Abstract
An association between progression of cervical disc degeneration and that of lumbar disc degeneration has been considered to exist. To date, however, this association has not yet been adequately studied. Age-related changes in the cervical intervertebral discs were evaluated by magnetic resonance imaging (MRI) in patients with lumbar disc herniation, and compared with the MRI findings of healthy volunteers without lower back pain. The purpose of this study was to clarify whether the prevalence of asymptomatic cervical disc degeneration is higher in patients with lumbar disc herniation than in healthy volunteers. The study was conducted on 51 patients who were diagnosed as having lumbar disc herniation and underwent cervical spine MRI. The patients consisted of 34 males and 17 females ranging in age from 21–83 years (mean 46.9 ± 14.5 years) at the time of the study. The control group was composed of 113 healthy volunteers (70 males and 43 females) aged 24–77 years (mean 48.9 ± 14.7 years), without neck pain or low back pain. The percentage of subjects with degenerative changes in the cervical discs was 98.0% in the lumbar disc herniation group and 88.5% in the control group (p = 0.034). The presence of lumbar disc herniation was associated significantly with decrease in signal intensity of intervertebral disc and posterior disc protrusion in the cervical spine. None of the MRI findings was significantly associated with the gender, smoking, sports activities, or BMI. As compared to healthy volunteers, patients with lumbar disc herniation showed a higher prevalence of decrease in signal intensity of intervertebral disc and posterior disc protrusion on MRI of the cervical spine. The result of this study suggests that disc degeneration appears to be a systemic phenomenon.
Keywords: Lumbar disc herniation, Asymptomatic volunteers, MRI, Cervical spine, Disc degeneration
Background
Intervertebral disc degeneration is known to occur as a result of natural aging under the influence of various environmental factors [3, 6, 7, 12]. We conducted magnetic resonance imaging (MRI) evaluation of 497 healthy individuals without symptoms related to the cervical spine, and reported that the prevalence of disc degeneration increased with age, with decrease in signal intensity of intervertebral disc being observed on the MR images in 17% of males and 12% of females aged 20–29 years, and in 86% of males and 89% of females aged 60–69 years [15]. We subsequently followed these subjects for minimum 10 years and reported factors involved in the progression of cervical disc degeneration in asymptomatic subjects [18, 19].
Sambrook et al. [20] conducted cervical and lumbar spine MRI of 174 monozygotic twins and 154 dizygotic twins and scored the findings of disc generation on the MR images. They reported that genetic factors had a greater influence than environmental factors in these cases. Battie et al. [2] conducted a longitudinal study of twins in multiple countries, including Canada, Finland and USA, the Twin Spine Study, and reported that genetic factors were more closely associated with disc degeneration than the conventionally known environmental factors, such as smoking, vibration, and automobile driving. MacGregor et al. [13] investigated 1,064 twins about neck pain and lower back pain, and reported that genetic factors were more closely associated with these symptoms than environmental factors.
Following recent advances in genetic studies, cartilage inter-layer protein (CILP) [21], COL11A1 [16], THBS2 [8], and aspirin D14 [22] have been reported as gene polymorphisms frequently associated with disc degeneration. Then, if disc degeneration was genetically predestined, there should be some association between degenerative changes of the lumbar and cervical discs. During clinical practice, we often encounter patients having both cervical and lumbar disc degenerative disease at the same time, and several clinical studies reported concomitant cervical and lumbar degenerative disorders suggesting association between cervical and lumbar disc degeneration. To date, however, the tandem cervical and lumbar spinal disorders were studied only in a patient group without appropriate control.
The present study was undertaken to evaluate the age-related changes in the cervical disc by MRI in patients with lumbar disc herniation, in comparison with those in healthy volunteers not complaining of lower back pain. The hypothesis tested was that the prevalence of asymptomatic cervical disc degeneration is higher in patients with lumbar disc herniation than in healthy individuals.
Subjects and methods
This study was carried out prospectively with the approval of the Ethics Committee of the participating facilities. All participants in the study were provided an adequate explanation regarding the study by the physician in charge during their outpatient visits, and written informed consent was obtained from each of the study participants.
