Abstract
Filarial parasites are known to induce a large range of immunoregulatory mechanisms, including the induction of alternatively activated macrophages and regulatory T cells. These mechanisms are used to evade and down‐modulate the host’s immune system, to support parasite survival. Several reports have focused on some of these mechanisms, in humans and murine models, but the complex immunoregulatory networks associated with filarial infections remain unclear. Recent publications have conferred a role for regulatory T cells in the ability of helminth parasites to modulate human immune responses, such cells promoting the induction of the non‐complement‐fixing immunoglobulin G4 (IgG4). High plasma concentrations of IgG4 have been reported in hypo‐responsive and asymptomatic cases of helminth infection. In both human lymphatic filariasis and onchocerciasis, the asymptomatic infections are characterised by high plasma concentrations of IgG4 (compared with those of IgE) and of the complement‐fixing antibodies IgG1, IgG2 and IgG3. In asymptomatic filarial infection, elevations in IgG4 are also often associated with high worm loads and with high plasma levels of the immunomodulatory interleukin‐10. Here, various aspects of the induction of IgG4 in humans and it roles in the immunomodulation of the human responses to filarial parasites are reviewed.
Infections with filarial nematodes remain a major public‐health problem, especially in tropical countries (Kazura and Bockarie, 2003; Gbakima et al., 2005; Ramaiah et al., 2005; Njepuome et al., 2009). According to recent estimations (Molyneux, 2003; WHO, 2004), >200 million people are infected with the parasites that cause lymphatic filariasis (Wuchereria bancrofti, Brugia malayi and B. timori), onchocerciasis (Onchocerca volvulus) or loiasis (Loa loa). These infections are often chronic and persist for many years. In most infected individuals, however, few, if any, symptoms develop, the relatively asymptomatic existence of filarial parasites in the host being the result of the ability of filarial nematodes to down‐modulate the host’s immune responses, in order to facilitate their own survival. Unfortunately, some infected individuals do develop serious immunopathology. The immunological and genetical factors influencing the direction that any filarial infection takes, towards immunopathology or to tolerance, are still not fully understood.
Induction of the non‐cytolytic and blocking immunoglobulin G4 (IgG4) is believed to represent one major mechanism used by filarial parasites to evade destruction by their host’s immune system. The immunoregulatory properties of IgG4 have been recently demonstrated (Van der Neut Kolfschoten et al., 2007). This immunoglobulin seems to have markedly different properties to those of other immunoglobulins of the G subclass. Although the blocking effects of IgG4 in allergic diseases are well known (Taylor et al., 2004; Akdis et al., 2005, 2006), the function of this immunoglobulin in filarial infections is not well documented. Some aspects of the immunoregulatory role of IgG4 in the pathology of three major filarial infections (lymphatic filariasis, onchocerciasis and loiasis) are reviewed below.
DISEASE AND THE SUBCLASSES OF IMMUNOGLOBULIN G
The four subclasses of IgG differ in both their physical and biological properties. It is, for example, well known that IgG1 antibody responses are prominent in humans with microbial infection. Although such responses are usually driven by Th1 cells, IgG1 is not specific to Th1. In fact, IgG1 responses more often correlate with IgE expression, which is mainly related to Th2. Unlike the situation in mice models, where clear Th1/Th2 polarizations can be observed, both Th1 and Th2 cytokines have been shown to be able to drive IgG1 in humans (Mosmann and Coffman, 1989; Kotowicz and Callard, 1993; Fujieda et al., 1995; Fear et al., 2004). In various experimental models, helminth antigens and allergens have been shown to induce IgG4 and IgE, both in vitro and in vivo (Lobos et al., 2003), whereas other parasite antigens, such as those of malarial parasites, induce IgG1 and IgG3 (Garraud et al., 1995, 2002, 2003a). These differences are often controlled by the cytokines that the antigens induce. Although naïve B cells produce IgG4 and IgE in the presence of interleukin‐4 (IL‐4) and interleukin‐13 (IL‐13), they produce IgG2 in the presence of interferon‐γ (IFN‐γ) and interleukin‐12 (IL‐12; Garraud et al., 2003b). In mice, interleukin‐21 (IL‐21) appears to have a critical role in delineating between the production of IgG1, the likely equivalent of human IgG4, and IgE (Ozaki et al., 2002; Aalberse et al., 2009). Similarly, IL‐21 has been implicated in the preferential secretion of IgG4 by human leucocytes (unfractionated peripheral‐blood mononuclear cells) that have been stimulated with phytohaemagglutinin (Wood et al., 2004). These findings, together with the fact that IL‐21 can be secreted by Th17 cells, indicate a possible role for Th17 cells in the regulation of the IgG subclasses (Wei et al., 2007; Jang et al., 2009; Liu et al., 2009). The possible modulation of B‐cell responses by Th17 cytokines remains to be studied. Interleukin‐10 (IL‐10), tumour growth factor‐β (TGF‐β) and other immunoregulatory cytokines are also often implicated in IgG4 production in humans (Satoguina et al., 2002; see Table).
Table.
