Abstract
The capacity of mesenchymal stem cells (MSCs) to differentiate into intervertebral disc (IVD)-like cells has been well described, but their ability to modulate the inflammatory processes in the IVD remains unclear. We found that tissue obtained by discectomy of degenerated and post-traumatic IVD contains significant amounts of IgG antibodies, a sign of lymphocyte infiltration. Further we investigated whether MSCs in vitro, which were characterized for their multilineage differentiation potential and may have immunomodulatory effects on IVD fragments. IVD fragments were co-cultured in contact with peripheral blood lymphocytes (PBLs) and MSCs, and as functional controls we used contact co-cultures of PBLs stimulated with pokeweed mitogen (2.5 μg/mL) and MSCs. The time course of lymphocyte proliferation (Alamar Blue), IgG (ELISA) and gene expression (RT-PCR) of anti-inflammatory cytokines (TGF-β1, IL-10) by MSCs and pro-inflammatory molecules (IL-1α, IL-1β and TNF-α) by the IVD fragments were analyzed. Depending on the response to the presence of MSCs, the IVD fragments (n = 13) were divided in two groups: responders (n = 9), where inflammation was inhibited by MSCs and non-responders (n = 4), where MSCs did not decrease inflammation. At 1 week in co-culture, MSCs reduced significantly the IgG production in the IVD responders group to 69% and PBLs proliferation to 57% of the control. MSCs expression of the anti-inflammatory TGF-β1 increased with time, while IL-10 was expressed only at day 1. IVD gene expression of TNF-α decreased constantly, whereas IL-1α and IL-1β expression increased. In conclusion, these data suggest that MSCs may modulate disc-specific inflammatory and pain status and aid regeneration of the host tissue.
Keywords: Mesenchymal stem cells, Intervertebral discs, Peripheral blood lymphocytes, Immunomodulation
Introduction
Chronic back pain is a common disorder which has been associated with degeneration of the intervertebral disc (IVD) [1]. The IVDs structure is finely regulated and failure in balancing the processes of matrix formation and degradation leads to degeneration of the tissue. During degeneration, the cell loss [2] and decrease in proteoglycan content lead to [3] progressive loss of space between vertebrae and instability, which triggers other events, such as and nerve and vessel ingrowth [4, 5]. The local production of matrix-degrading enzymes is a key event leading to degeneration, but it must be also considered in the context of the upstream molecules, such as cytokines, which regulate enzyme synthesis. IVD cells have a capacity to express a range of cytokines [6, 7], including pro-inflammatory molecules, such as both isoforms of interleukin-1, alpha (IL-1α) and beta (IL-1β) [8] and tumor necrosis factor alpha (TNF-α) [9]. It has been proposed that IL-1 can regulate IVD cell expression of key matrix-degrading enzymes in vitro [10] and in vivo [11], placing this cytokine as a main factor inducing degeneration.
In the healthy IVD, the expression of IL-1 is matched by its natural inhibitor IL-1Ra, but during degeneration IL-1α and Il-1β gene expression is upregulated, disrupting the balance with the inhibitor [8]. TNF-α is widely expressed in normal and degenerated IVDs, but data about this gene are controversial. Seguin et al. [12] described that, in bovine nucleus pulposus cells, TNF-α reduced synthesis of extracellular matrix and increased expression of matrix-degrading enzymes, while later Le Maitre et al. [13] showed that TNF-α had no impact on human matrix degrading activity and also found that native IVD cells expressed low levels of TNF-α receptor, which were further decreased during degeneration. Thus, TNF-α produced by IVD cells may have a pro-inflammatory effect either on the surrounding tissues (muscles, bones or nerves) or on lymphocytes which infiltrated the IVD tissue during traumatic or degenerative disruption of the vertebral end plates. These events can lead to swelling, acute and chronic discogenic pain, symptoms that currently are treated either conservatively with steroid and non-steroid anti-inflammatory drugs or surgically. Both approaches have their limitations, including various side effects and above all, treating the symptoms and not the underlying process of degeneration.
Recently, much attention has been given to the potential use of stem cells in regenerative treatment of the IVD. Mesenchymal stem cells (MSCs), characterized by their relatively easy availability, their capacity to proliferate rapidly and differentiate in vitro and in vivo, have been frequently associated with tissue engineering and cell-based therapies aimed to replace damaged tissues. MSCs have been used to repair injured tissue and to replace necrotic and apoptotic cells in many animal models of human diseases [14]. Despite the MSCs’ ability to differentiate both in vitro and in vivo, it has been shown that in the majority of cases, the observed improvements were not a clear result of the engraftments [15]. In one of the first human trials of MSC implantation [16], children affected by osteogenesis imperfecta had bone marrow transplantation and, after several years, they received MSCs from the same donor. The procedure resulted in substantial improvements, however, the amount derived from the donor cells detected in bone, skin and other tissue was equal just to 1%. Another example is that of a murine model for myocardial infarction, where intravenous administration of MSCs improved cardiac function after 3 weeks but only a few MSCs were detectable in the cardiac muscle [17]. Therefore, it is clear that MSCs do not repair damaged tissue only by differentiating, but also by interacting with the surrounding tissue via other mechanisms. For example, Nasef et al. demonstrated that, in cell contact co-culture of human MSCs and T lymphocytes, T cell proliferation is inhibited by the presence of MSCs [18]. Indeed, the immunomodulatory (regulating the level of an immune response) properties of MSCs by secreting cytokines were established in vitro [14] and in vivo [19] and also work has been done to clarify that they suppress the allogenic response of mixed lymphocyte reaction [20–22] and modify antigen-presenting cells maturation in vivo [23].
The objective of our study was to evaluate the immunosuppressive influences of MSCs in vitro on IVD fragments obtained from patients undergoing discectomy. We discovered that post-traumatic and degenerated discs contained significant amounts of IgG, probably produced by lymphocytes infiltrating during the breakdown of the end plates. We were further interested to investigate if MSCs in vitro can reduce the production antibodies, pro-inflammatory cytokines by the disc tissue and if, in a co-culture with disc fragments, the MSCs would be induced to express anti-inflammatory genes, such as transforming growth factor—beta 1 (TGF-β1) and interleukin-10 (IL-10) [18]. In addition, lymphocyte blastogenic transformation (lymphocyte proliferation) was measured as a primary immune reaction indicator.
