Abstract
Individuals with ADHD may self-medicate with nicotine, the main psychoactive ingredient in tobacco smoke, in order to reduce symptoms and negative moods associated with ADHD. ADHD medication (e.g., methylphenidate, atomoxetine) may mimic some of the effects of nicotine and may aid smoking cessation in smokers with ADHD. The present study examined if ADHD medication reduces smoking and withdrawal in non-treatment seeking smokers with ADHD. Fifteen adult smokers with ADHD participated in the study, which consisted of an experimental phase and field monitoring phase to examine the acute and extended effects, respectively, of ADHD medication. During the experimental phase, smokers were asked to complete a Continuous Performance Task (CPT) and the Shiffman-Jarvik smoking withdrawal questionnaire during the following four conditions: (1) ADHD medication + cigarette smoking, (2) ADHD medication + overnight abstinence, (3) placebo + cigarette smoking, and (4) placebo + overnight abstinence. During the field monitoring phase, participants were asked to provide salivary cotinine samples and complete electronic diaries about smoking, smoking urge, ADHD symptoms, and stress in everyday life for two days on ADHD medication and for two days on placebo. Results of the experimental phase showed that ADHD medication improved task performance on the CPT and reduced withdrawal during overnight abstinence. During the field monitoring phase, ADHD medication reduced salivary cotinine levels compared to placebo. In addition, the electronic diary revealed that ADHD medication improved difficulty concentrating during no smoking events and stress. The findings of the present study suggest that, along with other strategies, ADHD medication may be used to aid with smoking withdrawal and cessation in smokers with ADHD.
Keywords: Smoking cessation, Stimulant medication, Smoking, Tobacco use, Nicotine dependence
1. Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a frequent diagnosis for symptoms of inattention, impulsivity, and hyperactivity in children and the prevalence rates for adults range from 2 to 6% (Weiss and Murray, 2003). Individuals with ADHD are at an increased risk for early smoking initiation, have elevated smoking prevalence and reduced quit rates compared to the general population (Burke et al., 2001, Lambert and Hartsough, 1998, Milberger et al., 1997, Molina and Pelham, 2003, Pomerleau et al., 1995). Recent research has shown that nicotine, the main psychoactive ingredient of tobacco smoke, reduces clinical symptoms and negative moods in individuals with ADHD independent of smoking status (Gehricke et al., 2009). The effects of nicotine on ADHD symptoms may be similar to those found in response to ADHD medications such as methylphenidate (e.g., Ritalin, Concerta), atomoxetine (e.g., Strattera), and dextroamphetamine (e.g., Dexedrine, Adderall, and Vyvanse) (Conners et al., 1996, Gehricke et al., 2006, Levin et al., 1996, Potter and Newhouse, 2004). These medications for ADHD may be able to mimic some of the behavioral effects of nicotine and thus may aid smokers with ADHD during smoking cessation (Gehricke et al., 2009, Gehricke et al., 2007).
Covey and colleagues (Covey et al., 2009) found that an 11-week administration of OROS-methylphenidate (OMPH) led to a four-week complete abstinence rate of 42.9% in non-Caucasians with ADHD compared to 23.1% in Caucasians with ADHD. However, no information was provided on the motivation to quit, medication-induced task improvement during abstinence, and interactions with perceived stress, which may have contributed in one form or another to the differences in abstinence rates. In addition, Ray and colleagues (Ray et al., 2009) showed that the non-stimulant atomoxetine reduced smoking withdrawal symptoms during abstinence. Moreover, stress has been found to negatively affect abstinence rates in smokers (Businelle et al., 2010, Shaw and al'Absi, 2008) and ADHD medication may be useful to reduce withdrawal associated with abstinence, in particular during stress.
Overall, little is known about the effects of ADHD medication on smoking and withdrawal symptoms without the confounding effects of motivation to quit in smokers with ADHD. The goal of the present study was to provide more information on the interaction between ADHD medication administration, cigarette smoking, clinical symptoms and withdrawal in non-treatment seeking smokers with ADHD. The study consisted of an experimental phase and a subsequent field monitoring phase to examine the acute and extended effects, respectively, of ADHD medication. More specifically, the experimental phase examined the acute effects of ADHD medication compared to placebo and smoking compared to abstinence on task performance and withdrawal symptoms in a controlled setting. The field monitoring phase evaluated effects of two days of ADHD medication administration compared to two days of placebo on nicotine intake, smoking urge, and ADHD symptoms during everyday life. In addition, this phase examined the interactive effects of ADHD medication and smoking on smoking urge and symptoms of ADHD during stress compared to no stress. For the experimental phase, it was hypothesized that ADHD medication compared to placebo would improve task performance and reduce withdrawal symptoms during smoking abstinence. For the field monitoring phase, it was hypothesized that ADHD medication compared to placebo would reduce nicotine intake. In addition, it was expected that ADHD medication would reduce smoking urge and ADHD symptoms during no smoking events and stress.
