Abstract
The aim of this study was to evaluate the influence of smoking on the outcome of patients undergoing surgery for degenerative spinal diseases, and to examine whether smoking had a differential impact on outcome, depending on the fusion technique used. The cohort included 120 patients treated with two different fusion techniques (translaminar screw fixation and TLIF). They were categorised with regard to their smoking habits at the time of surgery and completed the Core Outcome Measures Index at baseline and follow-up (FU) (3, 12 and 24 months FU); at FU they also rated the global outcome of surgery. The distribution of smokers was comparable in the two groups. For the TS group, the greater the number of cigarettes smoked, the less the reduction in pain intensity from pre-op to 24 months FU; the relationship was not significant for the TLIF group. The percentage of good global outcomes declined with time in the TS smokers such that by 24 months FU, there was a significant difference between TS smokers and TS-non-smokers. No such difference between smokers and non-smokers was evident in the TLIF group at any FU time. In conclusion, the TS technique was more vulnerable to the effects of smoking than was TLIF: possibly the more extensive stabilisation of the 360° fusion renders the environment less susceptible to the detrimental effects on bony fusion of cigarette smoking.
Keywords: Degenerative lumbar disease, Cigarette smoking, Transforaminal interbody fusion, Translaminar screw fixation
Introduction
There is general agreement in the literature that cigarette smoking is detrimental to bone healing [1, 2] and specifically to spinal fusion. It has been demonstrated that the rate of non-union or pseudoarthrosis after spinal fusion is up to four times higher in smokers than non-smokers [3–6] although the specific pathophysiologic mechanism responsible is still unclear. Although the major mechanism seems to be related to the severe vasoconstrictive effect of nicotine on the microvasculature, which hinders angioblastic activity during bone graft healing [7], smoking has also been associated with calcitonin resistance [8] and impaired osteoblastic function [9]. Possibly related to its effect on solid fusion, smoking has also been found to be a strong negative predictor of patient satisfaction with treatment after lumbar fusion surgery [10, 11]. Nonetheless, this might also represent an independent effect, since smoking is associated with poorer outcome in the operative treatment of other lumbar spinal disorders, even when fusion is not involved; a number of studies have shown that smoking habit has a negative influence on outcome in the surgical treatment of lumbar disc surgery [12–14].
The influence of smoking in relation to the extensiveness and invasiveness of the fixation technique used to achieve a solid fusion is still unclear: theoretically, the more solid the basis for fusion provided by the fixation technique, the less should be its susceptibility to harmful environmental factors, such as smoking.
In this study, we examined the influence of smoking on the outcome of two different fixation techniques commonly used to achieve a solid fusion: translaminar screw fixation (TS) and transforaminal interbody fusion (TLIF). TS, introduced and popularised by Magerl [15], has been shown to provide a solid fusion with less extensive surgery when compared with TLIF, which is widely considered the gold standard for spine fusion [16]. There is nonetheless evidence to suggest there may be an increased risk of re-operation due to non-union with TS compared with pedicle screw instrumentation [17]. Assuming a weakness of TS to provide solid fusion, compared with TLIF, we hypothesised that the outcome of TS might be more susceptible to the detrimental effect of smoking and that this may be reflected in a technique-dependent difference in patient-rated outcome between smokers and non-smokers.
The aim of this study was to evaluate the influence of smoking on the outcome of patients undergoing surgery for degenerative spinal diseases and to examine whether smoking had a differential impact on outcome, depending on the fusion technique used. The study represents a secondary analysis of the data collected in a previous investigation in which the outcome of the two different fusion techniques per se was compared [18].
Methods
Patients
A retrospective analysis of prospectively collected data was performed.