The subjects were 51 patients who were diagnosed as having lumbar disc herniation on the basis of clinical symptoms and MRI findings and also underwent cervical spine MRI within 6 months of the diagnosis of lumbar disc herniation. There were 34 males and 17 females, aged 21–83 years (mean 46.9 ± 14.5 years) at the time of the study. The herniation level was L1/2 in 1 patient, L2/3 in 1 patient, L3/4 in 1 patient, L4/5 in 21 patients and L5/S1 in 27 patients. The inclusion criteria were: (1) subjects should be asymptomatic about cervical spine, (2) absence of a history of cervical spine disease, head disease or trauma and absence of systemic inflammatory disease, and (3) absence of lumbar spondylolisthesis or scoliosis. Lumbar disc herniation was treated conservatively in 14 of the 51 patients and surgically in the remaining 37 patients. The operative procedure was microdiscectomy (including endoscopic microdiscectomy) in 23 patients and fusion in the remaining 14 patients (Table 1).
Table 1.
Profile of 51 subjects
| Herniation group (n = 51) | Control (n = 113) | ||
|---|---|---|---|
| Age | Mean | 46.9 ± 14.5 (21–83) | 48.9 ± 14.7 (24–77) |
| Age distribution | <40 | 19 (37.3%) | 32 (28.3%) |
| ≥40 | 32 (62.7%) | 81 (71.7%) | |
| Gender | Male | 34 (66.7%) | 70 (61.9%) |
| Female | 17 (33.3%) | 43 (38.1%) | |
| Level of lumbar disc herniation | L4–5 | 21 (41.2%) | |
| L5–S1 | 17 (54.9%) | ||
| Others | 3 (5.9%) | ||
| Treatment | Conservative treatment | 14 (27.5%) | |
| Surgery | 37 (72.5%) | ||
| Herniotomy | 23 (62.2%) | ||
| Spinal fusion | 14 (37.8%) | ||
Before undertaking an MRI of the cervical spine, a spine surgeon conducted examination of the cervical spine, including neurological examination. Patients with neck pain, weakness in the upper extremities, sensory disturbance or abnormal deep tendon reflexes of the upper extremities were excluded from the study.
Both the cervical and lumbar spine MRI in all patients were carried out using a 1.5-T superconductive imager (Achieva, Koninklijke Philips Electronics N.V.) under the following settings: sagittal T1-weighted fast spin-echo imaging [repetition time (TR)/echo time (TE) 620/10, echo train length 3, thickness of slice 3.5 mm, field of view (FOV) 23 cm, matrix size 336 × 218, number of excitation (NEX) 6]; sagittal T2-weighted fast spin-echo image (TR/TE 3000/100, echo train length 25, NEX 8, other conditions identical to those for the T1-weighted sagittal imaging); and axial T1- and T2-weighted fast spin-echo imaging (TR/TE 3000/100, thickness of slice 3 mm, FOV 18 cm, other conditions identical to those above).
Of the 223 asymptomatic volunteers on cervical spine reported previously, 113 were enrolled in the control group for this study. There were 70 males and 43 females aged 24–77 years (mean 48.9 ± 14.7 years) at the time of the study. Inclusion criteria for the control group were: the subjects had no symptom in the cervical or lumbar spine; and they had no history of cranial or cervical spinal diseases, or cervical trauma. Significant difference was not recognized in age distribution or gender between the two groups. The cervical spine MRI in this control group was performed using a 1.5-T superconductive imager (Signa Excite HD 1.5T, General Electronic Company) under the following settings: sagittal T1-weighted fast spin-echo imaging (TR/TE 380/8.2, echo train length 2, thickness of slice 4 mm, FOV 24 cm, matrix size 256 × 192, NEX 3); sagittal T2-weighted fast spin-echo imaging (TR/TE 5000/100, echo train length 16, NEX 3; other conditions identical to those for the T1-weighted sagittal imaging); and transverse T1- and T2-weighted fast spin-echo imaging (TR/TE 5000/102, thickness of slice 5 mm, FOV 16 cm, other conditions identical to those above).