2. Methods
2.1. Participants
Fifteen adult smokers with ADHD were recruited from local treatment centers and clinical practices via announcements and referrals by treating clinicians (see Table 1 for sample characteristics). Each participant was assessed according to DSM-IV-TR criteria (American Psychiatric Association, 2000) with the Structured Clinical Interview for DSM-IV (SCID; First, 1995) and the QUEST method (Wigal et al., 2007). The QUEST is a semi-structured clinical interview, which utilizes the DSM-IV-TR symptoms for ADHD with age-appropriate probes. Participants were excluded if they were treated for any chronic illness such as heart disease, irregular heartbeat, hypertension, diabetes, skin allergies or skin diseases, even if currently controlled by medication. All female participants were asked to undergo a pregnancy test prior to participation in the study to ensure that they were not pregnant.
Table 1.
Sample characteristics
| Characteristics | Valuea | Standard Deviation |
|---|---|---|
| Ages, years | 27.2 | 8.6 |
| Male Gender | 87% | |
| Caucasian | 87% | |
| Education, years | 13.1 | 1.7 |
| Employed | 73% | |
| DSM-IV ADHD Symptoms | ||
| Inattentive | 6.8 | 2.1 |
| Hyperactive-Impulsive | 6.1 | 2.1 |
| Combined | 12.9 | 2.6 |
| Comorbid Disorders | 1.6 | 1.9 |
| Number of cigarettes/day | 16.5 | 10.1 |
| Age started smoking regularly, years | 14.1 | 2.7 |
| Number of quit attempts | 2.6 | 1.8 |
| Fagerström Test for Nicotine Dependence | 6.3 | 1.2 |
Note. Values are means, unless noted otherwise.
Smokers were defined as individuals who have been smoking at least 10 cigarettes per day with 0.5 mg of nicotine per cigarette for at least two years. Cigarette smoking history and habits were assessed with the California Tobacco Survey (Davis, 2005) and salivary cotinine. Nicotine dependence was assessed with the Fagerström Test for Nicotine Dependence. Each participant was asked to abstain from smoking at least 8 hours prior coming to the laboratory, which was validated with expired carbon monoxide using a Portable CO Analyzer (National Draeger). The standard cut-off level for participation in the study was 8 ppm. Average sleep duration was 7.95 hours (SD = 1.29) and did not differ between the monitoring days. Abstinence from drugs of abuse prior to and during participation in the study was verified with commercially available urine drug screens (Integrated E-Z Split Key Cups 10 Panel; Drugformation.com).
All participants were taking ADHD medication on a regular basis (see Table 2 for type and dosage of medication) to ensure that ADHD symptoms caused significant impairment in functioning. Participants were asked to wash out their medication prior to participation. The medication wash out period was approximately five half-lives, i.e., 18 hours for OMPH, 25 hours for Atomoxetine, 40 hours for Dextroamphetamine, 50 hours for Lisdexamfetamine, and 50 hours for Amphetamine, Dextroamphetamine Mixed Salts. Five half-lives were used in previous research (e.g., Bush et al., 1999) and two to three days is standard procedure.
Table 2.
Medications used by participants
| Medication | Number of Subjects | Dosage (mg/day) | ||
|---|---|---|---|---|
| Mean | Minimum | Maximum | ||
| Dextroamphetamine (D-AMPH) | 1 | 30 | 30 | 30 |
| Amphetamine, D-AMPH Mixed Salts | 7 | 19 | 10 | 40 |
| Atomoxetine | 4 | 36 | 25 | 40 |
| OMPH | 1 | 54 | 54 | 54 |
| Lisdexamfetamine | 2 | 30 | 30 | 30 |
2.2. Procedures
The study was approved by the Institutional Review Board of the University of California, Irvine. Participants were asked to attend an orientation session prior to participating in the study. During the orientation session, participants learned more about the study procedures, medication and placebo administration. Following the equipment demonstration, all questions by the participants were answered and informed consent forms were signed. In addition, a set of demographic and psychosocial measures were completed and the SCID (First, 1995) and QUEST (Wigal et al., 2007) administered. Participants then scheduled the two 2-day monitoring sessions. Participants were asked to abstain from medications and drugs at least 12 hours prior to and during their participation in the study. See Figure 1 for an overview of the study procedures.
Fig. 1.
