Abstract
Idiopathic or functional abdominal pain (FAP) is common in school-age children and typically reflects a functional gastrointestinal disorder (FGID). FGIDs in adults have been distinguished by enhanced responses of the central nervous system to pain stimuli, known as central sensitization, This study investigated whether adolescents and young adults with a history of pediatric FAP (n = 144), compared with well control subjects (n = 78), showed enhanced central sensitization demonstrated by greater temporal summation (wind-up) to brief, repetitive heat pulses. We also assessed the role of gender and trait anxiety in wind-up to heat pain. Women with a history of FAP showed greater wind-up to heat pain than men with a history of FAP (P < .05) and well control subjects of both genders (P < .05). Results were similar for FAP participants whose abdominal pain was ongoing at follow-up and those whose pain had resolved. Although anxiety was significantly higher in the FAP group compared with control subjects (P < .01) and in women compared with men (P < .05), anxiety did not explain the increased wind-up observed in women with a childhood history of FAP. Results suggest that women with a pediatric history of FAP may have a long-term vulnerability to pain associated with enhanced central nervous system responses to pain stimuli.
Keywords: Chronic pain, Pediatrics, Irritable bowel syndrome, Central sensitization, Wind-up
1. Introduction
Idiopathic or functional abdominal pain (FAP) is common in school-age children [2,6,16,20] and typically reflects a functional gastrointestinal disorder (FGID) such as irritable bowel syndrome, functional dyspepsia, or abdominal migraine [3]. FGIDs are among several chronic pain disorders distinguished by enhanced responses of the central nervous system to pain stimuli or central sensitization [28,33].
One putative neurobiological mechanism of central sensitization is temporal summation of nociceptive signals, often referred to as wind-up [10,17,24]. Noxious stimuli activate nociceptors that transmit pain information to the spinal cord via Aδ and C-fibers. With repetitive stimulation, C-fibers may produce long-lasting excitatory postsynaptic potentials that summate to increase activity of dorsal horn neurons. This can result in changes at the synapse affecting how pain information is processed [10,32] and may be associated with the initiation or maintenance of chronic pain [18,32,33]. Changes in the somatosensory system associated with central sensitization may persist even after the triggering injury or inflammation resolves [32]. Thus, it is possible that chronic or recurrent pain in childhood would be reflected in central sensitization in adulthood, even if the original pediatric pain condition has resolved. No studies to date, however, have prospectively investigated the relation of pediatric pain to central sensitization in adulthood.
The purpose of this study was to investigate thermal wind-up in patients with a history of pediatric FAP. Patients were adolescents and adults who had previously presented to a pediatric gastroenterology clinic for evaluation of chronic or recurrent abdominal pain and whose evaluation revealed no underlying explanatory disease. The current long-term follow-up investigation occurred 4 to 15 years after patients’ initial evaluation. A well comparison group was recruited at the same time as FAP patients and also was followed up prospectivety. We hypothesized that wind-up to heat pain stimuli would be greater in former pediatric FAP patients compared with well control subjects. Additionally, we predicted that FAP patients with an ongoing course of abdominal pain, defined by the presence of an FGID at follow-up, would demonstrate even greater wind-up than former FAP patients whose abdominal pain was resolved at follow-up.
Several studies indicate that women display greater acute pain sensitivity and elevated temporal summation to pain stimuli compared with men [14,22]. Therefore, we hypothesized that women, particularly those with a history of FAP, would show greater thermal wind-up than men. Given evidence that trait anxiety may contribute to gender differences in temporal summation [15,22], we also assessed this construct to rule out potential confounding effects on wind-up findings.
2. Methods
2.1. Sample
FAP participants were recruited from a database of former patients who had presented to the Vanderbilt Pediatric Gastroenterology Clinic for evaluation of abdominal pain when they were 8 to 16 years old and enrolled in one of several studies conducted by Walker et al. between 1993 and 2004 [29–31]. A total of 367 participants whose medical evaluation (reviewed by a gastroenterologist associated with the study) yielded no evidence of organic disease were eligible for the current study. Forty-eight patients declined to participate, 3 were excluded because they had received a diagnosis of inflammatory bowel disease in the intervening years, and 175 participants completed a telephone survey but did not participate in the current laboratory study that required a visit to the medical center. Thus, 144 participants constituted the FAP sample. These participants were recruited on average 9.1 years (±3.62 years) after their pediatric FAP evaluation. The FAP sample had a mean age of 20.5 years (±3.8 years) at the time of the current study, was 55.0% female, and was primarily Caucasian (91.0%). Participants who completed the telephone survey only were slightly older (mean = 22.5, SD = 4.53 years) than those who completed the current laboratory study (t = −3.16, P < .01), but did not differ significantly regarding gender.