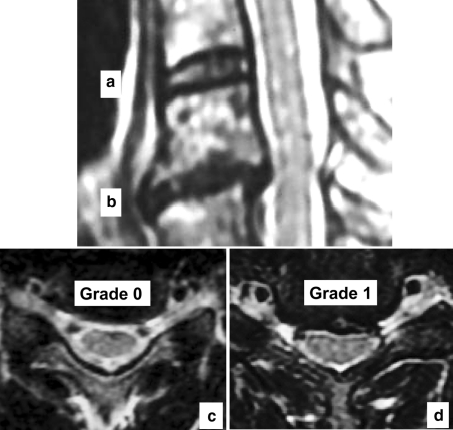
The cervical intervertebral levels evaluated were six levels from C2/3 to C7/T1. Each level was assessed for (1) decrease in signal intensity of intervertebral disc, (2) posterior disc protrusion, and (3) disc space narrowing, according to modified Matsumoto’s classification [15] (Table 2, Fig. 1).
Table 2.
Grading system for MR evaluation
| Decrease in signal intensity of intervertebral disc |
| Grade 0: as bright as or slightly less bright than cerebrospinal fluid |
| Grade 1: Markedly darker than cerebrospinal fluid |
| Grade 2: no signal |
| Posterior disc protrusion |
| Grade 0: no protrusion |
| Grade 1: disc material protruding beyond the posterior margin of the vertebral body without cord compression |
| Grade 2: beyond vertebral body with cord compression |
| Disc space narrowing |
| Grade 0: 100–75% of height of upper healthy disc |
| Grade 1: 75–50% of height of upper healthy disc |
| Grade 2: <50% of height of upper healthy disc |
Fig. 1.
a, b Cervical spine MRI T2-weighted sagittal image: the intervertebral disc a is grade 0 in decrease in signal intensity of intervertebral disc, and grade 0 in disc space narrowing. The intervertebral disc b was : grade 1 in decrease in signal intensity of intervertebral disc and grade 1 in disc space narrowing. c Cervical spine MRI T2-weighted axial image shows grade 0 in posterior disc protrusion. d Cervical spine MRI T2-weighted axial image shows grade 1 in posterior disc protrusion
The MRI scans were interpreted by one experienced neuroradiologist in a blinded and independent manner. The final result was determined from the result obtained by the neuroradiologist. However, to check the reliability of the ratings, the scans were also interpreted by a spine surgeon, and the inter-observer reliability was analyzed by calculating the Kappa coefficient. In terms of the inter-observer reliability, values of the Kappa coefficient of over 0.75 are considered to be excellent, values of over 0.40 and <0.75 are considered to be fair to good, and values below 0.40 are considered to be poor.
Statistical analysis
Chi-square test was used to compare the data between the lumbar disc herniation group and the control group; p < 0.05 was regarded as statistically significant. Logistic regression analysis was carried out with presence/absence of lumbar disc herniation (herniation group vs. control group), age, gender, smoking habit, periodic sports activities engaged in, and the presence/absence of obesity (BMI less than 25 vs. over 25) serving as the covariables, and the MRI findings of disc degeneration serving as the dependent variables. The statistical analyses were performed using Dr. SPSSII for Windows (SPSS Inc, Tokyo, Japan).
Results
The percentage of subjects with at least one finding of disc degeneration on the MR images differed significantly between the herniation group (98.0%, 50/51 patients) and the control group (88.5%, 100/113 volunteers) (p = 0.034).
Analysis of the prevalence of each finding of disc degeneration on MRI revealed that the prevalence of decrease in signal intensity of intervertebral disc was 94.1% (48/51) in the herniation group and 82.3% (93/113) in the control group (p = 0.033), that of posterior disc protrusion was 92.2% (47/51) in the herniation group and 79.6% (90/113) in the control group (p = 0.034), and that of disc space narrowing was 17.6% (9/51) in the herniation group and 29.2% (33/113) in the control group (N.S.) (Table 3).
