Summary
Background
Trial findings show cognitive behaviour therapy (CBT) and graded exercise therapy (GET) can be effective treatments for chronic fatigue syndrome, but patients' organisations have reported that these treatments can be harmful and favour pacing and specialist health care. We aimed to assess effectiveness and safety of all four treatments.
Methods
In our parallel-group randomised trial, patients meeting Oxford criteria for chronic fatigue syndrome were recruited from six secondary-care clinics in the UK and randomly allocated by computer-generated sequence to receive specialist medical care (SMC) alone or with adaptive pacing therapy (APT), CBT, or GET. Primary outcomes were fatigue (measured by Chalder fatigue questionnaire score) and physical function (measured by short form-36 subscale score) up to 52 weeks after randomisation, and safety was assessed primarily by recording all serious adverse events, including serious adverse reactions to trial treatments. Primary outcomes were rated by participants, who were necessarily unmasked to treatment assignment; the statistician was masked to treatment assignment for the analysis of primary outcomes. We used longitudinal regression models to compare SMC alone with other treatments, APT with CBT, and APT with GET. The final analysis included all participants for whom we had data for primary outcomes. This trial is registered at http://isrctn.org, number ISRCTN54285094.
Findings
We recruited 641 eligible patients, of whom 160 were assigned to the APT group, 161 to the CBT group, 160 to the GET group, and 160 to the SMC-alone group. Compared with SMC alone, mean fatigue scores at 52 weeks were 3·4 (95% CI 1·8 to 5·0) points lower for CBT (p=0·0001) and 3·2 (1·7 to 4·8) points lower for GET (p=0·0003), but did not differ for APT (0·7 [−0·9 to 2·3] points lower; p=0·38). Compared with SMC alone, mean physical function scores were 7·1 (2·0 to 12·1) points higher for CBT (p=0·0068) and 9·4 (4·4 to 14·4) points higher for GET (p=0·0005), but did not differ for APT (3·4 [−1·6 to 8·4] points lower; p=0·18). Compared with APT, CBT and GET were associated with less fatigue (CBT p=0·0027; GET p=0·0059) and better physical function (CBT p=0·0002; GET p<0·0001). Subgroup analysis of 427 participants meeting international criteria for chronic fatigue syndrome and 329 participants meeting London criteria for myalgic encephalomyelitis yielded equivalent results. Serious adverse reactions were recorded in two (1%) of 159 participants in the APT group, three (2%) of 161 in the CBT group, two (1%) of 160 in the GET group, and two (1%) of 160 in the SMC-alone group.
Interpretation
CBT and GET can safely be added to SMC to moderately improve outcomes for chronic fatigue syndrome, but APT is not an effective addition.
Funding
UK Medical Research Council, Department of Health for England, Scottish Chief Scientist Office, Department for Work and Pensions.
Introduction
Chronic fatigue syndrome is characterised by chronic disabling fatigue in the absence of an alternative diagnosis.1 Myalgic encephalomyelitis is thought by some researchers to be the same disorder and by others as different with separate diagnostic criteria.1,2 The prevalence of chronic fatigue syndrome is between 0·2% and 2·6% worldwide, dependent on the definition used.1 Prognosis is poor if untreated.3
Specific therapies can improve outcomes. The UK National Institute for Health and Clinical Excellence (NICE) recommend cognitive behaviour therapy (CBT) and graded exercise therapy (GET).2 Although this recommendation was supported by systematic reviews,4–7 supporting evidence remains restricted to small trials.4–7 Surveys by patients' organisations in the UK have reported that CBT and GET are sometimes harmful, and have recommended pacing and specialist health care.8,9
We designed the pacing, graded activity, and cognitive behaviour therapy: a randomised evaluation (PACE) trial10 to compare pacing, defined as adaptive pacing therapy (APT), CBT, and GET, when added to specialist medical care (SMC) with SMC alone. We sought evidence of benefit and harm. We also aimed to compare APT against CBT and GET and examine these comparisons in subgroups satisfying different diagnostic criteria for chronic fatigue syndrome and myalgic encephalomyelitis. We postulated that CBT and GET would be more effective than would APT and SMC, and that APT would be more effective than SMC alone.
Methods
Study design and participants
PACE was a parallel, four group, multicentre, randomised trial, with outcomes assessed up to 52 weeks after randomisation for patients with chronic fatigue syndrome.10 We recruited 641 participants from consecutive new outpatients attending six specialist chronic fatigue syndrome clinics in the UK National Health Service between March 18, 2005, and Nov 28, 2008, and completed outcome data collection in January, 2010.
Several diagnostic criteria exist for chronic fatigue syndrome and myalgic encephalomyelitis.11–13 We selected participants in accordance with Oxford criteria for chronic fatigue syndrome.11 These criteria require fatigue to be the main symptom, accompanied by significant disability, in the absence of an exclusionary medical or psychiatric diagnosis (psychosis, bipolar disorder, substance misuse, an organic brain disorder, or an eating disorder).11 All participants were medically assessed by the specialist clinic doctors to exclude alternative diagnoses.2,12 Research assessors used the structured clinical interview from the Diagnostic and Statistical Manual of Mental Disorders IV to diagnose exclusionary and comorbid psychiatric disorders (ie, mood and anxiety disorders).10,14
Other eligibility criteria consisted of a bimodal score of 6 of 11 or more on the Chalder fatigue questionnaire15 and a score of 60 of 100 or less on the short form-36 physical function subscale.16 11 months after the trial began, this requirement was changed from a score of 60 to a score of 65 to increase recruitment.
We excluded patients who were younger than 18 years or at significant risk of self-harm, unable to attend hospital appointments, unable to speak and read English, had medical needs that made participation inappropriate, had previously received a trial treatment for their present illness at a PACE trial clinic (we initially excluded anyone who had received a trial treatment, but found the nature of treatment given elsewhere hard to establish).10 Participants were also assessed by international criteria for chronic fatigue syndrome,12 requiring four or more accompanying symptoms, and the London criteria13 for myalgic encephalomyelitis (version 2), requiring postexertional fatigue, poor memory and concentration, symptoms that fluctuate, and no primary depressive or anxiety disorder (interpreted as an absence of any such disorder).
We obtained separate written informed consent for assessment and entry into the trial. The West Midlands Multicentre Research Ethics Committee (MREC 02/7/89) approved the study.
Randomisation and masking
Participants were allocated to treatment groups through the Mental Health and Neuroscience Clinical Trials Unit (London, UK), after baseline assessment and obtainment of consent. A database programmer undertook treatment allocation, independently of the trial team. The first three participants at each of the six clinics were allocated with straightforward randomisation. Thereafter allocation was stratified by centre, alternative criteria for chronic fatigue syndrome12 and myalgic encephalomyelitis,13 and depressive disorder (major or minor depressive episode or dysthymia),14 with computer-generated probabilistic minimisation. Once notified of treatment allocation by the Clinical Trials Unit, the research assessor informed the participant and clinicians. One therapist was available for every therapy per centre, with few exceptions. Specialist medical care doctors were allocated by convenience. As with any therapy trial, participants, therapists, and doctors could not be masked to treatment allocation and it was also impractical to mask research assessors. The primary outcomes were rated by participants themselves. The statistician undertaking the analysis of primary outcomes was masked to treatment allocation.
Procedures
Panel 1 shows treatment strategies and webappendix p 1 shows characteristics of treating clinicians. Therapy leaders (one per therapy and with substantial experience in treatment of chronic fatigue syndrome) trained therapists until they were deemed competent to provide trial treatments. Individual therapy supervision was provided once every month, and by group every 3 months.24 All treatment sessions were recorded acoustically. Two independent clinicians, who were masked to allocated treatment, rated recordings of a randomly chosen sample of the tenth (or nearest) session of 62 (13%) of 480 participants (two sessions for every therapist, when available) for therapy type, adherence to the manual (7-point Likert scale), and therapeutic alliance between therapist and participant (7-point Likert scale). These clinicians recorded when masking had failed, such as when the treatment was mentioned by name. All doctors received training in specialist medical care, and we assessed competence and monitored manual adherence for most. We defined ten sessions of therapy or three sessions of specialist medical care alone as adequate treatment for the per-protocol analysis. We recorded number of treatment sessions attended, active withdrawals from treatment, additional treatments received, and dropouts from follow-up.
Panel 1. Treatments provided.
Overview
We standardised treatments by provision of manuals for doctors, therapists, and participants. At least three sessions of specialist medical care were offered to participants during the 12 months, and more were offered if clinically indicated. Up to 14 therapy sessions were offered during the first 23 weeks; the first four were once a week and subsequently they were once every 2 weeks. An additional booster session was offered at 36 weeks. No other additional sessions were offered. Most treatments were delivered face-to-face but some were provided by telephone. Treatment was provided individually although participants could be accompanied if they wanted.
