Abstract
In animal models of degenerative lumbar disease, inducible nitric oxide synthase (iNOS) is expressed in macrophages and Schwann cells following compression of the cauda equina. We previously reported that NO metabolites (nitrite plus nitrate: [NOx]) in the cerebrospinal fluid (CSF) correlate with postoperative pain relief in patients with degenerative lumbar disease and with neurologic recovery rate postoperatively or after conservative treatment in patients with spinal cord injury. The objective of the present study was to examine the relationship between [NOx] and neurologic severity, and recovery in degenerative cervical and lumbar diseases. Two hundred fifty-seven cases, including 85 patients with cervical compression myelopathy (CCM), 25 with cervical disc herniation (CDH), 70 with lumbar canal stenosis (LCS), and 77 with lumbar disc herniation (LDH), were examined. The CSF [NOx] was measured using the Griess method. Severity of neurologic impairment and clinical recovery was assessed using the Japanese Orthopedic Association score and Hirabayashi’s method. [NOx] in CCM and LCS, but not CDH and LDH groups, was significantly higher than that in controls, and correlated with postoperative recovery rates, but not with preoperative neurologic severity. [NOx] significantly correlated with neurologic recovery following surgery for CCM and LCS.
Keywords: Nitric oxide, Degenerative cervical diseases, Degenerative lumbar diseases, Cerebrospinal fluid, Neurologic severity, Neurologic recovery
Introduction
Degenerative spinal diseases, such as cervical spondylotic myelopathy or lumbar canal stenosis, often require surgical management now that the life expectancy is becoming longer. The ability to predict the surgical outcome prognosis is therefore important for patients with these diseases. Multiple factors are considered to be useful prognostic predictors, such as preoperative neurologic severity [1], symptom duration [2, 3], and the transverse area of the compressed spinal cord [2, 3].
Nitric oxide (NO), plays a physiological role in neuronal signal transmission [4] and vessel dilation [5], and is involved in the secondary injury that occurs following spinal cord injury (SCI) [6, 7]. We previously reported that preoperative NO metabolite concentrations (nitrite plus nitrate: [NOx]) in the cerebrospinal fluid (CSF) were significantly higher in 25 patients with degenerative lumbar diseases than in controls, and correlated with postoperative pain relief [8]. Furthermore, the [NOx] in patients with SCI correlates with neurologic severity and recovery rate postoperatively, or with conservative treatment [9]. In animal models, inducible NO synthase (iNOS) is expressed in macrophages and Schwann cells at the cauda equina compression site [10] and in macrophages in the early stages after SCI [7]. Excessive NO production derived from iNOS has cytotoxic effects [11] and induces neuronal apoptosis secondary to neural degeneration and neurodysfunction [12]. Apoptotic cells are also observed in spinal cord after cauda equina or chronic spinal cord compression [13, 14]. NO, therefore, may be produced by chronic compression of the cauda equina as well as the spinal cord with induction of neuronal apoptosis, causing neurologic dysfunction. In the present study, we examined whether the CSF [NOx] in patients with chronic or subacute compression of the cauda equina or the spinal cord, such as lumbar canal stenosis, lumbar disc herniation, chronic compressive myelopathy, or cervical disc herniation, correlates with neurologic severity and postoperative recovery.