Table 3.
Number of the discs with positive MRI findings at each intervertebral level
| Herniation group (n = 51) | Control (n = 113) | p value | |
|---|---|---|---|
| Decrease in signal intensity | 48 (94.1) | 93 (82.3) | 0.033* |
| Posterior disc protrusion | 47 (92.2) | 90 (79.6) | 0.034* |
| Disc space narrowing | 9 (17.6) | 33 (29.2) | 0.082 |
Parentheses indicate percentage
* Statistical significance
Then, the prevalence of each finding was analyzed by age. The prevalence of decrease in signal intensity of intervertebral disc was 84.2% (16/19) in subjects under 40 years old and 100.0% (32/32) in subjects over 40 years of age in the herniation group, and those in the control group were 68.8% (22/32) and 87.7% (71/81), respectively. The prevalence of posterior disc protrusion was 78.9% (15/19) in subjects under 40 years old and 100.0% (32/32) in subjects over 40 years of age in the herniation group, and those in the control group were 62.5% (20/32) and 86.4% (70/81), respectively. The prevalence of disc space narrowing was 21.1% (4/19) in subjects under 40 years old and 15.6% (5/32) in subjects over 40 years of age in the herniation group, and those in the control group were 15.6% (5/32) and 34.8% (28/81), respectively (Table 4). Thus, decrease in signal intensity of intervertebral disc (p = 0.047) and posterior disc protrusion (p = 0.016) were seen more frequently in subjects over the age of 40 than in those under 40 in the herniation group, and all of decrease in signal intensity of intervertebral disc (p = 0.021), posterior disc protrusion (p = 0.006) and disc space narrowing (p = 0.035) were seen more frequently in subjects over 40 years old than in those under 40 in the control group.
Table 4.
Age and positive MRI findings
| Herniation group (n = 51) | Control (n = 113) | |||||
|---|---|---|---|---|---|---|
| Age < 40 (n = 19) | Age ≥ 40 (n = 32) | p value | Age < 40 (n = 32) | Age ≥ 40 (n = 81) | p value | |
| Decrease in signal intensity | 16 (84.2) | 32 (100.0) | 0.047* | 22 (68.8) | 71 (87.7%) | 0.021* |
| Posterior disc protrusion | 15 (78.9) | 32 (100.0) | 0.016* | 20 (62.5) | 70 (86.4) | 0.006* |
| Disc space narrowing | 4 (21.1) | 5 (15.6) | 0.447 | 5 (15.6) | 28 (34.8) | 0.035* |
Parentheses indicate percentage
* Statistical significance
When the prevalence of each abnormality was analyzed by the gender, the prevalence of decrease in signal intensity of intervertebral disc was 91.2% (31/34) in the males and 100.0% (17/17) in the females of the herniation group, and 82.9% (58/70) in the males and 81.4% (35/43) in the females of the control group. The prevalence of posterior disc protrusion was 91.2% (31/34) in the males and 94.1% (16/17) in the females of the herniation group, and 84.3% (59/70) in the males and 72.1% (31/43) in females of the control group. The prevalence of disc space narrowing was 17.6% (6/34) in the males and 17.6% (3/17) in the females of the herniation group, and 28.6% (20/70) in the males and 30.2% (13/43) in the females of the control group. Thus, no significant gender-related differences were observed in the prevalence of any of these findings.
In the logistic regression analysis, age was found to be significantly associated with decrease in signal intensity of intervertebral disc [odds ratio (OR), 1.117; 95% confidence interval (CI), 1.060–1.177], posterior disc protrusion (OR, 1.092; 95% CI, 1.045–1.142), and disc space narrowing (OR, 1.045; 95% CI, 1.017–1.073) of the cervical spine. The presence of lumbar disc herniation was significantly associated with a decrease in signal intensity of intervertebral disc (OR, 5.333; 95% CI, 1.340–21.229; p = 0.018) and posterior disc protrusion (OR, 3.673; 95% CI, 1.105–12.212; p = 0.034) on the cervical spine MRI.
