Abstract
Sirtuins (class III histone deacetylase) are evolutionarily conserved NAD+-dependent enzymes that catalyze the deacetylation of acetyl-lysine residues of histones and other target proteins. Because of their associations in various pathophysiological conditions, the identification of small molecule modulators has been of significant interest. In the present study, virtual screening was carried out with NCI Diversity Set II using crystal structure of hSIRT2 (PDB ID: 1J8F) as a model for the docking procedure to find potential compounds, which were then subjected to experimental tests for their in vitro SIRT2 inhibitory activity. One of the 40 compounds tested, NSC671136 (IUPAC name: 6-Acetyl-4-oxo-1,3-diphenyl-2-thioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-5-yl 2,4-dichlorobenzoate) has structurally unique scaffold, showed strong inhibitory activity towards SIRT2 with IC50 of ~8.7 μM and to a lesser extent on SIRT1 activity. The reported compound is substantially potent compared to the published SIRT2 inhibitors and serves as an excellent base for future lead development.
Keywords: Sirtuins, SIRT2, Deacetylase, Virtual screening, Inhibitor
Introduction
Sirtuins are class III histone deacetylase enzymes that are unique with respect to their requirement of NAD as a co-substrate for catalysis (Imai et al. 2000). They are evolutionarily conserved from bacteria to mammals and share no sequence similarity with the classical HDAC enzymes (Yang and Seto 2007). Their role has been reported in diverse cellular phenomena like gene silencing, genomic stability, stress resistance, cell division, metabolism and apoptosis (Lavu et al. 2008; Milne and Denu 2008; Picard et al. 2004). In addition, by favorable regulation of stress management, and energy homeostasis they have been shown to influence longevity in several species (Tissenbaum and Guarente 2001; Cohen et al. 2004). Because of their diverse functions in normal physiology and diseased conditions, they generated enormous interest as potential therapeutic targets for pharmacologic intervention.
Sirtuins possess an approximately 275 amino acid catalytic core region, showing high degree of structural similarity among known sirtuin structures (Sanders et al. 2010). The catalytic core region takes on an elongated shape and can be distinguished into a larger Rossmann-fold domain and a smaller zinc binding domain. The loop regions that connect the two domains form an extended cleft between the two domains where NAD and acetyl lysine containing substrates bind the enzyme (Sanders et al. 2010; Min et al. 2001). The amino acids involved in catalysis and the reactive groups of both the substrates are buried within a hydrophobic tunnel in the cleft (Sanders et al. 2010). Some sirtuins posses significant extensions flanking the conserved core region at N and C termini. Evidence suggests that these extensions may promote substrate binding specificity (Cuperus et al. 2000).
The mammalian ortholog of yeast Sir2, SIRT1, is primarily a nuclear sirtuin but evidence shows that it also exits in the cytoplasm. Being the most extensively studied of all the sirtuins various SIRT1 targets have been identified (at least 34 distinct proteins) with roles in diverse processes including metabolism, stress response, differentiation and circadian rhythms. Concomitant with the pleotrophic nature of its activity SIRT1 has been implicated in several pathological conditions including metabolic, neurodegenerative diseases and cancer (Lavu et al. 2008; Milne and Denu 2008; Picard et al. 2004).
SIRT2 constantly moves in and out of nucleus, with a Crm-dependent nuclear export signal maintaining the majority of the protein in the cytoplasm (North and Verdin 2007), but becomes predominantly localized to nucleus during mitosis where it plays a prominent role in regulating mitotic exit from the cell cycle (Dryden et al. 2003). Primarily SIRT2 deacetylates lysine-40 of α Tubulin, both in vitro and in vivo (Inoue et al. 2007; North et al. 2003). In addition, P53 has also been found to be one of the substrates of SIRT2 (Jin et al. 2008) opening the door for cancer treatment by SIRT2 inhibition. SIRT2 was also found to block the entry to chromosome condensation in glioma cell lines in response to mitotic stress caused by microtubule inhibitors (MTIs). Accordingly, inhibition of SIRT2 can be used in combination to cancer chemotherapy by MTIs. Further, Inhibition of SIRT2 showed neuroprotection in cellular models of Huntington’s and Parkinson’s diseases (Outeiro et al. 2007).