Specialist medical care (SMC)
SMC was provided by doctors with specialist experience in chronic fatigue syndrome (webappendix p 1). All participants were given a leaflet explaining the illness and the nature of this treatment. The manual was consistent with good medical practice, as presently recommended.2 Treatment consisted of an explanation of chronic fatigue syndrome, generic advice, such as to avoid extremes of activity and rest, specific advice on self-help, according to the particular approach chosen by the participant (if receiving SMC alone), and symptomatic pharmacotherapy (especially for insomnia, pain, and mood).
Adaptive pacing therapy (APT)
APT was based on the envelope theory of chronic fatigue syndrome.17,18 This theory regards chronic fatigue syndrome as an organic disease process that is not reversible by changes in behaviour and which results in a reduced and finite amount (envelope) of available energy. The aim of therapy was to achieve optimum adaptation to the illness, hence APT. This adaptation was achieved by helping the participant to plan and pace activity to reduce or avoid fatigue, achieve prioritised activities and provide the best conditions for natural recovery.13,17,18 Therapeutic strategies consisted of identifying links between activity and fatigue by use of a daily diary, with corresponding encouragement to plan activity to avoid exacerbations, developing awareness of early warnings of exacerbation, limiting demands and stress, regularly planning rest and relaxation, and alternating different types of activities, with advice not to undertake activities that demanded more than 70% of participants' perceived energy envelopes. Increased activities were encouraged, if the participant felt able, and as long as they did not exacerbate symptoms.
Because this treatment had not been described in a manual, we created and piloted manuals for therapists and patients on the basis of previous descriptions,13,17 what pilot patients and clinicians reported as helpful, and with the advice of experienced therapists. Westcare and Action for ME helped in the design of the therapy and endorsed the final manuals.18 APT was provided by occupational therapists (webappendix p 1).
Cognitive behaviour therapy (CBT)
CBT was done on the basis of the fear avoidance theory of chronic fatigue syndrome. This theory regards chronic fatigue syndrome as being reversible and that cognitive responses (fear of engaging in activity) and behavioural responses (avoidance of activity) are linked and interact with physiological processes to perpetuate fatigue. The aim of treatment was to change the behavioural and cognitive factors assumed to be responsible for perpetuation of the participant's symptoms and disability. Therapeutic strategies guided participants to address unhelpful cognitions, including fears about symptoms or activity by testing them in behavioural experiments. These experiments consisted of establishing a baseline of activity and rest and a regular sleep pattern, and then making collaboratively planned gradual increases in both physical and mental activity. Furthermore, participants were helped to address social and emotional obstacles to improvement through problem-solving. Therapy manuals were based on manuals used in previous trials.19–21 CBT was delivered mainly by clinical psychologists and nurse therapists (webappendix p 1).
Graded exercise therapy (GET)
GET was done on the basis of deconditioning and exercise intolerance theories of chronic fatigue syndrome. These theories assume that the syndrome is perpetuated by reversible physiological changes of deconditioning and avoidance of activity. These changes result in the deconditioning being maintained and an increased perception of effort, leading to further inactivity. The aim of treatment was to help the participant gradually return to appropriate physical activities, reverse the deconditioning, and thereby reduce fatigue and disability. Therapeutic strategies consisted of establishment of a baseline of achievable exercise or physical activity, followed by a negotiated, incremental increase in the duration of time spent physically active. Target heart rate ranges were set when necessary to avoid overexertion, which eventually aimed at 30 min of light exercise five times a week. When this rate was achieved, the intensity and aerobic nature of the exercise was gradually increased, with participant feedback and mutual planning. The most commonly chosen exercise was walking. The therapy manual was based on that used in previous trials.22,23 GET was delivered by physiotherapists and one exercise physiologist (webappendix p 1).
We undertook assessments at baseline and 12 weeks (mid-therapy), 24 weeks (post-therapy), and 52 weeks after randomisation. Primary outcomes were also assessed at the time of dropouts, and used when no other outcome data were available. The research assessors did the assessments, usually face-to-face in clinic. Most measures were self-rated by the participant. Because masking of research assessors to treatment allocation after randomisation was impractical, we relied on participant ratings to keep observer bias to a minimum.
Outcomes
The two participant-rated primary outcome measures were the Chalder fatigue questionnaire (Likert scoring 0, 1, 2, 3; range 0–33; lowest score is least fatigue)15 and the short form-36 physical function subscale (version 2; range 0–100; highest score is best function).16 Before outcome data were examined, we changed the original bimodal scoring of the Chalder fatigue questionnaire (range 0–11) to Likert scoring to more sensitively test our hypotheses of effectiveness. The two primary outcome measures15,16 are valid and reliable and have been used in previous trials.4–7
For safety outcomes, we included non-serious adverse events, serious adverse events, serious adverse reactions to trial treatments, serious deterioration, and active withdrawals from treatment.10 Adverse events were defined as any clinical change, disease, or disorder reported, whether or not related to treatment. Three scrutinisers (two physicians and one liaison psychiatrist who all specialised in chronic fatigue syndrome) reviewed all adverse events and reactions, independently from the trial team, and were masked to treatment group, to establish whether they were serious adverse events. Scrutinisers were then unmasked to treatment allocation to establish if any serious adverse events were serious adverse reactions. Serious deterioration in health was defined as any of the following outcomes: a short form-36 physical function score decrease of 20 or more between baseline and any two consecutive assessment interviews;16 scores of much or very much worse on the participant-rated clinical global impression change in overall health scale at two consecutive assessment interviews;25 withdrawal from treatment after 8 weeks because of a participant feeling worse; or a serious adverse reaction.
For secondary outcomes, we used the clinical global impression scale to assess change from baseline in overall health.25 This 7-point scale was condensed into three categories: negative change (very much worse or much worse), minimum change (a little worse, no change, or a little better), and positive change (much better or very much better). We also assessed overall disability with the work and social adjustment scale,26 6-min walking ability (distance in m walked),27 Jenkins scale score for disturbed sleep,28 hospital anxiety and depression scale score,29 number of chronic fatigue syndrome symptoms, and individual symptoms of postexertional malaise and poor concentration or memory, as in the international criteria.12 These secondary outcomes were a subset of those specified in the protocol, selected in the statistical analysis plan as most relevant to this report. After participants had been told their treatment allocation, but before treatment began, they rated how logical their proposed treatment seemed and how confident they were that it would help them (5-point Likert scale with moderately and extremely condensed into a positive response to help with interpretation). At 52 weeks, participants rated satisfaction with treatment received on a 7-point scale, condensed into three categories to aid interpretation (satisfied, neutral, or dissatisfied).
Statistical analysis
We calculated sample sizes assuming 60% response to CBT at 52 weeks, 50% response to GET, 25% response to APT, and 10% response to SMC.10 We assumed APT to be at least as effective as in previous trials of relaxation and flexibility therapies.20,22 For a two-sided test with 5% significance level and 90% power, we calculated that the number of participants needed to compare SMC with APT was 135, SMC with GET was 80, and SMC with CBT was 40. We increased group size to 150 per group to allow for 10% dropout, to provide equality between groups, and for secondary analyses. The statistical analysis plan was finalised, including changes to the original protocol, and was approved by the trial steering committee and the data monitoring and ethics committee before outcome data were examined.
We used continuous scores for primary outcomes to allow a more straightforward interpretation of the individual outcomes, instead of the originally planned composite measures (50% change or meeting a threshold score).10,30 We prorated primary outcomes scales only when there were at most two items per scale missing (nine participants for Chalder fatigue questionnaire and 11 for short form-36). Prorating involved calculating the mean value of the item scores present and replacing the missing values with that score.
We summarised continuous variables with mean (SD) or median (IQR) and categorical variables with frequencies and proportions. Differentiation of treatment compared independent ratings of therapy sessions with actual treatment. We calculated the inter-rater reliability (κ and 95% CI) between the two assessors. We used Kruskal-Wallis tests for comparisons of therapy received, therapeutic alliance, and manual adherence. We compared categorical variables with Fisher's exact test.
A clinically useful difference between the means of the primary outcomes was defined as 0·5 of the SD of these measures at baseline,31 equating to 2 points for Chalder fatigue questionnaire and 8 points for short form-36. A secondary post-hoc analysis compared the proportions of participants who had improved between baseline and 52 weeks by 2 or more points of the Chalder fatigue questionnaire, 8 or more points of the short form-36, and improved on both. In another post-hoc analysis, we compared the proportions of participants who had scores of both primary outcomes within the normal range at 52 weeks. This range was defined as less than the mean plus 1 SD scores of adult attendees to UK general practice of 14·2 (+4·6) for fatigue (score of 18 or less) and equal to or above the mean minus 1 SD scores of the UK working age population of 84 (−24) for physical function (score of 60 or more).32,33
We estimated differences between treatment groups for both primary outcomes with mixed linear regression models with Kenward-Roger adjusted standard errors. Covariates were treatment group, baseline value of outcome, time, and stratification factors (centre, present depressive disorder, and alternative criteria for chronic fatigue syndrome and myalgic encephalomyelitis; all as stratified at entry). Time by treatment interaction terms were included to allow extraction of contrasts at 52 weeks. Models for the primary outcomes and the clinical global impression incorporated random intercepts and slopes over time by participant and main health-care practitioner (doctor or therapist who saw the participant most frequently, or, if equal, the first practitioner to see the participant) to allow for clustering of outcomes within practitioner. We calculated intraclass correlation coefficients, adjusted for baseline outcomes, using one-way random effects analysis of covariance at 52 weeks within every treatment group. Unadjusted and Bonferroni corrected p values are provided for five comparisons for both primary outcomes. Comparisons of primary outcomes across treatment groups by alternative criteria for chronic fatigue syndrome and myalgic encephalomyelitis, and comorbid depressive disorder included the treatment by criteria or disorder interaction terms. Because some errors were made in stratification at randomisation, we used true status variables rather than status at stratification as covariates.
