Abstract
Lateral transpsoas interbody fusion (LTIF) is a minimally invasive technique that permits interbody fusion utilizing cages placed via a direct lateral retroperitoneal approach. We sought to describe the locations of relevant neurovascular structures based on MRI with respect to this novel surgical approach. We retrospectively reviewed consecutive lumbosacral spine MRI scans in 43 skeletally mature adults. MRI scans were independently reviewed by two readers to identify the location of the psoas muscle, lumbar plexus, femoral nerve, inferior vena cava and right iliac vein. Structures potentially at risk for injury were identified by: a distance from the anterior aspect of the adjacent vertebral bodies of <20 mm, representing the minimum retraction necessary for cage placement, and extension of vascular structures posterior to the anterior vertebral body, requiring anterior retraction. The percentage of patients with neurovascular structures at risk for left-sided approaches was 2.3% at L1–2, 7.0% at L2–3, 4.7% at L3–4 and 20.9% at L4–5. For right-sided approaches, this rose to 7.0% at L1–2, 7.0% at L2–3, 9.3% at L3–4 and 44.2% at L4–5, largely because of the relatively posterior right-sided vasculature. A relationship between the position of psoas muscle and lumbar plexus is described which allows use of the psoas position as a proxy for lumbar plexus position to identify patients who may be at risk, particularly at the L4–5 level. Further study will establish the clinical relevance of these measurements and the ability of neurovascular structures to be retracted without significant injury.
Keywords: Lateral transpsoas interbody fusion, Minimally invasive surgery, Lumbar plexus anatomy, Spine surgery complications
Introduction
Lateral transpsoas interbody fusion (LTIF) is a minimally invasive surgical technique that permits anterior column interbody fusion utilizing cages designed for placement via a direct lateral transpsoas approach. The risk to adjacent neurovascular structures (lumbar plexus, femoral nerve, great vessels) during LTIF surgery has not been well characterized. The operative corridor through the psoas is established by bluntly splitting the psoas fibers and retracting the anterior portion of the psoas anteriorly, and the posterior psoas and lumbar plexus posteriorly. Although the lumbar plexus and femoral nerves are typically located in the posterior aspect of the psoas musculature, these structures may be at risk during LTIF in patients with anomalous lumbar plexus anatomy (plexus too anterior in psoas), variant psoas anatomy (psoas too anterior relative to disc), or small discs when measured in the anteroposterior dimension. Because the operative corridor is directly lateral to the disc, the great vessels including the aorta, vena cava and iliac vessels are typically not visualized or at risk. Anatomic variants of the vena cava and right common iliac vein may place these structures at risk given the more-posterior location of the right-sided venous structures in comparison to the left side.
Several published studies have characterized the location of the lumbar plexus within the psoas muscle using cadaveric dissection. Moro et al. [5] described the location of the lumbar plexus, the genitofemoral nerve and nerve roots from L1 to the L5–S1 disc space with respect to the anterior to posterior axis of the vertebral bodies based on the dissection of only six cadavers. Similarly, Benglis et al. [1] evaluated the location of the lumbar plexus with respect to the approach used for LTIF in three cadavers and suggested that the lumbar plexus may be at risk when approaching the L4–5 interspace. These studies were performed using a small number of specimens which compromises generalization of the results and limits description of anatomic outliers.
A number of anatomic studies have been published in the anesthesia literature which describes lumbar plexus anatomy for the purpose of placing regional nerve blocks. Farny et al. [2] and Kirchmair et al. [3] both report on cadaver dissections of the lumbar plexus and the surrounding psoas muscle. While these studies carefully documented the location of the plexus as well as the location of the femoral and obturator nerves, results are reported with respect to distances more useful for regional anesthesia than LTIF such as the distance of these structures from the skin of lower back.