The apo-structure of human SIRT2 catalytic core domain is known (Finnin et al. 2001). Till date, several small-molecule SIRT2 modulators have been identified and these inhibitors are predicted to alter the catalytic activity by competing with either the NAD+/acetyl-lysine peptide-binding site, or binding to other unknown allosteric sites on the enzymes. The NAD+-dependent deacetylase activity is inhibited by the NAD+ hydrolysis product nicotinamide. HDAC inhibitors hold great promise as new anticancer agents, and a number of these inhibitors have already entered into clinical trials (Johnstone 2002; Marks et al. 2004; McLaughlin and La Thangue 2004). Because of the diverse processes that class III HDACs (sirtuins) regulate there is an increasing interest in the development of their modulators. Some small molecule inhibitors for sirtuins have already been described in the literature (Tervo et al. 2004; Kiviranta et al. 2006; 2007). The crystal structure of SIRT2 was used as a starting point for virtual screening with NCI Diversity Set II towards finding structurally diverse potential compounds. Here, we report a novel nucleoside analog, NSC671136 (IUPAC name: 6-Acetyl-4-oxo-1,3-diphenyl-2-thioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-5-yl 2,4-dichlorobenzoate), a very potent SIRT2 inhibitor (hereafter, NSC671136).
Materials and methods
Virtual screening calculations
The AutoDock 4.0 (AD4) software as implemented through the graphical user interface called AutoDockTools (ADT) (Morris et al. 1998; Huey et al. 2007) was used to dock ligands to SIRT2. The human SIRT2 crystal structure was used as a model for the docking procedure (PDB ID: 1J8F; Finnin et al. 2001). The charge values for all atoms were generated automatically by ADT. The docking area was assigned visually around the acetylated peptide binding site. A grid of 40 × 40 × 40 Å with 0.375 Å spacing was calculated around the docking area for 13 ligand atom types using AutoGrid4. For virtual screening, compound structures of the NCI Diversity Set II were prepared using the ZINC database server (http://zinc.docking.org/upload.shtml) to take into account the different protomeric and tautomeric states of each compound. All the ligands were then converted in the AutoDock format file (pdbqt format). For each ligand, 150 separate docking calculations were performed. Each docking calculation consisted of 1.75 million energy evaluations using the Lamarckian genetic algorithm local search (GALS) method. The GALS method evaluates a population of possible docking solutions and propagates the most successful individuals from each generation into the subsequent generation of possible solutions. A low-frequency local search according to the method of Solis and Wets is applied to docking trials to ensure that the final solution represents a local minimum. All dockings described in this paper were performed with a population size of 150, and 300 rounds of Solis and Wets local search were applied with a probability of 0.06. A mutation rate of 0.02 and a crossover rate of 0.8 were used to generate new docking trails for subsequent generations, and the best individual from each generation was propagated to the next generation. The docking results from each of the eight calculations were clustered on the basis of root-mean square deviation (rmsd) between Cartesian coordinates of the atoms and were ranked on the basis of free energy of binding. The top-ranked compounds were visually inspected for good chemical geometry. Pictures of the modeled ligand/enzyme complex were produced by PyMol (http://www.pymol.org).
Compounds determined by AD4 to have low binding energies to SIRT2 were requested in a group of 40 and received from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute (http://dtp.nci.nigh.gov/branches/dscb/repo_request.html). Chemical compounds were dissolved in DMSO to 10 mM final concentration and stored at room temperature.