Materials and methods
Isolation of MSCs from adult human bone marrow
Bone marrow was obtained from the iliac crest of the donors during surgery after informed consent by a procedure approved by the ethics committee of canton Luzern. Bone marrow aspirates (three donors, average age 48 years) were diluted in 3.8% sodium citrate and 1× phosphate buffered saline (PBS) and filtered through a 100-μm cell strainer (Falcon, BD Bioscience). Mononuclear cells were separated by Ficoll gradient centrifugation (density 1.077 g/mL; GE Healthcare) in a Leucosep tube (Greiner) at 800 g for 20 min. Cells were washed once with PBS, centrifuged again, re-suspended in 10 mL PBS and counted using trypan blue dye in a Neubauer chamber (C-Chip Typ Neubauer, Zeiss). Cells were placed in a T150 tissue culture flask (TPP) in NH-expansion medium (Miltenyi) at 37°C in a humid atmosphere containing 5% CO2. After 2 days, non-adherent cells were discarded, whereas adherent cells were cultured in DMEM/F12 + GlutaMAX, supplemented with 10% fetal bovine serum (FBS) (100 units/mL) penicillin/(100 mg/mL) streptomycin, 2.5 ng/mL amphotericin B (all GIBCO) and 5 ng/mL recombinant bFGF (Peprotech) at 37°C in a humid atmosphere containing 5% CO2, with medium changed three times a week. At 70% confluence, cells were harvested by dissociation with 0.05% trypsin and expanded to another passage. MSCs were cryopreserved at −150°C in a medium containing 45% DMEM/F12, 45% FBS and 10% dimethyl sulfoxide (DMSO).
MSCs in vitro characterization and differentiation into osteogenic, chondrogenic and adipogenic phenotype
Mesenchymal stem cells were characterized by colony forming unit (CFU) assay, cellular morphology and immunostaining with mesenchymal stem cell markers CD105 and Stro-1. CFU assay was achieved by seeding cells at 1 × 102/dish in 10-cm dishes (FALCON, PRIMARIA Tissue Culture Dish) in DMEM/F12 containing 10% FBS (100 units/mL) penicillin/(100 mg/mL) streptomycin, 2.5 ng/mL amphotericin B and 10 ng/mL basic fibroblast growth factor (Peprotech) for 2 weeks.
Cells were stained with antibodies for cell surface epitopes CD105 (SH2 or endoglin) [24] and Stro-1, both mesenchymal stem cell markers. Cells were incubated for 1 h with the monoclonal mouse antibodies (1:10 dilution, P3D1, Developmental Studies Hybridoma Bank) followed by fluorescein isothiocyanate (FITC)-conjugated antimouse IgG secondary antibody (1:15 dilution, Bethyl).
The potential of MSCs to differentiate into chondrogenic, osteogenic and adipogenic lineage was investigated to characterize cell populations (n = 3). Cells in monolayer were plated at 5 × 105 cells/cm2 for the adipogenic and chondrogenic differentiation, and 5 × 103 cells/cm2 for the osteogenic differentiation, in distinct six-well culture plates. Cultures were then stimulated with the appropriate differentiation medium according to the conditions described below. As negative control, cells were grown in basal medium: DMEM/F12, 5% FBS (100 units/mL) penicillin/(100 mg/mL) streptomycin and 2.5 ng/mL amphotericin B.
Chondrogenic differentiation
Cells were stimulated for 4 weeks in basal medium supplemented with 40 ng/mL dexamethasone, 50 mg/mL l-proline, 50 mg/mL l-ascorbic acid 2-phosphate (all Sigma-Aldrich), 1× insulin–transferrin–selenium (Gibco) and 5 ng/mL human recombinant TGF-β1 (Peprotech). Proteoglycans (glycosaminoglycans) were stained with Alcian Blue (Sigma-Aldrich). Aggrecan gene expression was determined as chondrogenesis marker.
Osteogenic differentiation
Cells were stimulated for 2 weeks in basal medium supplemented with 0.05 mM l-ascorbic acid 2-phosphate, 10 mM β-glycerophosphate (Sigma-Aldrich) and 100 nM dexamethasone. Von Kossa stain was used to identify mineralization in the cell culture: under light illumination, silver phosphate was degraded to silver (black stain). Osteopontin gene expression was determined as osteogenesis marker.
Adipogenic differentiation
Cells were cultured for 3 weeks under two different culture conditions: adipogenesis inducing medium—basal medium supplemented with 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 0.5 mM indomethacin and 170 mM insulin (all Sigma-Aldrich); and adipogenesis maintenance medium—basal medium supplemented with 170 mM insulin. Lipid droplets were revealed by staining with Oil Red O (Sigma-Aldrich). Leptin gene expression was determined as adipogenic marker.
Intervertebral disc fragments harvest
Following informed consent, 13 human intervertebral disc (IVD) samples were obtained from 8 donors who underwent disc surgery (Table 1). Harvested IVD fragments were washed twice in PBS and cultured in DMEM/F12 + GlutaMAX, supplemented with 10% FBS (100 units/mL) penicillin/(100 mg/mL) streptomycin and 2.5 ng/mL amphotericin B. Fragments were cut manually to sections with the same size and co-cultured with MSCs and PBLs. Separate parts from the same disc fragment were distributed to both control and treated sample groups.
Table 1.
Demographic details of donors
| Sample | Sex | Donar age (years) | Type of sample | Initial IgG content (ng/mL) | CD45 RNA | Thompson grading scale | Type of operation |
|---|---|---|---|---|---|---|---|
| 1 | M | 25 | AF | 7 | − | IV | DDD L5/S1. Stabilization and decompression |
| 2 | M | 25 | NP | 30 | + | IV | DDD L5/S1. Stabilization and decompression |
| 3* | F | 70 | AF | 14 | + | II | Trauma. Th12/L1 ventral spondylodesis |
| 4 | F | 70 | NP | 20 | + | II | Trauma. Th12/L1 ventral spondylodesis |
| 5* | M | 37 | MD | 646 | + | V | DDD Discectomy L5/S1 |
| 6 | M | 55 | MD | 63 | − | IV | DDD. Spondylodesis. C3/4 |
| 7* | F | 39 | AF | 14 | + | II | Trauma. Stenosis. Vertebrectomy C4/5 |
| 8 | F | 39 | NP | 30 | + | II | Trauma. Stenosis. Vertebrectomy C4/5 |
| 9 | F | 41 | AF | 6 | + | V | DDD. Decompression L3/4 |
| 10* | F | 41 | NP | 24 | + | V | DDD. Decompression L3/4 |
| 11 | M | 44 | MD | 727 | + | IV | DDD. Microdiscectomy L5/S1 |
| 12 | M | 29 | AF | 811 | − | III | Trauma. L1/L2 |
| 13 | M | 29 | NP | 3036 | + | III | Trauma. Th12/L1 |
AF annulus fibrosus, NP nucleus pulposus, DDD degenerative disc disease
* Not responding samples
Separation of peripheral blood lymphocytes
Human peripheral blood lymphocytes (n = 13) (PBLs) were obtained from healthy volunteers (average age 35 years), one donation for each experiment. PBLs were isolated from 15 mL peripheral blood by centrifugation at 800 g for 20 min on a Ficoll gradient (density 1.077 g/mL; GE Healthcare) in a Leucosep tube (Greiner). Cells were washed once with PBS, counted using trypan blue dye in a Neubauer chamber (C-Chip Typ Neubauer, Zeiss) and used immediately in co-culture.