In this study, we utilize high resolution MRI images and a large patient cohort to answer three questions clinically relevant to surgeons performing LTIF: (1) What is the average location of the lumbar plexus within the psoas muscle and with respect to the anterior aspect of adjacent vertebral bodies at each lumbar interspace level?; (2) Do the average locations of the vena cava and right iliac vein place them at risk of injury in right sided LTIF approaches?; and (3) What proportion of patients have anomalous anatomic relationships which could endanger either the lumbar plexus or right-sided vascular structures during LTIF?
Materials and methods
We retrospectively reviewed consecutive MRI scans of the lumbosacral spine in 43 skeletally mature adults performed in a single 1.5 Tesla MRI scanner. The study population included 26 females, 17 males with an overall average age of 50 years. In order to limit the inclusion of patients who could have sagittal and coronal plane deformity that might affect anatomic relationships, we excluded patients with: scoliosis >10°, spondylolisthesis greater than grade 1, and prior spine fusion and/or instrumentation. Because of effects on psoas size and appearance [4, 6], we also excluded patients with significant hip osteoarthritis or prior hip arthroplasty as well as patients with prior retroperitoneal surgery.
All images were acquired on a superconducting closed 1.5 T magnet. Sagittal T1, T2, and T2 weight fat suppressed sequences were obtained at 3–3.5 mm slice intervals without a gap using a matrix of 256–512 × 224–256. Axial T2 weighted images were obtained continuously through the spine, appropriately angled to the disc spaces at 3–3.5 mm slice intervals without a gap utilizing a matrix of 256 × 224. Coronal T2 weighted images were obtained at 3 mm slice intervals without a gap utilizing a matrix of 256 × 256.
MRI scans were independently reviewed by two readers, a fellowship trained musculoskeletal radiologist and a 5th-year orthopedic chief resident. One trial reading round was performed and the results were reviewed to assist in standardizing image interpretation prior to beginning the study. After measurements for the study were completed, differences in measurements of more than 5 mm were settled by consensus. Differences of opinion when attempting to reach consensus were adjudicated by a senior musculoskeletal radiologist.
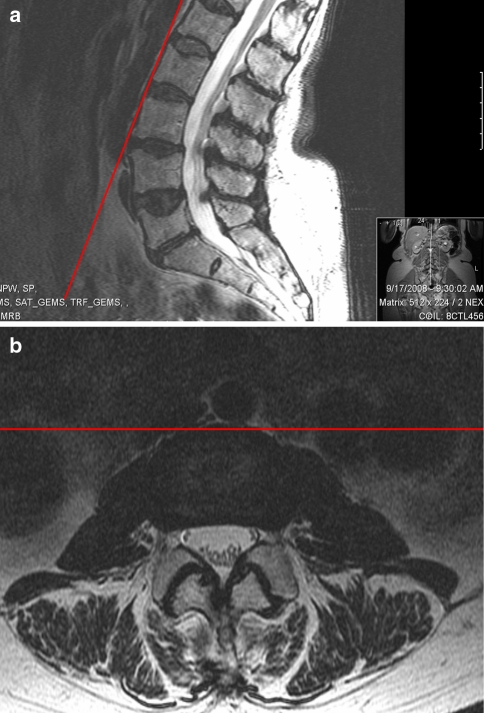
The reference plane for many measurements in the study was a coronal plane at each disc space defined by line connecting the anterior border of adjacent vertebral bodies which will be referred to as the anterior intervertebral plane (Fig. 1). The anterior borders of adjacent vertebral bodies are the principle landmarks used when performing the LTIF approach using fluoroscopic guidance (Fig. 2). Axial T2 MRI cuts were reviewed at the mid-disc space level from L1 through S1 in order to identify the anterior edge of the psoas muscle, the anterior aspect of the lumbar plexus, the location of the femoral nerve beginning the L3–4 level and the position of the posterior edge of either the inferior vena cava or right iliac vein depending on level with respect to the anterior vertebral reference plane described above (Fig. 3). Reported distances for structures posterior to the anterior intervertebral line were given a positive value while distances of structures anterior to the anterior intervertebral line were assigned a negative value. The anterior to posterior diameter of the psoas muscle and the distance from the lateral aspect of the disc space to the medial border of the lumbar plexus was also measured at each disc space (Fig. 3).