Cloning and expression of hSIRT1 and hSIRT2
The human SIRT1 and SIRT2 full length clones were purchased from Saf Labs and the regions spaning 195–530 aa (SIRT1) and 34–356 aa (SIRT2) were amplified. The resulting amplicons and the pET-28a bacterial expression vector (Novagen) were digested with NdeI and XhoI endonucleases, purified using gel extraction kit (GE) and ligated by T4 DNA ligase (Invitrogen). The ligation mixture was transformed into DH5α competent cells and selected on LB-agar plates containing 30 μg/ml kanamycin sulfate. The obtained clones were confirmed by sequencing using standard T7 forward and reverse primers. The recombinant plasmids were transformed into E. coli strain, Rossetta (Novagen), and the transformants were selected on LB-agar plates containing appropriate antibiotics (30 μg/ml kanamycin, 25 μg/ml chloramphenicol). The fresh colonies were inoculated into 1L of fresh LB medium and grown at 37°C, 180 rpm until OD600 reached a value of about 0.6. At this point, expression was induced by adding IPTG to the final concentration of 0.5 mM, and the bacteria were further cultivated at 30°C for 4 h. Harvest the cells by centrifugation at 6,000 rpm for 3–5 min.
Purification of hSIRT1 and hSIRT2
The cell pellets from expression were resuspended in pre-cooled lysis buffer (20 mM Tris-Hcl, pH 7.4, 500 mM NaCl, 10 mM β-mercaptoethanol, 5% glycerol) and lysed by sonication. The cell lysate was centrifuged at 13,000 rpm for 30 min to remove the unbroken cell and debris. The supernatant was loaded into Ni–NTA affinity column equilibrated with the lysis buffer. Non-specifically bound proteins were removed by washing with lysis buffer containing 20 mM imidazole. Protein was eluted with lysis buffer containing 250 mM imidazole. The obtained fractions were analysed by 12% SDS-PAGE and fraction with the highest protein concentration were pooled. Imidazole was removed by buffer exchange with the lysis buffer containing 150 mM NaCl and the resultant protein was concentrated further. The concentrated protein was loaded onto a size-exclusion column (Superdex S-75 (16/60), GE healthcare) equilibrated with 20 mM Tris-HCl pH 8.0, 150 mM NaCl, 5% glycerol and 4 mM DTT at 4°C. Protein purity was checked by 12% SDS-PAGE and fraction containing purified hSIRT1 and hSIRT2 were collected and concentrated with Amicon Ultra filter (cut-off 10 kDa, Millipore) to 5 mg/ml.
Biochemical assays
The biochemical assay for recombinant human SIRTs was based on the SIRT Fluorimetric Drug Discovery Kit (AK-555, AK-556 Biomol, Plymouth Meeting, PA). In this assay, aminomethylcoumarin (AMC) conjugated acetyl lysine is deacetylated by SIRT enzymes in the presence of NAD, followed by the addition of a developer solution containing proteolytic enzyme that cleaves the peptide and releases the fluorescent AMC. All the reactions were carried out in 96 well white plate supplied by the kit, in a reaction buffer comprising 50 mM Tris/Cl, pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 1 mg/ml BSA in the presence of 2% DMSO. Initially, compounds were diluted in the reaction buffer placed in the reaction wells at concentrations ranging from 5 μM to 1 mM, followed by addition of enzyme to a total volume of 25 μl. The reaction was initiated by the addition of 25 μl of 2× substrate solution containing 25 μM Fluor De Lys Peptide substrate and 500 μM NAD. After 1 h incubation 50 μl of developer solution containing 2 mM nicotinamide was added to stop the reaction. Readings were taken in PerkinElmer VICTOR 1420 Multilabel Plate Reader with excitation set at 355 nm and emission measured at 460 nm. IC50 measurement and curve fitting were performed using GraphPad Prism software.