Immunoglobulins and their corresponding stimuli and T‐cell responses
| Immunoglobulin | Cytokines | Antigens | Corresponding T‐cell response |
|---|---|---|---|
| IgG1 | (Interferon‐γ, interleukin‐12) | Bacteria, viruses | Th1 and Th2 |
| IgG2 | Interferon‐γ, interleukin‐12 | Th2 and Th1 | |
| IgG3 | – | – | Th2 and Th1 |
| IgG4 | Interleukin‐4, ‐10, ‐13 and ‐21 | Large parasites, allergens | Th2, regulatory T cells |
| IgE | Interleukin‐4 and ‐13 | Large parasites, allergens | Th2 |
| IgA | – | – | |
| IgM | – | – |
IMMUNOREGULATORY PROPERTIES OF IMMUNOGLOBULIN G4
IgG represents one of the most important classes of immunoglobulin, molecules of this class being secreted relatively late in an immune response, after the B cells have gone through affinity maturation, allowing a greater affinity of antibodies during persistent antigen exposure. IgG molecules are also secreted by memory B cells during secondary immune responses. Although all IgG molecules have the same overall structure, there are many points of difference between IgG4 and the other members of the IgG class (Burton et al., 1986; Simpson et al., 1990; Yoshida et al., 2006; Petrie and Zúñiga‐Pflücker, 2007; Wang et al., 2008) and these differences are not only structural but also functional (Burton et al., 1986; Simpson et al., 1990). While IgG1, IgG2 and IgG3 are able to fix and activate complement, IgG4 has no affinity for complement and is therefore unable to activate any of the protective immune mechanisms that involve complement. Furthermore, in contrast to IgG1 and IgG3, IgG4 cannot induce antibody‐dependent cell‐mediated cytotoxicity (ADCC) after binding to the FcγRIII molecule, CD16, found on the surface of neutrophils and eosinophils (Dafa’alla et al., 1992). IgG4 is also known to compete with IgE for the antibody fixation sites on mast cells and eosinophils (Zola et al., 1978; Aalberse et al., 2009). While IgE induces such cells to degranulate, IgG4 has no or few downstream effects and can even inhibit complement activation by other antibodies, as demonstrated in a phospholipase‐A model (Van der Zee et al., 1986; Aalberse et al., 2009). Another property of IgG4 was described only a few years ago. A day after injecting nude mice with blood‐derived or recombinant IgG4 antibodies against the major birch‐pollen antigen (Betv1) and with similar antibodies against a cat allergen (Feld1), Van der Neut Kolfschoten et al. (2007) demonstrated the development of IgG4 molecules with double specificity (i.e. to both Betv1 and Feld1). This observation indicated that an IgG4 molecule is a dynamic molecule that can swap a heavy chain and attached light chain (half‐molecule) for a heavy–light chain‐pair from another molecule (Van der Neut Kolfschoten et al., 2007; Aalberse et al., 2009). Such an exchange of Fab arms is probably responsible for some of the immunoregulatory properties of IgG4 seen in a rhesus‐monkey model of auto‐immune myasthenia gravis — a disease in which auto‐antibodies against an acetylcholine receptor (AChR) induce muscle weakness (Losen et al., 2005). By injecting rhesus monkeys with a human AChR‐specific antibody (IgG1‐637 — a patient‐derived antibody that induces AChR degradation), auto‐immune myasthenia gravis can be induced (Graus et al., 1997). Monkeys co‐injected with IgG4‐637, however, remain healthy, indicating a protective role for IgG4 in at least one inflammatory disease (Losen et al., 2005). A dynamic arm‐exchange mechanism might play a crucial role in the protective role of IgG4 again lymphoedema in lymphatic filariasis and allergic diseases (Hussain and Ottesen, 1986).
CORRELATION OF IGG4 LEVELS WITH THE ABSENCE OF PATHOLOGY IN HUMAN LYMPHATIC FILARIASIS
Human lymphatic filariasis, also known as elephantiasis, is caused by three species of filarial parasites: W. bancrofti, B. malayi and B. timori. While most people who are infected with one of these parasites remain asymptomatic, some develop visible manifestations such as lymphoedema of the limbs and/or the swelling of the scrotum and penis known as hydrocele (Pfarr et al., 2009). Infection with any of these nematodes is associated with a strong polarization of the host’s immune response towards Th2, with prominent secretion of IL‐4 and IL‐5 and elevated levels of IgG4 and IgE (Hussain et al., 1981; Ottesen et al., 1985; Hussain and Ottesen, 1986; Mahanty et al., 1993). Ottesen et al. (1985), for example, observed an extreme elevation of both total and, particularly, filarial‐antigen‐specific IgG4 in humans infected with W. bancrofti. Such elevations in IgG4 indicate the importance of this immunoglobulin in the human immune response to filarial infection (Noordin et al., 2003; Jiraamonnimit et al., 2009); most (50%–95%) of the antifilarial IgG in cases of W. bancrofti filariasis belonging to this subclass (Ottesen et al., 1985). Several experimental observations indicate that it is the parasites’ microfilariae that are the main inducers of IgG4. Shiny et al. (2009) recently reported a 54‐year‐old man from Haiti who, although initially microfilaraemic (with 100 microfilariae/ml blood) and seropositive for antifilarial IgG4, became amicrofilaraemic (and persistently seronegative for antifilarial IgG4) after treatment with diethylcarbamazine but developed lymphoedema in one leg approximately 1 year later. In this patient, titres of antifilarial IgG1 began to increase after the development of the lymphoedema, peaking after a further 2 years (Shiny et al., 2009). Similar observations were made by Kurniawan et al. (1993) while comparing antibody secretion in individuals resident in an area of Indonesia that was endemic for lymphatic filariasis caused by B. malayi. In this investigation, antibody levels to antigens extracted from adult B. malayi were determined for each of the IgG subclasses as well as for IgM and for IgE. The predominant isotype of antifilarial antibody was found to be IgG4, which, in asymptomatic microfilaraemics, represented 88% of the total IgG. Interestingly, the patients in this Indonesian study who had chronic disease (elephantiasis) were generally amicrofilaraemic and had substantially higher levels of IgG1, IgG2 and IgG3 but, on average, 3.4‐fold lower levels of specific IgG4 than the asymptomatic microfilaraemics. Kurniawan et al. (1993) also found that, in contrast to the trends in IgG4, the levels of antifilarial IgE were, on average, 4.5‐fold higher in the cases of elephantiasis than in the asymptomatic carriers. Taken together, these observations clearly demonstrated a protective role for IgG4 that seems to inhibit the development of elephantiasis. In their investigations of sera from patients with W. bancrofti filariasis, Hussain et al. (1992) found that, among the four IgG subclasses, only the levels of IgG4 correlated with the blocking activity observed in histamine‐release assays. In addition, the blocking activity detected in the sera showing high levels of histamine inhibition could be abolished by the selective depletion of the IgG4 in such sera, using anti‐IgG4 affinity columns (Hussain et al., 1992).