Fig. 1.
Anterior intervertebral plane (red line) for L2–3 drawn on both sagittal (a) and axial (b) plane images
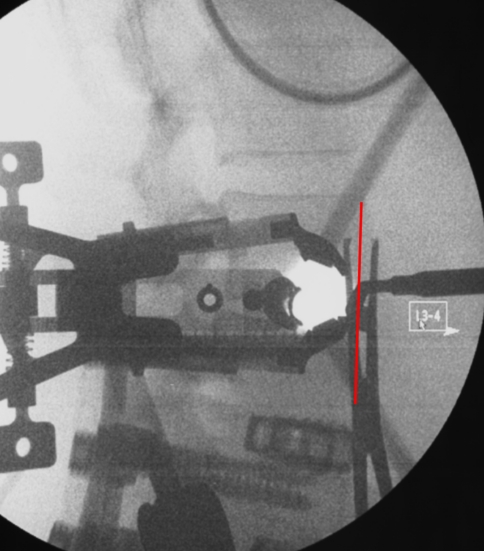
Fig. 2.
Intraoperative fluoroscopic image demonstrating retractor system and location of approach in reference to anterior intervertebral plane (red line)
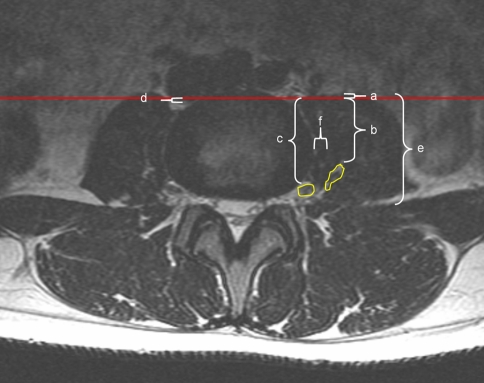
Fig. 3.
Axial MRI image at L3–4 demonstrating measurements in relationship to the anterior intervertebral plane: a the anterior edge of the psoas muscle (extending just anterior to the intervertebral plane in this image), b the anterior aspect of the lumbar plexus, c the location of the femoral nerve beginning the L3–4 level, d the position of the posterior edge of either the inferior vena cava or right iliac vein depending on level, e the anteroposterior diameter of the psoas muscle. The diagram also includes measurement (f), the transverse distance between the lateral aspect of the intervertebral disc and the medial aspect of the lumbar plexus
All distances are reported in millimeters in order to provide reference distances which can be compared with implant sizes and the amount of exposure needed to place standard interbody implants. The proportion of patients with neural structures at risk at each level was calculated as the proportion of patients whose lumbar plexus or femoral nerve was <20 mm from the anterior intervertebral plane of the left side and the closer of the anterior intervertebral plane and the posterior aspect of the retroperitoneal vasculature on the right side. The value 20 mm was chosen to equal the sum of the anterior to posterior diameter of a commonly used 18 mm interbody cage (XLIF, Nuvasive Inc., San Diego, CA) and a 2 mm allotment for the width of the retractor used to place the cage and represents the minimum distance through which this procedure can be done without neural retraction which may result in iatrogenic injury. These values were calculated at each level from L1–L5. L5–S1 was not included in this portion of the study because the LTIF procedure is not performed at this level.
Statistical analysis was performed using an α value of 0.05. Average distances between structures were compared at different spinal levels using Student’s t test. Inter- and intraobserver reliability was evaluated using the intraclass correlation coefficient (ICC). The ICC values were graded using previously described semiquantitative criteria [5]: excellent for a value of 0.90–1.0, good for 0.70–0.89, fair/moderate for 0.50–0.69, low for 0.25–0.49, and poor for 0.0–0.24.