Results and discussion
Docking studies
All the known sirtuin structures contain a conserved catalytic core of 270 aa with variable N- and C-terminal regions. The catalytic core consists of large classical Rossmann-fold domain and a small zinc binding domain (Fig. 1a). The interface between these two domains contains large groove which is generally subdivided into three pockets, based on the interaction of adenine, ribose and nicotinamide, which are parts of the NAD+ cofactor. The acetylated peptide substrate (histone or non-histone substrates) binds to the SIRT2 protein at the above mentioned large groove (Cosgrove et al. 2006). The acetylated lysine residue binds to the conserved hydrophobic pocket, near to the NAD+ cofactor binding region. Since the substrate and the cofactor binding regions are highly conserved between human and bacterial sirtuin, the apo-form of the human SIRT2 crystal structure (Finnin et al. 2001) (PDB ID: 1J8F) was used to obtain potential inhibitors by virtual screening method.
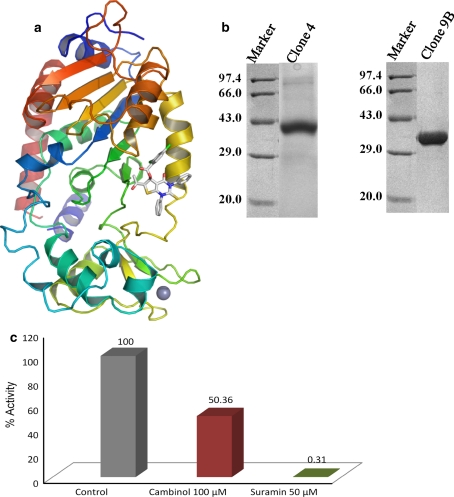
Fig. 1.
The biologically active hSIRT1 and hSIRT2. a The tertiary structure of the HDAC domain of hSIRT2 (PDB ID: 1J8F), used for docking studies, is drawn as ribbon model, colored from blue (N-terminus) to red (C-terminus). The ligand, NSC671136, shown by sticks, binds at the putative binding site of hSIRT2. The grey sphere represents the Zn2+ ion. b An SDS-PAGE profile of the purified HDAC domain of hSIRT1 (left panel) and hSIRT2 (right panel). Lane 1 on each panel represents the molecular weight marker, and lane 2 on each panel indicates hSIRT1 and hSIRT2, respectively. Electrophoresis was done with a 12% SDS polyacrylamide gel, which was stained with Coomassie Brilliant Blue. c Functional assay for hSIRT2. HDAC fluorimetric assay was performed using hSIRT2 protein produced in our lab in the presence of either 100 μM cambinol or 50 μM suramin. Activity of the SIRT2 protein was reduced by half in the presence of 100 μM cambinol and to totally negligible levels in the presence of 50 μM suramin
The new version of AutoDock (AD4) program (Huey et al. 2007; Morris et al. 1998) was used to perform virtual ligand screening (VLS) of the National Cancer Institute (NCI) Diversity Set II against human SIRT2 crystal structure. The VLS results were sorted on the basis of their predicted binding free energies (ΔGAD4), which ranged from -5.0 to -9.31 kcal/mol, and according to the cluster size for each docking conformations. Solutions with a cluster size lower than 20 out of 100 individuals were discarded. The predicted binding conformations for the selected compounds from this pool were visually checked carefully. The potential 40 compounds were obtained from NCI (The NCI/DTP Open Chemical Repository) to perform the binding assay.
Expression and purification of hSIRT1 and hSIRT2
The ability to produce large quantities of correctly folded hSIRT1 and hSIRT2 is an essential prerequisite for both in vitro screening and structure studies. Here, an E. coli expression system was chosen, since it is a more rapid and easier way of expressing relatively large amounts of proteins compared to the eukaryotic system available. The choice of a suitable fusion protein system and E. coli strain, as well as an extensive optimization of expression and purification condition, resulted in obtaining 4.0 mg of pure soluble protein from 1 L of culture. Coomassie Brilliant Blue-stained SDS-PAGE analysis indicated that the recombinant hSIRT2 was greater than 95% purity and the major contaminants were removed (Fig. 1b, right panel). In the case of hSIRT1, it showed about 85% purity (Fig. 1b, left panel). The hSIRT1 and hSIRT2 proteins were finally concentrated to about 5.0 mg/ml, which were subsequently used for in vitro screening against NCI compounds using SIRT2 fluorescent activity assay/discovery kit (AK-556; BIOMOL).
