches provide robust and mutually-supportive evidence for changes in gene expression in the heart during metamorphosis.
To determine if the cardiac actin genes are co-expressed in the adult heart, or are expressed in different subsets of the heart musculature (such as the cardiac tube versus the ventral longitudinal fibers), we analyzed actin-lacZ expression in fillets of adult abdomens using immunofluorescence. We counter-stained the preparations with a fluorescent conjugate of phalloidin, in order to visualize the different subsets of cardiac cells. The samples were analyzed by confocal microscopy, in order to distinguish expression in the more ventral longitudinal fibers, from expression of the lacZ transgenes in the persistent cardiac tube (Figure 4).
Figure 4. Act57B and Act87E are expressed in overlapping, but not identical, patterns in the dorsal vessel.
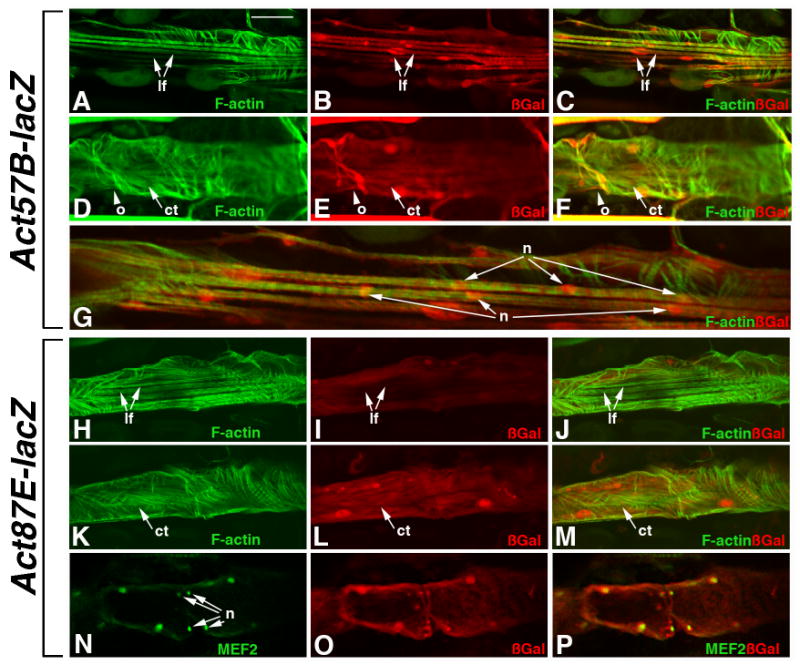
Adult transgenic animals were filleted and stained for the accumulation of F-actin (A-M) or MEF2 (N-P) in Green, to visualize the heart (Ht). Samples were counterstained for accumulation of ßGal in Red (all panels). A-G: Act57B-lacZ. ß-Gal was observed in the ventral longitudinal fibers (lf, A-C), as well as in the cardiac tube (ct, D-F). ß-Gal also accumulated in the cells forming the ostia (o, arrowhead). At higher magnification, single myofibers were observed associated with multiple nuclei (n, panel G). H-P: Act87E-lacZ. ß-Gal accumulation was not observed in the longitudinal fibers (H-J), but was readily observed in the cardiac tube (K-M). Counterstaining for MEF2 also confirmed that Act87E-lacZ was expressed in the cells of the ostia, given the enrichment of ß-Gal in ostial cell nuclei (n; N-P). Bar 30μm for A-F and H-P; 15μm for G.
For Act57B-lacZ, we observed robust expression in the longitudinal fibers, and we also noted that ß-Gal accumulation in these fibers became enriched in the nuclei (Figure 4 A-C). When the focus was upon the cardiac tube, there was also accumulation of ß-Gal, expressed from the Act57B-lacZ transgene (Figure 4 D-F). In addition, we noted that the enrichment of ß-Gal in the nuclei of the cardiac tube included the smaller nuclei corresponding to the adult ostia (arrowhead in Figure 4 D-F; Molina and Cripps, 2001). Thus, Act57B-lacZ is expressed in all of the major cell types of the adult Drosophila heart.
We have previously suggested that the adult cardiac longitudinal fibers were syncytial, since there appeared to be far more nuclei populating the longitudinal fibers, than there were separable fibers. Since there was enrichment of ß-Gal in the nuclei of cells expressing the lacZ transgenes, we studied Act57B-lacZ expression in the longitudinal fibers more closely, to determine if we could gain insight into this possibility. Indeed, we were readily able to observe instances where single muscle fibers, as identified by phalloidin staining, were closely associated with multiple ß-Gal-positive nuclei (Figure 4 G). This finding strongly supported the hypothesis that the longitudinal cardiac fibers are multinucleate, and provided us an opportunity to investigate the origins of these fibers more closely (see Section 2.3).
For the expression of Act87E-lacZ, a striking and interesting difference was observed when compared to Act57B-lacZ. At a focal plane that included the longitudinal fibers, we did not observe ß-Gal accumulation above background levels of staining, indicating that the transgene was not expressed in these imaginal fibers (Figure 4 H-J). Nevertheless, robust Act87E-lacZ expression was observed in the cardiac tube (Figure 4 K-M). These findings were consistent with our observation in Figure 3, which showed that expression of Act87E was located throughout the circumference of the cardiac tube.