Materials and methods
Subjects
Written informed consent was obtained from all subjects at each participating institute (Niigata Central Hospital, Niigata Rosai Hospital, Tachikawa Hospital, Kariwagun General Hospital, Akita Red Cross Hospital, and Nagaoka Red Cross Hospital). The study group comprised 257 cases, including 85 patients with cervical compression myelopathy (CCM group; 53 males, 32 females, age range [mean]: 33–83 [63 ± 14] years, duration of symptoms: 20 ± 4 months), 25 with cervical disc herniation (CDH group; 17 males, 8 females, age range: 30–73 [49 ± 11], duration of symptoms: 7 ± 2 months), 70 with lumbar canal stenosis (LCS group; 35 males, 35 females, age range: 40–83 [66 ± 10], duration of symptoms: 13 ± 2 months), and 77 with lumbar disc herniation (LDH group; 48 males, 29 females, age range: 21–81 [46 ± 17], duration of symptoms: 2 ± 0.3 months) (Table 1). The diagnosis in those patients was determined by two orthopedic surgeons based on radiological and neurological findings. The CCM group comprised 66 patients with cervical spondylotic myelopathy and 19 patients with ossification of the posterior longitudinal ligament. All CCM patients underwent cervical surgery (anterior spinal fusion: 19 cases, laminoplasty: 61, laminectomy: 3, laminoplasty and laminectomy: 2). The postoperative follow-up period ranged from 6 to 24 months with a mean of 12 months. Of the 25 CDH patients, 20 underwent surgery (anterior spinal fusion: 9, foraminotomy: 6, laminoplasty and foraminotomy: 5 cases). The postoperative follow-up period ranged from 7 to 27 months with a mean of 15 months. All the LCS patients underwent surgery (laminectomy: 49 cases, posterior lumbar interbody fusion: 21). The postoperative follow-up period ranged from 6 to 12 months with a mean of 9 months. All LDH patients underwent herniotomy. The postoperative follow-up period ranged from 6 to 12 months with a mean of 8 months. Patients with both degenerative cervical and lumbar diseases were excluded based on neurologic findings and radiologic examination, such as magnetic resonance (MR) imaging and myelography. Patients with spinal trauma, previous spinal surgery, or chronic inflammatory diseases, such as rheumatoid arthritis, were excluded.
Table 1.
Patient summary
| Diagnosis | n | Age range | Mean of age | Procedure | |
|---|---|---|---|---|---|
| Control | Healthy volunteers: 6 Inguinal herniation: 10 S/P fracture: 33 and so ona |
53 | 18–76 | 50 | |
| DCD | CCM (CSM 66, OPLL 19) | 85 | 33–83 | 63 | ASF: 19, laminoplasty: 61 Laminectomy: 3 Laminoplasty and laminectomy: 2 |
| CDH | 25 | 30–73 | 50 | ASF: 9, foraminotomy: 6 Laminoplasty and foraminotomy: 5 |
|
| DLD | LCS | 70 | 40–83 | 66 | Laminectomy: 49, PLIF: 21 |
| LDH | 77 | 21–81 | 46 | Herniotomy: 77 |
aThe patients were undergoing the removal of metal instrumentation following a lower extremity fracture
DCD degenerative cervical diseases, DLD degenerative lumbar diseases
The control group (34 males, 19 females; age range [mean]: 18–76 [49 ± 17]) comprised 53 cases comprising 6 healthy volunteers that had neither pain nor neurologic disorders, and 47 patients with inguinal herniation (n = 10) or who were undergoing the removal of metal instrumentation following a lower extremity fracture (n = 33), uterine prolapse (n = 1), hallux valgus (n = 1), soft tissue tumor in the leg (n = 1), and pigmented villonodular synovitis (n = 1). The CSF in the control group was obtained just before lumbar anesthesia.
CSF collection and measurement of NO metabolites
CSF (1–2 ml) was collected using a spinal needle (23–25 G) during preoperative myelography. Needle puncture was performed at the L3/4 or L4/5 level. The number of needle placements was one in almost all cases. The CSF specimens were stored at −20°C for later measurement of [NOx]. [NOx] measurement was performed within 6 months after CSF collection. CSF [NOx] was measured based on the Griess method [15], as reported previously [16]. The assay was performed with an NO analyzer (ENO-20, Eicom Corp, Kyoto, Japan). The minimum detectable level of NO2− (nitrite) and NO3− (nitrate) was 0.01 μM. The reproducibility and accuracy of this system was confirmed previously [16].
Comparison of [NOx] among control, CCM, CDH, LCS, and LDH groups
The [NOx] was compared among the control, CCM, CDH, LCS, and LDH groups (Fig. 1). The correlations between [NOx] and age, [NOx] and duration of symptoms were examined in each group.
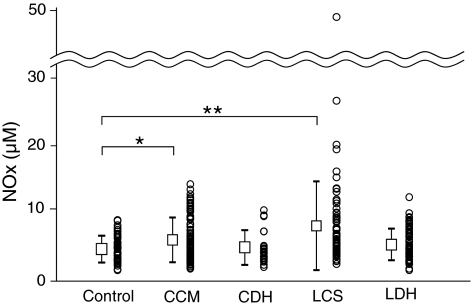
Fig. 1.