ROLE OF IGG4 IN THE PATHOLOGY OF HUMAN ONCHOCERCIASIS
The causative agent of human onchocerciasis is the filarial nematode O. volvulus. The adult parasites reside mainly in subcutaneous nodules (Brattig, 2004). The adult females produce thousands of microfilariae that migrate in the skin and to other tissues, causing various pathological manifestations (Korten et al., 2010a). Migration of the microfilariae into the eyes can cause blindness, the most severe manifestation of the disease. Most infected individuals are hypo‐responsive (in the generalized form of the disease) and present with high loads of microfilariae and adult worms. In contrast, a small number of infected individuals present with low parasitaemia but severe and chronic dermatitis (in the hyper‐reactive form of the disease, sometimes known as sowda). Untreated cases of sowda are usually able to eliminate their microfilaraemias by mounting a strong, local and systemic Th2 immune response (Brattig et al., 2002; Hoerauf and Brattig, 2002; Brattig, 2004) but this strong immune reaction is often associated with severe immunopathology. The humoral response in such cases is dominated by the production of both antigen‐specific and total IgE, IgG1 and IgG3. High IgG4 production is, however, the hallmark of the other, hypo‐reactive cases.
More IgG4‐positive plasma cells are detectable in onchocercomas from the hypo‐reactive cases of onchocerciasis than in those from hyper‐reactive patients (Brattig et al., 2010; Korten et al., 2010a). The results of a study by Dafa’alla et al. (1992) illustrated the prominence of IgG4 in human onchocerciasis and the role of such immunoglobulin in the survival of microfilariae. Dafa’alla et al. (1992) compared the IgG responses to O. gutturosa and O. volvulus and concluded that IgG4 secretion, in response to O. volvulus or O. gutturosa, was considerably higher than IgG1, IgG2 or IgG3 secretion (the ELISA used giving mean ‘corrected’ optical densities of 0.84, 0.27, 0.24 and 0.28, respectively). They also demonstrated that IgG4 levels were positively correlated with microfilaraemia, whereas IgG3 levels showed a negative association with the microfilarial load. The high levels of IgG4 antibody in individuals with high microfilarial loads indicate a role for this isotype in inhibiting microfilarial clearance. Dafa’alla et al. (1992) suggested that IgG4 competed with other antibodies, probably IgE and IgG3, for adherence to the microfilariae and thereby inhibited the ADCC known to be involved in microfilarial destruction (Subrahmanyam et al., 1978; Haque et al., 1981).
IGG4 IN HUMAN LOIASIS
Loiasis is a skin and eye disease caused by Loa loa filarial worms. The adult worms produce microfilariae that can be found in blood and other body fluids and in the lung (Agbolade and Akinboye, 2001; Padgett and Jacobsen, 2008). The main clinical sign is the ‘Calabar swelling’, which is oedema in the subcutaneous tissue caused by maturing larvae migrating away from the site where they were injected by a feeding vector fly. Migration of the worms through the eye causes severe eye pain, inflammation and sometimes blindness (Boussinesq, 2006). In Central and West Africa, individuals with high loads of L. loa microfilariae are at risk of developing serious neurological reactions after treatment with the diethylcarbamazine or ivermectin used in mass treatments for the elimination of onchocerciasis (Pion et al., 2005). Loiasis differs from the other filarial diseases of humans in that most infected subjects are without circulating microfilariae (i.e. they have ‘occult’ loiasis). Most cases of loiasis are also asymptomatic (Agbolade and Akinboye, 2001; Padgett and Jacobsen, 2008).
In a study in two villages in south–eastern Gabon (one with high‐intensity transmission of L. loa and one with low‐intensity transmission), Akue et al. (2002) found that plasma concentrations of microfilaria‐ and adult‐worm‐specific IgG4 were significantly higher in the microfilaraemics than in the amicrofilaraemic villagers. In contrast, levels of IgG1 specific to the third‐stage larvae or microfilariae of L. loa were significantly higher in the amicrofilaraemic subjects than in the microfilaraemic. These observations indicate that L. loa microfilariae are at least partially responsible for the preferential production of IgG4 in human loiasis. The absence of microfilariae is often associated with the production of the more immunocompetent immunoglobulins IgG1 and IgE, which often appear associated with the development of immunopathology. Curiously, in an earlier study in Gabon by the same research group, similarly high levels of IgG4 expression were found in subjects with and without L. loa microfilaraemias (Akue et al., 1994) and it seems possible that transmission intensity has a major effect on the antibody responses to L. loa infection (Akue et al., 1994, 2002).