None of the three findings was found to be significantly associated with the gender, smoking habit, sports activities or BMI (Table 5).
Table 5.
Logistic regression analyses of MR findings
| Decrease in signal intensity | Posterior disc protrusion | Disc space narrowing | ||||
|---|---|---|---|---|---|---|
| p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | |
| Age | 0.000* | 1.117 (1.060–1.177) | 0.000* | 1.092 (1.045–1.142) | 0.001* | 1.045 (1.017–1.073) |
| Gender (male) | 0.991 | 1.006 (0.343–2.952) | 0.148 | 2.052 (0.775–5.436) | 0.681 | 0.850 (0.391–1.847) |
| Smoking | 0.402 | 0.630 (0.214–1.815) | 0.510 | 0.707 (0.252–1.983) | 0.675 | 0.834 (0.356–1.953) |
| Sport | 0.489 | 1.684 (0.385–7.368) | 0.338 | 0.552 (0.163–1.862) | 0.813 | 1.150 (0.362–3.650) |
| BMI | 0.909 | 0.933 (0.234–3.063) | 0.440 | 1.575 (0.497–4.992) | 0.389 | 1.438 (0.629–3.290) |
| Lumbar disc herniation | 0.018* | 5.333 (1.340–21.229) | 0.034* | 3.673 (1.105–12.212) | 0.187 | 0.560 (0.237–1.325) |
* Statistical significance
Kappa coefficient, calculated for the ratings given by two readers was 0.87 for decrease in signal intensity of intervertebral disc, 0.87 for posterior disc protrusion, and 0.53 for disc space narrowing. Thus, the inter-observer reliability was good to excellent.
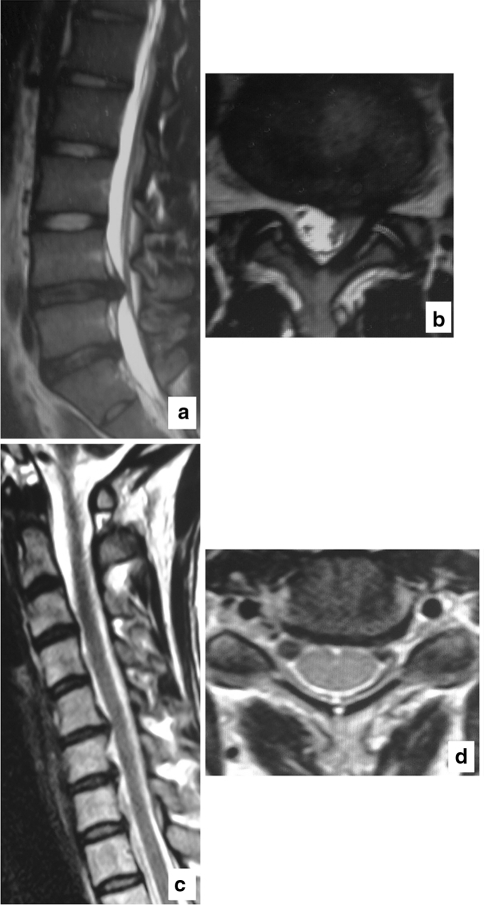
Case presentation: 36-year-old female with lumbar disc herniation at L4-5 (Fig. 2).
Fig. 2.
A 36-year-old female with lumbar disc herniation at L4–5 on the left side but with no symptom related to the cervical spine. a Lumbar spine MRI T2-weighted sagittal image: disc herniation with protrusion into the spinal canal is observed at L4–5. b Lumbar spine MRI T2-weighted axial image: L4–5 level. Disc herniation is observed on the left side with nerve root compression. c Cervical spine MRI T2-weighted sagittal image: reduced disc intensity visible from C2–3 through C6–7 levels. Posterior disc protrusion noted at C5–6 and C6–7 levels. Disc space narrowing was observed at C5–6 and C6–7 levels. d Cervical spine MRI T2-weighted axial image: C5–6 level. Large disc protrusion is observed
Discussion
Few published reports have focused on the relationship between the degenerative changes in the cervical and lumbar spine in the same healthy individual. Master et al. [14] evaluated the spondylopathic changes of the cervical and lumbar spine using 234 cadaver specimens. They found spondylopathic changes in 80% of all the specimens and reported a statistically significant association between the spondylopathic changes of the cervical spine and those of the lumbar spine. Lee et al. [11] measured the diameter of the spinal canal in the cervical and lumbar spine in 440 cadavers using digital calipers, and found narrowing of the spinal canal at both levels in 0.9–5.4% of all cadavers. On the basis of these results, they reported that narrowing of the cervical spinal canal was significantly associated with that of the lumbar spine.