The study was nested within our Spine Center patient outcome database in connection with the Spine Society of Europe Spine Tango data acquisition system. The inclusion criteria and recruitment procedure have been described in detail before [18]. Briefly, patients referred to the Spine Center were equally distributed to the surgeons in a 2-week term. All patients fitting the admission criteria were automatically eligible for study and were marked as such in the database. Other than this, there was absolutely no difference in the subsequent clinical practice or documentation procedure for these patients. Since the translaminar screw fixation is suitable only for monosegmental and occasionally bisegmental degenerative changes in the lumbar spine, the inclusion criteria for the study were selected accordingly: mono/bisegmental degenerative disc disease, facet syndrome or degenerative spondylolisthesis. In addition, the completion of self-rated questionnaires necessitated that the patient had a good understanding of written German. The only exclusion criteria were previous surgery other than discectomy, and lack of willingness to give informed consent to surgery and/or questionnaire completion. 120 patients satisfied the admission criteria; 57 in the TS group (1 surgeon) and 63 in the TLIF group (4 surgeons).
Surgical procedures
Each surgeon consistently used his/her pre-stated, preferred method (TS or TLIF) for all his patients fitting the inclusion criteria. This procedure assured optimal technical performance and motivation for the surgery.
Both procedures were initiated with a standard midline approach and classical dissection to expose the involved segments of the lumbar spine. Decompression with an undercutting technique was applied as indicated. Autologous bone, harvested via a separate approach at the posterior iliac crest, was used in all patients of the TS group and in 91% of the TLIF group. Allograft was used to augment or replace autologous bone in 11% patients in the TS group and in 44% patients in the TLIF group. Bone substitute was not used in any patients in the TS group, and was used in just one patient in the TLIF group.
The translaminar screws were inserted under direct visual control using a percutaneous stab incision if necessary [19, 20]. The screws perforate the surface of the superior and inferior facets in their centres, thereby preventing motion. The TLIF procedure was performed according to a standard technique. The procedure started with the bilateral insertion of the pedicle screws. Once the screws were in place, parallel distraction was applied to facilitate cage insertion. A one-sided facetectomy was performed to achieve sufficient access to the posterolateral aspect of the disc. The intervertebral disc was removed through a rectangular incision of the posterior annulus. The end plates were decorticated and the bone graft inserted into the anterior part of the disc space. Finally, through the opening in the annulus, the cage (Devex, Depuy Spine) was inserted and placed into the posterior part of the intervertebral space to maintain the width of the foramen. Finally, the distraction was released and a moderate compression applied to establish lordosis and to prevent cage migration.
Documentation forms and questionnaires
The Spine Society of Europe (SSE) Spine Tango Surgery forms were used to document information about the surgery: these data have already been reported [18].
Before and 3, 12 and 24 months after surgery, patients were requested to complete the multidimensional Core Outcome Measures Index (COMI) questionnaire [21]. On each occasion, the questionnaires were sent to the patients to complete at home, to ensure that the information given was free of care-provider influence. The COMI is a multidimensional index consisting of validated questions covering the domains of pain (leg and back pain intensity, each measured separately on a 0–10 graphic rating scale), function, symptom-specific well being, general quality of life, and social and work disability. The COMI was originally developed based on the recommendations for a short series of Core Outcome questions by an Expert Group in the field of Low Back Pain Outcome measurement [22] and subsequently validated as an outcome instrument by three research groups [21, 23–25]. In addition to the COMI questions answered both before and after surgery, at 3, 12 and 24 months' FU, there were further questions inquiring about the global outcome of surgery (“overall, how much did the operation help your back problem?”; 5 response categories from “helped a lot” to “made things worse”).
Patients were categorized with regards to their smoking habits based on the general admission report completed by residents on the first day of the patient’s hospitalisation. The six categories were: non-smoker; ≤5 cigarettes/day; 6–10/day; 11–15/day; 16–20/day; >20/day. Smoking data were available for 110/120 (92%) patients (53 TS, 57 TLIF) and these comprised the study groups of interest in the present investigation. Gender distribution was almost identical in the two groups, but TS were significantly older than TLIF and had significantly greater co-morbidity (higher ASA scores) (Table 1). Pre-op questionnaires were completed by 108/110 (98%) patients; FU questionnaires were completed by 109/110 (99%) patients after 3 months, 102/110 (92.7%) patients after 12 months and 99/110 (90%) patients after 24 months.