In conclusion, it is likely that, in general, the presence of L. loa microfilariae actively down‐regulates IgG1 levels while inducing IgG4, changes which, in turn, promote the survival of the microfilariae and adult worms.
CELLULAR MECHANISMS OF PREFERENTIAL IGG4 INDUCTION IN FILARIASIS
The mechanisms used by filarial parasites to suppress a host’s immune responses are diverse and multiform. Although the preferential induction of IgG4 is one important arm of this immunoregulatory network, the mechanisms that lead to IgG4 production are still not fully characterised. It is known that microfilariae can induce two immunoregulatory cytokines (TGF‐β and IL‐10) as well as IL‐10‐producing and CD4(+)CD25(+)FOXP3(+) regulatory T cells (Taylor et al., 2009; Korten et al., 2010b; Metenou et al., 2010). Since regulatory T cells and IL‐10 are known to promote the production of IgG4 by B cells (Satoguina et al., 2005, 2008), microfilariae may well be directly responsible for IgG4 production. The induction of IgG4 by regulatory T cells has also been observed in allergy models. Meiler et al. (2008), for example, demonstrated that circulating CD4(+)CD25(+)FOXP3(+)regulatory T cells and allergen‐specific, IL‐10‐secreting, type‐1 regulatory T cells from healthy individuals were able to induce IgG4, while suppressing IgE production in peripheral‐blood mononuclear cells and purified B‐cell cultures.
In their recent study, Satoguina et al. (2008) showed that both soluble factors and cell‐contact‐dependent mechanisms are involved in the induction of IgG4 by regulatory T cells. By using clones of human IL‐10‐producing, antigen‐specific regulatory T cells, in co‐culture with autologous B cells, it was demonstrated that the blocking of GITR (glucocorticoid‐induced tumour‐necrosis‐factor receptor‐related protein) or GITR ligand (GITR‐L) selectively prevented IgG4 production, as did the inhibition of IL‐10 or TGF‐β. In addition, the prevention of IgG4 induction with an anti‐GITR antibody could be reversed using an excess of recombinant IL‐10 but not by using recombinant TGF‐β (Satoguina et al., 2008). These observations indicated that IL‐10 plays the terminal role in a cascade of signals, including GITR, GITR‐L and TGF‐β, that leads to the production of IgG4.
It may be the IgG4/IgE or IgG4/IgG ratio that is one of the main criteria determining whether a filarial infection heads toward immunoregulation (and an asymptomatic state) or immunopathology. Hence, IL‐10 from microfilariae‐induced regulatory T cells might, in the context of an immunological synapse, induce B cells to secrete IgG4 preferentially, promoting parasite tolerance. Toll‐like receptors that are known to be expressed on regulatory T cells (Nyirenda et al., 2009; Urry et al., 2009) might be able to modulate the IgG4 induction.
CONCLUDING REMARKS
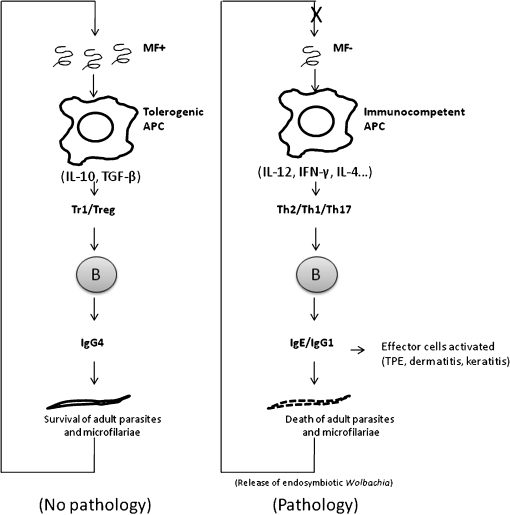
Filarial parasites are responsible for peripheral‐T‐cell tolerance, at least partially through the induction of IL‐10‐producing regulatory T cells and the recruitment and expansion of naturally occurring regulatory T cells. Natural CD4(+)CD25(+)FOXP3(+) regulatory T cells and antigen‐induced, IL‐10‐producing, type‐1 regulatory T cells modulate the host’s immune responses by enhancing the production of non‐cytolytic IgG4 antibodies. The IgG4 molecules are capable of inhibiting IgE‐ and IgG‐mediated effector mechanisms. This humoral regulation makes an important contribution to the avoidance of pathology (e.g. filarial lymphoedema, onchocercal dermatitis, keratitis and/or tropical pulmonary eosinophilia). In the absence of immunoregulation, the host’s immunocompetent antigen‐presenting cells activate effector T‐cells, which, in turn, induce B cells to produce cytolytic antibodies (IgG1, IgG2, IgG3 and IgE). These antibodies, through different effector mechanisms (e.g. complement activation and ADCC), induce parasite death and the subsequent release of antigens from endosymbobiotic Wolbachia bacteria. These bacterial antigens contribute to the induction of a strong immune reaction and, subsequently, to the development of pathology (see Figure). A better understanding of the genetic and immunological factors that induce the immunoregulatory mechanisms seen in human filariasis would surely contribute to the design of more efficient and safe therapies against filarial infections.
FIG.