Well control subjects of similar age and gender were recruited from a database of healthy school children without abdominal pain who participated in the original studies conducted by Walker et al. Of 161 eligible control participants invited to participate in the ongoing follow-up study, 4 declined participation, 4 were excluded because they met criteria for a functional gastrointestinal disorder at follow-up, and 75 completed the telephone survey but did not participate in the current laboratory study. Thus, 78 participants constituted the well control sample. These participants were recruited for the current study on average 7.0 years (±1.96 years} after participating in the original studies. The well control sample had a mean age of 17.6 years (±2.64) at the time of the current study, was 52.6% female, and was primarily Caucasian (96.2%). Participants who completed the telephone survey only were slightly older (mean = 19.8, SD = 3.70 years) than those who completed the laboratory study (t = −3.16, P < .01), but did not differ regarding gender. Well control participants were somewhat younger than FAP participants (t (220} = −6.05, P < .01), but did not differ on any other demographic variables.
2.2. Procedure
The Vanderbilt University Institutional Review Board approved all procedures. After providing informed consent, participants were interviewed over the telephone about their physical and emotional health. Subjects then participated in a laboratory study at the Vanderbilt University Pediatric Clinical Research Center that involved a psychophysiological assessment protocol designed to evaluate pain responsiveness. Before testing, participants were asked to refrain from taking any analgesic medications for the 24-hour period preceding the laboratory visit, and at the time of testing, participants were surveyed regarding any recent medication use.
2.3. Thermal pain task
2.3.1. Pain threshold and tolerance
Subjects underwent quantitative sensory testing with a computer-controlled Medoc Thermal NeuroSensory Analyzer {TSA-II, Medoc, Inc, Ramat, Israel). This device applied heat stimuli to the nondominant ventral forearm using a 30 × 30-mm Peltier thermistor probe as reported in prior studies [4,8,13]. Two separate series of trials were used to determine heat pain threshold and heat pain tolerance using an ascending method of limits [7], Practice trials using the Medoc equipment were conducted before the actual procedure to familiarize the participants with the task instructions and equipment. After initial thermal task training, participants underwent a cognitive stressor (serial subtraction task) before proceeding with the thermal pain task. This sequence of tasks was designed to enhance physiological arousal before the pain task to maximize any group differences in endogenous pain modulatory systems [5]. A series of 4 pain threshold trials was conducted, followed by a series of 4 pain tolerance trials, with the probe applied to a slightly different target, site on the arm for each trial to avoid local sensitization. Means of the last 3 trials in each series were computed for data analysis [13]. For pain threshold trials, the probe started at an adaptation temperature of 32°C, with the temperature increasing at a ramp rate of 0.5°C/s until the participant indicated via mouse click that their pain threshold had been reached. For each tolerance trial, the probe started at an adaptation temperature of 40°C, with the temperature increasing at a ramp rate of 0.5°C/s until the participant terminated the trial via mouse click to indicate maximum pain tolerance had been reached. For both thermal pain threshold and tolerance trials, the interstimulus interval was 25 seconds.
2.3.2. Temporal summation (wind-up)
Wind-up assessment trials with the TSA-II unit were conducted using commercially available software (TPS-CoVAS version 3.19, Medoc Inc) that administered a standardized oscillating thermal stimulation protocol designed specifically to assess C-fiber mediated temporal summation. This protocol is based on that which has been used to assess wind-up in several previous studies [8,13,14], During wind-up assessment, 2 sequences of 10 heat pulses each were applied to the ventral forearm, with the thermode in a fixed position throughout each sequence. The thermode was moved to a different nonoverlapping site on the same arm for the subsequent sequence of trials with a 1-minute interval between sequences. Within each pulse sequence, 10 successive pulses (0.5-second duration) were administered from a baseline temperature of 40°C at a frequency of 0.5 Hz, a frequency known to elicit C-fiber mediated wind-up in the dorsal horn [10]. Two sequences using 2 different stimulus intensities (47°C and 48°C) were used to maximize the likelihood of producing measurable results [13]. At the peak of every heat pulse within each sequence, subjects were asked to provide a verbal numeric pain intensity rating using a 0 to 100 scale with which they had previously been familiarized (anchored with 0 = no pain and 100 = worst possible pain). Participants were instructed that the procedure would be stopped if they reported a score of 100 or if they expressed a desire to stop before all 10 heat pulses were administered. The slopes of the lines fitted to the series of 10 pain ratings (or less if termination occurred early) at each stimulus intensity {47°C and 48°C) were used as indices of wind-up, as in our prior work [8], To derive these measures for use in primary analyses, within-subject regressions were conducted using the series of 10 pain ratings during each wind-up trial regressed on a dummy coded variable with a fixed interval of 1 (range = 1 to 10). This produced a slope (b) for each individual reflecting the degree of wind-up. These slopes were used as the dependent variables for wind-up in primary analyses described below. For a participant’s data to be included in analysis of wind-up slopes, a minimum of 3 wind-up pain ratings at a given target temperature were required. The number of participants who failed to meet this criterion (eg, who terminated trials early by reporting a score of 100) or who otherwise requested to stop wind-up testing before the 10th trial was quantified for analysis.