This study was approved by our Institutional Review Board.
Results
Position of lumbar plexus
The distance from the anterior intervertebral plane to the anterior border of the lumbar plexus averaged 30.8 mm at L1–2, 28.6 mm at L2–3, 28.2 mm at L3–4, 22.1 mm at L4–5 and 0.4 at L5–S1, respectively (Table 1). With the exception of the difference between L2–3 and L3–4, all differences between levels were statistically significant from one another (P < 0.02).
Table 1.
Anatomic structure distance by level
| L1–2 | L2–3 | L3–4 | L4–5 | L5–S1 | |
|---|---|---|---|---|---|
| Distance between anterior intervertebral plane and lumbar plexus (b) | 30.8 | 28.6 | 28.2 | 22.1 | 0.4 |
| Distance between lateral disc and medial lumbar plexus (f) | 2.7 | 3.2 | 4.7 | 11.2 | |
| Distance between anterior intervertebral plane and femoral nerve (b) | 33.5 | 26.8 | 6.2 | ||
| Distance between anterior intervertebral plane and right IVC/iliac vein (d) | −4.3 | −1.3 | 0 | 2.1 | 4.9 |
| Distance between anterior intervertebral plane and anterior psoas (a) | 8.1 | 1.8 | −3.8 | −11.6 | −31.5 |
Values are in mm. Letters in parenthesis are Fig. 3 labels
The distance from the lateral aspect of the intervertebral disc to the medial aspect of the lumbar plexus averaged 2.7 mm at L2–3, 3.2 mm at L3–4, 4.7 mm at L4–5 and 11.2 mm at L5–S1 (Table 1). Differences between measurements at L3–4 and L4–5 and L4–5 and L5–S1 were statistically significant (P < 0.01).
Position of femoral nerve
The distance from the anterior intervertebral plane to the anterior border of the femoral nerve averaged 33.5 mm at L3–4, 26.8 mm at L4–5 and 6.2 mm at L5–S1. These values were all statistically significantly different from one another (P < 0.01) (Table 1).
Position of right-sided vasculature
The position of either the IVC or right common iliac vein with respect to the anterior intervertebral plane measured −4.3 mm at L1–2, −1.3 mm at L2–3, even with the anterior intervertebral plane at L3–4, +2.1 mm at L4–5 and +4.9 mm at L5–S1 (Table 1). Differences between measurements at L3–4 and L4–5 and L4–5 and L5–S1 were statistically significant (P < 0.01).
Position of psoas muscle
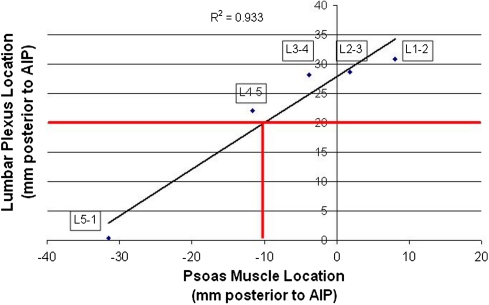
The position of the psoas muscle with respect to the anterior intervertebral plane measured 8.1 mm at L1–2, 1.8 mm at L2–3, −3.8 mm at L3–4, −11.6 mm at L4–5 and −31.5 mm at L5–S1 (Table 1). Differences between measurements at all levels were statistically different from one another (P < 0.01). The average anterior to posterior diameter of the psoas muscle increased significantly from L1–2 (32.8 mm) to L2–3 (43.4 mm) to L3–4 (48.8 mm < 0.01) before becoming statistically stable in diameter at L4–5 (48.0 mm) and L5–S1 (49.3 mm). The position of the anterior edge of the psoas muscle in comparison with the position of the anterior aspect of the lumbar plexus had a Pearson correlation coefficient of 0.933 when measured in relationship to the AIP (Fig. 4).
Fig. 4.