Simplified view of the induction and regulatory properties of IgG4 in human filariasis. Adult filarial parasites produce microfilariae (MF) that are responsible for the recruitment and induction of Foxp3(+) and interleukin‐10‐producing regulatory T cells (Treg), probably by the manipulation of antigen‐presenting cells (APC). Natural CD4(+)CD25(+)FOXP3(+) Treg and antigen‐induced, interleukin‐10‐producing, regulatory cells of type 1 (Tr1) interact with B cells and enhance the production of non‐cytolytic IgG4 while inhibiting the induction of other IgG and IgE. This humoral regulation contributes to the avoidance of pathology [e.g. filarial lymphoedema, onchocercal dermatitis, keratitis and tropical pulmonary eosinophilia (TPE)]. In the absence of immunoregulation, immunocompetent APC activate effector T‐cells (Th) which, in turn, induce B cells to produce cytolytic IgG1, IgG2, IgG3 and IgE. These antibodies induce various effector mechanisms (such as complement activation and antibody‐dependent cell‐mediated cytotoxicity), provoking parasite death and the release of antigens from endosymbobiotic Wolbachia. These bacterial antigens contribute to the induction of a strong immune reaction and to the subsequent development of pathology. IL‐10, Interleukin‐10; TGF‐β, tumour growth factor‐β; IL‐12, interleukin‐12; IFN‐γ, interferon‐γ; IL‐4, interleukin‐4.
Acknowledgments
The authors are grateful to the German Research Foundation (DFG) for its financial support (via grant Ho2009/8–1).
REFERENCES
- 1.Aalberse R. C., Stapel S. O., Schuurman J., Rispens T. Immunoglobulin G4: an odd antibody. Clinical and Experimental Allergy. 2009;39:469–477. doi: 10.1111/j.1365-2222.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 2.Agbolade M., Akinboye D. O. Loa loa and Mansonella perstans infections in Ijebu north, western Nigeria: a parasitological study. Japanese Journal of Infectious Diseases. 2001;54:108–110. [PubMed] [Google Scholar]
- 3.Akdis M., Blaser K., Akdis C. A. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. Journal of Allergy and Clinical Immunology. 2005;116:961–969. doi: 10.1016/j.jaci.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Akdis M., Blaser K., Akdis C. A. T regulatory cells in allergy. Chemical Immunology and Allergy. 2006;91:159–173. doi: 10.1159/000090279. [DOI] [PubMed] [Google Scholar]
- 5.Akue J. P., Egwang T. G., Devaney E. High levels of parasite‐specific IgG4 in the absence of microfilaremia in Loa loa infection. Tropical Medicine and Parasitology. 1994;45:248246. [PubMed] [Google Scholar]
- 6.Akue J. P., Devaney E., Wahl G., Moukana H. Expression of filarial‐specific IgG subclasses under different transmission intensities in a region endemic for loiasis. American Journal of Tropical Medicine and Hygiene. 2002;66:245–250. doi: 10.4269/ajtmh.2002.66.245. [DOI] [PubMed] [Google Scholar]
- 7.Boussinesq M. Loiasis. Annals of Tropical Medicine and Parasitology. 2006;100:715–731. doi: 10.1179/136485906X112194. [DOI] [PubMed] [Google Scholar]
- 8.Brattig N. W. Pathogenesis and host responses in human onchocerciasis: impact of Onchocerca filariae and Wolbachia endobacteria. Microbes and Infection. 2004;6:113–128. doi: 10.1016/j.micinf.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Brattig N. W., Lepping B., Timmann C., Büttner D. W., Marfo Y., Hamelmann C., Horstmann R. D. Onchocerca volvulus‐exposed persons fail to produce interferon‐γ in response to O. volvulus antigen but mount proliferative responses with interleukin‐5 and IL‐13 production that decrease with increasing microfilarial density. Journal of Infectious Diseases. 2002;185:1148–1154. doi: 10.1086/339820. [DOI] [PubMed] [Google Scholar]
- 10.Brattig N. W., Tenner‐Racz K., Korten S., Hoerauf A., Büttner D. W. Immunohistology of ectopic secondary lymph follicles in subcutaneous nodules from patients with hyperreactive onchocerciasis (sowda) Parasitology Research. 2010 doi: 10.1007/s00436-010-1912-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton D. R., Gregory L., Jefferis R. Aspects of the molecular structure of IgG subclasses. Monographs in Allergy. 1986;19:7–35. [PubMed] [Google Scholar]
- 12.Dafa’alla T. H., Ghalib H. W., Abdelmageed A., Williams J. F. The profile of IgG and IgG subclasses of onchocerciasis patients. Clinical and Experimental Immunology. 1992;88:258–263. doi: 10.1111/j.1365-2249.1992.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fear D. J., McCloskey N., O’Connor B., Felsenfeld G., Gould H. J. Transcription of Ig germline genes in single human B cells and the role of cytokines in isotype determination. Journal of Immunology. 2004;173:4529–4538. doi: 10.4049/jimmunol.173.7.4529. [DOI] [PubMed] [Google Scholar]
- 14.Fujieda S., Zhang K., Saxon A. IL‐4 plus CD40 monoclonal antibody induces human B cells γ subclass‐specific isotype switch: switching to γ1, γ3, and γ4, but not γ2. Journal of Immunology. 1995;155:2318–2328. [PubMed] [Google Scholar]