MSCs, PBLs and IVD fragments co-culture
Triplicates of MSCs and PBLs were co-cultured in contact in a 96-well plate (TPP) at the ratio of 1–10 in 200 μL of RPMI + GlutaMAX, supplemented with 10% FBS (100 units/mL) penicillin/(100 mg/mL) streptomycin and 2.5 ng/mL amphotericin B at 37°C in a humid atmosphere containing 5% CO2. MSCs were cultured at the density of 2 × 105 cells/well. As a positive control, PBLs activated by 2.5 μg/mL pokeweed mitogen (lectin from Phytolacca americana, Sigma-Aldrich) were used, and the negative control was untreated PBLs. Because different fragments from the same sample may not be equivalent, to avoid inter-fragment bias, IVD fragment triplicates were derived from three separate fragments, cut into three cubes (approx. 3 × 3 × 3 mm size), one cube for each test group. IVD fragments were co-cultured with non-activated PBL, with or without MSCs; as a reference, disc fragments were cultured alone.
Alamar Blue proliferation assay
Alamar Blue (LucernaChem) at a concentration of 10% was added to the cell culture medium of each PBL sample. The cell suspension culture of PBL and the Alamar Blue were mixed in a new 96-well plate to exclude MSCs from the assay. After 4 h of incubation at 37°C, fluorescence was measured (λex = 535 and λem = 595 nm) using a multiwall plate fluorimeter (DTX 880-Multimode detector, Beckman-Coulter). The gradient of defined cell concentrations served as a reference, and assays were run in triplicate for each sample.
IgG quantification by ELISA
Polystyrene plates (96-well, Beckman-Coulter) were coated with 1 μg/mL Protein A (Zymed, Lubio Science) diluted in PBS and incubated overnight at 4°C. Plates were washed three times by an automated plate washer (Beckman-Coulter) with PBS, followed by blocking of the additional binding sites in the wells by incubating for 1 h with 5% Top Block in PBS (Lubio Science).
Culture media containing IgG was collected from every cell culture and IVD fragment culture sample and then incubated for 2 h in the protein A-coated plates in triplicate for each sample. Goat antihuman IgG horseradish peroxidase (HRP)-conjugated secondary antibodies (Bethyl) were added to each well at the dilution of 1:5,000 in 5% Top Block + 5% FBS and incubated for 1 h at room temperature. Dilutions of purified human IgG (Zymed) were used as standards.
Citrate buffer, pH 5, containing the HRP substrate O-phenylenediamide (Zymed) and 0.3% H202, was added in each well, and after approximately 10 s the enzyme reaction was stopped by adding 0.1 M H2SO4. The optical density [25] at 450 nm was read by a plate reader (DTX 880, Multimode detector, Beckman-Coulter). The raw data were normalized to a standard curve and used for calculation of total human IgG.
RNA isolation, cDNA synthesis and real-time PCR
Total mRNA was isolated using TriFast RNA isolation reagent (PeqLAB) according to the manufacturer’s instructions with modifications. In brief, 200 μL chloroform (AppliChem) was added for 1 mL of TriFast to each sample, and sample tubes were centrifuged at 10,000 rpm for 10 min to enable phase separation. The aqueous phase was transferred to a new tube and 750 μL of isopropanol and 2 μL polyacryl carrier (Molecular Research Center, Inc.) was added to aid precipitation of RNA, centrifuged at 14,000 rpm for 10 min and RNA pellets were washed in 70% ethanol, dried and re-suspended in RNAse-free water. Total RNA was stored at −80°C.
The collected IVD fragments were frozen at −80°C and pulverized with a stainless steel mortar and pestle, kept on dry ice. Finally, the IVD fragment powder was dissolved in 1 mL of TriFast, and RNA was isolated as described above.
cDNA was prepared from total RNA using VILO cDNA Synthesis Kit (Invitrogen). RNA (≤500 ng) was used for cDNA synthesis in a reaction volume of 20 μL. cDNA was diluted 1:10 with RNAse-free water and stored at −20°C.
For real-time (RT)-PCR, DNA template (5 μL) was mixed with the PCR reaction solution (IQ SYBR Green Supermix, Bio Rad). The analyzed genes were CD45, GAPDH, IL-1α, IL-1β, leptin, osteopontin, TNF-α, TGF-β1 and IL-10. Primers, listed in Table 2, were used at a concentration of 0.25 nΜ in PCR reactions, carried out in triplicates in a final volume of 25 μL in 96-well low profile PCR polypropylene plates (Bio Rad). To check the specificity of the amplification products, a melting curve analysis was carried out after each reaction.
Table 2.