To determine if Act87E-lacZ is active in ostia cells of the cardiac tube in addition to myocardial cells, we assessed nuclear ß-Gal enrichment in the heart tube alongside MEF2, a transcription factor expressed in the dorsal vessel (Lilly et al., 2005). We found that ß-Gal accumulated in the folds of the cardiac wall corresponding to the ostia, and that there was significant enrichment of ß-Gal in the ostia nuclei, which are readily identified since they are smaller than the myocardial nuclei (Figure 4 N-P; Molina and Cripps, 2001).
Thus, Act87E-lacZ is expressed in all the cells of the cardiac tube, but it is not expressed in the cells forming the longitudinal muscle fibers. Together, the expression patterns of our reporters for Act57B and Act87E define regulatory modules that clearly distinguish between the persistent larval cells, versus the imaginal components of the adult heart.
In order to define the timecourse of Act87E-lacZ expression during cardiac remodeling, we studied ß-gal accumulation at different time points during pupal development (Figure 5). There was no expression of Act87E-lacZ in the larval dorsal vessel, although there were traces of ß-Gal adjacent to the dorsal vessel, associated with alary muscles (Figure 5A). The first traces of expression were observed around 24 hours after puparium formation (hr APF) in some cells of the cardiac tube (Figure 5B). Act87E-lacZ expression was much stronger at 48 hr APF and this stronger expression coincided with the elaboration of the cardiac myofibrillar structure, where the network of spirally-arranged myofibrils was becoming more dense (Figure 5C). Act87E-lacZ expression was also strong at 72 hr APF (Figure 5D). Thus, the activation of Act87E-lacZ expression coincided with the commencement of myofibrillar remodeling that is characteristic of the metamorphosing cardiac tube.
Figure 5. Temporal expression of Act87E-lacZ in the dorsal vessel.
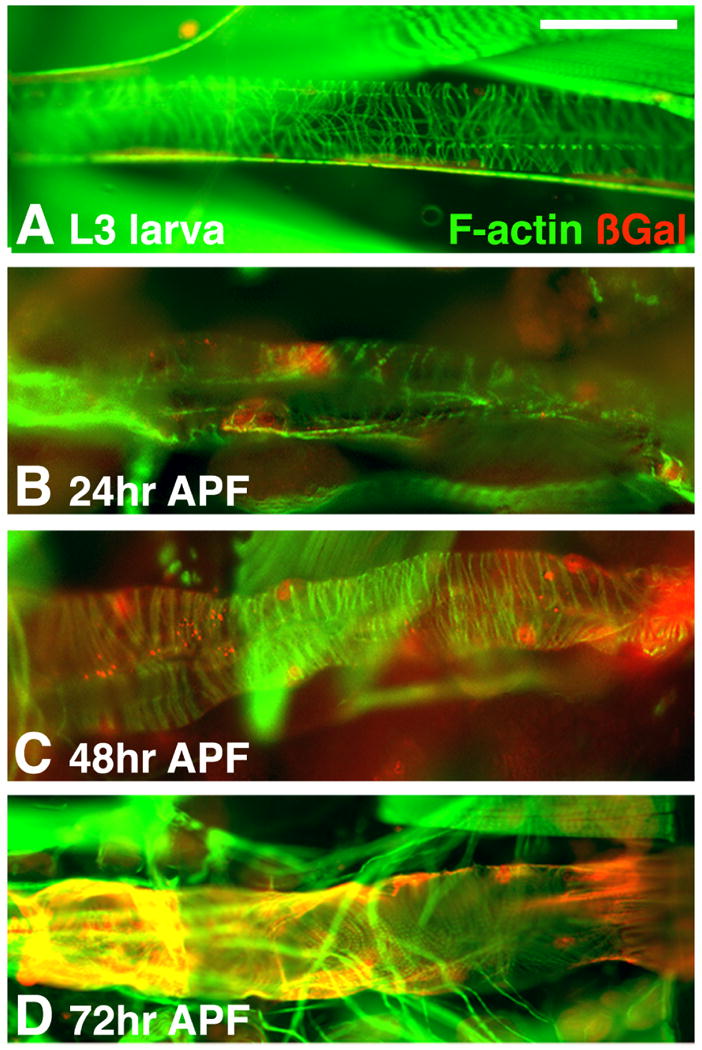
Act87E-lacZ transgenic animals, at the indicated ages, were stained for the presence of F-actin, to visualize the cardiac tube; and for accumulation of ß-gal, in order to define the onset of reporter gene expression. Act87E-lacZ was not expressed at the larval stage (A), nor at 24 hr APF (B). Earliest transgene expression was detected by 48 hr APF (C), and continued at a high level through 72 hr APF (D) and into adulthood (see Figures 3 and 4 for adult stains). Bar, 30μm.
2.3 Nature and origin of the longitudinal cardiac muscles
In order to gain insight into the origin of the longitudinal fibers, we sought to identify markers that are expressed in these cells. Our studies above had demonstrated that the longitudinal fibers are characterized by several small imaginal nuclei per fiber, suggesting that the fibers are syncytial, a characteristic of both skeletal muscles and a subset of visceral muscles. To assess this characteristic further, we studied in adults the expression of the duf-n-lacZ enhancer trap, that labels founder cell nuclei of fusing skeletal muscles (Ruiz-Gomez et al., 2000). Following fusion, duf-n-lacZ expression can be observed in all nuclei of the fused cell.