We calculated adverse event and reaction rates by dividing the number of events by person-years of follow-up multiplied by 100, and compared rate differences (95% CI) between treatment groups.
We analysed changes in clinical global impression scale using binary logistic generalised estimating equations regression with an exchangeable working correlation and bootstrapped standard errors. We analysed the number of chronic fatigue syndrome symptoms with ordinary least squares linear regression, and the presence of specific chronic fatigue syndrome symptoms with logistic regression. We analysed secondary outcomes with mixed linear regression models with random participant intercepts and slopes over time, apart from the walking test, which had random intercepts only. Covariates in the models were otherwise the same, except for clinical global impression (not measured at baseline) and chronic fatigue syndrome symptoms (measured only at 52 weeks).
We excluded participants from the intention-to-treat population for whom we had no primary outcome data in the final analysis, which used restricted maximum likelihood. The per-protocol analysis excluded participants who were ineligible after randomisation, treated at a second centre, or did not received adequate treatment, adjusting for actual stratification factors. Statistical analyses were done with Stata version 10, SAS version 9.1, and SPSS version 18.
This trial is registered at http://isrctn.org, number ISRCTN54285094.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All named authors had access to the data, commented on drafts, and approved the final report. Members of the writing group had responsibility for submitting the report, and PDW had final responsibility for the decision to submit for publication.
Results
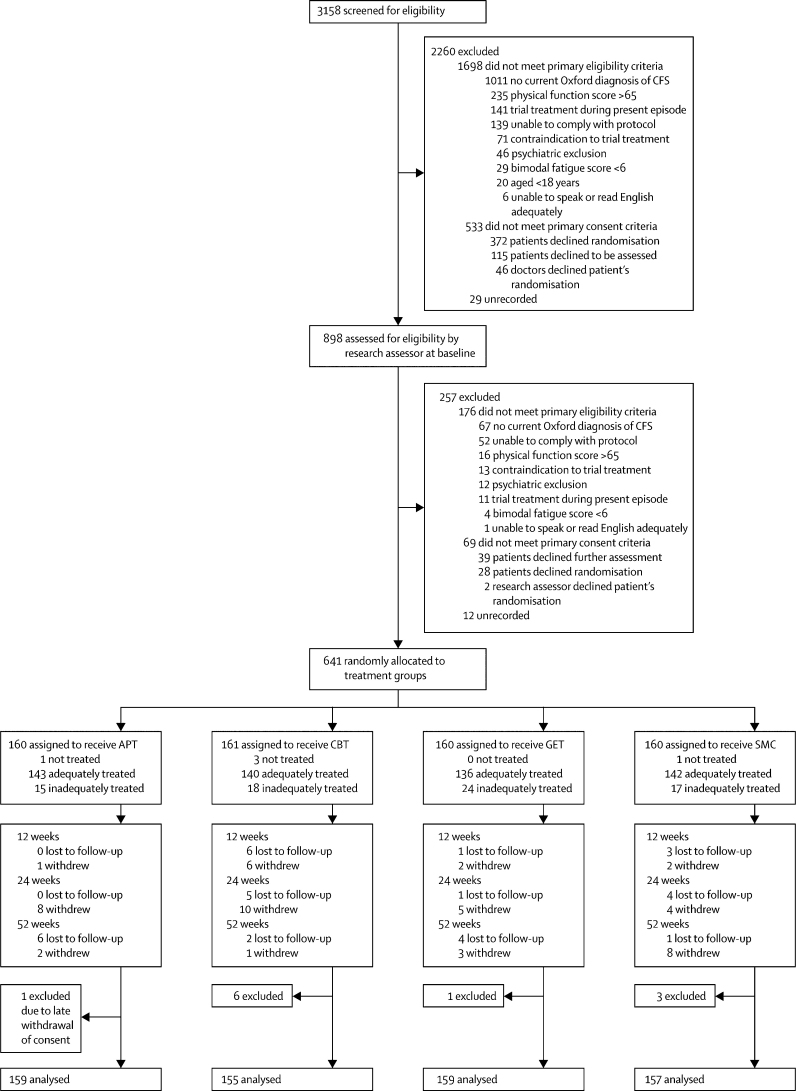
Figure 1 shows the trial profile. Briefly, 898 (28%) of 3158 patients screened for eligibility progressed to baseline screening and 641 (71%) participants were recruited (figure 1). The commonest reason for exclusion from initial clinician screening was failure to meet Oxford criteria for chronic fatigue syndrome (1011 participants). 745 (74%) of these excluded patients did not have chronic fatigue syndrome and the rest did not meet Oxford criteria despite having clinician-diagnosed chronic fatigue syndrome. Table 1 shows baseline characteristics of participants, which were much the same between groups, apart from a shorter duration of illness in the SMC group than was noted in the other groups. 33 (5%) of 640 participants were lost to follow-up, but rates did not differ between groups (p=0·30; figure 1). Ten of these 33 participants had no outcome data, and were therefore excluded from the final analysis. Primary outcomes were assessed at the time of dropout for three participants and included in the final analyses. Research assessors recorded primary outcomes (eg, dictated over the telephone) on 74 (4%) of 1920 occasions.
Figure 1.
CONSORT trial profile
CFS=chronic fatigue syndrome. APT=adaptive pacing therapy. CBT=cognitive behaviour therapy. GET=graded exercise therapy. SMC=specialist medical care alone. The numbers of participants per centre ranged from 63 to 135.
Table 1.
Baseline demographics and clinical characteristics
| Adaptive pacing therapy (n=159) | Cognitive behaviour therapy (n=161) | Graded exercise therapy (n=160) | Specialist medical care alone (n=160) | Overall (n=640) | ||
|---|---|---|---|---|---|---|
| Demographic data | ||||||
| Age (years) | 39 (11) | 39 (12) | 39 (12) | 37 (11) | 38 (12) | |
| Female | 121 (76%) | 129 (80%) | 123 (77%) | 122 (76%) | 495 (77%) | |
| White | 146 (92%) | 151 (94%) | 148 (93%) | 150 (94%) | 595 (93%) | |
| Any ME group membership | 31 (19%) | 26 (16%) | 25 (16%) | 23 (14%) | 105 (16%) | |
| Clinical data | ||||||
| International CFS criteria12 | ||||||
| As randomised | 99 (62%) | 100 (62%) | 98 (61%) | 100 (63%) | 397 (62%) | |
| Actual | 107 (67%) | 106 (66%) | 106 (66%) | 108 (68%) | 427 (67%) | |
| London ME criteria13 | ||||||
| As randomised | 89 (56%) | 90 (56%) | 89 (56%) | 89 (56%) | 357 (56%) | |
| Actual | 81 (51%) | 84 (52%) | 84 (53%) | 80 (50%) | 329 (51%) | |
| Any depressive disorder | ||||||
| As randomised | 55 (35%) | 55 (34%) | 54 (34%) | 55 (34%) | 219 (34%) | |
| Actual | 54 (34%) | 52 (32%) | 54 (34%) | 53 (33%) | 213 (33%) | |
| Any psychiatric disorder* | 75 (47%) | 75 (47%) | 73 (46%) | 77 (48%) | 300 (47%) | |
| Duration of illness (months) | 33 (16–69) | 36 (16–104) | 35 (18–67) | 25 (15–57) | 32 (16–68) | |
| Body-mass index (kg/m2) | 25·9 (5·5) | 25·4 (5·2) | 25·5 (4·6) | 25·1 (4·5) | 25·5 (5·0) | |
Data are mean (SD), n (%), or median (IQR). ME=myalgic encephalomyelitis. CFS=chronic fatigue syndrome.
Psychiatric disorders included any depressive disorder and any anxiety disorder, including phobias, obsessive-compulsive disorder, and post-traumatic stress disorder.