Human marker genes used in quantitative RT-PCR (F forward, R reverse)
| Gene | Primer nucleotide sequence (5′–3′) | Product size (bp) |
|---|---|---|
| Aggrecan | F—AGGCTATGAGCAGTGTGACG | 125 |
| R—GCACGCCATAGGTCCTGA | ||
| CD45 | F—CAGTTTCCCCCATTGACAACC | 120 |
| R—CAGAGGCATTAAGGTAGGCATC | ||
| GAPDH | F—TGGACTCCACGACGTACTCA | 102 |
| R—GGAAGCTTGTCATCAATGGAA | ||
| IL-1α | F—AACCAGTGCTGCTGAAGGA | 97 |
| R—TTCTTAGTGCCGTGAGTTTCC | ||
| IL-1β | F—CTGTCCTGCGTGTTGAAAGA | 70 |
| R—TTGGGTAATTTTTGGGATCTACA | ||
| IL-10 | F—GATGCCTTCAGCAGAGTGAA | 105 |
| R—GCAACCCAGGTAACCCTTAAA | ||
| Leptin | F—TTGTCACCAGGATCAATGACA | 71 |
| R—GTCCAAACCGGTGACTTTCT | ||
| Osteopontin | F—GAGGGCTTGGTTGTCAGC | 129 |
| R—CAATTCTCATGGTAGTGAGTTTTCC | ||
| TNF-α | F—CAGCCTCTTCTCCTTCCTGAT | 123 |
| R—GCCAGAGGGCTGATTAGAGA | ||
| TGF-β1 | F—TGGAGACAGGGGCTTTTATTT | 121 |
| R—CTCCAGCCTCCTTAGATCACA |
MSCs modulation of IVD fragments
To further understand the influence of MSCs on IVD, we co-cultured MSCs (2 donors, average age 43 years) and IVD fragments (2 donors, average age 44 years) in contact for 14 days in the absence of peripheral blood lymphocytes. MSCs were plated at two densities: 10,000 cells/well (2,780 cells/cm2) and 50,000 cells/well (13,904 cells/cm2) in a 12-well plate. Media from samples were collected after 7 and 14 days of co-culture and IgG production was evaluated. IVD fragments without MSCs were considered as reference.
Statistical analysis
To compare gene expression, IgG quantification and cell proliferation were used non-parametric Mann–Whitney–Wilcoxon U test for dependent variables, because ANOVA would assume normal distribution of the data, which cannot be guaranteed in this data set. Values are reported as mean ± SD. A significance value of p < 0.05 was specified. Data analysis was performed with SPSS for Windows 14.0 (SPSS Inc.).
Results
MSCs characterization and differentiation
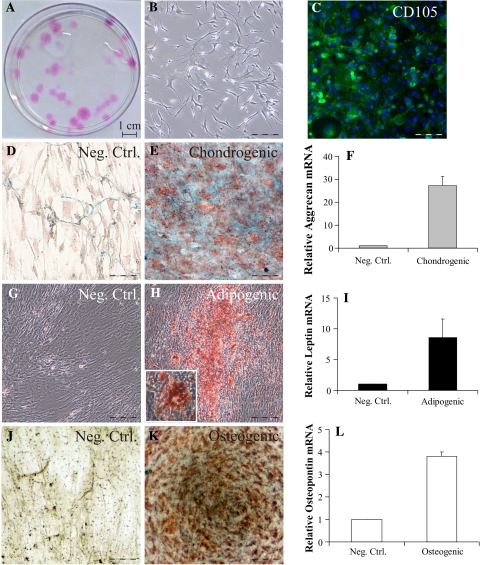
The bone marrow extracted MSCs were initially characterized by the ability to create colony forming units (CFU), by their characteristic elongated fibroblastic cellular morphology and by immunostaining with mesenchymal stem cell marker CD105 (Fig. 1a–c) and Stro-1 (data not shown). Further, MSCs were also histologically tested for their ability to differentiate chondrogenic (Fig. 1d, e), adipogenic (Fig. 1g, h) and osteogenic (Fig. 1j, k) phenotypes, colored, respectively, by Alcian Blue, Oil Red O and von Kossa staining. The induced differentiation was further confirmed by gene expression of specific markers by RT-PCR analysis of aggrecan (chondrogenesis), leptin (adipogenesis) and osteopontin (osteogenesis) (Fig. 1f, i, l).
Fig. 1.
Characterization and differentiation of MSCs in vitro. MSCs were characterized by colony forming units (CFU) ability (a), morphology (b) and by immunostaining with antibodies against CD105 (c). MSCs were differentiated in chondrogenic phenotype and stained by Alcian Blue, counterstained with nuclear fast red (d, e), adipogenic phenotype was stained by Oil Red O (g, h fat-containing vacuoles magnification in the inset), while osteogenesis was revealed by Von Kossa staining (j, k). Differentiation was confirmed by gene expression analysis of respective differentiation markers: aggrecan (chondrogenic, Fig. 1f), leptin (adipogenic, Fig. 1i) and osteopontin (osteogenic, Fig. 1l). Data are represented as fold increase normalized to negative control (entire scale bar 125 μm)
MSCs elicit different immunomodulation depending on IVD source
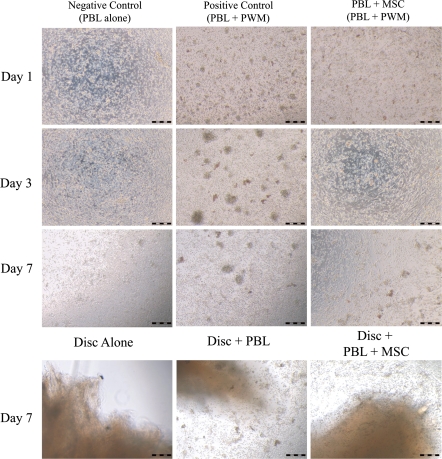
Human IVD fragments (n = 13) were co-cultured in contact with PBLs or PBLs and MSCs for 1 week. As a control of the inducibility of the lymphocytes, PBLs stimulated by pokeweed mitogen (PBLs + PWD) were used and the immunomodulation properties of the MSCs were controlled by the stimulated PBLs, co-cultured with MSCs (PBLs + PWD/MSC). Lymphocytes blasts transformation reactions were observed microscopically in the positive control samples (PBL + PWD) at days 3 and 7 (Fig. 2), but blasts diminished significantly when MSCs were present in the co-culture. IVD fragments co-cultured with lymphocytes lead to identical blast morphology at day 7 and again, MSCs in co-culture reduced the intensity of the blastogenic transformation.
Fig. 2.