Table 1.
Baseline characteristics of the patients in each group with smoking data
| Group TS | Group TLIF |
p value for comparison TS versus TLIF |
|
|---|---|---|---|
| Number of patients | N = 53 | N = 57 | |
| Gender | 69.8% women | 68.4% women | 0.87 |
| Age | 66.9 ± 10.3 yrs | 57.5 ± 13.7 yrs | <0.0001 |
| Comorbidity (ASA categories) | 2.0% ASA 1 | 40.4% ASA 1 | <0.0001 |
| 12.0% ASA 2 | 46.2% ASA 2 | ||
| 86.0% ASA 3 | 13.5% ASA 3 | ||
| Number of surgeons | N = 1 | N = 4 |
p values marked bold are significant at p < 0.05
Statistical analyses
Descriptive data are presented as mean ± standard deviations (SD).
The significance of the difference between the smokers and non-smokers for continuous, normally distributed data were analysed using unpaired Student’s t tests. Two-way analysis of variance (ANOVA) was used to examine the difference in COMI item/overall scores over time between the smoking groups and the treatment groups. Contingency analyses were used to analyse the association between smoking status and categorical variables.
Spearman Rank correlation coefficients corrected for ties were used to indicate the strength of the relationship between the number of cigarettes smoked per day and the change in pain intensity/COMI score from pre-op to 24 months follow-up.
The global outcome was dichotomised into “good” (=operation helped, or helped a lot) and “poor” (=operation only helped a little, did not help, made things worse) for the purposes of some of the subsequent analyses.
Statistical significance was accepted at the p < 0.05 level.
Results
Distribution of smokers
30/110 (27.3%) patients smoked; 16.6% had a daily cigarette consumption of <5 cigarettes; 33.3%, 6–10 cigarettes; 16.7%, 11–15 cigarettes; 16.7%, 16–20 cigarettes and 16.7%, >20 cigarettes.
The distribution of smokers was comparable in the TS and TLIF groups (23 and 32%, respectively; p = 0.29).
Baseline scores
The baseline scores for the smokers and non-smokers, for each of the COMI domains, are shown in Table 2. Pain scores showed a tendency to be higher in the non-smokers than the smokers, but the difference did not acquire significance (p = 0.14–0.19); there were no significant differences between the smokers and non-smokers for any of the other domains.
Table 2.
Baseline symptoms in the non-smokers and smokers, as determined from the COMI