Relationship between anterior aspect of the psoas muscle and anterior aspect of lumbar plexus in references to anterior intervertebral plane
Anatomic structures with respect to cage placement
The percentage of patients with neural structures at risk on the left side (referencing off only the anterior intervertebral plane) was 2.3% at L1–2, 7.0% at L2–3, 4.7% at L3–4 and 20.9% at L4–5 (Table 2). On the right side (taking into account the position of potentially interfering vasculature), the percentage of patients with neural structures at risk was 7.0% at L1–2, 7.0% at L2–3, 9.3% at L3–4 and 44.2% at L4–5 (Table 2).
Table 2.
Percentage of patients with neurovascular structures at risk by side and level
| L1–2 | L2–3 | L3–4 | L4–5 | |
|---|---|---|---|---|
| Left (%) | 2.3 | 7 | 4.7 | 20.9 |
| Right (%) | 7 | 7 | 9.3 | 44.3 |
Inter- and intraobserver reliability
ICC for interobserver reliability averaged 0.78: 0.74 for lumbar plexus position and 0.82 for femoral nerve position. ICC for intraobserver reliability was 0.87 and 0.85 overall for the two observers.
Discussion
Several previous studies have suggested that the risk for neurovascular injury during LTIF is higher at L4–5 [1, 7]. This is the first study to estimate the percentage of patients vulnerable to iatrogenic neurovascular injury during LTIF surgery. We found that when utilizing a “typical” 20 mm operative corridor, the neurovascular structures would be vulnerable to injury at L4–5 in 21 and 44% of patients when using left- and right-sided approaches, respectively. These data demonstrate the importance of preoperative planning in LTIF surgery and suggest that a significant percentage of patients may not be ideal LTIF candidates based on their neurovascular anatomy.
It is important to point out that in our study, many patients who might be considered “vulnerable to injury” could safely undergo LTIF surgery without incident. Our study documents the percentage of patients whose neurovascular structures lie within a typical 20 mm operative corridor. The common iliac vein can be retracted anteriorly and the lumbar plexus may be retracted posteriorly without injury. For the purposes of this study, we felt compelled to consider these patients to be “at risk” for injury because some degree of retraction would be required to obtain adequate access, and no safe thresholds of retraction have been established for neural or venous structures in the literature. These data serve not to predict what proportion of patients would be injured in surgery but rather to inform surgeons of the percentage of patients whose anatomy predisposes them to injury at various levels and sides. Our data clearly show that a high percentage of patients will require neurovascular retraction during LTIF surgery performed at L4–5 and especially on the right side.
Nervous structures analyzed in this report are small and most-easily identified by scanning multiple contiguous images to locate the structure of interest. We mitigated our learning curve in identifying structures by performing a limited reading round before the study began in earnest to standardize measurement practices. We performed all measurements for the study in duplicate and reviewed all measurements which differed by 5 mm or more to arrive at a consensus decision regarding the location of a structure in order to increase the precision of our anatomic characterization. The values presented for ICC were taken from initial reading rounds prior to establishment of consensus for discrepant values. ICC values describing interobserver reliability overall was 0.78 which represents good reliability based on the semiquantitative scale described above. A similar study performed by Regev et al. [7] arrived at a modestly lower but similar ICC of 0.73, described by that study as representing excellent reliability. Intraobserver reliability in our study was slightly higher with ICCs of 0.85 and 0.87 for our two observers. Together with the results of Regev et al. [7], our study shows that the structures of interest can be reliably identified by both radiologists and spinal surgeons.