Microphotographs of lymphocyte blast stimulation in PBLs culture, PBLs + PWD and PBLs + PWD/MSCs. Pictures were taken after 1, 3 and 7 days of co-culture. On the bottom, photos of IVD fragments cultured alone, with PBLs and with PBLs/MSCs after 1 week of co-culture (entire scale bar 250 μm. PBLs peripheral blood leukocytes, MSCs mesenchymal stem cells, PWD pokeweed, IVD intervertebral disc)
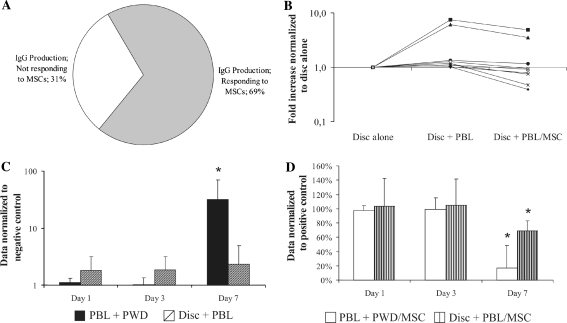
Depending on the effects of MSCs on IgG production after 1 week of co-culture with PBLs, IVD were divided in two groups: responders and non-responders (Fig. 3a). Approximately, 70% of the tested IVD samples (n = 9), the responders group, decreased their production of IgG in contact PBLs by more than one-third upon co-culture with MSCs. In contrast, the remaining 30% (n = 4), non-responders, did not show any reaction to co-culture, neither stimulated PBLs, nor were influenced by MSCs (data not shown). We further focused our attention on the group responding to MSCs immunomodulation (n = 9). IgG production in co-culture with IVD fragments was characterized by a large inter-sample variability among different donors (Fig. 3b). IVD fragments in co-culture with PBL increased in average 2.4-fold the production of IgG when compared with disc fragments alone, while in the presence of MSCs in the same co-culture, the increase corresponded just to 1.5, demonstrating the repressive action of MSCs.
Fig. 3.
IgG production by PBLs in direct co-culture with IVD and MSCs. After 7 days, 70% of IVD analyzed responded to IVD stimulation and MSC immunosuppressive action (a) (data normalized to disc alone). IVD and PBLs co-culture produced unequal increase in IgG production among different samples (individual samples depicted by a line). The addition of MSCs in co-cultures reduced this increase by more than one-third on an average (b). IgG production by PBLs stimulated by PWD and in co-culture with IVD. Only after 7 days, IgG production was strongly stimulated by PWD (32-fold increase when compared with day 1) (c), IVD immediately doubled the IgG production by PBLs (data normalized to negative control of the respective day). After 7 days, MSCs reduced by 83% and by 31% the IgG production by PWD and, respectively, IVD stimulated PBLs (d) (data normalized to positive control of the respective day; represented as a mean ± SD, *p < 0.05; when compared with day 1)
It was shown that the effect of pokeweed mitogen on IgG production by PBLs has completely different kinetics when compared with the effect of IVD fragments on IgG production. From days 1–3, PBLs stimulated with PWD did not synthesize significantly different amounts of IgG from the untreated control (no PWD), but after 1 week, there was dramatic increase in IgG (p < 0.05, Fig. 3c) corresponding to the expected time used by lymphocytes to synthesize IgG. On the other hand, when compared with IVD cultured alone, the production of IgG in the IVD and PBLs co-culture was increased to 180% when compared with negative control during days 1 and 3, while after 1 week, IgG detected in the medium was 235%. After 1 week, the presence of MSCs in co-cultures significantly reduced the production of IgG by PBLs stimulated by PWD and IVD. There, pokeweed mitogen activated PBLs reduced the synthesis of IgG by 83% (p < 0.05) and IVD induced PBLs reduced IgG by 31% (p < 0.05) (Fig. 3d). During days 1 and 3, MSCs did not affect the IgG amount in both co-cultures of IVD and PBLs, and stimulated PBLs.
MSCs reduced significantly PBLs proliferation in IVD co-cultures
Mesenchymal stem cells presence in co-culture significantly inhibited the proliferative response of PBLs to PWD and IVD fragments, as measured by Alamar Blue metabolic activity assay (Fig. 4a). Over the time course of the experiments, PWD stimulated PBLs proliferation to 150% during the first 3 days and to 177% after 1 week. Co-culture with IVD fragments did not affect PBL proliferation, except at day 1 with an increment in proliferation of 111%. Both PBLs and IVD data were normalized to PBLs cultured without PWD.
Fig. 4.
Alamar Blue assay of the metabolic activity of PBLs stimulated by PWD and in co-culture with IVD (a). PBLs were constantly stimulated by PWD (1.5-fold increase when compared with negative control); while IVD did not significantly promote PBLs proliferation (data normalized to negative control of the respective day). In the presence of MSCs, the stimulated PBLs reduced their proliferation by 0.48 at day 3 and by 0.6 at day 7. PBLs in co-culture with IVD and MSCs increased their proliferation at day 1 to 1.37, at day 3 to 1.18, but finally dropped to 0.57 after 1 week (b) (data normalized to respective positive control, represented as a mean ± SD; *p < 0.05 compared with day 1)
The addition of MSCs to cultures significantly inhibited the proliferation of PWD and IVD stimulated PBLs after 1 week (Fig. 4b). At the first day of co-culture, MSCs might have influenced slightly the proliferation of stimulated PBLs—a reduction by only 6% when compared with PBLs + PWD. However, the effect of MSCs after 3 days was clearly visible, reducing proliferation with 48% (p < 0.05) and after 1 week, proliferation was further reduced by 60% (p < 0.05). In contrast, PBLs co-cultured with IVD tissue and MSCs initially were stimulated by the MSCs to proliferate, and increased by 37% at day 1 when compared with PBLs in co-culture with IVD (set as 100%) and by 18% after 3 days of co-culture. However, this was a trend of a constant decrease and after 1 week in co-culture with MSCs and IVD, PBL proliferation was reduced by 43% when compared with the control (p < 0.05).