In duf-n-lacZ adults, we visualized ß-gal accumulation, and counterstained for markers that label either muscle nuclei (anti-MEF2) or muscle fibers (phalloidin). In those preparations that were counterstained for MEF2 accumulation (Figure 6 A,B), four major cardiac nucleus types were observed. First, a set of nuclei were ß-Gal positive and MEF2-negative. Based upon their size and location, these were pericardial cell nuclei (pcc in Figure 6B). Second, a set of cells whose nuclei were MEF2-positive and ß-Gal negative. Based upon their location and size, these were the nuclei of Tin and Svp cells, that form the cardiac tube (labeled ct in Figure 6B). The third and fourth sets of nuclei were positive for both MEF2 and ß-Gal. They were observed over the ventral surface of the cardiac tube, in the location of the longitudinal fibers. These nuclei varied only in their relative size, with a minority of the nuclei slightly larger than the rest of the nuclei (labeled ip and sip, respectively in Figure 6B). In Drosophila skeletal muscle fibers, the larger nuclei usually represent those that originated from the founder cells (Bate, 1990).
Figure 6. Expression of duf-n-lacZ in the adult heart.
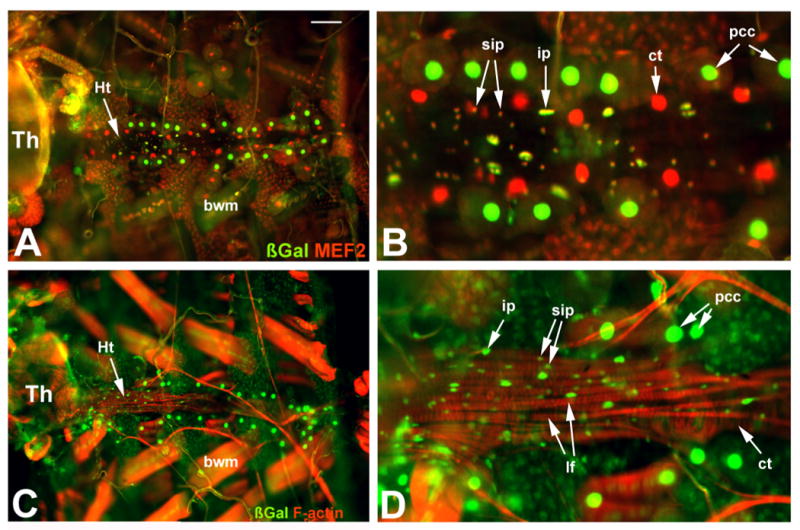
rp298 (duf-n-lacZ) transgenic adults were dissected and stained for accumulation of ß-Gal and MEF2 (A, B), or for ß-Gal and F-actin (C,D). Panels B and D are higher magnification views of panels A and C, respectively. The heart (Ht) runs from left to right (anterior to posterior) across the middle of the samples. A, B: ß-Gal (green) and MEF2 (red) display complex patterns of accumulation: the nuclei of pericardial cells (pcc) accumulate ß-Gal but not MEF2; nuclei of cells forming the cardiac tube (ct) accumulate MEF2 but not ß-Gal; the nuclei of cells forming the longitudinal fibers accumulate both ß-Gal and MEF2, and can be characterized as arising from either large (ip) or small (sip) imaginal precursor nuclei. C, D: For the longitudinal fibers (lf), their nuclei are ß-Gal positive, including both the larger and the smaller nuclei (ip and sip, respectively). Th, thorax; bwm, body wall muscle; ct, cardiac tube. Bar, 25μm for A and C; 10μm for B and D.
To localize more effectively the nuclei that were both MEF2-positive and ß-Gal-positive, we analyzed duf-n-lacZ expression in preparations that were counterstained with fluorescent phalloidin (Figure 6 C,D). Using this approach, we confirmed that the larger and smaller nuclei were nuclei of the longitudinal fibers. This finding suggested that the longitudinal fibers were of skeletal muscle origin, given their multinucleate nature. While visceral muscles in Drosophila also fuse to form syncytia, the visceral fibers are generally binucleate, whereas skeletal muscle fibers have greater numbers of nuclei per cell, much like we observed for the cardiac longitudinal fibers. More importantly, the identification of duf-n-lacZ as a marker for these cells afforded us the opportunity to identify their origin and development.
We next studied duf-n-lacZ expression in cells associated with the dorsal vessel, from the larval stage through to adulthood. These preparations were counter-stained for MEF2 accumulation, to visualize the cardiac cells. Through analysing a large number of time points, and multiple samples for each time point, we ultimately focused upon a group of cells located just posterior to the major lymph glands. This group of cells comprised two clusters, one on either side of the dorsal vessel. At the late larval stage, each cluster contained about 15 cells. In general appearance and location, these cells corresponded to the secondary lymph glands. However, there were generally 3-4 pairs of secondary lymph glands, while there was only one paired cluster of cells corresponding to these duf-n-lacZ positive cells. Thus, if these cells were the precursors to the imaginal cardiac fibers, they represented a unique and previously un-described set of cells associated with the larval dorsal vessel.