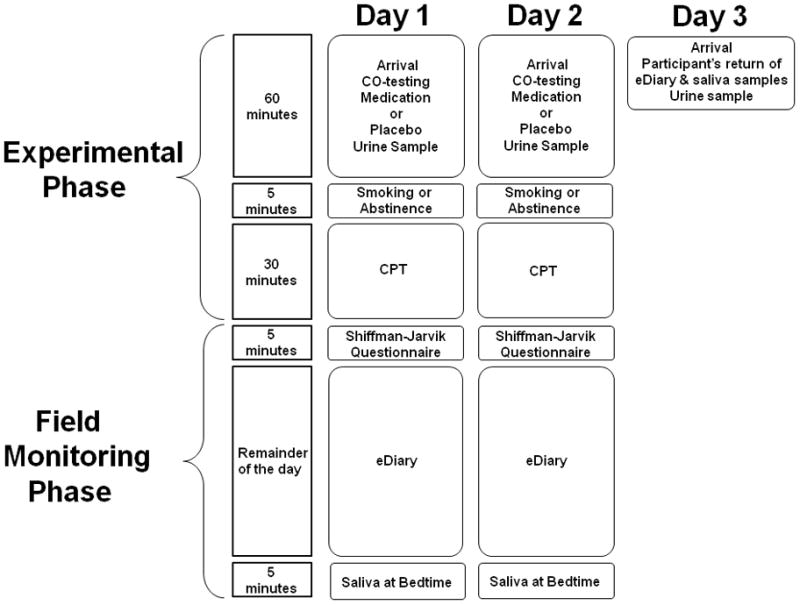
Overview over study procedures.
2.2.1. Experimental Phase
Participants were asked to abstain from smoking overnight and prior to arrival at the laboratory. On the first day of each 2-day period, the participant met with research staff in the morning (6 am to 11 am) and received a medication or placebo pill. A urine drug test and a medication and drug use questionnaire were administered in order to assess when participants took their last dose of ADHD medication and if they took any other drugs prior to participation. Participants were asked to exhale into the carbon monoxide analyzer to validate that they did not smoke prior to Continuous Performance Task (CPT) administration and diary set up. The electronic diary was set up on a Palm Pilot. This took approximately 30 minutes and was followed by another 30 minutes of watching a video, playing a computer game, or reading magazines in order to allow for medication to become effective. Then, the participant was asked to smoke a cigarette or to abstain from smoking prior to the CPT. During the smoking condition, the participant was asked to smoke a cigarette followed by the CPT for 30 minutes and subsequent administration of the Shiffman-Jarvik withdrawal questionnaire. During the abstinence condition, the participant was asked to abstain from smoking and complete the CPT and Shiffman-Jarvik questionnaire. After completion of the CPT, the participant left for the day after all questions were clarified. On the second day of the 2-day period, the participant filled out the medication and drug use questionnaire, completed a urine drug and expired carbon monoxide test and received the same medication or placebo pill as on the first day. The Palm Pilot with an electronic diary was provided and the participant was asked to watch a video, play a computer game, or read magazines until 60 minutes after medication or placebo pill administration. Again, the participant was asked to smoke a cigarette or to abstain from smoking prior to the CPT and administration of the Shiffman-Jarvik questionnaire. Smoking and abstinence conditions were applied in counterbalanced order. If the participant did the smoking condition on the first day, he or she did the abstinence condition on the second day. If the participant did the abstinence condition on the first day, he or she did the smoking condition on the second day. The participant left for the day after all questions were answered. On the third day, the participant met with the research team to collect the Palm Pilot and saliva samples. Each 2-day monitoring sequence was separated by at least 5 days and occurred on the same days of the week. Participants received $100 for each 2-day monitoring session.
2.2.2. Field Monitoring Phase
After the completion of the CPT, participants left for the day to continue with the monitoring of smoking urge, ADHD symptoms, and stress in everyday life using eDiaries. Smoking events were monitored by asking participants to initiate the eDiary after completion of smoking a cigarette. The eDiary was programmed to emit a sound every 45 minutes (+/− 10 minutes) that signaled the participant to fill out the diary in order to obtain information on smoking urge, ADHD symptoms and stress during nonsmoking events throughout waking hours. Similar to previous studies (Gehricke et al., 2009; Gehricke et al., 2006), participants were asked to provide a daily saliva sample at the end of each day and store the specimen in the freezer.
2.2.3. Medication administration
The study medication was prescribed by a board-certified psychiatrist (V.C.) and encapsulated by a local pharmacy. For the ADHD medication condition, participants received their usual dosage of their usual medication in the morning of each monitoring day. The advantage of administering the subject’s usual dosage of medication is that we believe ADHD symptoms to be sufficiently treated since the participant is not asking for changes in medication and/or dosage. Because individuals with ADHD vary in drug and dose responsiveness, administering a fixed dose of medication may not be effective or may introduce undue side effects from an overdose. For the placebo condition, a placebo pill instead of the usual dosage was provided in the morning of each day. In order to keep the Principal Investigator and participant blind to the medication condition, medication and placebo pills were administered by staff who were not otherwise in contact with the participants. However, participants were able to correctly identify medication in 63% and placebo in 78% of the time in a post-recording questionnaire.