- 15.Garraud O., Nkenfou C., Bradley J. E., Perler F. B., Nutman T. B. Identification of recombinant filarial proteins capable of inducing polyclonal and antigen‐specific IgE and IgG4 antibodies. Journal of Immunology. 1995;155:1316–1325. [PubMed] [Google Scholar]
- 16.Garraud O., Perraut R., Diouf A., Nambei W. S., Tall A., Spiegel A., Longacre S., Kaslow D. C., Jouin H., Mattei D., Engler G. M., Nutman T. B., Riley E. M., Mercereau‐Puijalon O. Regulation of antigen‐specific immunoglobulin G subclasses in response to conserved and polymorphic Plasmodium falciparum antigens in an in vitro model. Infection and Immunity. 2002;70:2820–2827. doi: 10.1128/IAI.70.6.2820-2827.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garraud O., Mahanty S., Perraut R. Malaria‐specific antibody subclasses in immune individuals: a key source of information for vaccine design. Trends in Immunology. 2003a;24:30–35. doi: 10.1016/s1471-4906(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 18.Garraud O., Perraut R., Riveau G., Nutman T. B. Class and subclass selection in parasite‐specific antibody responses. Trends in Parasitology. 2003b;19:300–304. doi: 10.1016/s1471-4922(03)00139-9. [DOI] [PubMed] [Google Scholar]
- 19.Gbakima A. A., Appawu M. A., Dadzie S., Karikari C., Sackey S. O., Baffoe‐Wilmot A., Gyapong J., Scott A. L. Lymphatic filariasis in Ghana: establishing the potential for an urban cycle of transmission. Tropical Medicine and International Health. 2005;10:387–392. doi: 10.1111/j.1365-3156.2005.01389.x. [DOI] [PubMed] [Google Scholar]
- 20.Graus Y. F., de Baets M. H., Parren P. W., Berrih‐Aknin S., Wokke J., van Breda Vriesman P. J., Burton D. R. Human anti‐nicotinic acetylcholine receptor recombinant Fab fragments isolated from thymus‐derived phage display libraries from myasthenia gravis patients reflect predominant specificities in serum and block the action of pathogenic serum antibodies. Journal of Immunology. 1997;158:1919–1929. [PubMed] [Google Scholar]
- 21.Haque A., Ouaissi A., Joseph M., Capron M., Capron A. IgE antibody in eosinophil‐ and macrophage‐mediated in vitro killing of Dipetalonema viteae microfilariae. Journal of Immunology. 1981;127:716–725. [PubMed] [Google Scholar]
- 22.Hoerauf A., Brattig N. Resistance and susceptibility in human onchocerciasis — beyond Th1 vs. Th2. Trends in Parasitology. 2002;18:25–31. doi: 10.1016/s1471-4922(01)02173-0. [DOI] [PubMed] [Google Scholar]
- 23.Hussain R., Ottesen E. A. IgE responses in human filariasis. IV. Parallel antigen recognition by IgE and IgG4 subclass antibodies. Journal of Immunology. 1986;136:1859–1863. [PubMed] [Google Scholar]
- 24.Hussain R., Hamilton R. G., Kumaraswami V., Adkinson Jr N. F., Ottesen E. A. IgE responses in human filariasis. I. Quantitation of filaria‐specific IgE. Journal of Immunology. 1981;127:1623–1629. [PubMed] [Google Scholar]
- 25.Hussain R., Poindexter R. W., Ottesen E. A. Control of allergic reactivity in human filariasis. Predominant localization of blocking antibody to the IgG4 subclass. Journal of Immunology. 1992;148:2731–2737. [PubMed] [Google Scholar]
- 26.Jang E., Cho S. H., Park H., Paik D. J., Kim J. M., Youn J. A positive feedback loop of IL‐21 signaling provoked by homeostatic CD4+CD25‐ T cell expansion is essential for the development of arthritis in autoimmune K/BxN mice. Journal of Immunology. 2009;182:4649–4656. doi: 10.4049/jimmunol.0804350. [DOI] [PubMed] [Google Scholar]
- 27.Jiraamonnimit C., Wongkamchai S., Boitano J., Nochot H., Loymek S., Chujun S., Yodmek S. A cohort study on anti‐filarial IgG4 and its assessment in good and uncertain MDA‐compliant subjects in brugian filariasis endemic areas in southern Thailand. Journal of Helminthology. 2009;83:351–360. doi: 10.1017/S0022149X09352669. [DOI] [PubMed] [Google Scholar]
- 28.Kazura J. W., Bockarie M. J. Lymphatic filariasis in Papua New Guinea: interdisciplinary research on a national health problem. Trends in Parasitology. 2003;19:260–263. doi: 10.1016/s1471-4922(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 29.Korten S., Hoerauf A., Kaifi J. T., Büttner D. W. Low levels of transforming growth factor‐beta (TGF‐beta) and reduced suppression of Th2‐mediated inflammation in hyperreactive human onchocerciasis. Parasitology. 2010a doi: 10.1017/S0031182010000922. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korten S., Kaifi J. T., Büttner D. W., Hoerauf A. Transforming growth factor‐beta expression by host cells is elicited locally by the filarial nematode Onchocerca volvulus in hyporeactive patients independently from Wolbachia. Microbes and Infection. 2010b;12:555–564. doi: 10.1016/j.micinf.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Kotowicz K., Callard R. E. Human immunoglobulin class and IgG subclass regulation: dual action of interleukin‐4. European Journal of Immunology. 1993;23:2250–2256. doi: 10.1002/eji.1830230930. [DOI] [PubMed] [Google Scholar]