At the larval stage, these cardiac imaginal precursors did not accumulate MEF2, but were clearly distinct from: other lymph gland cells, from the cardiac tube nuclei, and from the pericardial cells, based upon patterns of antibody staining (Figure 7A). Once the animal entered the pupal stage, two major alterations occurred. Firstly, the duf-n-lacZ lymph gland cells began to disperse and increase in number (Figure 7B). Secondly, these cells began to express Mef2, commencing about 36 hrs APF (Figure 7C). Subsequently, the imaginal precursors spread across the ventral surface of the heart to form the longitudinal fibers (Figure 7D, E). Thus, through visualizing these cells during the pupal stage, we were able to confirm that the duf-n-lacZ-expressing lymph gland cells observed at the larval stage indeed gave rise to the adult longitudinal fibers.
Figure 7. Expression of duf-n-lacZ in the larval and pupal dorsal vessel.
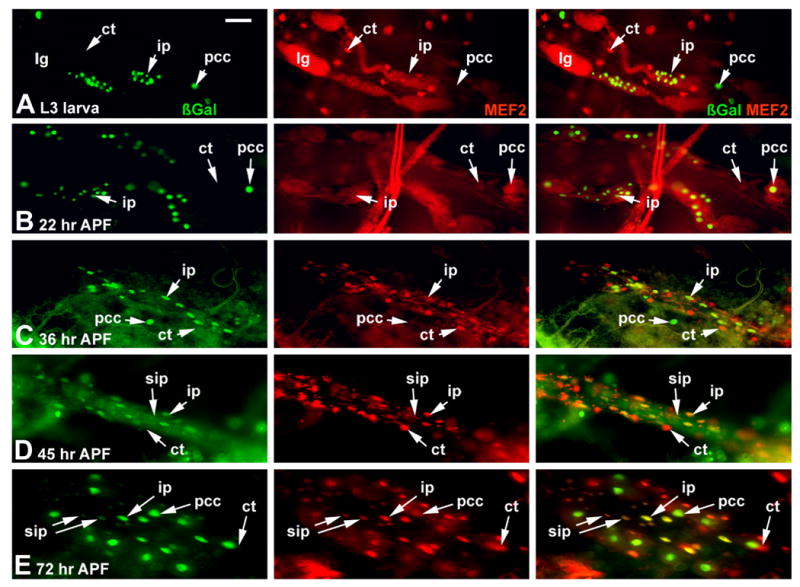
rp298 transgenic animals were dissected at the indicated developmental stages, and assessed for accumulation of ß-gal from the transgene (green), and of MEF2 as a marker of muscle nuclei (red). A: at the late larval stage, ß-gal is present in the pericardial cells (pcc) and in a small group of cells which we label imaginal cardiac precursors (ip). MEF2 is not present in these ip cells at this stage, but can be detected in cells of the cardiac tube (ct). ß-gal does not accumulate in lymph gland cells (lg), and MEF2 is present only at background levels in these cells. B-E: As development proceeds, the ip cells disperse and spread along the cardiac tube. Smaller nuclei also become visible as MEF2-positive cells at around 36 hr APF, which later also become ß-gal positive, presumably through fusing with the ip cells. Bar, 25μm.
Given the lymph gland -like nature of these cells, we next determined if they expressed the serpent (srp) gene, a marker of lymph gland and pericardial cells (Figure 8). During the larval stage, we confirmed that Srp accumulated in pericardial cells and the lymph glands. In addition, Srp was detected in the duf-n-lacZ-expressing cardiac precursors, consistent with their location close to the lymph glands and the pericardial cells, and suggesting that they have a similar developmental origin (Figure 8A). After pupariation, Srp accumulation tracked within the imaginal precursors cells through 16 hr and 24 hr APF (Figure 8B,C). However, by 36hr APF, Srp was not detected in these cells as they began to spread over the ventral surface of the cardiac tube (Figure 8D).
Figure 8. The gain of myogenic potential in the imaginal precursor cells coincides with a loss of Serpent (Srp) accumulation.
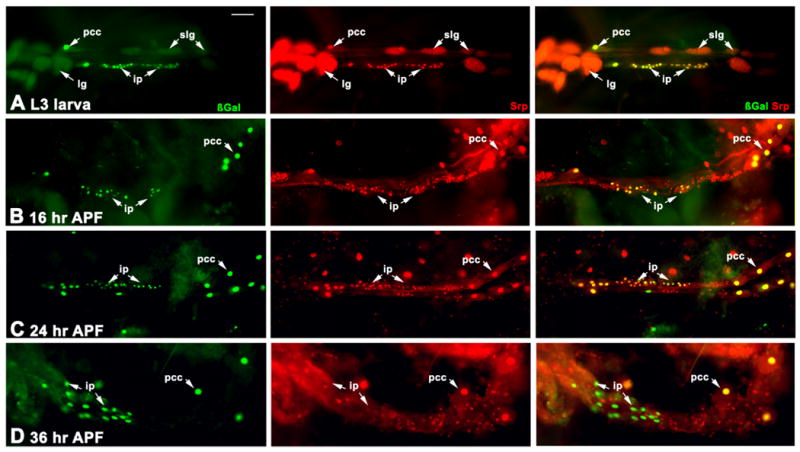
rp298 transgenic animals were dissected at the indicated developmental stages, and assessed for accumulation of ß-gal from the transgene (green), and of Srp as a marker of pericardial and lymph gland cell nuclei (red). A: At the larval stage, the ip cells are strongly Srp-positive, as are the pericardial cells and the lymph glands. B-D: As metamorphosis proceeds, the ip cells first disperse along the cardiac tube, yet by 36 hr APF lose Srp accumulation. By contrast, the pericardial cells remain Srp and ß-gal positive throughout this time period. Bar, 25μm.