Referencing measurements from the anterior intervertebral plane provides a clinically useful frame of reference—anterior cage placement is a conservative strategy which maximizes distances from posterior nervous structures while maintaining an intact ALL. The distance from the anterior extent of the lumbar plexus to the anterior intervertebral plane averaged 30.8 mm at L1–2, 28.6 mm at L2–3, 28.2 mm at L3–4, and 22.1 mm at L4–5. While cranial levels demonstrate larger safety margins, the L4–5 level in particular has only a small margin between the posterior edge of an ideally placed 18 mm deep cage and the anterior aspect of the lumbar plexus. In fact, we found that 20% of patients would require retraction of the lumbar plexus at L4–5 to place a standard XLIF cage with a left-sided approach, avoiding potentially interfering vascular structures which may be encountered with a right-sided approach. Considering the location of right-sided venous structures which often extend posterior to the anterior intervertebral plane, the percentage of patients who will require retraction of neurovascular structures reaches 44% for right-sided approach at L4–5. The tolerance of the lumbar plexus for displacement during retraction and the mobility of this structure within the psoas muscle have not been investigated. Although hip flexion weakness after LTIF has been described [1], larger clinical series are necessary to quantify the degree to which lower extremity weakness and paresthesias are seen after LTIF to shed light as to the clinical significance of these findings.
The findings of the present study echo the work done by Regev et al. [7] who identified the L4–5 level as presenting the highest risk for iatrogenic neurovascular injury because of the relatively anterior position of the lumbar plexus and the relatively posterior location of the retroperitoneal vascular structures. Unfortunately, the operative corridor is reported by Regev et al. as a percentage of the vertebral body diameter, which renders the data less clinically useful. There are wide variations in vertebral body diameters between individuals [8] and therefore our data more clearly convey the proportion of patients vulnerable to neurovascular injury during “typical” LTIF surgery utilizing currently available implants. Similarly, cadaveric studies by Moro et al. [5] and Benglis et al. [1] found the lumbar plexus location at L4–5 was relatively anterior to other levels although Benglis et al. described the position of the posterior aspect of the lumbar plexus in relationship to the posterior aspect of the adjacent endplate which seems less clinically relevant than referencing off the anterior vertebral body.
The femoral nerve moves anterolaterally at caudal lumbar vertebral levels to lie between the posterolateral surface of the psoas muscle and the anteromedial surface of the iliacus muscle. At the L3–4 level, the nerve averaged 33.5 mm posterior to the anterior intervertebral plane before moving further anteriorly at the L4–5 level to lie 26.8 mm posterior to this plane. While this location provides a larger margin of error compared to the lumbar plexus at the same level, three patients (7%) in our study had femoral nerves which were less than 20 mm posterior to the anterior intervertebral plane at L4–5 which could potentially put the femoral nerve at risk of injury from direct contact during dissection or via retraction while accessing the intervertebral space. Again, the tolerance of this nerve for retraction has not been quantitatively described and no large clinical series are available for evaluation of clinical significance of this relationship.
Establishing the location of the psoas muscle requires less practice and is initially less ambiguous than finding the location of the lumbar plexus. Considering this, we investigated the position of the lumbar plexus with respect to the position of the anterior edge of the psoas muscle. A high correlation was found between the position of the psoas muscle and the position of the anterior edge of the lumbar plexus with a correlation coefficient of 0.933, suggesting that the anterior edge of the psoas muscle can be used as a reliable proxy for lumbar plexus location. We performed linear regression of data points from each intervertebral level plotting average positions of the anterior edge of the psoas muscle versus anterior edge of the lumbar plexus. Based on the resulting best-fit line, if the anterior edge of the psoas muscle at L4–5 is found approximately 10 mm anterior to the anterior intervertebral plane, we can expect the lumbar plexus to be found approximately 20 mm posterior to the anterior intervertebral plane. A position 20 mm posterior to the anterior intervertebral plane corresponds to the maximum anterior lumbar plexus position before retraction of the lumbar plexus will be required for placement of an 18 mm cage. The trend line at the L4–5 level provides a slightly conservative estimate of the location of the lumbar plexus (trend line below average L4–5 value on Fig. 4), so a small amount of additional margin for error will be built into relying on a guideline that urges caution in the use of LTIF when the psoas muscle is 10 mm or more anterior to the anterior intervertebral plane. Psoas muscle position is more anterior than this guideline will result in average lumbar plexus locations which require retraction for standard cage placement.