2.3. Measures
2.3.1. Experimental Phase
2.3.1.1. Continuous Performance Task (CPT)
The CPT is a commonly used laboratory task to assess attention and inhibition in response to nicotine and medication in adults with ADHD (Conners et al., 1996, Levin et al., 2001, Levin et al., 1996, Riccio et al., 2001, Walker et al., 2000). This computerized task requires the participant to press the spacebar when any letter except “X” appears. The target letter “X” appears in 10% of the trials. Each trial consists of an individual letter that remains on the screen for approximately 250 milliseconds. There are 360 trials, grouped into 6 consecutive time blocks. Twenty trial sequences, each of three different interstimulus intervals (1, 2, and 4 sec) were presented in randomized order within each block. The task yielded the following measures for the entire test: Hit reaction time, number of omissions, and number of commissions. In the morning of each monitoring day, approximately 60 minutes after medication or placebo pill administration the participants were asked either to abstain from smoking or smoke a cigarette 5 minutes prior to starting the CPT. An approximate delay of one hour between ADHD medication administration and start of CPT was used previously (Barkley et al., 2005, Zillessen et al., 2001).
2.3.1.2. Smoking withdrawal
The Shiffman-Jarvik questionnaire (Shiffman and Jarvik, 1976) was designed to measure withdrawal from cigarette smoking and was completed after each CPT assessment. This questionnaire yields a total score of withdrawal and the following subscales: cravings, arousal, appetite, psychological and physiological withdrawal.
2.3.2. Field Monitoring Phase
2.3.2.1. Salivary cotinine
To assess nicotine intake, salivary cotinine was obtained by asking the participants to provide a saliva sample (NUNC Brands, Roskilde, Denmark) and store the daily specimens in their home freezer. The samples were provided each night before the participant went to bed. The frozen samples were collected when the participant met research personnel after each 2-day monitoring session.
2.3.2.2. eDiary
The eDiary, administered via handheld computer (Palm Z22), monitored cigarette smoking (yes, no) and stress (yes, no). In addition, the eDiary assessed smoking urge and ADHD symptoms including difficulty concentrating, restlessness, forgetfulness, impulsivity, and impatience using seven point scales (0 = not at all present to 6 = very strong). Each diary item appeared on a separate screen and participants had to activate the “next” button at the bottom of the screen to get to the next item. eDiary items were originally identified based on DSM-IV criteria and then pilot tested in a study of the interactive effects of nicotine and ADHD medication in adult smokers with ADHD (Gehricke et al., 2006).
Smoking events were monitored by asking participants to initiate the eDiary after completion of smoking a cigarette. After a smoking event, the diary was programmed to signal the participant once per hour on average (i.e., every 45 ± 10 minutes) during waking hours to monitor no smoking events. Filling out the diary took approximately 1–2 minutes and participants were expected to have a 50% response rate at minimum. For smoking events, participants initiated the eDiary on average 15.42 (SD = 5.45) during 2 days of ADHD medication compared to 10.83 (SD = 5.52) during 2 days on placebo. For no smoking events, the average response rate to the eDiary was 9.75 times (SD = 5.71) during ADHD medication compared to 8.58 times (SD = 7.93) during placebo.
2.3. Statistical analyses
All statistical testing was conducted with SPSS 17.0 using one-tailed tests to examine the a priori directional hypotheses.
2.3.1. Experimental Phase
A four-way repeated measure ANOVA was used to analyze differences between the four laboratory conditions (i.e., medication with smoking, medication with abstinence, placebo with smoking and placebo with abstinence) in each of the CPT outcome measures (i.e., hit reaction time, number of omissions, and number of commissions). Friedman’s Two-Way Analysis of Variance by Ranks was used to analyze differences between condition for the Shiffman-Jarvik smoking withdrawal questionnaire (total score and subscales). Significant differences between conditions were decomposed with post-hoc t-tests and Wilcoxon tests, respectively. The alpha level was set at .05.
2.3.2. Field Monitoring Phase
To study differences in nicotine intake between medication and placebo conditions, average salivary cotinine for each 2-day monitoring period was analyzed with a 2 (medication versus placebo) repeated measure ANOVA. The alpha level was set at .05. In addition, 2 (medication versus placebo) by 2 (smoking versus no smoking) by 2 (stress versus no stress) linear mixed model ANOVAs were used to examine self-reported smoking urge, difficulty concentrating, restlessness, forgetfulness, impulsivity, and impatience in the eDiary. Significant interactions were decomposed with t-tests. The alpha level was set at .017 to account for multiple testing in the eDiary (Cross and Chaffin, 1982).
3. Results
3.1. Experimental Phase
3.1.1. CPT
Significant differences between the four conditions (i.e., medication + smoking, medication + abstinence, placebo + smoking, placebo + abstinence) were found for errors of omission t-scores (F (3, 12) = 8.08, p < .001). Post-hoc t-tests revealed that medication + smoking, medication + abstinence and placebo + smoking reduced errors of omission compared to placebo + abstinence (t(14) ≤ 2.99, p ≤ .006), see Figure 2 for t-scores. No significant differences emerged for reaction time and errors of commission. Table 3 shows reaction times and errors of omission and commission.
Fig. 2.