Comparison of the CSF nitric oxide metabolite concentrations ([NOx]) among the control, cervical compression myelopathy (CCM), cervical disc herniation (CDH), lumbar canal stenosis (LCS), and lumbar disc herniation (LDH) groups. The [NOx] in patients with CCM and LCS was significantly higher than that in controls. Values are expressed as means ± SD, and each datum (vertical scatter-plots). * p < 0.05, ** p < 0.01
In the CCM group, the 80 patients were divided into two groups, the central cord syndrome group and transverse lesion syndrome group, based on the Crandall classification [17]. The [NOx] in the two groups was compared. Of 85 CCM patients, MR imaging was performed in 81 cases. The numbers of disc levels with spinal cord compression were examined on sagittal and axial MR images and classified as: 1 level, 2 levels, 3 levels, and 4 or more levels. The [NOx] was compared among these four groups. The [NOx] in the CCM groups with and without high signal intensity on T2-weighted MR images was also compared. The [NOx] was compared between groups with and without high signal intensity on T2-weighted MR images. Three patients were excluded because of poor quality MR images. Two spine surgeon blind to the patient clinical information performed the radiologic evaluation. The CDH group was subdivided into two groups (myelopathy and radiculopathy groups), in which the [NOx] was compared. In addition, the CDH group was subdivided into three groups (central, paramedian, and intraforaminal) due to differences in the localization of the disc protrusion.
Evaluation of neurologic severity and recovery in the CCM, LCS, and LDH groups
Neurologic severity was assessed using the Japanese Orthopedic Association (JOA) score. The JOA scoring system for cervical myelopathy comprises four categories: upper limb function, lower limb function, sensory disturbance, and urinary and bladder function [18] (Table 2). The JOA score for the lumbar diseases also comprises four categories: subjective symptoms, clinical signs, restriction of activities of daily living, and urinary bladder function [19] (Table 3). To investigate the relationship between [NOx] and neurologic severity, the [NOx] in the CCM, LCS, and LDH groups was compared with the total JOA score. The [NOx] in the CDH group was not included in this comparison because the symptoms in patients with CDH are accompanied by myelopathy and/or radiculopathy. In fact, the cervical JOA score is only applicable for cervical myelopathy. Surgical outcomes were evaluated by Hirabayashi’s recovery rate, which was calculated using the following equation: recovery rate (%) = {JOA score at follow-up–initial JOA score}/{full score–initial JOA score} × 100. The [NOx] in the CCM, LCS, and LDH groups was compared with Hirabayashi’s recovery rate.
Table 2.
The Japanese Orthopaedic Association’s evaluation system for cervical myelopathy (total 17 points)
| I. Upper extremity function |
| 0. Impossible to eat with either chopsticks or spoon |
| 1. Possible to eat with spoon, but not with chopsticks |
| 2. Possible to eat with chopsticks, but inadequately |
| 3. Possible to eat with chopsticks, but awkwardly |
| 4. Normal |
| II. Lower extremity function |
| 0. Impossible to walk |
| 1. Need cane or aid on flat ground |
| 2. Need cane or aid on stairs |
| 3. Possible to walk without cane or aid, but slowly |
| 4. Normal |
| III. Sensory disturbance |
| A. Upper extremity |
| 0. Apparent sensory loss |
| 1. Minimal sensory loss |
| 2. Normal |
| B. Lower extremity |
| 0. Apparent sensory loss |
| 1. Minimal sensory loss |
| 2. Normal |
| C. Trunk |
| 0. Apparent sensory loss |
| 1. Minimal sensory loss |
| 2. Normal |
| IV. Bladder function |
| 0. Complete retention |
| 1. Severe disturbance |
| Inadequate evacuation of the bladder, straining, dibbling of urine |
| 2. Mild disturbance |
| Urinary frequency, urinary hesitancy |
| 3. Normal |
Table 3.