Table 2 shows details of treatments received. Participants allocated to SMC alone received more sessions, but there were no differences in the number of SMC sessions or therapy sessions received between the other three groups. There were no differences between groups in the proportions who had received adequate treatment (85% or more in every group). Participants' expectations were high for APT and GET, but lower for CBT and SMC (table 2). Most of those who received a therapy were satisfied with treatment (82% or more for the three therapies), but fewer were satisfied with SMC (50%). Number of treatment dropouts did not differ between groups (p=0·50; table 2). The two independent therapy assessors rated 58 (94%) of 62 and 57 (92%) of 62 therapy sessions as being the one allocated; only one (2%) session was rated by both assessors as different from that allocated. The inter-rater reliability (κ; 95% CI) was 0·86 (0·75–0·97). The independent assessors were unmasked in 25 (40%) of 62 sessions that they listened to. All three therapies were rated as adhering well to the manuals. Therapeutic alliance median scores were high and the same across therapies.
Table 2.
Treatment details
| Adaptive pacing therapy (n=159) | Cognitive behaviour therapy (n=161) | Graded exercise therapy (n=160) | Specialist medical care alone (n=160) | p value* | |
|---|---|---|---|---|---|
| Treatment received | |||||
| Therapy sessions attended† | 13 (12–15) | 14 (12–15) | 13 (12–14) | .. | 0·17 |
| Specialist medical care sessions attended‡ | 3 (3–4) | 3 (3–4) | 3 (3–4) | 5 (3–6) | 0·0001 |
| Adequate treatment§ | 143 (90%) | 140 (87%) | 136 (85%) | 142 (89%) | 0·56 |
| Antidepressant at baseline | 63 (40%) | 57 (35%) | 74 (46%) | 66 (41%) | .. |
| Antidepressant at 24 weeks¶ | 53 (34%) | 45 (29%) | 61 (40%) | 60 (39%) | 0·19 |
| Antidepressant at 52 weeks¶ | 41 (27%) | 47 (31%) | 48 (31%) | 61 (39%) | 0·11 |
| Hypnotic at baseline | 6 (4%) | 9 (6%) | 6 (4%) | 5 (3%) | .. |
| Hypnotic at 24 weeks¶ | 3 (2%) | 7 (5%) | 5 (3%) | 6 (4%) | 0·61 |
| Hypnotic at 52 weeks¶ | 5 (3%) | 4 (3%) | 3 (2%) | 7 (5%) | 0·62 |
| Non-allocated treatment | 8 (5%) | 4 (3%) | 7 (4%) | 22 (14%) | 0·0005 |
| Dropouts from treatment | 11 (7%) | 17 (11%) | 10 (6%) | 14 (9%) | 0·50 |
| Views before treatment | |||||
| Treatment is logical | 134 (84%) | 115 (71%) | 135 (84%) | 79 (49%) | <0·0001 |
| Confident about treatment | 114 (72%) | 91 (57%) | 112 (70%) | 65 (41%) | <0·0001 |
| Views after treatment | |||||
| Satisfied with treatment¶ | 128 (85%) | 117 (82%) | 126 (88%) | 76 (50%) | <0·0001 |
| Dissatisfied with treatment¶ | 4 (3%) | 7 (5%) | 2 (1%) | 17 (11%) | 0·0010 |
| Therapeutic alliance‖ | 6·5 (6·0–6·5) | 6·5 (5·5–6·8) | 6·5 (5·5–7·0) | .. | 0·96 |
| Adherence to manual** | 6·0 (6·0–6·5) | 6·0 (5·0–6·5) | 6·5 (6·0–6·5) | .. | 0·35 |
Data are median (IQR) or n (%).
p values across all groups.
86% of sessions were received face-to-face and 14% by telephone.
94% of sessions were received face-to-face and 6% by telephone.
Adequate treatment was ten or more sessions of therapy or three or more sessions of specialist medical care alone.
Percentages exclude missing data.
Scored 1–7 (1=poor, 7=excellent).
Scored 1–7 (1=not at all, 7=very much so).
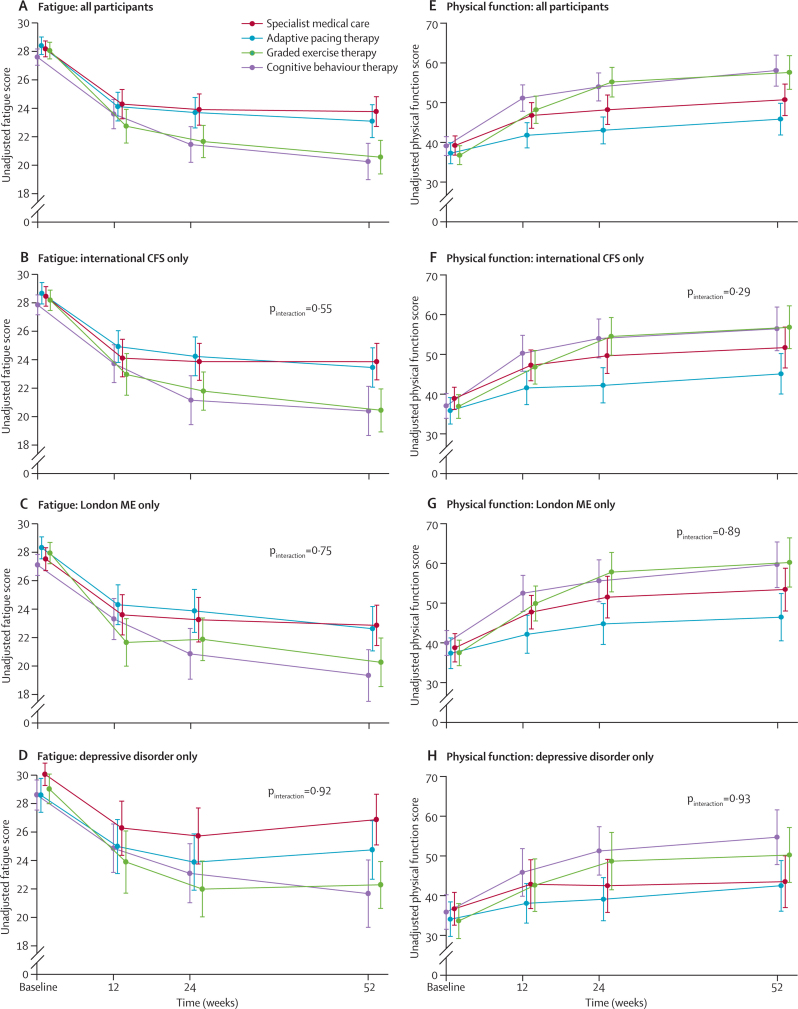
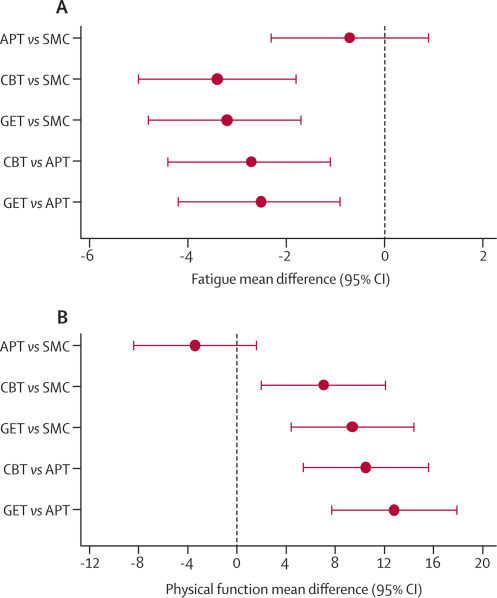
Table 3 shows baseline and outcomes data, and figure 2 shows profiles for the primary outcomes. In the final-adjusted models (figure 3), participants had less fatigue and better physical function after CBT and GET than they did after APT or SMC alone. Outcomes after APT were no better than they were after SMC. Allowing for clustering effects caused by participants attending the same main practitioner had little effect on these results; intraclass correlation coefficients ranged from −0·02 to 0·11 for fatigue, and −0·01 to 0·03 for physical function. Participant subgroups meeting international criteria for chronic fatigue syndrome, London criteria for myalgic encephalomyelitis, and depressive disorder criteria did not differ in the pattern of treatment effects (figure 2; all pinteractions were non-significant).
Table 3.