- 32.Kurniawan A., Yazdanbakhsh M., van Ree R., Aalberse R., Selkirk M. E., Partono F., Maizels R. M. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. Journal of Immunology. 1993;150:3941–3950. [PubMed] [Google Scholar]
- 33.Liu Z., Yang L., Cui Y., Wang X., Guo C., Huang Z., Kan Q., Liu Z., Liu Y. Il‐21 enhances NK cell activation and cytolytic activity and induces Th17 cell differentiation in inflammatory bowel disease. Inflammatory Bowel Disease. 2009;15:1133–1144. doi: 10.1002/ibd.20923. [DOI] [PubMed] [Google Scholar]
- 34.Lobos E., Nutman T. B., Hothersall J. S., Moncada S. Elevated immunoglobulin E against recombinant Brugia malayi γ‐glutamyl transpeptidase in patients with bancroftian filariasis: association with tropical pulmonary eosinophilia or putative immunity. Infection and Immunity. 2003;71:747–753. doi: 10.1128/IAI.71.2.747-753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Losen M., Stassen M. H. W., Martínez‐Martínez P., Machiels B. M., Duimel H., Frederik P., Veldman H., Wokke J. H. J., Spaans F., Vincent A., de Baets M. H. Increased expression of rapsyn in muscles prevents acetylcholine receptor loss in experimental autoimmune myasthenia gravis. Brain. 2005;128:2327–233. doi: 10.1093/brain/awh612. [DOI] [PubMed] [Google Scholar]
- 36.Mahanty S., King C. L., Kumaraswami V., Regunathan J., Maya A., Jayaraman K., Abrams J. S., Ottesen E. A., Nutman T. B. IL‐4‐ and IL‐5‐secreting lymphocyte populations are preferentially stimulated by parasite‐derived antigens in human tissue invasive nematode infections. Journal of Immunology. 1993;151:3704–3711. [PubMed] [Google Scholar]
- 37.Meiler F., Klunker S., Zimmermann M., Akdis C. A., Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll‐like receptors. Allergy. 2008;63:1455–1463. doi: 10.1111/j.1398-9995.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 38.Metenou S., Dembele B., Konate S., Dolo H., Coulibaly S. Y., Coulibaly Y. I., Diallo A. A., Soumaoro L., Coulibaly M. E., Sanogo D., Doumbia S. S., Traoré S. F., Mahanty S., Klion A., Nutman T. B. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. Journal of Immunology. 2010;184:5375–5382. doi: 10.4049/jimmunol.0904067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molyneux D. Lymphatic filariasis (elephantiasis) elimination: a public health success and development opportunity. Filaria Journal. 2003;2:13.. doi: 10.1186/1475-2883-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Advances in Immunology. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 41.Njepuome N. A., Hopkins D. R., Richards Jr F. O., Anagbogu I. N., Pearce P. O., Jibril M. M., Okoronkwo C., Sofola O. T., Withers Jr P. C., Ruiz‐Tiben E., Miri E. S., Eigege A., Emukah E. C., Nwobi B. C., Jiya J. Y. Nigeria’s war on terror: fighting dracunculiasis, onchocerciasis, lymphatic filariasis, and schistosomiasis at the grassroots. American Journal of Tropical Medicine and Hygiene. 2009;80:691–698. [PubMed] [Google Scholar]
- 42.Noordin R., Shenoy R. K., Rahman R. A. Comparison of two IgG4 assay formats (ELISA and rapid dipstick test) for detection of brugian filariasis. Southeast Asian Journal of Tropical Medicine and Public Health. 2003;34:768–770. [PubMed] [Google Scholar]
- 43.Nyirenda M. H., O’Brien K., Sanvito L., Constantinescu C. S., Gran B. Modulation of regulatory T cells in health and disease: role of toll‐like receptors. Inflammation and Allergy Drug Targets. 2009;8:124–129. doi: 10.2174/187152809788462581. [DOI] [PubMed] [Google Scholar]
- 44.Ottesen E. A., Skvaril F., Tripathy S. P., Poindexter R. W., Hussain R. Prominence of IgG4 in the IgG antibody response to human filariasis. Journal of Immunology. 1985;134:2707–2712. [PubMed] [Google Scholar]
- 45.Ozaki K., Spolski R., Feng C. G., Qi C. F., Cheng J., Sher A., Morse III H. C., Liu C., Schwartzberg P. L., Leonard W. J. A critical role for IL‐21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 46.Padgett J. J., Jacobsen K. H. Loiasis: African eye worm. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102:983–989. doi: 10.1016/j.trstmh.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Petrie H. T., Zúñiga‐Pflücker J. C. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annual Review of Immunology. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 48.Pfarr K. M., Debrah A. Y., Specht S., Hoerauf A. Filariasis and lymphoedema. Parasite Immunology. 2009;31:664–672. doi: 10.1111/j.1365-3024.2009.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pion S. D., Demanou M., Oudin B., Boussinesq M. Loiasis: the individual factors associated with the presence of microfilaraemia. Annals of Tropical Medicine and Parasitology. 2005;99:491–500. doi: 10.1179/136485905X51300. [DOI] [PubMed] [Google Scholar]
- 50.Ramaiah K. D., Vijay Kumar K. N., Ravi R., Das P. K. Situation analysis in a large urban area of India, prior to launching a programme of mass drug administrations to eliminate lymphatic filariasis. Annals of Tropical Medicine and Parasitology. 2005;99:243–252. doi: 10.1179/136485905X29701. [DOI] [PubMed] [Google Scholar]