Patients with severe neurological symptoms associated with both the cervical and lumbar spine have been reported as cases of tandem spinal stenosis (TSS), and have been treated primarily by surgery [1, 4, 10, 17]. In 1964, Teng and Papatheodoru [23] published the first report of a case of cervical spondylosis, with cervical myelopathy, as well as lumbar disc degeneration. They recommended myelography of the entire spine when dealing with cases of severe cervical spondylosis, even if they are asymptomatic about lumbar spine. Epstein et al. [5] analyzed the clinical symptoms of cases with narrowing of the spinal canal at the level of the cervical and lumbar segments of the spine and pointed out the necessity of detailed neurological examination and also testing to rule out motor neuron disease and peripheral neuropathy, because the symptoms of this disease are not typical. Jacobs et al. [9] studied the myelographic findings of the lumbar spine in patients having undergone surgical treatment of cervical disc degenerative disease, reporting that patients with degenerative disease of the cervical spine often had findings of degeneration of the lumbar spine (disc protrusion in 39% and nerve root compression in 50% of all cases).
These previous studies have suggested that degeneration of the cervical spine and lumbar spine may be closely associated with each other. However, they lacks in control subjects for comparison. In the present study, MRI was used which is the most sensitive imaging modality for evaluation of disc degeneration and we compared MR findings of cervical discs of patients with lumbar disc herniation with those of healthy asymptomatic subjects.
In the present MRI study, the presence of cervical disc degenerative changes was higher in the herniation group (98.0%) than in the control group (88.5%), even though all patients in the herniation group were asymptomatic regarding cervical spine. The prevalence of degenerative changes increased with aging, consistent with previous reports [3, 6, 7, 15, 18, 19]. When compared to healthy volunteers with no symptoms arising from the cervical spine, patients with lumbar disc herniation showed a significantly higher prevalence of decrease in signal intensity of intervertebral disc and posterior disc protrusion on MRI of the cervical spine. These results strongly suggest that patients with lumbar disc herniation are also likely to show degenerative changes of spinal segments other than those of the lumbar spine, and that some factors in the individual (e.g., genes) may stimulate disc degeneration in both the cervical and lumbar spine at the same time. On the other hand, disc degeneration was not associated with the smoking habit, sports activities that the subjects engaged in, or the presence/absence of obesity, although these factors have been previously reported to be associated with disc degeneration.
Disc space narrowing was not significantly associated with existence of herniation. Disc space narrowing represents an advanced stage of disc degeneration [18]. While decrease in signal intensity of intervertebral disc and posterior disc protrusion was observed in 80–90% of the subjects, disc space narrowing was observed only in about 20%. This low prevalence rate of disc space narrowing may be attributable to the negative association between lumbar disc herniation and disc space narrowing of the cervical spine.
One of the limitations of this study was that the MRI machine and pulse sequence, differed among the multiple facilities. Because decrease in signal intensity of intervertebral disc may vary particularly greatly depending on the type of MRI machines and pulse sequences used, we attempted to minimize the device-associated error by using an original grading system, such as comparison of the intensity of the cerebrospinal fluid on T2-weighted images, in the present study. Also, the kappa coefficient for disc space narrowing was much smaller than those for decrease in signal intensity of intervertebral disc and posterior disc protrusion. Disc space narrowing of grade 1 was defined as 50–75% loss of disc height compared with the upper intervertebral disc. Most subjects exhibited marginal disc space narrowing, i.e., 70–80% loss of disc height, making judgment by the two observers difficult. However, the kappa coefficient for disc space narrowing was 0.53, which was considered to be fair to good rather than poor.