Based upon these observations, we propose that the longitudinal fibers of the adult Drosophila heart arise from an unique subset of lymph gland-like cells. These cells undergo characteristic transitions in location and patterns of gene expression as they generate the longitudinal fibers, characteristics that will be discussed in greater detail below.
3. Discussion
Historically, the Drosophila heart has been considered a relatively simple linear tube; however, there is now compelling evidence that it is a complex organ, comprising numerous distinct cell types, and capable of undergoing significant remodeling in response to normal developmental triggers. Here, we emphasize this plasticity in the fly cardiac tissue, by studying its metamorphosis at the cellular, molecular, and developmental levels.
3.1. Cardiac inflow tracts in the Drosophila adult
An interesting facet of cardiac specification and differentiation in Drosophila is how early patterning events lay the groundwork for developmental fates much later in development. Specifically, the embryonic and larval heart occupies the most posterior segments (A6-A8), and the three sets of Svp cells in this chamber function as inflow tracts prior to metamorphosis. Even though four sets of anterior Svp cells are specified and occupy the aorta, they do not function in controlling hemolymph flow into or out of the cardiac tube. We showed previously that these more anterior Svp cells are destined to form the adult ostia, and that posterior Svp cells are histolyzed (Molina and Cripps, 2001). However the situation is more complex than this: firstly, the most anterior pair of ostia of the larval heart persist, to form the most posterior ostia in the adult heart, thus the two most posterior larval Svp cell groups are lost (Monier et al., 2005); secondly, while the adult heart was classically thought to contain four sets of inflow tracts (Miller, 1950), an additional thoracic inflow tract was recently described (Wasserthal, 2007); and thirdly, we show here that the thoracic inflow tract also forms from the Svp lineage of cells. Therefore, this set of Svp cells corresponds to the most anterior group of those cells observed in the embryo, specified by the combined actions of Ubx and Antp (Monier et al., 2004; Ryan et al., 2005). Clearly, a blueprint of Drosophila cardiac fate is laid down during embryonic development, that is realized at later stages of the life cycle. Key to realizing these fates are the developmental cues that control metamorphosis.
3.2. Unique patterns of gene expression in the adult heart
Recently, a detailed analysis of gene expression in the cardiac tube during cardiac metamorphosis was presented, identifying a large number of genes that are up-regulated as the heart tube changes. In large part, this response is controlled by hormonal cues induced by the steroid hormone 20-hydroxyecdysone (Monier et al., 2005; Zeitouni et al., 2007). Here, we demonstrate that one of the genes affected by this reprogramming is Act87E, whose expression is not detectable in the larval cardiac tube, but is detected as the adult heart forms. Monier et al. (2005) showed that increased myofibrillogenesis commences around 36 hr APF, which corresponds to the time window where we show that Act87E expression is initiated. Through identification of an Act87E promoter fragment that recapitulates this developmental switch in expression, we identify sequences through which these signals must act. Further, the differences in temporal and spatial actin expression patterns speak to complex regulatory evens that must differentially impact these genes. Indeed, MEF2 is a potent and direct regulator of Act57B expression during development (Kelly et al., 2002), however there are no functional binding sites for MEF2 in the Act87E promoter fragment that we identified (data not shown).
There is clear specialization in the patterns of gene expression in the remodeling cardiac tube, where the larval cardiac actin is encoded by Act57B, and the adult heart accumulates transcripts arising from both Act57B and Act87E. These alterations in actin gene expression in the remodeling heart are strongly reminiscent of the isoform switches that occur during mammalian heart maturation. In mammals, the predominant fetal actin expressed in the heart is encoded by the alpha-skeletal actin gene; later in development, the predominant actin isoform is generated from the alpha-cardiac actin locus (see Robbins, 2000 for a review).
In mice, this isoform switch accounts directly for altered cardiac physiology (Hewett et al., 1994). It is not clear if differential expression of Act87E versus Act57B has a functional significance, since their coding potentials are almost identical – only two amino acid changes distinguish Actin57B from Actin87E. Nevertheless, our findings provide opportunities to assess the regulation of cardiac actin gene switching in this invertebrate system.
3.2. Origin and nature of the longitudinal cardiac fibers
Even though MEF2 is not a direct regulator of Act87E, Mef2 expression correlates closely with the formation of the longitudinal fibers, that is achieved by a group of imaginal cardiac precursor cells. Our studies have uncovered what appears to be a trans-differentiation event, from lymph gland-like cells into myoblasts. This finding is highly novel, and speaks to a number of outstanding questions in the field of Drosophila cardiac development.
Firstly, our findings define for the first time a source of the adult myoblasts forming the longitudinal cardiac fibers, a source which has been elusive since the formation of these fibers was first described in detail (Molina and Cripps, 2001).
Secondly, we demonstrate that the longitudinal fibers are likely to be specialized skeletal muscles, since they are multinucleate, and arise from the fusion of several cells to a founder cell. While this layer of cells bears some resemblance to the dorsal diaphragm observed in other insect species, a specific function for these longitudinal fibers still has yet to be identified. Now that we have identified the origin of these cells, it should be possible to ablate them and determine if this has phenotypic consequences.