Primary outcomes of fatigue and physical function
|
Fatigue* |
Physical function† |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adaptive pacing therapy | Cognitive behaviour therapy | Graded exercise therapy | Specialist medical care alone | Adaptive pacing therapy | Cognitive behaviour therapy | Graded exercise therapy | Specialist medical care alone | ||
| Baseline | 28·5 (4·0); n=159 | 27·7 (3·7); n=161 | 28·2 (3·8); n=160 | 28·3 (3·6); n=160 | 37·2 (16·9); n=159 | 39·0 (15·3); n=161 | 36·7 (15·4); n=160 | 39·2 (15·4); n=160 | |
| 12 weeks | 24·2 (6·4); n=153 | 23·6 (6·5); n=153 | 22·8 (7·5); n=153 | 24·3 (6·5); n=154 | 41·7 (19·9); n=153 | 51·0 (20·7); n=153 | 48·1 (21·6); n=153 | 46·6 (20·4); n=154 | |
| 24 weeks | 23·7 (6·9); n=155 | 21·5 (7·8); n=148 | 21·7 (7·1); n=150 | 24·0 (6·9); n=152 | 43·2 (21·4); n=155 | 54·2 (21·6); n=148 | 55·4 (23·3); n=150 | 48·4 (23·1); n=152 | |
| 52 weeks | 23·1 (7·3); n=153 | 20·3 (8·0); n=148 | 20·6 (7·5); n=154 | 23·8 (6·6); n=152 | 45·9 (24·9); n=153 | 58·2 (24·1); n=148 | 57·7 (26·5); n=154 | 50·8 (24·7); n=152 | |
| Mean difference (95% CI) from SMC (52 weeks) | −0·7 (−2·3 to 0·9) | −3·4 (−5·0 to −1·8) | −3·2 (−4·8 to −1·7) | .. | −3·4 (−8·4 to 1·6) | 7·1 (2·0 to 12·1) | 9·4 (4·4 to 14·4) | .. | |
| Unadjusted p values | 0·38 | 0·0001 | 0·0003 | .. | 0·18 | 0·0068 | 0·0005 | .. | |
| Bonferroni adjusted p values | 0·99 | 0·0006 | 0·0013 | .. | 0·89 | 0·0342 | 0·0025 | .. | |
| Mean difference (95% CI) from APT (52 weeks) | .. | −2·7 (−4·4 to −1·1) | −2·5 (−4·2 to −0·9) | .. | .. | 10·5 (5·4 to 15·6) | 12·8 (7·7 to 17·9) | .. | |
| Unadjusted p values | .. | 0·0027 | 0·0059 | .. | .. | 0·0002 | <0·0001 | .. | |
| Bonferroni adjusted p values | .. | 0·0136 | 0·0294 | .. | .. | 0·0012 | 0·0002 | .. | |
| Number improved from baseline‡ | 99 (65%) | 113 (76%) | 123 (80%) | 98 (65%) | 75 (49%) | 105 (71%) | 108 (70%) | 88 (58%) | |
Data are mean scores (SD) or n (%), unless otherwise stated. Comparisons of differences across groups made at 52 weeks are from the final adjusted models, so are slightly different from unadjusted values. p values for comparisons are unadjusted, with Bonferroni values adjusted for five comparisons for every primary outcome.
Chalder fatigue questionnaire (range 0–33, 0=best).15
Short form-36 physical function subscale score (range 0–100, 100=best).16
Participants improved from baseline by two or more points for fatigue and eight or more for physical function.
Figure 2.
Physical function subscale and fatigue questionnaire scores by treatment group
Data are unadjusted means (95% CI). pinteraction is the p-value of the interaction between treatment and criteria or disorder from the adjusted model. CFS=chronic fatigue syndrome. ME=myalgic encephalomyelitis. (A–D) Lowest fatigue score is best. (E–H) Highest physical function score is best.
Figure 3.
Primary outcome treatment differences for fatigue (A) and physical function (B) at 52 weeks
(A) Negative values for fatigue favour the first treatment group in each pair of comparisons. (B) Positive values for physical function favour the first treatment group in each pair of comparisons. APT=adaptive pacing therapy. SMC=specialist medical care. CBT=cognitive behaviour therapy. GET=graded exercise therapy.
64 (42%) of 153 participants in the APT group improved by at least 2 points for fatigue and at least 8 points for physical function at 52 weeks, compared with 87 (59%) of 148 participants for CBT, 94 (61%) of 154 participants for GET, and 68 (45%) of 152 participants for SMC. More participants improved after CBT compared with APT (p=0·0033) or SMC (p=0·0149), and more improved with GET compared with APT (p=0·0008) or SMC (p=0·0043); APT did not differ from SMC (p=0·61; webappendix p 2).
25 (16%) of 153 participants in the APT group were within normal ranges for both primary outcomes at 52 weeks, compared with 44 (30%) of 148 participants for CBT, 43 (28%) of 154 participants for GET, and 22 (15%) of 152 participants for SMC. More participants were within normal ranges after CBT than APT (p=0·0057) or SMC (p=0·0014), and more were within normal ranges with GET compared with APT (p=0·0145) or SMC (p=0·0040); APT did not differ from SMC (p=0·65).
Webappendix p 3 shows the per-protocol analysis. Differences between treatments were very similar to those of the final analysis, but magnitude was almost always higher in the per-protocol analysis.
Table 4 shows safety outcomes. Non-serious adverse events were common. Participants who received CBT reported slightly fewer such events than did those in the APT (p=0·0081) and SMC (p=0·0016) groups. Serious adverse events and serious deterioration were uncommon; serious adverse reactions were rare. There were more serious adverse events in the GET group than there were in the SMC group (p=0·0433). Rates of serious adverse reactions and serious deterioration did not differ between treatment groups. Webappendix pp 4–5 shows a summary of serious adverse events and serious adverse reactions.
Table 4.
Safety outcomes
| Adaptive pacing therapy (n=159) | Cognitive behaviour therapy (n=161) | Graded exercise therapy (n=160) | Specialist medical care alone (n=160) | ||
|---|---|---|---|---|---|
| Non-serious adverse events | 949 | 848 | 992 | 977 | |
| Participants with non-serious adverse events | 152 (96%) | 143 (89%) | 149 (93%) | 149 (93%) | |
| Non-serious adverse events per 100 person-years | 597 (559–636) | 527 (492–563) | 620 (582–660) | 611 (573–650) | |
| Serious adverse events | 16 | 8 | 17 | 7 | |
| Participants with serious adverse events | 15 (9%) | 7 (4%) | 13 (8%) | 7 (4%) | |
| Serious adverse events per 100 person-years | 10·1 (5·8–16·3) | 5·0 (2·2–9·8) | 10·6 (6·2–17·0) | 4·4 (1·8–9·0) | |
| Serious adverse reactions | 2 | 4 | 2 | 2 | |
| Participants with serious adverse reactions | 2 (1%) | 3 (2%) | 2 (1%) | 2 (1%) | |
| Serious adverse reactions per 100 person-years | 1·3 (0·2–4·5) | 2·5 (0·7–6·4) | 1·3 (0·2–4·5) | 1·3 (0·2–4·5) | |
| Serious deterioration (composite)* | 13 (8%) | 14 (9%) | 10 (6%) | 15 (9%) | |
| Physical functioning reduction | 7 (4%) | 5 (3%) | 5 (3%) | 6 (4%) | |
| PCGI worse | 5 (3%) | 7 (4%) | 1 (<1%) | 10 (6%) | |
| Withdrawn due to worsening | 3 (2%) | 0 | 2 (1%) | 1 (<1%) | |
| Serious adverse reactions | 2 (1%) | 3 (2%) | 2 (1%) | 2 (1%) | |
| Differences in serious deterioration | |||||
| Comparison with specialist medical care | −1·2%; p=0·71 | −0·7%; p=0·83 | −3·1%; p=0·30 | .. | |
| Comparison with adaptive pacing therapy | .. | 0·5%; p=0·87 | −1·9%; p=0·51 | .. | |
Data are n, n (%), or rate (95% CI), unless otherwise stated. Adverse events were considered serious when they involved death, hospital admission, increased severe and persistent disability, self-harm, were life-threatening, or required an intervention to prevent one of these. There were no suspected unexpected serious adverse reactions. PCGI=participant-rated clinical global impression.
Serious deterioration composite is either of a short form-36 physical function subscale score reduction at two consecutive visits, a PCGI score of much worse or very much worse at two consecutive visits, withdrawal from treatment due to explicit worsening, or a serious adverse reaction; the numbers withdrawn from treatment due to worsening is a subset of all those withdrawing from treatment shown in table 2.
Table 5 shows data for the clinical global impression scale ratings. At 52 weeks, more patients rated themselves as much better or very much better in overall health after CBT and GET than did after APT and SMC. A minority (≤9% in every group) rated themselves as much worse or very much worse, which did not differ between groups.
Table 5.
Participant-rated clinical global impression of change in overall health
| Adaptive pacing therapy (n=159) | Cognitive behaviour therapy (n=161) | Graded exercise therapy (n=160) | Specialist medical care alone (n=160) | ||
|---|---|---|---|---|---|
| Change from baseline | |||||
| 12 weeks | 153 (96%) | 153 (95%) | 151 (94%) | 151 (94%) | |
| Positive change | 20 (13%) | 32 (21%) | 37 (25%) | 7 (5%) | |
| Minimum change | 126 (82%) | 113 (74%) | 111 (74%) | 133 (88%) | |
| Negative change | 7 (5%) | 8 (5%) | 3 (2%) | 11 (7%) | |
| 24 weeks | 155 (97%) | 149 (93%) | 148 (93%) | 151 (94%) | |
| Positive change | 37 (24%) | 56 (38%) | 54 (37%) | 28 (19%) | |
| Minimum change | 111 (72%) | 82 (55%) | 89 (60%) | 107 (71%) | |
| Negative change | 7 (5%) | 11 (7%) | 5 (3%) | 16 (11%) | |
| 52 weeks | 153 (96%) | 147 (91%) | 152 (95%) | 152 (95%) | |
| Positive change | 47 (31%) | 61 (41%) | 62 (41%) | 38 (25%) | |
| Minimum change | 96 (63%) | 77 (52%) | 80 (53%) | 100 (66%) | |
| Negative change | 10 (7%) | 9 (6%) | 10 (7%) | 14 (9%) | |
| Odds ratio (positive change vs negative or minimum changes) | |||||
| Compared with specialist medical care | 1·3 (0·8–2·1); p=0·31 | 2·2 (1·2–3·9); p=0·011 | 2·0 (1·2–3·5); p=0·013 | .. | |
| Compared with adaptive pacing therapy | .. | 1·7 (1·0–2·7); p=0·034 | 1·5 (1·0–2·3); p=0·028 | .. | |
Data are n (%) or odds ratio (95% CI). Comparisons made at 52 weeks were taken from the final adjusted models. Positive change was defined as very much better or much better. Minimum change was defined as a little better, no change, or a little worse. Negative change was defined as much worse or very much worse.