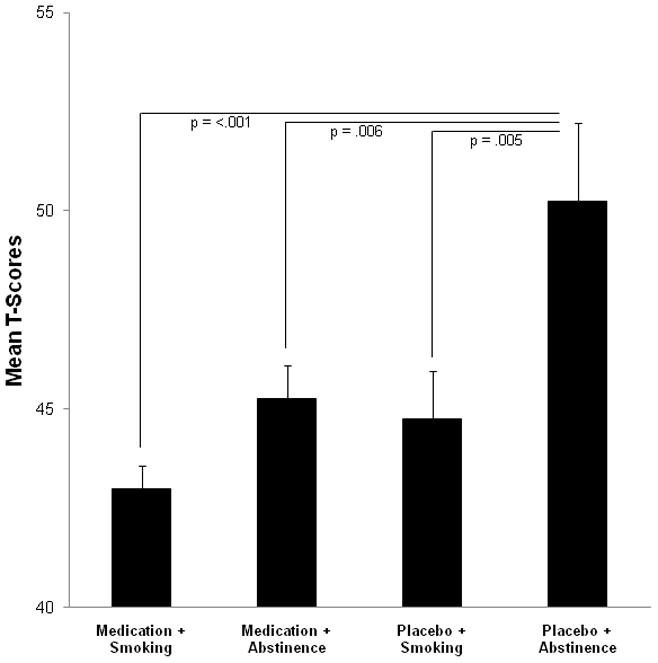
Mean errors of omission t-scores during the experimental phase.
Table 3.
Mean CPT data
| Medication + Smoking | Medication + Abstinence | Placebo + Smoking | Placebo + Abstinence | F | p ≤ | |
|---|---|---|---|---|---|---|
| Hit RT | 335.57 (11.15) | 339.34 (9.92) | 340.44 (14.53) | 351.35 (15.82) | 0.91 | 0.212 |
| Omission | 0.40 (0.15) | 1.08 (0.32) | 1.00 (0.43) | 2.80 (0.68) | 8.12 | 0.001 |
| Commission | 11.20 (1.49) | 11.67 (1.83) | 12.20 (2.18) | 13.00 (1.93) | 0.45 | 0.360 |
Note. Standard errors are in parentheses.
3.1.2. Shiffman-Jarvik Withdrawal Questionnaire
Friedman’s Two-Way Analysis of Variance by Ranks revealed significant differences between the four conditions for total withdrawal (χ2 = 8.28, p = .015) and physiological withdrawal scores (χ2= 9.00, p = .020) in the Shiffman-Jarvik questionnaire (see Figure 3 for total withdrawal scores). Post-hoc Wilcoxon tests showed that ADHD medication reduced total withdrawal scores (median = 91.37; p = .016) and physiological withdrawal (median = 6.33; p = .009) during medication + abstinence compared to placebo + abstinence (median = 102.00 and median = 8.64, respectively) but not during smoking conditions. No other subscales showed significant differences between conditions.
Fig. 3.
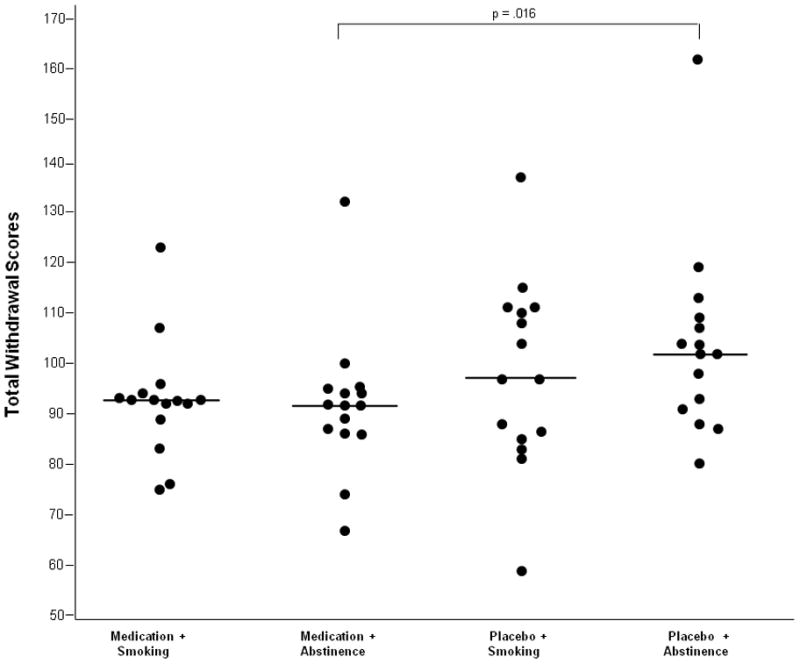
Median Shiffman-Jarvik total scores during the experimental phase.
3.2. Field Monitoring Phase
3.2.1. Salivary Cotinine
A 2 (medication versus placebo) repeated measure ANOVA showed a significant main effect in reducing salivary cotinine during medication compared with placebo (F(1, 14) = 3.32, p = .045). The significant differences between the means suggest that ADHD medication reduced nicotine intake compared to the placebo condition (see Figure 4).
Fig. 4.