| Non-smokers (N = 78) | Smokers (N = 29) | p value | |
|---|---|---|---|
| Back pain intensitya | 7.0 (2.3) | 6.1 (3.3) | 0.15 |
| Leg pain intensitya | 6.4 (2.8) | 5.6 (2.9) | 0.19 |
| Pain intensity worst symptom (back or leg)a | 7.6 (1.9) | 6.9 (2.9) | 0.14 |
| Functionb | 4.0 (0.9) | 4.0 (1.0) | 0.82 |
| Symptom-specific well being | 4.8 (0.6) | 4.7 (0.8) | 0.53 |
| General quality of life | 4.1 (0.8) | 4.2 (0.8) | 0.79 |
| Social disability | 4.3 (1.3) | 3.8 (1.6) | 0.13 |
| Work disability | 3.3 (1.8) | 3.5 (1.8) | 0.55 |
| COMI whole scorea | 7.9 (1.6) | 7.6 (2.1) | 0.51 |
Data are from N = 108 patients: smoking data was available for 110 patients, 2 of whom had no baseline COMI questionnaire data
a0–10 scale (higher score, worse status)
b1–5 scale (higher score, worse status)
Change in COMI scores up to 2 years post-op
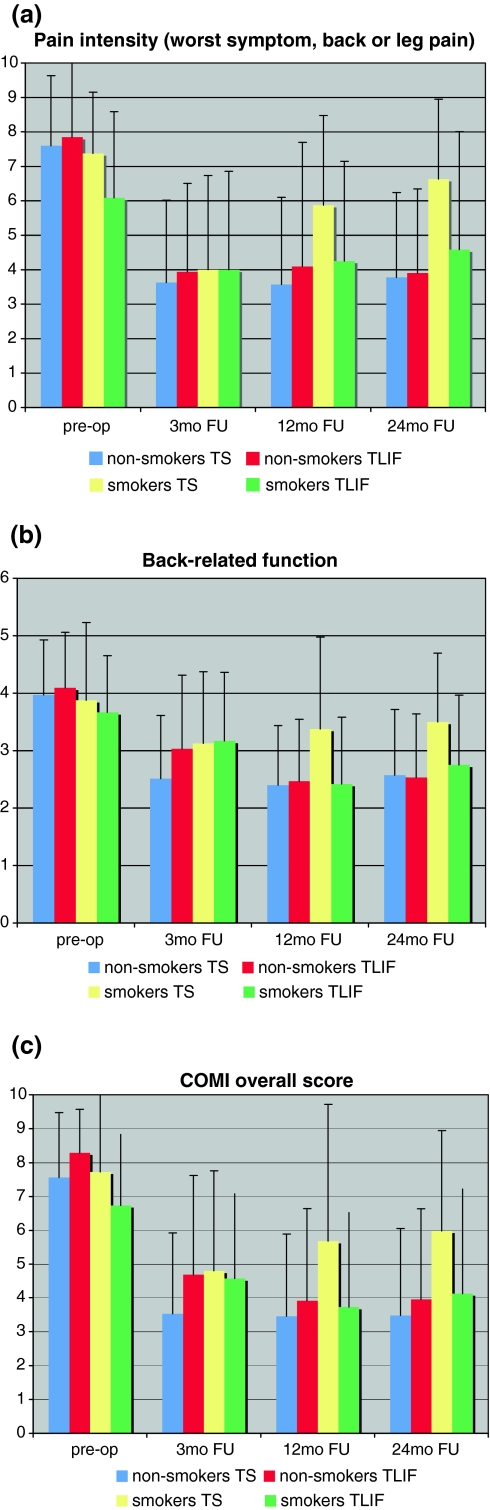
Figure 1 shows the scores for pain intensity (worst symptom, back pain or leg pain), back-related function, and the COMI overall score at baseline and at each of the follow-up time points. For these domains, the ANOVA revealed a significant interaction (p < 0.05) between the pattern of score-change over time and smoking status, with the smokers showing a worsening of status from 12 to 24 months FU, most markedly so in the group of TS smokers.
Fig. 1.
Changes in a pain intensity (worst of leg pain or back pain), b function, c COMI overall score at pre-op, 3 months FU, 12 months FU, and 24 months FU (p < 0.05 for the ANOVA interaction smoking status × change in scores over time)
Dose–response relationship
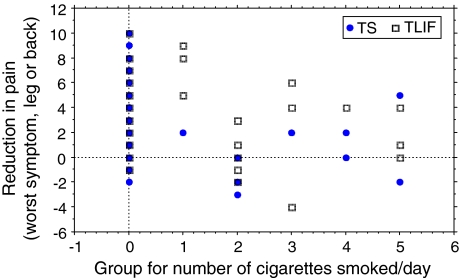
In the TS group, the greater the number of cigarettes smoked, the less the reduction in pain intensity for the worse symptom, leg or back pain (ρ = −0.41, p = 0.006) and COMI overall score (ρ = −0.27, p = 0.07) from pre-op to 24 months follow-up; the TLIF group showed relationships in the same direction, but they failed to reach significance (pain: ρ = −0.19, p = 0.18; COMI: ρ = −0.14, p = 0.33).
When both groups were considered together, the correlation between the number of cigarettes smoked and change score from pre-op to 24 months was significant for pain intensity (worst symptom) (ρ = −0.28, p = 0.006; Fig. 2), but not for COMI (ρ = −0.18, p = 0.08).
Fig. 2.