The Japanese Orthopaedic Association’s evaluation system for low back pain syndrome
| I. Subjective symptoms | |||
| Lower back pain | None | 3 | |
| Occasional mild pain | 2 | ||
| Frequent mild or occasional severe pain | 1 | ||
| Frequent or continuous severe pain | 0 | ||
| Leg pain and/or tingling | None | 3 | |
| Occasional slight symptom | 2 | ||
| Frequent slight or occasional severe symptom | 1 | ||
| Frequent or continuous severe symptom | 0 | ||
| Gait | Normal | 3 | |
| Able to walk farther than 500 m although it results in symptoms | 2 | ||
| Unable to walk father than 500 m | 1 | ||
| Unable to walk father than 100 m | 0 | ||
| II. Clinical signs | |||
| Straight leg raising test | Normal | 2 | |
| 30–70 | 1 | ||
| Less than 30 | 0 | ||
| Sensory disturbance | None | 2 | |
| Slight disturbance | 1 | ||
| Marked disturbance | 0 | ||
| Motor disturbance | Normal | 2 | |
| Slight weakness (MMT 4) | 1 | ||
| Marked weakness (MMT 3-0) | 0 | ||
| Severe | Moderate | None | |
|---|---|---|---|
| III. Restriction of activities of daily living | |||
| Turn over while lying | 0 | 1 | 2 |
| Standing | 0 | 1 | 2 |
| Washing face | 0 | 1 | 2 |
| Leaning forwards | 0 | 1 | 2 |
| Sitting (about 1 h) | 0 | 1 | 2 |
| Lifting or holding heavy objects | 0 | 1 | 2 |
| Waking | 0 | 1 | 2 |
| IV. Urinary bladder function | Normal | 0 | |
| Mild dysuria | −3 | ||
| Severe dysuria | −6 |
Data analysis
Data are expressed as means ± SD. Differences in means between two groups or between more than two groups were tested using the Mann–Whitney U test and one-way ANOVA, respectively. Correlations were assessed using Spearman’s ranked correlation coefficient. A p value of less than 0.05 was considered statistically significant.
Results
CSF [NOx] in Control, CCM, CDH, LCS, and LDH groups
The [NOx] in the CCM and LCS, but not the CDH and LDH groups was significantly higher than that in the control group (Fig. 1). There was no significant correlation between [NOx] and age, duration of symptoms in each group.
In addition, [NOx] did not significantly differ between patients with central cord syndrome (n = 34, [NOx]: 6.1 ± 0.6 μM) and those with transverse lesion syndrome (n = 46, 6.2 ± 0.4 μM). [NOx] did not significantly differ among the number of disc levels with spinal cord compression; 1 level (n = 18, 6.4 ± 0.7 μM), 2 levels (n = 28, 6.0 ± 0.6 μM), 3 levels (n = 28, 5.8 ± 0.6 μM), and more than 4 levels (n = 7, 5.8 ± 1.3 μM). The [NOx] was significantly higher in patients with high intensity lesions on T2-weighted MR images (n = 52) than in those without (n = 26, p < 0.05; Fig. 2).
Fig. 2.
Comparison of the CSF nitric oxide metabolite concentrations ([NOx]) between patients with and without high signal intensity on T2-weighted MR images in patients with cervical compression myelopathy. The [NOx] in patients with high signal intensity (n = 52) was significantly higher than in those without (n = 26). Values are expressed as means + SD. * p < 0.05
In the CDH group, [NOx] did not differ between the myelopathy (n = 12, 5.3 ± 0.8 μM) and the radiculopathy (n = 13, 4.7 ± 0.6 μM) groups. There was also no significant difference in the [NOx] among the central (n = 9, 5.6 ± 2.9 μM), paramedian (n = 6, 4.5 ± 1.7 μM), and intraforaminal (n = 10, 4.6 ± 2.2 μM) subgroups.
Correlation between [NOx] and neurologic severity
The [NOx] and JOA scores were not significantly correlated in the CCM, LCS, and LDH groups.
Correlation between the [NOx] and postoperative recovery rate
The [NOx] correlated significantly with the postoperative recovery rate in the LCS and CCM groups (Fig. 3), but not LDH groups. These findings indicate that [NOx] in patients with chronic compression of the cauda equina or spinal cord correlates with a postoperative recovery following decompressive surgery.
Fig. 3.
Correlations between the CSF nitric oxide metabolite concentrations ([NOx]) and postoperative Hirabayashi’s recovery rate. There was a significant negative correlation between the [NOx] and Hirabayashi’s recovery rate in patients with lumbar canal stenosis (LCS) (ar = −0.24, p < 0.05) and cervical compression myelopathy (CCM) (br = −0.31, p < 0.01)
Discussion
NO production under chronic compression of the cauda equina and spinal cord
The present study demonstrated that the [NOx] was significantly higher in the CCM and LCS groups than in the control group. The [NOx] in the CDH and LDH groups, however, was not higher than that in the control group. These findings suggest that the [NOx] increases with chronic, but not subacute, spinal cord or cauda equina compression. In an animal experiment, we previously demonstrated that iNOS is expressed in macrophages and Schwann cells at the cauda equina compression site in rats [10]. Levy et al. observed iNOS expression following chronic constriction injury to the sciatic nerve in rats [20]. NO may be produced by iNOS under chronic compression of the cauda equina or spinal cord. In addition to the experimental studies, Yumite et al. reported that CSF [NOx] is significantly higher in patients with LCS (n = 30) and LDH (n = 30) than in controls [21]. Watanabe et al. reported that CSF [NOx] was significantly higher in an LCS group (n = 28), but not an LDH group (n = 13), compared with a control group [22]. The present findings are consistent with Watanabe’s report.