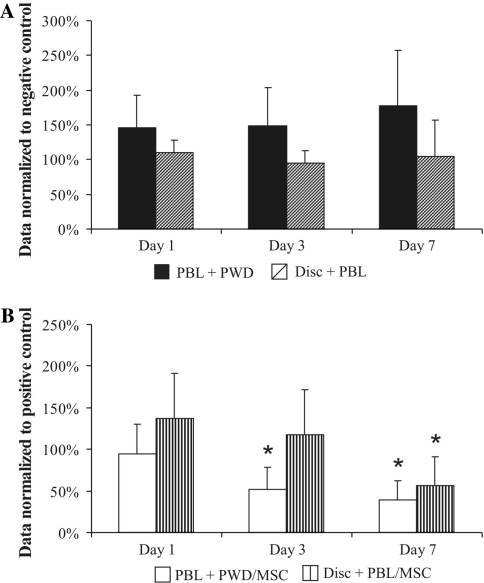
Expression of pro-inflammatory cytokine genes by PBLs
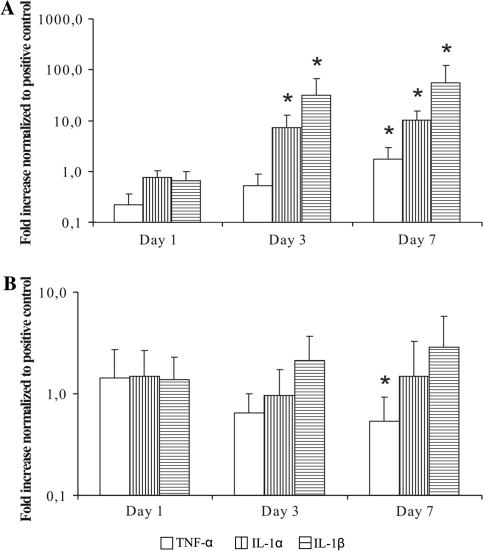
We further analyzed the gene expression of three cytokines typically involved in sustaining the inflammatory state, namely IL-1α, IL-1β and TNF-α. Quantitative real-time PCR was used to compare the effect on gene expression in PWD stimulated PBL (Fig. 5a) and IVD fragments (Fig. 5b) after co-culture with MSCs and PBL. Data were normalized to positive controls from the same day: PWD stimulated PBL and IVD fragments in co-culture with PBL, respectively. The presence of MSCs decreased the expression of these pro-inflammatory genes stimulated by PWD only at day 1, whereas during the time course of the experiment the expression, level of these genes increased constantly. Finally, gene expression levels of IL-1α, IL-1β and TNF-α were, respectively, increased significantly to 17, 21.4 and 56-fold increase when compared with the positive control at day 7 (p < 0.05). In contrast, MSCs induced the gene expression of IL-1α, IL-1β and TNF-α in the IVD fragment samples at day 1 to 137–149%, however, the trend for the separate genes throughout the duration of experiment was different for each. TNF-α gene expression was progressively decreasing, and after 1 week, the expression was reduced to 0.53 of the control (p < 0.05). The IL-1α gene expression went down to 0.98% at day 3, but up to 2.1-fold after 1 week. On the other hand, the pattern of expression of IL-1β was undoubtedly increasing during the time line of the experiments, and by week 1 reached 2.85-fold increases when compared with positive control.
Fig. 5.
Gene expression of IL-1α, IL-1β and TNF-α by stimulated PBL (a) and IVD (b), after 1, 3 and 7 days in co-culture with MSCs. Quantitative RT-PCR analysis (relative expression normalized to GAPDH and to positive control of the respective day represented as a mean ± SD, *p < 0.05 compared with day 1)
Expression of anti-inflammatory cytokine genes by MSCs
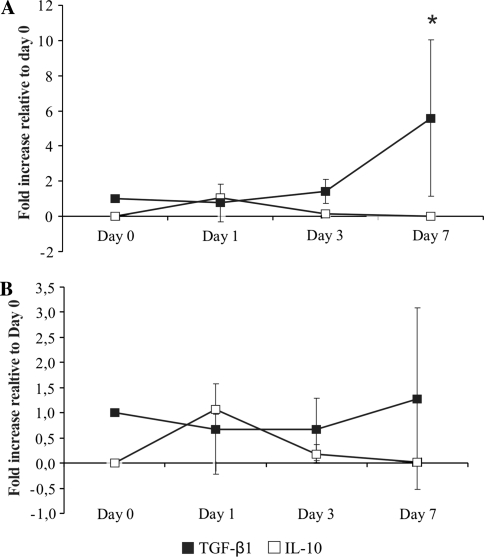
Gene expression of TGF-β1 and IL-10, cytokines involved in anti-inflammatory processes, was measured in the MSCs, which were in co-cultures with PWD stimulated PBLs or IVD fragments. The expression of TGF-β1 gene in MSCs decreased slightly on day 1 for both co-cultures when compared with day 0, but the pattern of expression of TGF-β1 switched later to up-regulation, which reached 5.58-fold increase for PBLs co-culture (p < 0.05) and 1.3-fold increase for IVD co-culture at day 7 (Fig. 6a). Although IL-10 is not expressed usually by MSCs in monolayer cultures, IL-10 gene expression was detectable at day 1 in both co-cultures (Fig. 6b).
Fig. 6.
Expression of TGF-β1 and IL-10 genes by MSCs in co-culture with stimulated PBLs (a) and IVD + PBLs (b), after 1, 3 and 7 days. Quantitative RT-PCR analysis (relative expression normalized to GAPDH compared with day 0, represented as a mean ± SD; *p < 0.05 compared with day 1)
MSCs modulation of IgG secretion by IVD fragments
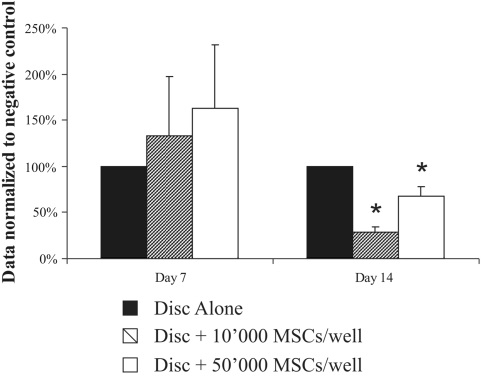
In contrast to the results obtained with co-cultures with PBLs, after 7 days, MSCs stimulated IVD secretion of IgG (133% increment at MSCs density of 10,000 cell/well and 163% at density of 50,000 cells/well) (Fig. 7). However, at day 14, the stimulation of IgG MSCs was replaced by the opposite effect after 14 days, and MSCs suppressed IgG secretion. IgG levels in the media were lower at MSCs density of 10,000 cell/well (72% reduction relative to control, p < 0.05) when compared with density of 50,000 cells/well (32% reduction, p < 0.05).
Fig. 7.