Enzyme inhibition
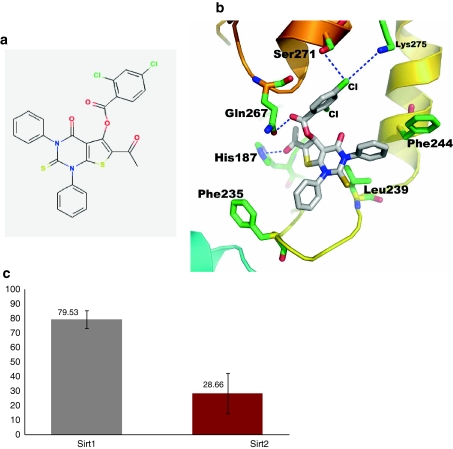
Prior to perform screening against NCI compounds, the HDAC activity of hSIRT2 was confirmed by florescence based HDAC assay using the BIOMOL discovery kit (Fig. 1c). We also have checked the inhibition activity against hSIRT2 for the known inhibitors cambinol and suramin (Fig. 1c). The behavior of inhibition activity for these two compounds is very similar to published report. We then performed the HDAC assay with the 40 compounds obtained from Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, against His-tagged hSIRT2. Initial screening was done at 500 μM inhibitor concentration. At this concentration, different compounds showed different potencies (data not shown) of which NSC671136 showed the greatest potency in terms of inhibition of enzyme activity. The compound NSC671136 corresponds to the IUPAC name of 6-Acetyl-4-oxo-1,3-diphenyl-2-thioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-5-yl 2,4-dichlorobenzoate (Fig. 2a). When we analyzed the structure of the modeled complex, the ligand nicely binds to the SIRT2 protein at the acetylated peptide binding pocket (Fig. 1a, 2b). The phenyl rings of the ligand are positioned in the hydrophobic pocket produced by the conserved Phe235 and Phe244 residues. The pyrimidin base may contribute p-interaction with Leu239. Intriguingly, the dichlorobenzoate moiety contributes hydrophilic interactions with the non-conserved residues, Gln267, Ser271 and Lys275. Also, it is interesting to note that, to our knowledge, this is the first identified nucleoside analog compound to inhibit the SIRT2 deacetylase activity.
Fig. 2.
Identification of NSC671136 as an inhibitor of hSIRT1 and hSIRT2. a The chemical structure of NSC671136. b A close-up view of a representative docked conformation of NSC671136 binds at the putative binding pocket of hSIRT2. The putative interacting amino acids at the binding pocket region are shown by sticks. c Comparison of NSC671136 mediated inhibition of hSIRT1 and hSIRT2 enzymes. Recombinant His-tagged human SIRT1 and SIRT2 proteins were assayed for deacetylase activity using the HDAC fluorescent activity assay. Results are expressed as the relative activity versus the control whose value is taken as 100% activity. All the values are the average of three independent experiments performed in duplicates
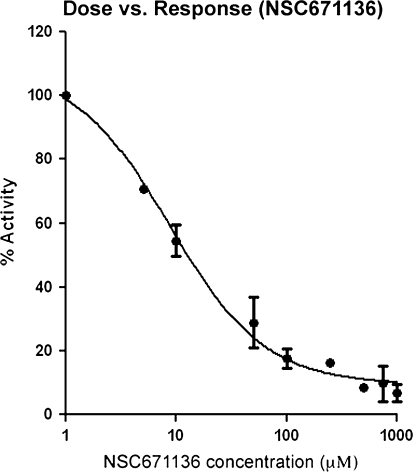
To test the specificity of NSC671136 towards SIRT2, the same assay was performed using both SIRT1 and SIRT2 at constant inhibitor concentration of 50 μM. As shown in Fig. 2c, NSC671136 inhibited SIRT1 (20% inhibition compared to control) albeit to a lesser extent when compared to SIRT2 (71% inhibition compared to control) at constant inhibitor concentration. This compound was taken further to determine IC50 value using the same assay kit. As shown in Fig. 3, NSC671136 shows a dose dependent inhibition of SIRT2 activity which rose to 72% at 50 μM concentration. IC50 was calculated to be 8.7 μM by GraphPad Prism software. The identified compound is more potent than Cambinol (IC50 of 59 μM) (Heltweg et al. 2006), Sirtinol (IC50 of 38 μM) (Cen 2009) and other published SIRT2 inhibitors (Huhtiniemi et al. 2008, Huber et al. 2010). These studies suggest that the NSC671136 compound may selectively inhibit hSIRT2 over hSIRT1 and thus, NSC671136 appears to be a promising initial hit geared up for further development.