Little is known, however, about the mechanisms underlying NO production in chronic spinal cord compression. Consistent with peripheral nerve compression [20], NO may continue to be produced secondary to the chronic compression of the spinal cord, such as in chronic compressive myelopathy.
Relationship between the NOx levels and neurologic severity
NO is not so toxic at physiologic concentrations. Excessive NO production, however, derived from iNOS has cytotoxic effects [6, 11] and induces neuronal apoptosis [12]. We previously reported that [NOx] correlates with neurologic severity in patients with SCI, suggesting that high NO concentrations induce neural dysfunction [9]. There is still controversy about the relation between [NOx] and neurologic severity in degenerative spinal diseases. Yumite et al. reported that [NOx] correlates with the JOA score in cervical degenerative diseases [21]. Watanabe et al. however, reported that [NOx] does not correlate with symptom severity in degenerative lumbar diseases [22]. In the present study, there was no correlation between [NOx] and neurologic severity in patients with degenerative lumbar diseases or CCM.
In patients with cervical compressive myelopathy, the [NOx] in patients with high signal intensity on T2-weighted MR images was significantly higher than that in patients without. It is generally known that high signal intensity on T2-weighted images reflects myelomalacia or cord gliosis secondary to long-standing compression of the spinal cord [23]. It is reported that neurologic findings in patients with increased signal intensity in the spinal cord on T2-weighted MR images are worse than in those without [24]. On the other hand, Wada et al. [25] reported that high intensity areas are not correlated with the severity of myelopathy or surgical outcome. In our study, the CSF [NOx] significantly correlated with the recovery rate. This finding may support the notion that high intensity lesions in MR images affect the recovery rate. Changes on MRI together with NOx analyses may help clinicians to better predict the size of the spinal cord injury and how this will influence recovery. The findings of the present study indicate that [NOx] may reflect the histopathologic changes in the spinal cord in patients with CCM despite the fact that [NOx] did not correlate with neurologic severity.
Significant correlation between CSF [NOx] and neurologic recovery in chronic compression of cauda equina and spinal cord
Excessive NO production derived from iNOS has cytotoxic effects and induces neuronal apoptosis [12]. Sekiguchi et al. [13] demonstrated that apoptosis of dorsal and ventral horn neurons increases after cauda equina compression in rats. Yamaura et al. [14] observed apoptotic oligodendrocytes in the spinal cord with chronic compression both in human autopsy samples and in a mouse model. Kim et al. suggested that oligodendrocyte apoptosis is an important determinant of morbidity in cervical spondylotic myelopathy [26]. Collectively, we hypothesized that high [NOx] induces neuronal apoptosis with irreversible changes, resulting in poor recovery following surgery. In fact, our previous report indicated that preoperative [NOx] significantly correlates with the degree of pain relief following surgical decompression for degenerative lumbar disease [8]. In the present study we reproduced this finding with a larger series of patients (Fig. 3a). In addition to the correlation with degenerative lumbar disease, [NOx] correlated with surgical outcome in CCM (Fig. 3b).
It is generally known that preoperative neurologic severity [1], duration of symptoms [2, 3], or transverse area at the compression site [2, 3] is a candidate of useful predictors. The present study demonstrated a significantly negative correlation between preoperative CSF [NOx] and postoperative recovery rate in patients with CCM as well as LCS. The r value was too low, however, to use [NOx] as a clinical predictor.
Limitations of the present study
The mean age differed between the groups, with the two groups with the highest NOx (LCS and CCM groups) having the highest mean age, which may mean that [NOx] is age-related. We previously reported, however, that [NOx] in young control subjects is higher than that in older control subjects [16]. There was no correlation between [NOx] and age among the entire patient population in the present study. The controls were age-matched for each group. Therefore, [NOx] in the CCM and LCS groups was not dependent on age, although the mean ages of the two groups was relatively high.