Despite these limitations, the present study was the first study to investigate age-related changes in the cervical spine by MRI in patients with lumbar disc herniation in comparison with healthy volunteers. The results of this study showed that disc degeneration in the cervical spine is more prevalent in patients with lumbar disc herniation than healthy subjects, calling the attention of spine care professionals to potential cervical lesions in patients with lumbar disc herniation.
Conclusion
MRI of cervical spine was performed in patients with lumbar disc herniation without any cervical spine symptoms, and a cross-sectional analysis was conducted on the MRI findings of disc degeneration.
Patients with lumbar discs herniation had a higher prevalence of decrease in signal intensity of intervertebral disc and posterior disc protrusion as compared with healthy volunteers on cervical spine MRI. Thus, disc degeneration appears to be a systemic phenomenon.
Acknowledgment
We express our cordial thanks to Daisuke Ichihara M.D. and Kazuhiro Chiba M.D. at Department of Orthopaedic Surgery of Keio University, Suketaka Momoshima M.D. at Department of Diagnostic Radiology of Keio University, Yuji Nishiwaki M.D. at Department of Preventive Medicine and Public Health of Keio University, Ken Ninomiya M.D. and Yukio Horiuchi M.D. at Department of Orthopaedic Surgery of Kawasaki Municipal Hospital, Takeshi Hashimoto M.D. at Department of Orthopaedic Surgery of Keio University Tsukigase Rehabilitation Center, Masahiko Watanabe M.D. at Department of Orthopaedic Surgery of Tokai University, Tomoo Inoue M.D. at Department of Orthopaedic Surgery of Kyorin University, Takeshi Takahata M.D. at Department of Orthopaedic Surgery of Isehara Kyodo Hospital, and Yoshiji Suzuki M.D. of the Omaezaki Municipal Hospital for their cooperation for this study. This study was supported by a grant from the General Insurance Association of Japan.
Contributor Information
Eijiro Okada, Email: e-okada@pg8.so-net.ne.jp.
Morio Matsumoto, Phone: +81-3-53633812, FAX: +81-3-33536597, Email: morio@sc.itc.keio.ac.jp.
Hirokazu Fujiwara, Email: hirokazu_fujiwara@yahoo.co.jp.
Yoshiaki Toyama, Email: toyama@sc.itc.keio.ac.jp.
References
- 1.Aydogan M, Ozturk C, Mirzanli C, et al. Treatment approach in tandem (concurrent) cervical and lumbar spinal stenosis. Acta Orthop Belg. 2007;73:234–237. [PubMed] [Google Scholar]
- 2.Battié MC, Videman T, Kaprio J, et al. The Twin Spine Study: contributions to a changing view of disc degeneration. Spine J. 2009;9:47–59. doi: 10.1016/j.spinee.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Boden SD, McCowin PR, Davis DO, et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:1178–1184. [PubMed] [Google Scholar]
- 4.Dagi TF, Tarkington MA, Leech JJ. Tandem lumbar and cervical spinal stenosis. Natural history, prognostic indices, and results after surgical decompression. J Neurosurg. 1987;66:842–849. doi: 10.3171/jns.1987.66.6.0842. [DOI] [PubMed] [Google Scholar]
- 5.Epstein NE, Epstein JA, Carras R, et al. Coexisting cervical and lumbar spinal stenosis: diagnosis and management. Neurosurgery. 1984;15:489–496. doi: 10.1227/00006123-198410000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Gore DR, Sepic SB, Gardner GM. Roentgengraphic findings of the cervical spine in asymptomatic people. Spine. 1986;11:521–524. doi: 10.1097/00007632-198607000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Gore DR. Roentgengraphic findings in the cervical spine in asymptomatic persons: a ten-year follow-up. Spine. 2001;26:2463–2466. doi: 10.1097/00007632-200111150-00013. [DOI] [PubMed] [Google Scholar]