Table 6 shows other secondary outcomes. At 52 weeks, participants in the CBT and GET groups had better outcomes than did participants in the APT and SMC groups for work and social adjustment scores, sleep disturbance, and depression (with the one exception that GET was no different from APT for depression). Anxiety was lower after CBT and GET than it was after SMC, but not than after APT. There were fewer chronic fatigue syndrome symptoms after CBT than there were after SMC. Poor concentration and memory did not differ between groups. Postexertional malaise was lower after CBT and GET than it was after APT and SMC. 6-min walking distances were greater after GET than they were APT and SMC, but were no different after CBT compared with APT and SMC. There were no differences in any secondary outcomes between APT and SMC groups (webappendix pp 6–9).
Table 6.
Secondary outcomes
| Adaptive pacing therapy (n=159) | Cognitive behaviour therapy (n=161) | Graded exercise therapy (n=160) | Specialist medical care alone (n=160) | ||
|---|---|---|---|---|---|
| Work and social adjustment scale | 150 (94%) | 143 (89%) | 144 (90%) | 151 (94%) | |
| Baseline score | 27·9 (6·1) | 27·4 (6·2) | 27·3 (6·3) | 26·9 (6·7) | |
| 52-week score | 24·5 (8·8) | 21·0 (9·6) | 20·5 (9·4) | 23·9 (9·2) | |
| Comparison with SMC | 0·1; p=0·93 | −3·6; p=0·0001 | −3·2; p=0·0006 | .. | |
| Comparison with APT | .. | −3·7; p=0·0001 | −3·3; p=0·0004 | .. | |
| 6-min walking test | 111 (70%) | 123 (76%) | 110 (69%) | 118 (74%) | |
| Baseline distance (m) | 314 (90) | 333 (86) | 312 (87) | 326 (95) | |
| 52-week distance (m) | 334 (117) | 354 (106) | 379 (100) | 348 (108) | |
| Comparison with SMC | −5·7; p=0·55 | −1·5; p=0·87 | 35·3; p=0·0002 | .. | |
| Comparison with APT | .. | 4·2; p=0·65 | 41·0; p<0·0001 | .. | |
| Jenkins sleep scale | 150 (94%) | 143 (89%) | 144 (90%) | 151 (94%) | |
| Baseline score | 12·1 (4·9) | 12·5 (4·9) | 11·7 (4·3) | 12·4 (5·0) | |
| 52-week score | 10·6 (4·8) | 9·9 (5·3) | 9·0 (4·8) | 11·0 (5·0) | |
| Comparison with SMC | −0·1; p=0·76 | −1·1; p=0·0216 | −1·4; p=0·0024 | .. | |
| Comparison with APT | .. | −0·9; p=0·0466 | −1·3; p=0·0062 | .. | |
| HADS depression scale | 149 (94%) | 143 (89%) | 144 (90%) | 151 (94%) | |
| Baseline score | 8·1 (3·9) | 8·3 (3·7) | 8·2 (3·6) | 8·0 (3·9) | |
| 52-week score | 7·2 (4·5) | 6·2 (3·7) | 6·1 (4·1) | 7·2 (4·7) | |
| Comparison with SMC | −0·6; p=0·11 | −1·4; p=0·0003 | −1·1; p=0·0035 | .. | |
| Comparison with APT | .. | −0·8; p=0·0382 | −0·5; p=0·18 | .. | |
| HADS anxiety scale | 149 (94%) | 143 (89%) | 144 (90%) | 149 (93%) | |
| Baseline score | 8·1 (4·2) | 8·1 (4·3) | 8·0 (4·2) | 7·9 (4·3) | |
| 52-week score | 7·5 (4·2) | 6·8 (4·2) | 7·1 (4·5) | 8·0 (4·4) | |
| Comparison with SMC | −0·7; p=0·0713 | −1·4; p=0·0003 | −1·0; p=0·0142 | .. | |
| Comparison with APT | .. | −0·7; p=0·0671 | −0·3; p=0·50 | .. | |
| Chronic fatigue syndrome symptom count | 151 (95%) | 145 (90%) | 144 (90%) | 149 (93%) | |
| Baseline | 4·8 (1·8) | 4·6 (1·8) | 4·6 (1·8) | 4·7 (1·7) | |
| 52 week | 3·8 (2·3) | 3·4 (2·3) | 3·4 (2·5) | 3·9 (2·2) | |
| Comparison with SMC | −0·1; p=0·62 | −0·5; p=0·0329 | −0·4; p=0·0916 | .. | |
| Comparison with APT | .. | −0·4; p=0·0986 | −0·3; p=0·23 | .. | |
| Poor concentration or memory | 151 (95%) | 145 (90%) | 144 (90%) | 149 (93%) | |
| Baseline (n [%] with symptoms) | 122 (77%) | 117 (73%) | 122 (76%) | 115 (72%) | |
| 52 weeks (n [%] with symptoms) | 93 (59%) | 73 (45%) | 76 (48%) | 90 (56%) | |
| Comparison with SMC | Odds ratio 1·0; p=0·97 | Odds ratio 0·6; p=0·0602 | Odds ratio 0·7; p=0·14 | .. | |
| Comparison with APT | .. | Odds ratio 0·6; p=0·0629 | Odds ratio 0·7; p=0·15 | .. | |
| Postexertional malaise | 151 (95%) | 145 (90%) | 144 (90%) | 149 (93%) | |
| Baseline (n [%] with symptoms) | 134 (84%) | 135 (84%) | 131 (82%) | 139 (87%) | |
| 52 weeks (n [%] with symptoms) | 100 (63%) | 79 (49%) | 71 (44%) | 101 (63%) | |
| Comparison with SMC | Odds ratio 1·0; p=0·86 | Odds ratio 0·6; p=0·0254 | Odds ratio 0·5; p=0·0026 | .. | |
| Comparison with APT | .. | Odds ratio 0·6; p=0·0380 | Odds ratio 0·5; p=0·0042 | .. | |
Data are number of completed questionnaires at 52 weeks (%), means (SD), or mean difference, unless otherwise stated. Comparisons across treatment arms at 52 weeks are from the final adjusted models. Webappendix pp 6–9 shows forest plots of mean differences (95% CI) and odds ratios (95% CI) for comparisons between groups. APT=adaptive pacing therapy. SMC=specialist medical care alone. HADS=hospital anxiety and depression scale.
Discussion
When added to SMC, CBT and GET had greater success in reducing fatigue and improving physical function than did APT or SMC alone. APT was no better than was SMC alone. Our findings were much the same for participants meeting the different diagnostic criteria for chronic fatigue syndrome and for myalgic encephalomyelitis, for those with depressive disorder, and after allowing for clustering effects. Other secondary outcomes showed a very similar pattern. There were no important differences in safety outcomes between treatment options.
Mean differences between groups on primary outcomes almost always exceeded predefined clinically useful differences for CBT and GET when compared with APT and SMC. In all comparisons of the proportions of participants who had either improved or were within normal ranges for these outcomes, CBT and GET did better than did APT or SMC alone. No more than 30% of participants were within normal ranges for both outcomes and only 41% rated themselves as much better or very much better in their overall health. We suggest that these findings show that either CBT or GET, when added to SMC, is an effective treatment for chronic fatigue syndrome, and that the size of this effect is moderate (panel 2).
Panel 2. Research in context.