In addition, the follow-up was not performed at the same time after surgery in the patients, making the recovery score difficult to interpret in patients decompressed because of myelopathy or cauda equina symptoms, because recovery is often slow and continues for at least 12 months in these patients (especially for myelopathy patients). In some previous papers, the postoperative follow-up period was about 1 year [2, 27]. The results of the present study, however, are consistent with the findings from our previous study with a similar follow-up period [8]. In the present study, mean Hirabayashi’s recovery rate in LCS and CSM was 54 and 44%, respectively. These data are comparable to the findings in other studies [3, 28]. The different follow-up time may have resulted in the smaller r value of the correlation between [NOx] and the recovery rate.
The present study is limited by the fact that patients with both CCM and LCS were excluded. Those patients commonly undergo decompressive surgery. Therefore, the [NOx] in patients with double lesions, such as cervical stenosis and LCS, should be examined in the future.
Acknowledgments
We thank Yoshiaki Tanaka for his technical assistance. This study was approved by the Ethics Committee of Niigata University.
Conflict of interest None.
References
- 1.Baba H, Furusawa N, Chen Q, Imura S, Tomita K. Anterior decompressive surgery for cervical ossified posterior longitudinal ligament causing myeloradiculopathy. Paraplegia. 1995;33:18–24. doi: 10.1038/sc.1995.5. [DOI] [PubMed] [Google Scholar]
- 2.Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96:173–179. doi: 10.3171/spi.2002.96.2.0173. [DOI] [PubMed] [Google Scholar]
- 3.Koyanagi T, Hirabayashi K, Satomi K, Toyama Y, Fujimura Y. Predictability of operative results of cervical compression myelopathy based on preoperative computed tomographic myelography. Spine. 1993;18:1958–1963. doi: 10.1097/00007632-199310001-00006. [DOI] [PubMed] [Google Scholar]
- 4.Shibuki K, Okada D. Endogenous nitric oxide release required for long-term synaptic depression in the cerebellum. Nature. 1991;349:326–328. doi: 10.1038/349326a0. [DOI] [PubMed] [Google Scholar]
- 5.Moncada S, Palmer RM, Higgs EA. The discovery of nitric oxide as the endogenous nitrovasodilator. Hypertension. 1988;12:365–372. doi: 10.1161/01.hyp.12.4.365. [DOI] [PubMed] [Google Scholar]
- 6.Hamada Y, Ikata T, Katoh S, Tsuchiya K, Niwa M, Tsutsumishita Y, Fukuzawa K. Roles of nitric oxide in compression injury of rat spinal cord. Free Radic Biol Med. 1996;20:1–9. doi: 10.1016/0891-5849(95)02017-9. [DOI] [PubMed] [Google Scholar]
- 7.Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K, Kiuchi K. Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain Res Mol Brain Res. 2000;85:114–122. doi: 10.1016/S0169-328X(00)00253-9. [DOI] [PubMed] [Google Scholar]
- 8.Kimura S, Watanabe K, Yajiri Y, Uchiyama S, Hasegawa K, Shibuki K, Endo N. Cerebrospinal fluid nitric oxide metabolites are novel predictors of pain relief in degenerative lumber diseases. Pain. 2001;92:363–371. doi: 10.1016/S0304-3959(01)00279-2. [DOI] [PubMed] [Google Scholar]
- 9.Hosaka N, Kimura S, Yamazaki A, Wang X, Denda H, Ito T, Hirano T, Endo N. Significant correlation between cerebrospinal fluid nitric oxide concentrations and neurologic prognosis in incomplete cervical cord injury. Eur Spine J. 2006;17:281–286. doi: 10.1007/s00586-007-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Kimura S, Kakita A, Hosaka N, Denda H, Ito T, Hirano T, Endo N. Nitric oxide in cerebrospinal fluid and local inducible nitric oxide synthase after cauda equina compression in rats. Neuroreport. 2006;17:1473–1478. doi: 10.1097/01.wnr.0000234746.35195.b0. [DOI] [PubMed] [Google Scholar]