Thirdly, we describe significant new findings with regard to the functions of lymph glands and pericardial cells associated with the dorsal vessel. As discussed earlier, the large clusters of primary lymph gland cells are known to be hematopoietic organs for the pupal and adult stages (Jung et al., 2005). However, all larvae also possess 2-3 sets of secondary lymph glands, as well as a line of pericardial cells, down each side of the dorsal vessel. The functions of these cell clusters have yet to be fully elucidated. Pericardial cells are thought to have kidney-like functions (Miller, 1950), and also are important to maintaining normal heart physiology (Fujioka et al., 2005). The secondary lymph glands have not been studied in detail. Our studies define a cluster of cells that are smaller than, and distinct from, some of the secondary lymph glands. Nevertheless these cells show some characteristics of both pericardial cells (expression of duf-n-lacZ, and of srp) and of lymph glands (expression of srp), and they eventually form the adult fibers. Interestingly, a recent paper by Togel et al. (2008) demonstrates that some Hand-GFP-expressing pericardial cells form the pulsatile organs of the adult. The pulsatile organs are small, contractile, diaphragm-like structures, that are thought to assist in circulation. That pericardial cells can trans-differentiate into specialized circulatory tissues lends support to our conclusion that lymph gland-like cells also form the longitudinal fibers of the adult heart.
Finally, we note that expression of MEF2 correlates with the differentiation of the new adult cardiac cells, and that the up-regulation of MEF2 coincides with a strong down-regulation of Srp accumulation. This observation is interesting in light of the finding that Srp is a potent repressor of embryonic myogenesis, where ectopic accumulation of Srp in the presumptive skeletal muscles causes a profound loss of muscle mass (Hayes et al., 2001). We propose that one means by which Srp achieves this repressive goal is through down-regulation of either MEF2 activity or Mef2 expression. However, how Srp might directly impact MEF2 has yet to be addressed.
In summary, our data provide significant new insight into mechanisms of cardiac remodeling, and raise a number of new avenues of investigation into the molecular basis of cardiac plasticity.
4. Experimental Procedures
4.1 Fly stocks and crosses
All crosses were carried out at 25°C. The y w strain was used as a control. Act57B-lacZ was described in Kelly et al. (2002), and Tg-lacZ was described in Iklé et al. (1998).
4.2 Generation of transgenic flies
The -344/+6 Act87E promoter was generated by PCR using the primers Act87E 5′+ (5′-GGGAATTCTATTAGAAAATCATTACAC) and Act87E Exon 1- (5′-GGCTCGAGCCAAATCAACACTTTCGCTGTAGAAC). Underlined sequence denote restriction sites added to aid in cloning. The product was cloned into pGEM-T Easy (Promega Corp.), before sub-cloning into pCaSpeR-hs-AUG-ßGal (CHAB, Thummel and Pirrotta, 1992). Generation of transgenic flies was as described by Rubin and Spradling (1982). Lines were made homozygous by standard genetic crosses, and at least three lines of the insert were assessed to ensure consistency in patterns of staining.
4.3 Histochemistry and immunofluorescence
Preparation and staining of larval and adult fillets were as described in Molina and Cripps (2001). Pupal samples were timed by marking the location of newly-pupariated animals, and maintaining the stocks at 25°C until dissection. Animals were removed from the pupal case and then dissected and processed in a Sylgard (Dow Corning Laboratories) coated petri dish as referenced above.
Frozen sections were generated by freezing flies in OCT compound (VWR Scientific Products) over dry ice, and then sectioning at 10μm thickness in a Minotome Plus cryotome (Triangle Biomedical Sciences, NC, USA). Sections were briefly fixed with 1.9 % v/v formaldehyde on ice for five minutes, before washing with phosphate buffered saline and exposure to the chromogenic solution. Paraffin sections for in situ hybridization were generated as described by Cripps et al. (1998) and Lovato et al (2001).
X-gal staining to detect ß-gal activity was carried out either in the dissection dishes for whole mounts, or under a cover slip for cryosections, using a standard staining solution (Ashburner, 1989).
Antibody staining was essentially as described by Patel (1994) and modified by Molina and Cripps (2002) for filleted samples. Primary antibodies used were: rabbit anti-MEF2 (Lilly et al., 1995), 1: 1,000; anti-ß-gal (mouse – Promega Corp.; rabbit – AbCam), 1:1,000; and rabbit anti-Serpent (Sam et al., 1996), 1:500. Secondary antibodies were Alexa- linked (Invitrogen/Molecular Probes), and used at 1:2,000. Alexa-linked Phalloidin (Invitrogen/Molecular Probes) was used at 1:500 and included with the secondary antibody.
Confocal images were captured using a BioRad MRC600 confocal microscope. All images shown are from single confocal planes.
4.4 In situ hybridization
The Act57B probes were described in Kelly et al. (2002). For Act87E, an 824bp DNA fragment representing the 3′UTR was generated by PCR using the primers 87E3′+II (5′-GGAATCGTCCACCGCAAGTGC) and 87E3′-(5′-CCGGTACCGAGCTCTTGTATTCCTAATTAG); the product was cloned into pGEM-T Easy. Riboprobes were made from this plasmid using standard techniques. In situ hybridization of these probes to de-paraffinized sections was as described by Lovato et al (2001).