- 51.Satoguina J., Mempel M., Larbi J., Badusche M., Loliger C., Adjei O., Gachelin G., Fleischer B., Hoerauf A. Antigen‐specific T regulatory‐1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis) Microbes and Infection. 2002;4:1291–1300. doi: 10.1016/s1286-4579(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 52.Satoguina J. S., Weyand E., Larbi J., Hoerauf A. T regulatory‐1 cells induce IgG4 production by B cells: role of IL‐10. Journal of Immunology. 2005;174:4718–4726. doi: 10.4049/jimmunol.174.8.4718. [DOI] [PubMed] [Google Scholar]
- 53.Satoguina J. S., Adjobimey T., Arndts K., Hoch J., Oldenburg J., Layland L. E., Hoerauf A. Tr1 and naturally occurring regulatory T cells induce IgG4 in B cells through GITR/GITR‐L interaction, IL‐10 and TGF‐β. European Journal of Immunology. 2008;38:3101–3113. doi: 10.1002/eji.200838193. [DOI] [PubMed] [Google Scholar]
- 54.Shiny C., Krushna N. S., Archana B., Farzana B., Narayanan R. B. Serum antibody responses to Wolbachia surface protein in patients with human lymphatic filariasis. Microbiology and Immunology. 2009;53:685–693. doi: 10.1111/j.1348-0421.2009.00172.x. [DOI] [PubMed] [Google Scholar]
- 55.Simpson L. L., Lake P., Kozaki S. Isolation and characterization of a novel human monoclonal antibody that neutralizes tetanus toxin. Journal of Pharmacology and Experimental Therapeutics. 1990;254:98–103. [PubMed] [Google Scholar]
- 56.Subrahmanyam D., Mehta K., Nelson D. S., Rao Y. V., Rao C. K. Immune reactions in human filariasis. Journal of Clinical Microbiology. 1978;8:228–232. doi: 10.1128/jcm.8.2.228-232.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor A., Verhagen J., Akdis C. A., Akdis M. T regulatory cells in allergy and health: a question of allergen specificity and balance. International Archives of Allergy and Immunology. 2004;135:73–82. doi: 10.1159/000080523. [DOI] [PubMed] [Google Scholar]
- 58.Taylor M. D., van der Werf N., Harris A., Graham A. L., Bain O., Allen J. E., Maizels R. M. Early recruitment of natural CD4+ Foxp3+ Treg cells by infective larvae determines the outcome of filarial infection. European Journal of Immunology. 2009;39:192–206. doi: 10.1002/eji.200838727. [DOI] [PubMed] [Google Scholar]
- 59.Urry Z., Xystrakis E., Richards D. F., McDonald J., Sattar Z., Cousins D. J., Corrigan C. J., Hickman E., Brown Z., Hawrylowicz C. M. Ligation of TLR9 induced on human IL‐10‐secreting Tregs by 1α,25‐dihydroxyvitamin D3 abrogates regulatory function. Journal of Clinical Investigation. 2009;119:387–398. doi: 10.1172/JCI32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van der Neut Kolfschoten M., Schuurman J., Losen M., Bleeker W. K., Martínez‐Martínez P., Vermeulen E., den Bleker T. H., Wiegman L., Vink T., Aarden L. A., de Baets M. H., van de Winkel J. G., Aalberse R. C., Parren P. W. Anti‐inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 61.Van der Zee J. S., van Swieten P., Aalberse R. C. Inhibition of complement activation by IgG4 antibodies. Clinical and Experimental Immunology. 1986;64:415–422. [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S. Y., Racila E., Taylor R. P., Weiner G. J. NK‐cell activation and antibody‐dependent cellular cytotoxicity induced by rituximab‐coated target cells is inhibited by the C3b component of complement. Blood. 2008;111:1456–1463. doi: 10.1182/blood-2007-02-074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei L., Laurence A., Elias K. M., O’Shea J. J. IL‐21 is produced by Th17 cells and drives IL‐17 production in a STAT3‐dependent manner. Journal of Biological Chemistry. 2007;282:34,605–34,610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wood N., Bourque K., Donaldson D. D., Collins M., Vercelli D., Goldman S. J., Kasaian M. T. IL‐21 effects on human IgE production in response to IL‐4 or IL‐13. Cellular Immunology. 2004;231:133–145. doi: 10.1016/j.cellimm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 65.World Health Organization Report on the mid‐term assessment of microfilaraemia reduction in sentinel sites of 13 countries of the Global Programme to Eliminate Lymphatic Filariasis. Weekly Epidemiological Record. 2004;79:358–365. [PubMed] [Google Scholar]
- 66.Yoshida M., Masuda A., Kuo T. T., Kobayashi K., Claypool S. M., Takagawa T., Kutsumi H., Azuma T., Lencer W. I., Blumberg R. S. IgG transport across mucosal barriers by neonatal Fc receptor for IgG and mucosal immunity. Springer Seminars in Immunopathology. 2006;28:397–403. doi: 10.1007/s00281-006-0054-z. [DOI] [PubMed] [Google Scholar]
- 67.Zola H., Garland L. G., Cox H. C., Adcock J. J. Separation of IgE from IgG subclasses using staphylococcal protein A. International Archives of Allergy and Immunology. 1978;56:123–127. doi: 10.1159/000232014. [DOI] [PubMed] [Google Scholar]