Fig. 3.
Dose vs response (IC50) curve of NSC671136 against His-tagged human SIRT2 enzyme. The curve fitting and IC50 determination of NSC671136 was performed using GraphPad Prism 5.0 software. Each data represents the average of triplicate samples
In conclusion, we purified bacterially expressed human sirtuins SIRT1 and SIRT2, and showed that it has NAD+-dependent deacetylase activity. In addition, we also have performed virtual screening of compounds from the NCI database to identify low micromolar inhibitors of therapeutic sirtuin proteins, SIRT1 and SIRT2. The compound, NSC671136 described here is the first nucleoside-analog scaffold inhibitor of these enzymes. Furthermore, fluorimetric assay revealed that the abovementioned inhibitor is more specific to hSIRT2 compared to hSIRT1, and hence, it may form the basis for development of potential inhibitors of hSIRT2 from this scaffold analog.
Acknowledgments
We thank Drs. Javed Iqbal and Devyani Haldar for allowed us to utilize their biological facilities as well as for their constant support during our experimental work at Institute of Life Sciences (ILS, a not-for-profit organization), University of Hyderabad campus, Gachibowli, Hyderabad.
Footnotes
Padavattan Sivaraman and Suresh Mattegunta contributed equally to this work.
References
- Cen Y. Sirtuins inhibitors: the approach to affinity and selectivity. Biochim Biophys Acta. 2009;1804:1635–1644. doi: 10.1016/j.bbapap.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Cosgrove MS, Bever K, Avalos JL, Muhammad S, Zhand X, Wolberger C. The structural basis of sirtuin substrate affinity. Biochemistry. 2006;45:7511–7521. doi: 10.1021/bi0526332. [DOI] [PubMed] [Google Scholar]
- Cuperus G, Shafaatian R, Shore D. Locus specificity determinants in the multifunctional yeast silencing protein Sir2. EMBO J. 2000;19:2641–2651. doi: 10.1093/emboj/19.11.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. 2003;23:3173–3185. doi: 10.1128/MCB.23.9.3173-3185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnin MS, Donigian JR, Pavletich NP. Structure of the histone deacetylase SIRT2. Nat Struct Biol. 2001;8:621–625. doi: 10.1038/89668. [DOI] [PubMed] [Google Scholar]
- Heltweg B, Gatbonton T, Schuler AD, Posakony J, Li H, Goehle S, Kollipara R, DePinho RA, Gu Y, Simon JA, Bedalov A. Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res. 2006;66:4368–4377. doi: 10.1158/0008-5472.CAN-05-3617. [DOI] [PubMed] [Google Scholar]
- Huber K, Schemies J, Uciechowska U, Wagner JM, Rumpf T, Lewrick F, Seuss R, Sippl W, Jung M, Bracher F. Novel 3-arylideneindolin-2-ones as inhibitors of NAD+-dependent histone deacetylases (sirtuins) J Med Chem. 2010;53:1383–1386. doi: 10.1021/jm901055u. [DOI] [PubMed] [Google Scholar]
- Huey R, Morris GM, Olson AJ, Goodsell DS. A semi-empirical free energy force field with charge-based desolvation. J Comput Chem. 2007;28:1145–1152. doi: 10.1002/jcc.20634. [DOI] [PubMed] [Google Scholar]