Acknowledgments
We are very grateful to Dr John Sparrow for critical reading of the manuscript, and in whose laboratory some of these findings were made. We thank Dr Kathryn Ryan for critical comments on the manuscript. We are grateful to Dr Bruce Paterson for providing the MEF2 antibody, and to Dr Debbie Hoshizaki for the Serpent antibody. KKKT was a recipient of an American Heart Association, Desert Mountain Affiliate predoctoral fellowship, and MRM was supported by the Initiatives to Maximize Student Diversity program, R25 GM060201, from the NIH. This project was supported by a Grant-in-Aid from the American Heart Association, Pacific Mountain Affiliate to RMC, and by R01 HL080545 from the NIH to RMC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner M. Drosophila: A Laboratory Handbook and Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Azpiazu N, Frasch M. tinman and bagpipe: Two homeobox genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes and Development. 1993;7:1325–1340. doi: 10.1101/gad.7.7b.1325. [DOI] [PubMed] [Google Scholar]
- Baker PW, Kelly Tanaka KK, Klitgord N, Cripps RM. Adult myogenesis in Drosophila melanogaster can proceed independently of Myocyte enhancer factor-2. Genetics. 2005;170:1747–1759. doi: 10.1534/genetics.105.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate M. The embryonic development of larval muscles in Drosophila. Development. 1990;110:791–804. doi: 10.1242/dev.110.3.791. [DOI] [PubMed] [Google Scholar]
- Bernstein SI, O'Donnell PT, Cripps RM. Molecular genetic analysis of muscle development, structure and function in Drosophila. Int Rev Cytol. 1993;43:63–152. doi: 10.1016/s0074-7696(08)61874-4. [DOI] [PubMed] [Google Scholar]
- Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Developmental Biology. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118:719–729. doi: 10.1242/dev.118.3.719. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Frasch M. Genetic determination of the Drosophila heart development. In: Rosenthal N, Harvey R, editors. Heart Development. Academic Press; New York: 1999. pp. 65–90. [Google Scholar]
- Cripps RM, Black BL, Zhao B, Lien CL, Schulz RA, Olson EN. The myogenic regulatory gene Mef2 is a direct target for transcriptional activation by Twist during Drosophila myogenesis. Genes Dev. 1998;12:422–434. doi: 10.1101/gad.12.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps RM, Olson EN. Control of cardiac development by an evolutionarily conserved transcriptional network. Developmental Biology. 2002;246:14–28. doi: 10.1006/dbio.2002.0666. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Wessells RJ, Han Z, Liu J, Fitzgerald K, Yusibova GL, Zamora M, Ruiz-Lozano P, Bodmer R, Jaynes JB. Embryonic even skipped-dependent muscle and heart cell fates are required for normal adult activity, heart function, and lifespan. Circ Res. 2005;97:1108–1114. doi: 10.1161/01.RES.0000191546.08532.B2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrberg EA, Mahaffey JW, Bond BJ, Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue specific manner. Cell. 1983;33:115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Garg V. Insights into the genetic basis of congenital heart disease. Cell Mol Life Sci. 2006;63:1141–1148. doi: 10.1007/s00018-005-5532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SA, Miller JM, Hoshizaki DK. serpent, a GATA-like transcription factor gene, induces fat-cell development in Drosophila melanogaster. Development. 2001;128:1193–1200. doi: 10.1242/dev.128.7.1193. [DOI] [PubMed] [Google Scholar]
- Hewett TE, Grupp IL, Grupp G, Robbins J. Alpha-skeletal actin is associated with increased contractility in the mouse heart. Circ Res. 1994;74:740–46. doi: 10.1161/01.res.74.4.740. [DOI] [PubMed] [Google Scholar]
- Iklé J, Elwell JA, Bryantsev AL, Cripps RM. Cardiac expression of the Drosophila Transglutaminase (CG7356) gene is directly controlled by Myocyte enhancer factor-2. Dev Dyn. 2008;237:2090–2099. doi: 10.1002/dvdy.21624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Kelly KK, Meadows SM, Cripps RM. Drosophila MEF2 is a direct regulator of Actin57B transcription in cardiac, skeletal, and visceral muscle lineages. Mech Dev. 2002;110:39–50. doi: 10.1016/s0925-4773(01)00586-x. [DOI] [PubMed] [Google Scholar]
- Lilly B, Zhao B, Ranganayakulu G, Paterson BM, Schulz RA, Olson EN. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science. 1995;267:688–93. doi: 10.1126/science.7839146. [DOI] [PubMed] [Google Scholar]
- Lo PCH, Frasch M. Establishing A-P polarity in the embryonic heart tube: a conserved function of Hox genes in Drosophila and vertebrates? Trends Cardiovasc Med. 2003;13:182–187. doi: 10.1016/s1050-1738(03)00074-4. [DOI] [PubMed] [Google Scholar]
- Lo P, Skeath J, Gajewski K, Schulz RA, Frasch M. Homeotic genes autonomously specify the anteroposterior subdivision of the Drosophila dorsal vessel into aorta and heart. Developmental Biology. 2002;251:307–319. doi: 10.1006/dbio.2002.0839. [DOI] [PubMed] [Google Scholar]