- 8.Hirose Y, Chiba K, Karasugi T, et al. A functional polymorphism in THBS2 that affects alternative splicing and MMP binding is associated with lumbar-disc herniation. Am J Hum Genet. 2008;82:1122–1129. doi: 10.1016/j.ajhg.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs B, Ghelman B, Marchisello P. Coexistence of cervical and lumbar disc disease. Spine. 1990;15:1261–1264. doi: 10.1097/00007632-199012000-00006. [DOI] [PubMed] [Google Scholar]
- 10.LaBan MM, Green ML. Concurrent (tandem) cervical and lumbar spinal stenosis: a 10-yr review of 54 hospitalized patients. Am J Phys Med Rehabil. 2004;83:187–190. doi: 10.1097/01.PHM.0000113405.48879.45. [DOI] [PubMed] [Google Scholar]
- 11.Lee MJ, Garcia R, Cassinelli EH, et al. Tandem stenosis: a cadaveric study in osseous morphology. Spine J. 2008;8:1003–1006. doi: 10.1016/j.spinee.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Lehto IJ, Tertti MO, Komu ME, et al. Age-related MRI changes at 0.1 T in cervical discs in asymptomatic subjects. Neuroradiology. 1994;36:49–53. doi: 10.1007/BF00599196. [DOI] [PubMed] [Google Scholar]
- 13.MacGregor AJ, Andrew T, Sambrook PN, et al. Structural, psychological, and genetic influences on low back and neck pain: a study of adult female twins. Arthritis Rheum. 2004;51:160–167. doi: 10.1002/art.20236. [DOI] [PubMed] [Google Scholar]
- 14.Master DL, Eubanks JD, Ahn NU. Prevalence of concurrent lumbar and cervical arthrosis: an anatomic study of cadaveric specimens. Spine. 2009;34:E272–E275. doi: 10.1097/BRS.0b013e318195d10b. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto M, Fujimura Y, Suzuki N, et al. MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br. 1998;80:19–24. doi: 10.1302/0301-620X.80B1.7929. [DOI] [PubMed] [Google Scholar]
- 16.Mio F, Chiba K, Hirose Y, et al. A functional polymorphism in COL11A1, which encodes the alpha 1 chain of type XI collagen, is associated with susceptibility to lumbar disc herniation. Am J Hum Genet. 2007;81:1271–1277. doi: 10.1086/522377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naderi S, Mertol T. Simultaneous cervical and lumbar surgery for combined symptomatic cervical and lumbar spinal stenoses. J Spinal Disord Tech. 2002;15:229–232. doi: 10.1097/00024720-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Okada E, Matsumoto M, Ichihara D, et al. Aging of the cervical spine in healthy volunteers: a 10-year longitudinal magnetic resonance imaging study. Spine. 2009;34(7):706–712. doi: 10.1097/BRS.0b013e31819c2003. [DOI] [PubMed] [Google Scholar]
- 19.Okada E, Matsumoto M, Ichihara D. Does the sagittal alignment of the cervical spine have an impact on disk degeneration? Minimum 10-year follow-up of asymptomatic volunteers. Eur Spine J. 2009;18:1644–1651. doi: 10.1007/s00586-009-1095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366–372. doi: 10.1002/1529-0131(199902)42:2<366::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Seki S, Kawaguchi Y, Chiba K, et al. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat Genet. 2005;37:607–612. doi: 10.1038/ng1557. [DOI] [PubMed] [Google Scholar]
- 22.Song YQ, Cheung KM, Ho DW, et al. Association of the asporin D14 allele with lumbar-disc degeneration in Asians. Am J Hum Genet. 2008;82:744–747. doi: 10.1016/j.ajhg.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng P, Papatheodorou C. Combined cervical and lumbar spondylosis. Arch Neurol. 1964;10:298–307. doi: 10.1001/archneur.1964.00460150068007. [DOI] [PubMed] [Google Scholar]