IgG secreted in the media after 1 and 2 weeks in co-culture of IVD with MSCs, 10,000 MSCs/well and 50,000 MSCs/well (data normalized to disc alone of the respective day, represented as a mean ± SD, *p < 0.05 compared with disc alone)
Discussion
We do not know why some people with disc degeneration have pain and some are pain free, but one of the possible explanations is the development of intradiscal inflammation. Previous studies have demonstrated that a variety of inflammatory mediators have been implicated in the degeneration of the IVD and discogenic pain, including nitric oxide [26], interleukins, matrix metalloproteinases, prostaglandin E2 [27] and a group of cytokines [13]. The analysis conducted showed that the IVD responses to such pro-inflammatory mediators are not always consistent and such pro-inflammatory molecules, if present in the disc when the integrity of the vertebral endplates is compromised by trauma or degeneration, will attract B lymphocytes and antibodies will be produced locally, inside the disc. Indeed, we observed the presence of significant amount of IgG antibodies when thoroughly washed human disc fragments were cultured in vitro (Table 1). Interestingly, within the same donor, the amount of antibodies detected in NP culture media was always greater than in the AF culture media. Therefore, we wanted to investigate if MSCs can lower the inflammation state of IVD material isolated from patients with disc herniation (protrusion, extrusion or sequestration), who underwent full or partial discectomy, when introduced in in vitro co-culture of human IVD fragments and peripheral blood lymphocytes. Based on their sensitivity to respond to MSCs, measured by the reduction of the amount of IgG produced after 1 week of co-culture, we divided the patient samples in two groups: responders and non-responders. 70% of the samples, the responders group, had reduction in IgG production when MSCs were present, while 30% did not. The experimental setup allowed us to give at least two possible explanations about the differences between the groups. First, it is possible that some people are genetically predisposed to respond to MSCs immunomodulation, while others are not. However, there were two donors who donated each separate annulus fibrosus and nucleus pulposus disc material, in which cases within the same donor one of the tissues was sensitive to MSC and the other was not. In such case, it is likely that the non-responding samples represent tissues that were not chronically inflamed and, therefore, were not sensitive to the presence of MSCs and PBL. However, due to ethical considerations, this hypothesis is difficult to test. One possible way to manage this issue in a further planned work is to establish a more controlled tissue sampling protocol at the operation theater level, where we can separate among samples taken directly from a sequester, from disc prolapse or from the surrounding disc tissues. Co-cultures of the responding IVD fragments and MSCs showed that inflammation measured by secreted IgG was reduced significantly after 2 weeks. IgG production by the IVD fragments was reduced by 75% when compared with control in the presence of 10,000 MSCs/well. Surprisingly, in the presence of 50,000 MSCs/well IgG inhibition was attenuated when compared with 10,000 MSCs/well demonstrating that stem cells are not to be treated as stoichiometric reagents and showing that MSCs suppressive effects were changing depending on the type of culture and co-culture proportions in a non-linear way. In addition, after just 1 week, the effect of MSCs on IVD (without added PBL) was different and MSCs promoted IgG production. This result is in contrast to co-cultures of IVD fragments and PBLs where already after 1 week, there was a reduction in the inflammatory response. An explanation may lie in that the lymphocytes which have infiltrated the disc tissue via the damaged endplate originate from the vertebral body (detected by the presence of IgG in all IVD samples and mRNA of the lymphocyte marker CD45, in most of the samples) and are not phenotypically identical to PBL. The vertebral body contains hematopoietic bone marrow and therefore among the infiltrated cells there are immature leukocytes and such leukocytes are known to be stimulated into differentiation in the presence of MSC, while PBLs are not.
To be confident that the stem cells used in the study were effective immunomodulators, we demonstrated that MSCs had always an immunomodulatory effect on pokeweed mitogen stimulated PBL, therefore isolating with certainty the responding from the non-responding IVD samples. Consistently after 1 week in co-culture, pokeweed activated PBLs proliferation decreased in the presence of MSCs and in 70% of the cases IVD activated proliferation too. In our experiments, we showed that MSCs had an effective role in decreasing IgG production in PBLs co-cultures. After 1 week, the amount of IgG present in the culture media was reduced by 83% when compared with positive controls. MSCs also had suppressive effects on PBLs and IVD co-cultures, reducing IgG production by 31%. The difference was that IVD fragments were not stimulating PBLs in such a pronounced way and to the same extent as PWD, hence the reduction was also smaller. However, such a quantitative comparison between PWD and IVD as IgG production stimuli makes not much biological sense, because PWD is an extremely powerful immunogenic plant-derived lectin, used in the laboratory practice exactly because of these properties, while IVD is a ‘normal’ tissue.
Interestingly, IVD fragments did not stimulate PBLs proliferation in co-culture, but in their presence IgG production was constantly doubled. This fast doubling of IgG may correspond to the activation of the already present and mature infiltrated in the IVD tissue B lymphocytes, while upon PWD stimulus PBLs produced substantial amounts of IgG only after 7 days. On the other hand, MSCs were able to stimulate PBL proliferation at day 1 showing that the biological effects of MSCs are broad, complex and also variable in time. Recently, it was demonstrated by expression profiling arrays that interactions between IVD cells and adipose-derived MSC are too complex and involve also change in pro-inflammatory mediators and targets in the MSC [28]. In conclusion, in our work, MSC co-culture with IVD fragments promoted a reduction in PBL proliferation and IgG production, both important events in inflammation processes, however, the interactions are complex and the underlying biological processes are unclear.
In an attempt to understand the underlying inflammation regulation mechanisms and dynamics, we also investigated the gene expression of pro-inflammatory cytokines, namely IL-1α, IL-1β and TNF-α in PBLs and IVD. These molecules were chosen, because their presence was already described in pathological IVD tissue [13]. When co-cultured with MSCs, PBLs reduced the expression of these cytokines surprisingly only at day 1. The following days, the expression was higher than in positive control demonstrating that MSCs had time limited suppressive influences on PBLs and after this they reacted in a counter-intuitive manner. On the other hand, the expression of these cytokines in the IVD tissue was upregulated at day 1, in the presence of MSCs in co-culture and the trend after 1 week differed for each molecule: TNF-α expression decreased, IL-1α was stabilized and IL-1β increased. The TNF-α decrease correlated with the reduction in IgG production and the PBLs’ loss of proliferation, but both isoforms of IL-1 seem not to be implied in this regulation in a simple correlation. Unfortunately, we were not able to make a sound model of what is happening based on this data, because of the many unknowns, for instance other interleukins, which have not been analyzed here, have been associated with degenerated disc, such as IL-17 [6] and it is also known that the interleukins show pleiotropic effects, i.e. they have different targets in various cells and tissues. We cannot rule out the possibility that proportion of the cytokines in the IVD samples come from the disc tissue instead from the infiltrated leukocytes, as both types of cells produce interleukins, however, it is clear that the presence of MSC suppresses inflammation in both cases and leads to a decrease in IgG (product only of the infiltrated leukocytes).