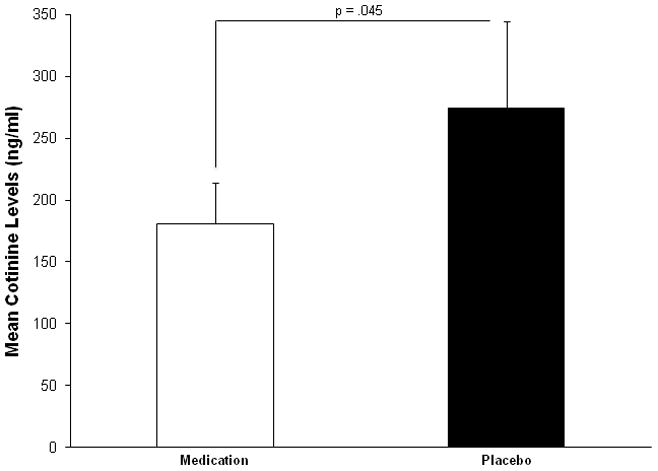
Mean cotinine levels during the field monitoring phase.
3.2.2. eDiary
As depicted in Figure 5, a 2 (medication versus placebo) by 2 (smoking versus no smoking) by 2 (stress versus no stress) linear mixed model ANOVA revealed a medication by smoking by stress interaction for difficulty concentrating (F(1,11) = 5.18, p = .012). Post-hoc t-tests showed that medication reduced difficulty concentrating compared to placebo under no smoking events and stress (t (1, 14) = 2.67, p = .006). In addition, medication reduced difficulty concentrating compared to placebo during smoking events and stress (t (1, 14) = 2.77, p = .004). Moreover, stress increased difficulty concentrating compared to no stress during smoking events when on medication (t (1, 14) = 2.62, p = .007) as well as during no smoking events when on placebo (t (1, 14) = 5.11, p < .001). No other comparisons were significant.
Fig. 5.
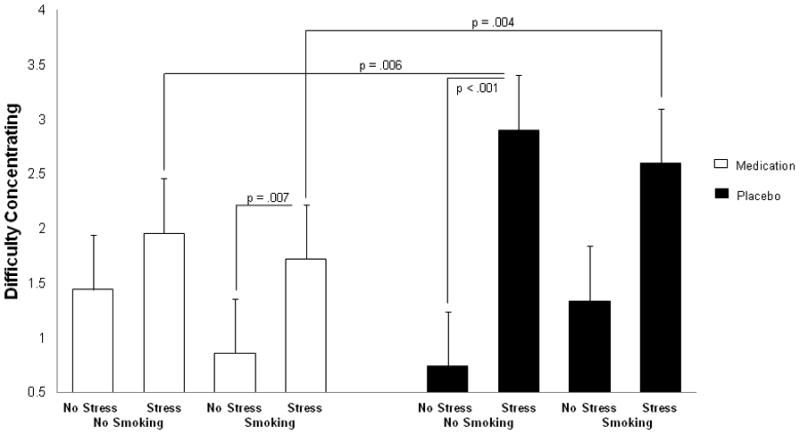
Mean difficulty concentrating ratings in the eDiary during the field monitoring phase.
Smoking by stress interactions were found for impulsivity (F(1,12) = 8.35, p = .006) and impatience (F(1, 10) = 7.40, p = .012). Decomposition of these interactions revealed significant increases during stress compared to no stress during no smoking events for impulsivity (mean 1.91, SE = 0.25 versus mean = 0.87, SE = 0.28; t (1, 14) = 1.03, p = 0.004) as well as impatience (mean = 3.30, SE = 0.40 versus mean 1.65, SE = 0.45; t (1, 14) = 1.65, p = .005).
Restlessness showed interactions for medication by stress (F (1, 10) = 6.14, p = .007) and smoking by stress (F(1, 10) = 10.02, p = .001). Post-hoc comparisons for the medication by stress interaction revealed that stress significantly increased restlessness compared to no stress under the placebo condition (mean = 2.60, SE = 0.27 versus mean 1.41, SE = 0.24; t (1, 14) = 1.18, p = .004). Post-hoc t-tests for the smoking by stress interaction showed that restlessness increased under stress compared to no stress during no smoking events (mean 2.45, SE = 0.23 versus mean = 1.18, SE = 0.26, t (1, 14) = 1.26 p < .001).
Smoking urge showed a main effect for smoking (F(1, 10) = 12.96, p = .002) and an interaction between medication and smoking (F (1,31) = 4.88, p = .017). Post-hoc t-test showed that no smoking events significantly increased smoking urge compared to smoking events (mean = 2.66, SE = 0.24 versus mean = 1.85, SE = 0.24; t (1, 14) = 12.96, p = .002). In addition, smoking urge was significantly increased during no smoking events compared to smoking events under medication (mean = 3.06, SE= 0.30 versus mean = 1.90, SE = 0.28; t (1, 14) = 2.83, p = .004).