One potential weakness of this study is that all magnetic resonance scans were performed with the patient in the supine position. The exact effect of this positioning on anatomic structures in comparison to the lateral decubitus position used for LTIF is uncertain. Practically, however, this difference of position is unavoidable as obtaining magnetic resonance imaging with patients in the lateral decubitus position with lateral flexion to mimic the surgical position which maximizes access between the inferior aspect of the ribs and superior aspect of the iliac crest would be impossible with currently available MRI machines.
A second weakness is that this study is based entirely on imaging—we do not have surgical corroboration of our findings regarding anatomic relationships.
The current study included patients with relatively undeformed spines. The results of this study may not apply to patients with scoliosis or spondylolisthesis of grade 2 or higher.
Conclusion
This study evaluated the position of vital neurovascular structures in relationship to the increasingly popular lateral transpsoas approach to the lumbar spine. In line with other studies, we found that the L4–5 level presented the highest risk for iatrogenic injury. At this level, the anterior edge of the lumbar plexus approached 20 mm with reference to the anterior intervertebral plane, a value below which would require retraction for placement of a commonly used intervertebral cage. Finally, the anterior edge of the psoas muscle may be used as a proxy for estimation of the position of the lumbar plexus—when the anterior edge of the psoas muscle is 10 mm anterior to the anterior intervertebral plane, the average lumbar plexus position will be approximately 20 mm posterior to the anterior intervertebral plane, at the threshold of the zone where cage placement will require lumbar plexus retraction. Further clinical studies are necessary to determine the clinical significance of these anatomic relationships.
References
- 1.Benglis DM, Vanni S, Levi AD. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J Neurosurg Spine. 2009;10:139–144. doi: 10.3171/2008.10.SPI08479. [DOI] [PubMed] [Google Scholar]
- 2.Farny J, Drolet P, Girard M. Anatomy of the posterior approach to the lumbar plexus block. Can J Anaesth. 1994;41:480–485. doi: 10.1007/BF03011541. [DOI] [PubMed] [Google Scholar]
- 3.Kirchmair L, Lirk P, Colvin J, Mitterschiffthaler G, Moriggl B. Lumbar plexus and psoas major muscle: not always as expected. Reg Anesth Pain Med. 2008;33:109–114. doi: 10.1016/j.rapm.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Laban MM. Atrophy and clinical weakness of the iliopsoas muscle: a manifestation of hip osteoarthritis. Am J Phys Med Rehabil. 2006;85:629. doi: 10.1097/01.phm.0000223222.55943.9a. [DOI] [PubMed] [Google Scholar]
- 5.Moro T, Kikuchi S, Konno S, Yaginuma H. An anatomic study of the lumbar plexus with respect to retroperitoneal endoscopic surgery. Spine. 2003;28:423–428. doi: 10.1097/00007632-200303010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Rasch A, Bystrom AH, Dalen N, Berg HE. Reduced muscle radiological density, cross-sectional area, and strength of major hip and knee muscles in 22 patients with hip osteoarthritis. Acta Orthop. 2007;78:505–510. doi: 10.1080/17453670710014158. [DOI] [PubMed] [Google Scholar]
- 7.Regev GJ, Chen L, Dhawan M, Lee YP, Garfin SR, Kim CW. Morphometric analysis of the ventral nerve roots and retroperitoneal vessels with respect to the minimally invasive lateral approach in normal and deformed spines. Spine (Phila Pa 1976) 2009;34:1330–1335. doi: 10.1097/BRS.0b013e3181a029e1. [DOI] [PubMed] [Google Scholar]
- 8.Sevinc O, Barut C, Is M, Eryoruk N, Safak AA. Influence of age and sex on lumbar vertebral morphometry determined using sagittal magnetic resonance imaging. Ann Anat. 2008;190:277–283. doi: 10.1016/j.aanat.2007.04.005. [DOI] [PubMed] [Google Scholar]