Systematic review
We searched the PubMed and Cochrane Library databases up to Nov 6, 2010, without language restrictions for full papers reporting randomised controlled trials, systematic reviews, and meta-analyses with the search terms “chronic fatigue syndrome”, “myalgic encephalomyelitis”, “myalgic encephalopathy” and “cognitive behaviour therapy”, “exercise”, “pacing”. We excluded trials of adolescents, education, and group interventions. Our search identified the two most recent systematic reviews,4,5 two meta-analyses,6,7 and two additional trials34,35 that were not included in these reviews. The reviews and meta-analyses concluded that cognitive behaviour therapy and graded exercise therapy are moderately effective treatments for chronic fatigue syndrome, and that limitations of previous trials included small size, an absence of data for safety outcomes, and high dropout rates.4–7 The findings from these studies concur with the UK National Institute for Health and Clinical Excellence guidelines.2
Interpretation
In the pacing, graded activity, and cognitive behaviour therapy: a randomised evaluation (PACE) trial, we affirm that cognitive behaviour therapy and graded exercise therapy are moderately effective outpatient treatments for chronic fatigue syndrome when added to specialist medical care, as compared with adaptive pacing therapy or specialist medical care alone. Findings from PACE also allow the following interpretations: adaptive pacing therapy added to specialist medical care is no more effective than specialist medical care alone; our findings apply to patients with differently defined chronic fatigue syndrome and myalgic encephalomyelitis whose main symptom is fatigue; and all four treatments tested are safe.
Our conclusions are supported by secondary outcomes, as both CBT and GET provided greater improvements than did APT and SMC for most outcomes. The objective walking test favoured GET over CBT, whereas CBT provided the largest reduction in depression. The comparatively greater reduction in postexertional malaise with both CBT and GET compared with the other two treatments is notable, since the risk of exacerbation of this symptom is commonly given as a reason to avoid treatments such as GET. The 47% prevalence of mood and anxiety disorders at baseline was much the same as that noted in previous trials in secondary care (38–56%).20,23,36 The equivalent use of antidepressants in the treatment groups implies that the differences in outcomes are unlikely to be attributable to these drugs.
There were no differences between groups in the proportions with serious deterioration or serious adverse reactions. The increased rate of serious adverse events with GET compared with SMC is unlikely to be important because serious adverse events were not thought by the independent scrutinisers to be related to treatment. Consequently, if these treatments are delivered as described, by similarly qualified and trained clinicians, patients need not be concerned about safety.37
The finding that APT when added to SMC was no more effective than SMC alone was contrary to our initial hypothesis. This finding might in part be caused by greater improvement after SMC than was expected. Suboptimum delivery of APT is an unlikely explanation because APT therapists were the most experienced; the therapeutic alliance and the adherence to manuals were rated highly in this group and participant satisfaction did not differ from that for other therapies. Since participants' confidence that APT would help them was much the same as for GET, and greater than that for CBT, they were unlikely to have been biased by negative expectations. The fundamental difference between APT and both CBT and GET is that APT encourages adaptation to the illness,13,17,18 whereas CBT and GET encourage gradual increases in activity with the aim of ameliorating the illness.2,4,7 Our results do not support pacing, in the form of APT, as a first-line therapy for chronic fatigue syndrome.
We plan to report relative cost-effectiveness of the treatments, their moderators and mediators, whether subgroups respond differently, and long-term follow-up in future publications. Our finding that studied treatments were only moderately effective also suggests research into more effective treatments is needed. The effectiveness of behavioural treatments does not imply that the condition is psychological in nature.
Our findings were strengthened by the small numbers of dropouts, high rates of acceptance of the treatments, use of manual-defined treatments provided by competent clinicians, high rates of participant satisfaction, adherence to manuals, and therapeutic alliance. The PACE findings can be generalised to patients who also meet alternative diagnostic criteria for chronic fatigue syndrome12 and myalgic encephalomyelitis13 but only if fatigue is their main symptom.11
Our trial had limitations. We excluded patients unable to attend hospital. Our results apply to patients referred to secondary care. SMC is not the same as usual medical care that might be provided by a family doctor; this study was not designed to compare SMC with usual medical care. Although more than 3000 patients attending clinics had to be screened to identify the 641 recruited, the commonest reason for exclusion at screening was not having chronic fatigue syndrome. We chose conventional criteria for defining clinically useful differences between treatments, although other thresholds could have been chosen.32 SMC was not as closely monitored or supervised as the other therapies, and participants receiving SMC alone had more sessions than did those in the therapy groups; this is unlikely to have affected comparisons between the groups. Masking of participants or clinicians to treatment allocation was not possible, and research assessors were also not masked. Primary outcomes were subjective and rated by participants. While this avoided investigator bias, it could be subject to other biases. Although participant-rated outcome measures could have been affected by expectations of treatment, which were highest for APT and GET, CBT was one of the two most effective treatments despite lower expectations.
Findings from the PACE trial suggest that individually delivered CBT and GET, when added to SMC, are more effective and as safe as APT added to SMC or SMC alone. Patients attending secondary care with chronic fatigue syndrome should be offered individual CBT or GET, alongside SMC.
Acknowledgments
Acknowledgments
The PACE trial was funded by the UK Medical Research Council (MRC G0200434), the Department of Health for England, the UK Department for Work and Pensions, and the Chief Scientist Office of the Scottish Government Health Directorates. The Department of Health and the Chief Scientist Office provided excess treatment and service support costs for the trial therapists and doctors. We thank the participants who took part in the PACE trial, staff from all the centres (including all PACE research nurses or assistants: Susan Begg, Matteo Cella, Sally Cregeen, Sarah Horne, Chris James, Julie Richards, Joanna Smee, and Vicky Toghill; data managers: Victoria Bates, Ann M Doust, Emma Hartley, Kate Lievesley, Sandy Smith, and Olga Zielona; therapists: Mary Barker, Jen Bobrow, Laura Butler, Nathan Butler, Richard-John Chippindall, Ruth Cowlishaw, Vincent Deary, Caroline J Heading, Lindsey Hume, Vicky Johnson, Sally Ludlam, Lorraine Maher-Edwards, Louise Mason, Christina Michailidou, Stacey Millet-Clay, Karen Shute, C M Simpson, Sheena E Spence, Valerie Suarez, Brendan Thomas, Tracey Turner, Rebecca Van Klinken, S M Wagner, Sue Wilkins, Giselle Withers, Fiona Wright, and Daniel Zahl; and specialist medical care doctors: Janet Andrews, Michael Broughton, Frauke Fehse, Eleanor Feldman, Janet Gray, Michael E Jones, Tara Lawn, Brian Marien, Tim Peto, Angharad Ruttley, Alastair Santhouse, Adrian Vos, and Simon Wessely. Kathy Fulcher, Tom Meade, C L Murphy, Anthony J Pinching, and Rajesh Shah contributed and provided advice about the study, and Kurt Kroenke, Jan Scott, Peter Tyrer, and Simon Wessely commented on an early draft of the report.
PACE trial group
Trial Steering Committee (independent members)—Janet Darbyshire (Chair), Jenny Butler, Patrick Doherty, Stella Law, M Llewelyn, and Tom Sensky. Observers—Sir Mansel Aylward (Department for Work and Pensions, London, UK), Sir Peter Spencer and Chris Clark (Action for ME, Bristol, UK), Stephen Stansfeld (Queen Mary University of London, London, UK), Alison Wearden (Fatigue Intervention by Nurses Evaluation trial), and members of analysis strategy group and writing and publication oversight committee. Data Monitoring and Ethics Committee—Paul Dieppe (initial Chair), Astrid Fletcher (final Chair), and Charlotte Feinmann. Independent assessors of the trial safety data—Hiroko Akagi, Alastair Miller, and Gavin Spickett. Independent assessors of therapy—Barbara Bowman and Deborah Fleetwood.
Contributors
The principal investigators (PDW, TC, and MS) designed the study and obtained funding. Design of the trial was further developed by the trial management group, trial steering committee, data monitoring and ethics committee, and the patients' organisation, Action for ME. The trial management group, chaired by PDW, included all the authors of this report and Chris Clark, Eleanor Feldman, Tim Peto, and Sir Peter Spencer. The analysis strategy group, chaired by MS, consisted of HLB, TC, JCD, KAG, ALJ, LP, PDW, and RW. The statistical analysis plan was written by the analysis strategy group and approved by the trial steering committee and data monitoring and ethics committee before the analysis was started. The trial analysis team, chaired by ALJ, consisted of HLB, TC, KAG, MS, PDW, and RW. ALJ, LP, and RW were trial statisticians who participated in the design of the study, with RW being the lead writer of the analysis plan. KAG did the main statistical analysis. TC designed and undertook the analysis of therapy differentiation, integrity, and alliance. The writing and publication oversight committee, chaired by MS, consisted of HLB, TC, KAG, ALJ, PM, LP, PDW, and RW. The trial managers were JCD initially, then HLB. The treatment leaders were JB, MB, DLC, LVC, and GM (JB and LVC in sequence for one treatment group), who designed the treatment manuals in collaboration with the principal investigators and trained and supervised the trial therapists and doctors. The centre leaders were BA, TC, Eleanor Feldman, GM, MM, HO, Tim Peto, MS, PDW, DW, and Simon Wessely. The centres were at St Bartholomew's Hospital, London; Western General Hospital, Edinburgh; King's College Hospital, London; John Radcliffe Hospital, Oxford; Royal Free Hospital, London and the Frenchay Hospital, Bristol (all UK).