4. Discussion
The findings of the study suggest that ADHD medication may aid in reducing smoking and nicotine withdrawal symptoms in smokers with ADHD. More specifically, the experimental phase showed that ADHD medication improves CPT performance and ameliorates withdrawal symptoms during smoking abstinence. In particular, errors of omission were reduced in the three medication and smoking conditions (i.e., medication + smoking, medication + abstinence, placebo + smoking) compared to the placebo with abstinence condition, which supports the notion that appropriate ADHD medication may mimic some of the behavioral effects of nicotine (Gehricke et al., 2009, Gehricke et al., 2007). Although the errors of omission were within the normal range and do not have direct clinical application, the findings suggest that participants were able to better sustain attention during the three medication and smoking conditions compared to the placebo with abstinence condition. These improvements in attention are similar to those found in response to methylphenidate in children with ADHD (Epstein et al., 2006; Solanto et al., 2009). In addition, ADHD medication with abstinence compared to placebo with abstinence significantly reduced total scores on the Shiffman-Jarvik withdrawal questionnaire and the physiological withdrawal subscale, corroborating previous findings (Ray et al., 2009).
The findings of the field monitoring phase complimented the results of the experimental phase by showing a reduction in salivary cotinine associated with medication administration, which suggests that ADHD medication may reduce nicotine intake in smokers with ADHD. This corroborates previous research (Covey et al., 2009), which revealed that ADHD medication might aid smoking cessation in smokers with ADHD based on expired CO and self-reported smoking abstinence.
Although ADHD medication did not ameliorate smoking urge, it reduced the intensity of difficulty concentrating in the eDiary during the field monitoring phase. More specifically, difficulty concentrating was reduced under medication during no smoking and stress events compared to placebo and no smoking and stress events. This finding suggests that medication may aid concentration during abstinence and stress. Medication also reduced difficulty concentrating during no stress compared to stress events when smoking. In addition, stress affected ADHD symptom ratings in the eDiary. Difficulty concentrating was reduced under no stress compared to stress events during placebo. Stress also increased impulsivity and impatience during no smoking events independent of medication. Moreover, restlessness was increased by stress, in particular during no smoking events.
The findings of the present study have to be interpreted with caution because of the small sample size, lack of control groups, short duration of the study, and administration of multiple doses of stimulant and non-stimulant medications with both immediate and extended release formulations. In addition, the reduced cotinine levels found in response to ADHD medication may be caused not only by reduced smoking frequency but also by changes in smoking topography and nicotine to cotinine metabolism. Moreover, impulsivity was not assessed in the experimental phase with a Go-NoGo paradigm. Another limitation was that the frequency of self-initiated diary entries (indicating a smoking event) was affected by medication condition, which may have made the diary recordings more reliable during medication compared to placebo administration. More research is necessary to replicate the findings and to study the long-term effects of ADHD medication treatment on tailored smoking cessation for smokers with ADHD.
Despite such caveats the study findings suggest that tailored smoking cessation for individuals with ADHD may be benefitted by ADHD medication to reduce nicotine intake and alleviate nicotine withdrawal during abstinence. Depending on age, a tailored smoking cessation may consist of ADHD medication, cognitive behavioral interventions (Perkins et al., 2008), and Nicotine Replacement Therapy (NRT) such as nicotine patches, nicotine gum, and nicotine inhaler. For smokers with ADHD who are minors, cognitive-behavioral intervention for smoking cessation should be linked with ADHD medication administration. For adult smokers with ADHD who have a longer history of smoking and who may have higher levels of nicotine dependence, NRT may be added to reduce severe nicotine withdrawal. However, given the effects of NRT and ADHD medication on cardiovascular activity (Gehricke et al., 2009, Gehricke et al., 2006), blood pressure should be monitored regularly during such intervention. Such individualized smoking cessation may increase the rates for smoking cessation and long-term abstinence in individuals with ADHD.
Acknowledgments
This study was supported by NIDA grant DA018752 and NCRR grant RR00827 to Jean-G. Gehricke. We thank Dr. Kenneth Steinhoff for his clinical contributions and Dr. Douglas Granger and Salimetrics for essaying the salivary cotinine samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Barkley RA, Murphy KR, O'Connell T, Connor DF. Effects of two doses of methylphenidate on simulator driving performance in adults with attention deficit hyperactivity disorder. J Safety Res. 2005;36:121–31. doi: 10.1016/j.jsr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Burke JD, Loeber R, Lahey BB. Which aspects of ADHD are associated with tobacco use in early adolescence? J Child Psychol Psychiatry. 2001;42:493–502. [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biological Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Businelle MS, Kendzor DE, Reitzel LR, Costello TJ, Cofta-Woerpel L, Li Y, et al. Mechanisms linking socioeconomic status to smoking cessation: a structural equation modeling approach. Health Psychol. 2010;29:262–73. doi: 10.1037/a0019285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacol Bull. 1996;32:67–73. [PubMed] [Google Scholar]
- Covey LS, Hu MC, Winhusen T, Weissman J, Berlin I, Nunes EV. OROS-methylphenidate or placebo for adult smokers with attention deficit hyperactivity disorder: racial/ethnic differences. Drug Alcohol Depend. 2009;110:156–9. doi: 10.1016/j.drugalcdep.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross EM, Chaffin WW. Use of binomial theorem in interpreting results of multiple tests of significance. Educational Psychology and Measurement. 1982;42:25–34. [Google Scholar]
- Davis B. California Tobacco Survey [electronic version] 2005 Questionnaire. 2005:1–16. from http://www.dhs.ca.gov/tobacco/documents/eval/Survey_CATS2005.pdf.