We also investigated if MSCs promoted the decrease in the inflammatory response in co-culture, by expressing themselves anti-inflammatory molecules, such as IL-10 and TGF-β1. The expression of these two molecules and secretion of them in the environment may explain potential mechanisms by which MSCs are able to reduce the inflammatory response. The results showed that gene expression trends were similar when MSCs were co-cultured with pokeweed or IVD stimulated PBLs. The expression of TGF-β1 was characterized by an initial slight decrease at day 1—when compared with day 0, but during the time course of the experiments, the expression was progressively increasing. On the other hand, IL-10 expression was detectable just at day 1.
The down-regulation of the inflammatory response in vitro of IVD fragments promoted by MSCs supported the already published data concerning the immunosuppressive properties of MSCs in other tissues, including the somewhat intimidating complexity of their modes of action. An important conclusion, however, is that the potential therapeutic application of MSCs for IVD regeneration reaches beyond the capacity of MSCs to differentiate into disc-like cells and generate new extracellular matrix. The reduction of inflammation could be a key element for inhibition of the activity of the enzymes involved in the process of tissue degradation, and possibly in pain control. These data suggest an avenue leading to a possible clinical application, where autologous, MSCs can be injected in a painful, but with low level of degeneration IVD, which may prove an effective substitute to more invasive surgical interventions.
Acknowledgments
This study was supported by Swiss Paraplegic Foundation. We thank Swiss Paraplegic Center for Clinical Support, Dr. Angela Frotzler for statistical advice and Dr. David Magnani for scientific assistance.
Conflict of interest None.
References
- 1.Pye SR, Reid DM, Smith R, Adams JE, Nelson K, Silman AJ, O’Neill TW. Radiographic features of lumbar disc degeneration and self-reported back pain. J Rheumatol. 2004;31:753–758. [PubMed] [Google Scholar]
- 2.Rannou F, Lee TS, Zhou RH, Chin J, Lotz JC, Mayoux-Benhamou MA, Barbet JP, Chevrot A, Shyy JY. Intervertebral disc degeneration: the role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am J Pathol. 2004;164:915–924. doi: 10.1016/S0002-9440(10)63179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O’Brien J, Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178–181. doi: 10.1016/S0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 5.Johnson WE, Evans H, Menage J, Eisenstein SM, El Haj A, Roberts S. Immunohistochemical detection of Schwann cells in innervated and vascularized human intervertebral discs. Spine (Phila Pa 1976) 2001;26:2550–2557. doi: 10.1097/00007632-200112010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Shamji MF, Setton LA, Jarvis W, So S, Chen J, Jing L, Bullock R, Isaacs RE, Brown C, Richardson WJ. Pro-inflammatory cytokine expression profile in degenerative and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62(7):1974–1982. doi: 10.1002/art.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paesold G, Nerlich AG, Boos N. Biological treatment strategies for disc degeneration: potentials and shortcomings. Eur Spine J. 2007;16:447–468. doi: 10.1007/s00586-006-0220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiler C, Nerlich AG, Bachmeier BE, Boos N (2005) Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine (Phila Pa 1976) 30:44–53. doi:00007632-200501010-00009 (discussion 54) [DOI] [PubMed]
- 10.Le Maitre CL, Freemont AJ, Hoyland JA. A preliminary in vitro study into the use of IL-1Ra gene therapy for the inhibition of intervertebral disc degeneration. Int J Exp Pathol. 2006;87:17–28. doi: 10.1111/j.0959-9673.2006.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxf) 2008;47:809–814. doi: 10.1093/rheumatology/ken056. [DOI] [PubMed] [Google Scholar]
- 12.Seguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976) 2005;30:1940–1948. doi: 10.1097/01.brs.0000176188.40263.f9. [DOI] [PubMed] [Google Scholar]
- 13.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1β and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11917–11923. doi: 10.1073/pnas.1834138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clin Pharmacol Ther. 2007;82:241–243. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- 16.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, Sensebe L, Zhang Y, Gorin NC, Thierry D, Fouillard L. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Exp. 2007;13:217–226. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz EM, Prather WR. Cytokines as the major mechanism of mesenchymal stem cell clinical activity: expanding the spectrum of cell therapy. Isr Med Assoc J. 2009;11:209–211. [PubMed] [Google Scholar]
- 20.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 21.Wei XF, Liu KY. [Inhibitory effects of human bone marrow mesenchymal stem cells and cord blood mononuclear cells on mixed lymphocyte response and PHA induction transformation] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2004;12:261–264. [PubMed] [Google Scholar]
- 22.Xuan M, Qiu GQ, Xie XB. [Immune modulatory effects of mesenchymal stem cells on T lymphocytes in mixed lymphocyte culture] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2006;22:433–435. [PubMed] [Google Scholar]
- 23.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 24.Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105) Biochem Biophys Res Commun. 1999;265:134–139. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- 25.Ichim TE, Alexandrescu DT, Solano F, Lara F, Campion RD, Paris E, Woods EJ, Murphy MP, Dasanu CA, Patel AN, Marleau AM, Leal A, Riordan NH (2009) Mesenchymal stem cells as anti-inflammatories: implications for treatment of Duchenne muscular dystrophy. Cell Immunol. doi:10.1016/j.cellimm.2009.10.006 [DOI] [PubMed]
- 26.Kohyama K, Saura R, Doita M, Mizuno K. Intervertebral disc cell apoptosis by nitric oxide: biological understanding of intervertebral disc degeneration. Kobe J Med Sci. 2000;46:283–295. [PubMed] [Google Scholar]
- 27.Kang JD, Stefanovic-Racic M, McIntyre LA, Georgescu HI, Evans CH. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine (Phila Pa 1976) 1997;22:1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 28.Gruber HE, Deepe R, Hoelscher GL, Ingram JA, Norton HJ, Scannell B, Loeffler BJ, Zinchenko N, Hanley EN, Tapp H. Human adipose-derived mesenchymal stem cells: direction to a phenotype sharing similarities with the disc, gene expression profiling, and coculture with human annulus cells. Tissue Eng Part A. 2010;16:2843–2860. doi: 10.1089/ten.tea.2009.0709. [DOI] [PubMed] [Google Scholar]