Dose–response relationship between group for number of cigarettes smoked and the reduction in pain intensity (worst of leg pain or back pain)) from preoperatively to 24 months FU (note, there are a number of overlapping points and hence the number of points shown does not equal the total number of data points contributing to the analysis)
Global outcome at follow-up
Table 3 shows the global outcomes for the smokers and non-smokers in each treatment group at 3, 12 and 24 months.
Table 3.
Proportion of good outcomes at each FU in each treatment group
| TS | TLIF | |||||
|---|---|---|---|---|---|---|
| Non-smokers (%) | Smokers (%) | p | Non-smokers (%) | Smokers (%) | p | |
| Percentage good at 3 months | 77.5 | 72.7 | 0.74 | 82.1 | 83.3 | 0.91 |
| Percentage good at 12 months | 75.0 | 50.0 | 0.14 | 75.0 | 75.0 | >0.99 |
| Percentage good at 24 months | 86.5 | 44.4 | 0.01 | 75.7 | 75.0 | >0.99 |
At 3 months' FU, the percentage good global outcomes (“operation helped/helped a lot”) did not differ between the smokers and non-smokers or between the surgical groups (all between 73 and 83%); however, with time, the outcome in the TS smokers declined, such that by 24 months' FU there was a significant difference between TS smokers and TS-non-smokers (44 vs. 87% good outcomes, respectively; p < 0.05). No such difference between smokers and non-smokers was evident in the TLIF group at any FU time (75 vs. 76% good outcomes at 24 months, respectively).
Discussion
In this study, we examined the influence of smoking on the patient-rated outcome of two different fixation techniques commonly used to achieve a solid fusion in lumbar spine surgery: translaminar screw fixation and transforaminal interbody fusion. Our previous study indicated that there were no significant differences between the two techniques for the mid-term patient-rated outcome [16]. From a biomechanical point of view, the combination of anterior support and rigid posterior fixation theoretically provides a more solid ground for fusion than posterior fusion with translaminar screws alone. Although this has not been clearly confirmed by in vitro biomechanical studies, it is suggested by the findings of the clinical studies of both Tuli et al. [17] and Anjarwalla et al. [26] in which the TS technique appeared to be associated with a lesser likelihood of fusion when compared with TLIF. Tuli reported an increased risk of re-operation for non-union in patients treated with TS when compared with those that were instrumented with pedicle screws. In examining four different fusion techniques—stand alone ALIF, ALIF plus TS, ALIF plus unilateral pedicle screw, and ALIF plus bilateral pedicle screw—Anjarwalla et al. found a significantly greater incidence of fusion at minimum 2 years of follow-up in the last group, suggesting that a 360° fusion provides the best conditions for promoting solid bony fusion. Interestingly, however, no differences were found in the fusion rate of the different fixation groups dependent on smoking habit.
We hypothesised that the different type of stability offered by TS, which provides exclusively posterior and posterolateral fusion, would render the technique more susceptible to detrimental environment factors such as smoking. And, indeed, the current findings appeared to support this hypothesis, if it can be assumed that the declining outcomes beyond 3 months reflect a greater incidence of non-union in the TS smokers. The initial results seemed to be fairly comparable for both techniques. The deterioration of the subjective results after 3 months in the TS smokers suggests that the differences occurred during the phase of calcification and biological stabilization when the mechanical stability provided by the implants decreases. Possibly the TLIF with its superior implant mass provides longer lasting “mechanical” stability than the translaminar screws alone.
The present study differs from many previous studies in that we focused exclusively on the role of smoking in relation to the clinical outcome, without actually measuring the rates of fusion/non-union or re-operation. Our reasoning for this was that ultimately it is the patients’ self-rated outcome and satisfaction with the procedure that is of greatest importance, regardless of the fusion status. Whilst verification of our proposed mechanism of action (namely, that fusion status mediates the association between smoking and fixation technique) would naturally serve to substantiate the hypothesis, it would require all study patients (regardless of their current satisfaction) to be exposed to the radiation associated with the CT imaging required to make accurate estimates of fusion, and yet would still not alter the clinical conclusions of the study.