- Epstein JN, Conners CK, Hervey AS, Tonev ST, Arnold LG, Abikoff HB, et al. Assessing medication effects in the MTA study using neuropsychological outcomes. J Child Psychol Psychiatry. 2006;47:446–56. doi: 10.1111/j.1469-7610.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer MG, Williams JBW. The structured clinical interview for DSM-IV axis I disorders. New York: New York State Psychiatric Institute; 1995. Patient edition. [Google Scholar]
- Gehricke JG, Hong N, Whalen CK, Steinhoff K, Wigal TL. Effects of transdermal nicotine on symptoms, moods, and cardiovascular activity in the everyday lives of smokers and nonsmokers with attention-deficit/hyperactivity disorder. Psychol Addict Behav. 2009;23:644–55. doi: 10.1037/a0017441. [DOI] [PubMed] [Google Scholar]
- Gehricke JG, Loughlin SE, Whalen CK, Potkin SG, Fallon JH, Jamner LD, et al. Smoking to self-medicate attentional and emotional dysfunctions. Nicotine Tob Res. 2007;9 (Suppl 4):S523–36. doi: 10.1080/14622200701685039. [DOI] [PubMed] [Google Scholar]
- Gehricke JG, Whalen CK, Jamner LD, Wigal TL, Steinhoff K. The reinforcing effects of nicotine and stimulant medication in the everyday lives of adult smokers with ADHD: A preliminary examination. Nicotine Tob Res. 2006;8:37–47. doi: 10.1080/14622200500431619. [DOI] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–44. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Canu W, March J. Effects of chronic nicotine and methylphenidate in adults with attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2001;9:83–90. doi: 10.1037/1064-1297.9.1.83. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Wilens T, Chu MP. Associations between ADHD and psychoactive substance use disorders. Findings from a longitudinal study of high-risk siblings of ADHD children. Am J Addict. 1997;6:318–29. [PubMed] [Google Scholar]
- Molina BS, Pelham WE., Jr Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Conklin CA, Levine MD. Cognitive-Behavorial Therapy for Smoking Cessation: a practical guidebook to the most effective treatments. New York: Routledge; 2008. [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7:373–8. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2004;176:182–94. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Ray R, Rukstalis M, Jepson C, Strasser A, Patterson F, Lynch K, et al. Effects of atomoxetine on subjective and neurocognitive symptoms of nicotine abstinence. J Psychopharmacol. 2009;23:168–76. doi: 10.1177/0269881108089580. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Waldrop JJ, Reynolds CR, Lowe P. Effects of stimulants on the continuous performance test (CPT): implications for CPT use and interpretation. J Neuropsychiatry Clin Neurosci. 2001;13:326–35. doi: 10.1176/jnp.13.3.326. [DOI] [PubMed] [Google Scholar]
- Shaw D, al'Absi M. Attenuated beta endorphin response to acute stress is associated with smoking relapse. Pharmacol Biochem Behav. 2008;90:357–62. doi: 10.1016/j.pbb.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 1976;50:35–9. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Solanto M, Newcorn J, Vail L, Gilbert S, Ivanov I, Lara R. Stimulant drug response in the predominantly inattentive and combined subtypes of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:663–71. doi: 10.1089/cap.2009.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AJ, Shores EA, Trollor JN, Lee T, Sachdev PS. Neuropsychological functioning of adults with attention deficit hyperactivity disorder. J Clin Exp Neuropsychol. 2000;22:115–24. doi: 10.1076/1380-3395(200002)22:1;1-8;FT115. [DOI] [PubMed] [Google Scholar]
- Weiss M, Murray C. Assessment and management of attention-deficit hyperactivity disorder in adults. CMAJ. 2003;168:715–22. [PMC free article] [PubMed] [Google Scholar]
- Wigal TL, Wigal SB, Steinhoff K, Kollins S, Newcorn JH, Steinberg-Epstein R, et al. Establishing a Clinical Diagnosis of ADHD in Adults: The QUEST Method. Advances in ADHD. 2007;2:17–24. [Google Scholar]
- Zillessen KE, Scheuerpflug P, Fallgatter AJ, Strik WK, Warnke A. Changes of the brain electrical fields during the continuous performance test in attention-deficit hyperactivity disorder-boys depending on methylphenidate medication. Clin Neurophysiol. 2001;112:1166–73. doi: 10.1016/s1388-2457(01)00535-1. [DOI] [PubMed] [Google Scholar]