Conflicts of interest
PDW has done voluntary and paid consultancy work for the UK Departments of Health and Work and Pensions and Swiss Re (a reinsurance company). DLC has received royalties from Wiley. JB was on the guideline development group of the National Institute for Health and Clinical Excellence guidelines for chronic fatigue syndrome and myalgic encephalomyelitis and has undertaken paid work for the insurance industry. GM has received royalties from Karnac. TC has done consultancy work for insurance companies and has received royalties from Sheldon Press and Constable and Robinson. MB has received royalties from Constable and Robinson. MS has done voluntary and paid consultancy work for government and for legal and insurance companies, and has received royalties from Oxford University Press. ALJ, BA, HLB, LVC, JCD, KAG, LP, MM, PM, HO, RW, and DW declare that they have no conflicts of interests.
Web Extra Material
References
- 1.Prins JB, van der Meer JW, Bleijenberg G. Chronic fatigue syndrome. Lancet. 2006;367:346–355. doi: 10.1016/S0140-6736(06)68073-2. [DOI] [PubMed] [Google Scholar]
- 2.National Institute for Health and Clinical Excellence Clinical guideline CG53. Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy): diagnosis and management. http://guidance.nice.org.uk/CG53 (accessed Nov 6, 2010).
- 3.Cairns R, Hotopf M. A systematic review describing the prognosis of chronic fatigue syndrome. Occup Med. 2005;55:20–31. doi: 10.1093/occmed/kqi013. [DOI] [PubMed] [Google Scholar]
- 4.Edmonds M, McGuire H, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2004;3 doi: 10.1002/14651858.CD003200.pub2. CD003200. [DOI] [PubMed] [Google Scholar]
- 5.Bagnall A-M, Hempel S, Chambers D, Orton V, Forbes C. The treatment and management of chronic fatigue syndrome (CFS)/myalgic encephalomyelitis (ME) in adults and children: update of CRD Report 22. http://www.york.ac.uk/inst/crd/CRD_Reports/crdreport35.pdf (accessed Nov 6, 2010).
- 6.Malouff JM, Thorsteinsson EB, Rooke SE, Bhullar N, Schutte NS. Efficacy of cognitive behavioral therapy for chronic fatigue syndrome: a meta-analysis. Clin Psychol Rev. 2008;28:736–745. doi: 10.1016/j.cpr.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Price JR, Mitchell E, Tidy E, Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database Syst Rev. 2008;3 doi: 10.1002/14651858.CD001027.pub2. CD001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Action for ME. ME 2008: what progress? Action for ME, 2008. http://www.afme.org.uk/res/img/resources/Survey%20Summary%20Report%202008.pdf (accessed Nov 6, 2010).
- 9.ME Association Managing my ME. What people with ME/CFS and their carers want from the UK's health and social services. http://www.meassociation.org.uk/?page_id=1345 (accessed Nov 6, 2010).
- 10.White PD, Sharpe MC, Chalder T, DeCesare JC, Walwyn R, on behalf of the PACE trial group Protocol for the PACE trial: a randomised controlled trial of adaptive pacing, cognitive behaviour therapy, and graded exercise, as supplements to standardised specialist medical care versus standardised specialist medical care alone for patients with the chronic fatigue syndrome/myalgic encephalomyelitis or encephalopathy. BioMed Cent Neurol. 2007;7:6. doi: 10.1186/1471-2377-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharpe MC, Archard LC, Banatvala JE. A report—chronic fatigue syndrome. J Roy Soc Med. 1991;84:118–121. doi: 10.1177/014107689108400224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves WC, Lloyd A, Vernon SD. The international chronic fatigue syndrome study group identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Serv Res. 2003;3:2. doi: 10.1186/1472-6963-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The London criteria . Report on chronic fatigue syndrome (CFS), post viral fatigue syndrome (PVFS) and myalgic encephalomyelitis (ME) The National Task Force; Westcare, Bristol: 1994. [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR axis I disorders, research version, patient edition with psychotic screen (SCID-I/P W/ PSY SCREEN) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- 15.Chalder T, Berelowitz G, Hirsch S, Pawlikowska T, Wallace P, Wessely S. Development of a fatigue scale. J Psychosom Res. 1993;37:147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 16.McHorney CA, Ware JE, Raczek AE. The MOS 36 item short form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Pesek JR, Jason LA, Taylor RR. An empirical investigation of the envelope theory. J Human Behav Soc Environ. 2000;3:59–77. [Google Scholar]
- 18.Action for ME Pacing for people with ME. http://www.afme.org.uk/res/img/resources/Pacing%20booklet%2019%20March%2007.pdf (accessed Nov 6, 2010).
- 19.Sharpe M, Hawton K, Simkin S. Cognitive behaviour therapy for the chronic fatigue syndrome: a randomised controlled trial. BMJ. 1996;312:22–26. doi: 10.1136/bmj.312.7022.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deale A, Chalder T, Marks I, Wessely S. Cognitive behavior therapy for chronic fatigue syndrome: a randomized controlled trial. Am J Psychiatry. 1997;154:408–414. doi: 10.1176/ajp.154.3.408. [DOI] [PubMed] [Google Scholar]
- 21.Prins JB, Bleijenberg G, Bazelmans E. Cognitive behaviour therapy for chronic fatigue syndrome: a multicentre randomised controlled trial. Lancet. 2001;357:841–847. doi: 10.1016/S0140-6736(00)04198-2. [DOI] [PubMed] [Google Scholar]
- 22.Fulcher KY, White PD. Randomised controlled trial of graded exercise in patients with the chronic fatigue syndrome. BMJ. 1997;314:1647–1652. doi: 10.1136/bmj.314.7095.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss-Morris R, Sharon C, Tobin R, Baldi JC. A randomized controlled graded exercise trial for chronic fatigue syndrome: outcomes and mechanisms of change. J Health Psychol. 2005;10:245–259. doi: 10.1177/1359105305049774. [DOI] [PubMed] [Google Scholar]
- 24.Cox DL, Araoz G. The experience of therapy supervision within a UK multi-centre randomized controlled trial. Learn Health Soc Care. 2009;8:301–314. [Google Scholar]
- 25.Guy W. ECDEU assessment manual for psychopharmacology. National Institute of Mental Health; Rockville, MD: 1976. pp. 218–222. [Google Scholar]
- 26.Mundt JC, Marks IM, Shear K, Griest JH. The work and social adjustment scale: a simple measure of impairment in functioning. Br J Psychiatry. 2002;180:461–464. doi: 10.1192/bjp.180.5.461. [DOI] [PubMed] [Google Scholar]
- 27.Butland RJA, Pang J, Gross ER, Woodcock AA, Geddes DM. Two, six, and 12 minute walking test in respiratory disease. BMJ. 1982;284:1607–1608. doi: 10.1136/bmj.284.6329.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkins CD, Stanton B, Niemcryk S, Rose R. A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol. 1988;41:313–321. doi: 10.1016/0895-4356(88)90138-2. [DOI] [PubMed] [Google Scholar]
- 29.Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;87:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 30.Senn S, Julious S. Measurement in clinical trials: a neglected issue for statisticians? Stats Med. 2009;28:3189–3209. doi: 10.1002/sim.3603. [DOI] [PubMed] [Google Scholar]
- 31.Guyatt GH, Osaba D, Wu AW. Methods to explain the clinical significance of health status measures. Mayo Clinic Proceedings. 2002;77:371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 32.Cella M, Chalder T. Measuring fatigue in clinical and community settings. J Psychosom Res. 2010;69:17–22. doi: 10.1016/j.jpsychores.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Bowling A, Bond M, Jenkinson C, Lamping DL. Short form 36 (SF-36) health survey questionnaire: which normative data should be used? Comparisons between the norms provided by the Omnibus Survey in Britain, The Health Survey for England and the Oxford Healthy Life Survey. J Publ Health Med. 1999;21:255–270. doi: 10.1093/pubmed/21.3.255. [DOI] [PubMed] [Google Scholar]
- 34.Wearden AJ, Dowrick C, Chew-Graham C. Fatigue Intervention by Nurses Evaluation (FINE) trial writing group and the FINE trial group. Nurse led, home based self help treatment for patients in primary care with chronic fatigue syndrome: randomised controlled trial. BMJ. 2010;340:c1777. doi: 10.1136/bmj.c1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jason LA, Torres-Harding S, Friedberg F. Non-pharmacologic interventions for CFS: a randomized trial. J Clin Psychol Med Settings. 2007;14:275–296. [Google Scholar]
- 36.Wearden AJ, Morriss RK, Mullis R. Randomised, double-blind, placebo-controlled treatment trial of fluoxetine and graded exercise for chronic fatigue syndrome. Br J Psychiatry. 1998;172:485–490. doi: 10.1192/bjp.172.6.485. [DOI] [PubMed] [Google Scholar]
- 37.Heins MJ, Knoop H, Prins JB, Stulemeijer M, van der Meer JWM, Bleijenberg G. Possible detrimental effects of cognitive behaviour therapy for chronic fatigue syndrome. Psychother Psychosom. 2010;79:249–256. doi: 10.1159/000315130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.