- Huhtiniemi T, Suuronen T, Valtteri MR, Wittekindt C, Lahtela-Kakkonen M, Jarho E, Walle′n EAA, Salminen A, Poso A, Leppänen J. Oxadiazone-carbonylaminothioureas as SIRT1 and SIRT2 inhibitors. J Med Chem. 2008;51:4377–4380. doi: 10.1021/jm800639h. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hiratsuka M, Osaki M, Oshimura M. The molecular biology of mammalian SIRT proteins: SIRT2 in cell cycle regulation. Cell Cycle. 2007;6:1011–1108. doi: 10.4161/cc.6.9.4219. [DOI] [PubMed] [Google Scholar]
- Jin YH, Kim YJ, Kim DW, Baek KH, Kang BY, Yeo CY, Lee KY. Sirt2 interacts with 14–3-3 beta/gamma and down-regulates the activity of p53. Biochem Biophys Res Commun. 2008;368:690–695. doi: 10.1016/j.bbrc.2008.01.114. [DOI] [PubMed] [Google Scholar]
- Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- Kiviranta PH, Leppanen J, Kyrylenko S, Salo HS, Lahtela-Kakkonen M, Tervo AJ, Wittekindt C, Suuronen T, Kuusisto E, Jarvinen T, Salminen A, Poso A, Wallen EA. N,N′-Bisbenzylidenebenzene-1,4-diamines and N,N′-Bisbenzylidenenaphthalene-1,4-diamines as Sirtuin Type 2 (SIRT2) Inhibitors. J Med Chem. 2006;49:7907–7911. doi: 10.1021/jm060566j. [DOI] [PubMed] [Google Scholar]
- Kiviranta PH, Leppanen J, Rinne VM, Suuronen T, Kyrylenko O, Kyrylenko S, Kuusisto E, Tervo AJ, Jarvinen T, Salminen A, Poso A, Wallen EA. N-(3-(4-Hydroxyphenyl)-propenoyl)-amino acid tryptamides as SIRT2 inhibitors. Bioorg Med Chem Lett. 2007;17:2448–2451. doi: 10.1016/j.bmcl.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins—novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- Marks PA, Richon VM, Miller T, Kelly WK. Histone deacetylase inhibitors. Adv Cancer Res. 2004;91:137–168. doi: 10.1016/S0065-230X(04)91004-4. [DOI] [PubMed] [Google Scholar]
- McLaughlin F, La Thangue NB. Histone deacetylase inhibitors open new doors in cancer therapy. Biochem Pharmacol. 2004;68:1139–1144. doi: 10.1016/j.bcp.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Milne JC, Denu JM. The Sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol. 2008;12:11–17. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Min J, Landry J, Sternglanz R, Xu RM. Crystal structure of a SIR2 homolog-NAD complex. Cell. 2001;105:269–279. doi: 10.1016/S0092-8674(01)00317-8. [DOI] [PubMed] [Google Scholar]
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Algorithm and empirical binding free energy function. J Comput Chem. 1998;19:1639–1662. doi: 10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B. [DOI] [Google Scholar]
- North BJ, Verdin E. Interphase nucleocytoplasmic shuttling and localization of SIRT2 during mitosis. PLoS ONE. 2007;2:e784. doi: 10.1371/journal.pone.0000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/S1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders BD, Jackson B, Marmorstein R. Structural basis for sirtuin function: what we know and what we don’t, Biochim. Biophys Acta. 2010;1804:1604–1616. doi: 10.1016/j.bbapap.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo AJ, Kyrylenko S, Niskanen P, Salminen A, Leppanen J, Nyronen TH, Jarvinen T, Poso A. An in silico approach to discovering novel inhibitors of human sirtuin type 2. J Med Chem. 2004;47:6292–6298. doi: 10.1021/jm049933m. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Seto E. HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]