- 11.Dawson VL, Dawson TM, Bartley DA, Dawson TM, Bartley DA, Uhl GR, Synder SH. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci. 1993;13:2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estévez AG, Spear N, Manuel SM, Radi R, Henderson CE, Barbeito L, Beckman JS. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J Neurosci. 1998;18:923–931. doi: 10.1523/JNEUROSCI.18-03-00923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekiguchi M, Kikuchi S, Robert RM. Experimental spinal stenosis: relationship between degree of cauda equina compression, neuropathology, and pain. Spine. 2004;29:1105–1111. doi: 10.1097/00007632-200405150-00011. [DOI] [PubMed] [Google Scholar]
- 14.Yamaura I, Yone K, Nakahara S, Nagamine T, Baba H, Uchida K, Komiya S. Mechanism of destructive pathologic changes in the spinal cord under chronic mechanical compression. Spine. 2002;27:21–26. doi: 10.1097/00007632-200201010-00008. [DOI] [PubMed] [Google Scholar]
- 15.Griess JP. On a new series of bodies in which nitrogen is substituted for hydrogen. Philos Trans R Soc Lond. 1964;154:667–731. [Google Scholar]
- 16.Kimura S, Watanabe K, Yajiri Y, Motegi T, Masuya Y, Shibuki K, Uchiyama S, Homma T, Takahashi H. Cerebrospinal fluid nitric oxide metabolites in painful diseases. Neuroreport. 1999;10:275–279. doi: 10.1097/00001756-199902050-00013. [DOI] [PubMed] [Google Scholar]
- 17.Crandall PH, Batzdorf U. Cervical spondylotic myelopathy. J Neurosurg. 1966;25:57–66. doi: 10.3171/jns.1966.25.1.0057. [DOI] [PubMed] [Google Scholar]
- 18.Japanese Orthopaedic Association (1976) Criteria on the evaluation of the treatment of cervical myelopathy (in Japanese). J Japanese Orthop Assn 50:Addenda 5
- 19.Inoue S, Kataoka O, Tajima T. Assessment of treatment for low back pain. J Japanese Orthop Assn. 1986;60:909–911. [Google Scholar]
- 20.Levy D, Höke A, Zochodne DW. Local expression of inducible nitric oxide synthase in an animal model of neuropathic pain. Neurosci Lett. 1999;260:207–209. doi: 10.1016/S0304-3940(98)00982-3. [DOI] [PubMed] [Google Scholar]
- 21.Yumite Y, Takeuchi K, Harada Y, Ogawa N, Inoue H. Concentration of nitric oxide (NO) in spinal fluid of chronic spinal disease. Acta Medica Okayama. 2001;55:219–228. doi: 10.18926/AMO/31996. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe T, Kato S, Sato K, Nagata K. Nitric oxide regulation system in degenerative lumbar disease. Kurume Med J. 2005;52:39–47. doi: 10.2739/kurumemedj.52.39. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi M, Yamashita T, Sakamoto Y, Kojima R. Chronic cervical cord compression: clinical significance of increased signal intensity on MR images. Radiology. 1989;173:219–224. doi: 10.1148/radiology.173.1.2781011. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda Y, Miyazaki K, Tada K, Yasuda A, Nakayama T, Murakami H, Matsuo M. Increased MR signal intensity due to cervical myelopathy. Analysis of 29 surgical cases. J Neurosurg. 1992;74:887–892. doi: 10.3171/jns.1991.74.6.0887. [DOI] [PubMed] [Google Scholar]
- 25.Wada E, Ohmura M, Yonenobu K. Intramedullary changes of the spinal cord in cervical spondylotic myelopathy. Spine. 1995;20:2226–2232. doi: 10.1097/00007632-199510001-00009. [DOI] [PubMed] [Google Scholar]
- 26.Kim DH, Vaccaro AR, Henderson FC, Benzel EC. Molecular biology of cervical myelopathy and spinal cord injury: role of oligodendrocyte apoptosis. Spine J. 2003;3:510–519. doi: 10.1016/S1529-9430(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 27.Lee DY, Lee SH. Spinous process splitting laminectomy for lumbar canal stenosis: a critical appraisal. Minim Invasive Neurosurg. 2008;51:204–207. doi: 10.1055/s-2008-1073137. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe K, Hosoya T, Shiraishi T, Matsumoto M, Chiba K, Toyama Y. Lumbar spinous process–splitting laminectomy for lumbar canal stenosis. J Neurosurg Spine. 2005;3:405–408. doi: 10.3171/spi.2005.3.5.0405. [DOI] [PubMed] [Google Scholar]