The results of the study highlight the complexity of choosing a specific operative technique for the individual patient. Our data indicate that smoking habit should be considered as a reliable predictor of outcome, determining (in part) the surgical technique to be used. Furthermore, the findings indicate that there is an even greater impetus to suggest smoking cessation in candidates for spine surgery if for any reason (e.g. age, comorbidity, other “technical” reasons) a less invasive technique, such as TS is planned. In addition to the higher perioperative risks [27], higher wound-related complications, and higher rate of non-union associated with smoking, even poorer results in terms of patient-rated outcome should be expected if TS is chosen as the fusion technique in smokers.
Limitations of the present study include the observational nature of its design and the relatively low number of patients in the “smokers” subgroups; the results could be strengthened using a larger cohort study or a randomised controlled trial of the two types of stabilisation in which participants are pre-stratified by smoking status.
Conclusion
The study confirms a fixation technique-dependent relationship between smoking and patient-rated outcome in spine surgery. The TS technique was more vulnerable to the effects of smoking than was TLIF: possibly the more extensive stabilisation of the 360° fixation renders the environment less susceptible to the detrimental effects on bony fusion of cigarette smoking, with subsequent effects on patient-rated outcome.
Smoking habit proved to be at least one factor influencing outcome. The outcome of the two fixation techniques differed after analysing the results with respect to smoking habit.
Conflict of interest
None.
References
- 1.Read RC. Presidential address. Systemic effects of smoking. Am J Surg. 1984;148(6):706–711. doi: 10.1016/0002-9610(84)90421-5. [DOI] [PubMed] [Google Scholar]
- 2.Krupski WC. The peripheral vascular consequences of smoking. Ann Vasc Surg. 1991;5(3):291–304. doi: 10.1007/BF02329389. [DOI] [PubMed] [Google Scholar]
- 3.Silcox DH, IIIrd et al. (1995) The effect of nicotine on spinal fusion. Spine (Phila Pa 1976) 20(14):1549–1553 [DOI] [PubMed]
- 4.Blumenthal SL, et al. The role of anterior lumbar fusion for internal disc disruption. Spine (Phila Pa 1976) 1988;13(5):566–569. doi: 10.1097/00007632-198805000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Brown CW, Orme TJ, Richardson HD. The rate of pseudarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine (Phila Pa 1976) 1986;11(9):942–943. doi: 10.1097/00007632-198611000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Hanley EN Jr, Levy JA (1989) Surgical treatment of isthmic lumbosacral spondylolisthesis. Analysis of variables influencing results. Spine (Phila Pa 1976), 14(1):48–50 [DOI] [PubMed]
- 7.Daftari TK, et al. Nicotine on the revascularization of bone graft. An experimental study in rabbits. Spine (Phila Pa 1976) 1994;19(8):904–911. doi: 10.1097/00007632-199404150-00007. [DOI] [PubMed] [Google Scholar]
- 8.Hollo I, Gergely I, Boross M. Smoking results in calcitonin resistance. JAMA. 1977;237(23):2470. doi: 10.1001/jama.237.23.2470b. [DOI] [PubMed] [Google Scholar]
- 9.de Vernejoul MC et al. (1983) Evidence for defective osteoblastic function. A role for alcohol and tobacco consumption in osteoporosis in middle-aged men. Clin Orthop Relat Res (179):107–115 [PubMed]
- 10.Glassman SD, et al. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine (Phila Pa 1976) 2000;25(20):2608–2615. doi: 10.1097/00007632-200010150-00011. [DOI] [PubMed] [Google Scholar]