- Lovato TL, Meadows SM, Baker PW, Sparrow JC, Cripps RM. Characterization of muscle actin genes in Drosophila virilis reveals significant molecular complexity in skeletal muscle types. Insect Mol Biol. 2001;10:333–340. doi: 10.1046/j.0962-1075.2001.00270.x. [DOI] [PubMed] [Google Scholar]
- Lovato TL, Nguyen TP, Molina MR, Cripps RM. The Hox gene abdominal-A specifies heart cell fate in the Drosophila dorsal vessel. Development. 2002;129:5019–5027. doi: 10.1242/dev.129.21.5019. [DOI] [PubMed] [Google Scholar]
- Medioni C, Senatore S, Salmand PA, Lalevee N, Perrin L, Semeriva M. The fabulous destiny of the Drosophila heart. Curr Opin Genetics Dev. 2009;19:518–525. doi: 10.1016/j.gde.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Miller A. The internal anatomy and histology of the imago. In: Demerec M, editor. Biology of Drosophila. Wiley; New York, NY: 1950. [Google Scholar]
- Molina MR, Cripps RM. Ostia, the inflow tracts of the Drosphila heart, develop from a genetically distinct subset of cardial cells. Development. 2001;109:51–59. doi: 10.1016/s0925-4773(01)00509-3. [DOI] [PubMed] [Google Scholar]
- Monier B, Astier M, Semeriva M, Perrin L. Steroid-dependent modification of Hox function drives myocyte reprogramming in the Drosophila heart. Development. 2005;132:5283–5293. doi: 10.1242/dev.02091. [DOI] [PubMed] [Google Scholar]
- Neely GG, Kuba K, Cammarato A, et al. A global in vivo Drosophila RNAi screen identifies NOT3 as a conserved regulator of heart function. Cell. 2010;141:142–153. doi: 10.1016/j.cell.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K, Reeves NL, Wessells RJ, Fink M, Chen HS, Akasaka T, Yasuda S, Metzger JM, Giles W, Posakony JW, Bodmer R. KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proc Natl Acad Sci U S A. 2007;104:3943–3948. doi: 10.1073/pnas.0609278104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NH. Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. Methods Cell Biol. 1994;44:445–87. doi: 10.1016/s0091-679x(08)60927-9. [DOI] [PubMed] [Google Scholar]
- Perrin L, Monier B, Ponzielli R, Astier M, Semeriva M. Drosophila cardiac tube organogenesis requires multiple phases of Hox activity. Developmental Biology. 2004;272:419–431. doi: 10.1016/j.ydbio.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Ponzielli R, Astier M, Chartier A, Therond A, Semeriva M. Heart tube patterning in Drosophila requires integration of axial and segmental information provided by the Bithorax Complex genes and Hedgehog signaling. Development. 2002;129:4509–4521. doi: 10.1242/dev.129.19.4509. [DOI] [PubMed] [Google Scholar]
- Rizki TM. The circulatory system and associated cells and tissues. In: Ashburner M, Wright TRF, editors. The Genetics and Biology of Drosophila. 2b. Academic Press; New York: 1978. pp. 397–452. [Google Scholar]
- Robbins J. Remodeling the cardiac sarcomere using transgenes. Ann Rev Physiol. 2000;62:261–287. doi: 10.1146/annurev.physiol.62.1.261. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Zhou ZJ, Tang ML, Meller S, Chen JW, Bellen H, Kimbrell DA. Identification of immune system and response genes, and novel mutations causing melanotic tumor formation in Drosophila melanogaster. Genetics. 1996;143:929–940. doi: 10.1093/genetics/143.2.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–53. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gomez M, Coutts N, Price A, Taylor MV, Bate M. Drosophila dumbfounded: a myoblast attractant essential for fusion. Cell. 2000;102:189–198. doi: 10.1016/s0092-8674(00)00024-6. [DOI] [PubMed] [Google Scholar]
- Ryan KM, Hoshizaki DK, Cripps RM. Homeotic selector genes control the patterning of seven-up cells in the Drosophila dorsal vessel. Mech Dev. 2005;122:1023–1033. doi: 10.1016/j.mod.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Sam S, Leise W, Hoshizaki DK. The serpent gene is necessary for progression through the early stages of fat body development. Mech Dev. 1996;60:197–205. doi: 10.1016/s0925-4773(96)00615-6. [DOI] [PubMed] [Google Scholar]
- Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Thummel CS, Pirrotta V. New pCaSpeR P element vectors. Drosophila Information Service. 1992;71:150. [Google Scholar]
- Togel M, Pass G, Paululat A. The Drosophila wing hearts originate from pericardial cells and are essential for wing maturation. Dev Biol. 2008;318:29–37. doi: 10.1016/j.ydbio.2008.02.043. [DOI] [PubMed] [Google Scholar]
- Tobin SL, Cook PJ, Burn TC. Transcripts of individual Drosophila actin genes are differentially distributed during embryogenesis. Dev Genet. 1990;11:15–26. doi: 10.1002/dvg.1020110104. [DOI] [PubMed] [Google Scholar]
- Wasserthal LT. Drosophila flies combine periodic heart beat reversal with a circulaion in the anterior body mediated by a newly discovered anterior pair of ostial valves and “venous” channels. J Exp Biol. 2007;210:3707–3719. doi: 10.1242/jeb.007864. [DOI] [PubMed] [Google Scholar]
- Zeitouni B, Senatore D, Aknin C, Semeriva M, Perrin R. Signaling pathways involved in adult heart formation revealed by gene expression profiling. PLoS Genetics. 2007;3:1907–1920. doi: 10.1371/journal.pgen.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]