- 11.Andersen T, et al. Smoking as a predictor of negative outcome in lumbar spinal fusion. Spine (Phila Pa 1976) 2001;26(23):2623–2628. doi: 10.1097/00007632-200112010-00018. [DOI] [PubMed] [Google Scholar]
- 12.Woertgen C, et al. Does the choice of outcome scale influence prognostic factors for lumbar disc surgery? A prospective, consecutive study of 121 patients. Eur Spine J. 1997;6(3):173–180. doi: 10.1007/BF01301432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manniche C, et al. Analysis of preoperative prognostic factors in first-time surgery for lumbar disc herniation, including Finneson’s and modified Spengler’s score systems. Dan Med Bull. 1994;41(1):110–115. [PubMed] [Google Scholar]
- 14.Weir BK. Prospective study of 100 lumbosacral discectomies. J Neurosurg. 1979;50(3):283–289. doi: 10.3171/jns.1979.50.3.0283. [DOI] [PubMed] [Google Scholar]
- 15.Magerl F, WB (1985) Translaminäre Verschraubung der Intervertebralgelenke. Springer, Berlin, pp 315–317
- 16.Aepli M, Mannion AF, Grob D. Translaminar screw fixation of the lumbar spine: long-term outcome. Spine (Phila Pa 1976) 2009;34(14):1492–1498. doi: 10.1097/BRS.0b013e3181a0934f. [DOI] [PubMed] [Google Scholar]
- 17.Tuli J, et al. A comparison of long-term outcomes of translaminar facet screw fixation and pedicle screw fixation: a prospective study. J Neurosurg Spine. 2007;7(3):287–292. doi: 10.3171/SPI-07/09/287. [DOI] [PubMed] [Google Scholar]
- 18.Grob D, et al. A prospective, cohort study comparing translaminar screw fixation with transforaminal lumbar interbody fusion and pedicle screw fixation for fusion of the degenerative lumbar spine. J Bone Joint Surg Br. 2009;91(10):1347–1353. doi: 10.1302/0301-620X.91B10.22195. [DOI] [PubMed] [Google Scholar]
- 19.Grob D, Humke T. Translaminar screw fixation in the lumbar spine: technique, indications, results. Eur Spine J. 1998;7(3):178–186. doi: 10.1007/s005860050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humke T, et al. Translaminar screw fixation of the lumbar and lumbosacral spine. A 5-year follow-up. Spine (Phila Pa 1976) 1998;23(10):1180–1184. doi: 10.1097/00007632-199805150-00021. [DOI] [PubMed] [Google Scholar]
- 21.Mannion AF, et al. Outcome assessment in low back pain: how low can you go? Eur Spine J. 2005;14(10):1014–1026. doi: 10.1007/s00586-005-0911-9. [DOI] [PubMed] [Google Scholar]
- 22.Deyo RA, et al. Outcome measures for low back pain research. A proposal for standardized use. Spine (Phila Pa 1976) 1998;23(18):2003–2013. doi: 10.1097/00007632-199809150-00018. [DOI] [PubMed] [Google Scholar]
- 23.Ferrer M, et al. Validation of a minimum outcome core set in the evaluation of patients with back pain. Spine (Phila Pa 1976) 2006;31(12):1372–1379. doi: 10.1097/01.brs.0000218477.53318.bc. [DOI] [PubMed] [Google Scholar]
- 24.Mannion AF, et al. Pain measurement in patients with low back pain. Nat Clin Pract Rheumatol. 2007;3(11):610–618. doi: 10.1038/ncprheum0646. [DOI] [PubMed] [Google Scholar]
- 25.White P, Lewith G, Prescott P. The core outcomes for neck pain: validation of a new outcome measure. Spine (Phila Pa 1976) 2004;29(17):1923–1930. doi: 10.1097/01.brs.0000137066.50291.da. [DOI] [PubMed] [Google Scholar]
- 26.Anjarwalla N, Morcom RK, Fraser RD. Supplementary stabilization with anterior lumbar intervertebral fusion—a radiologic review. Spine (Phila Pa 1976) 2006;31(11):1281–1287. doi: 10.1097/01.brs.0000217692.90624.ab. [DOI] [PubMed] [Google Scholar]
- 27.Warner DO. Tobacco dependence in surgical patients. Curr Opin Anaesthesiol. 2007;20(3):279–283. doi: 10.1097/ACO.0b013e3280c60c3b. [DOI] [PubMed] [Google Scholar]