2.4. Study questionnaires
2.4.1. Rome III Diagnostic Questionnaire for functional gastrointestinal disorders
The Rome III Questionnaire [11] was developed by the Rome Foundation Board based on the Rome III criteria for functional gastrointestinal disorders. This study administered only the 24 questionnaire items that assess symptom criteria for functional gastrointestinal disorders (FGIDs) associated with abdominal pain: irritable bowel syndrome, functional dyspepsia, abdominal migraine, functional abdominal pain, and functional abdominal pain syndrome. Participants’ responses were scored to determine whether they currently met symptom criteria for each of the FGIDs at follow-up. If a participant met criteria for an FGID at follow-up, the outcome of their FAP was considered to be ongoing. Failure to meet criteria for an FGID at follow-up indicated a “resolved FAP” outcome for participants.
2.4.2. Spielberger State Trait Anxiety Inventory-Trait Form (STAI-T)
The STAI-T is a widely used standardized measure of the tendency to experience anxiety symptoms [23]. Higher total scores on this measure were indicative of greater tendency to experience anxiety.
2.5. Data analysis
Two-by-two analyses of covariance (ANCOVAs) were used to test the impact of FAP history (FAP versus well) and gender on the following dependent variables: 47°C and 48°C wind-up scores, pain threshold, pain tolerance, and anxiety score. ANCOVA was also used to determine whether wind-up scores differed by FAP outcome (resolved FAP versus ongoing FAP) and gender. Age was entered as the covariate in all of these analyses. A-final set of ANCOVAs was used to control for effects of anxiety while testing the effects of FAP history and gender on wind-up scores. One-way analyses of variance (ANOVAs) and paired t-tests were used for analyses of simple effects as well as planned and post-hoc comparisons.
3. Results
3.1. Temporal summation of heat pain
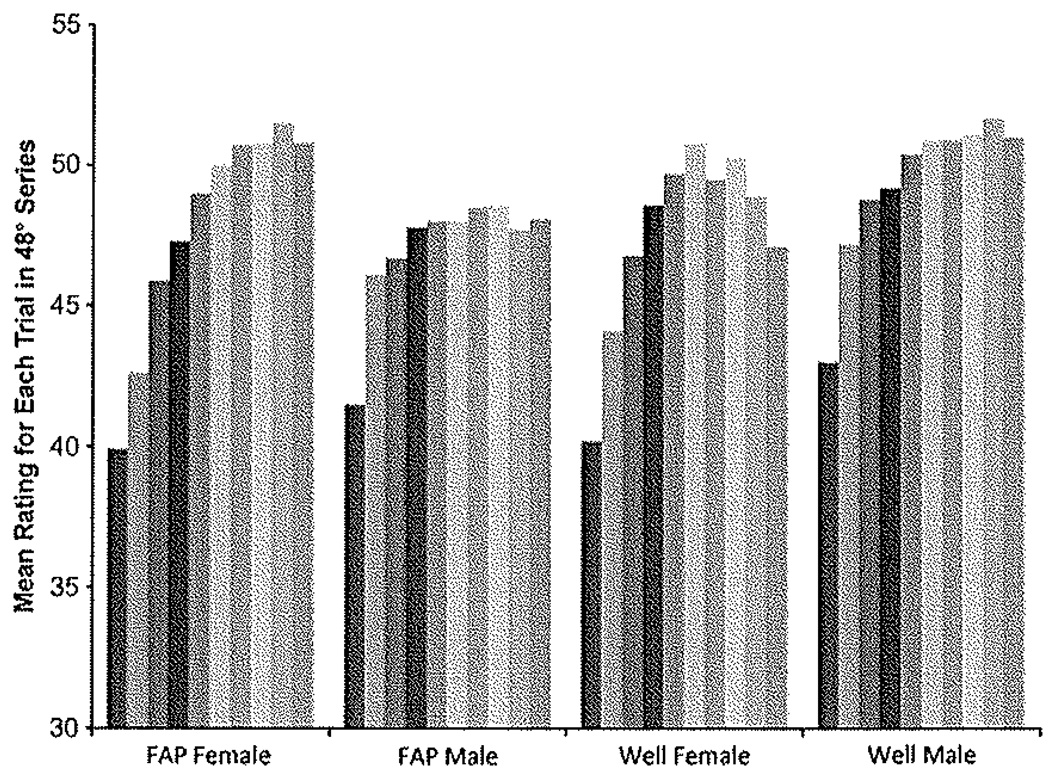
No main effects were shown for FAP history or gender on wind-up scores, but a significant gender-by-FAP history interaction was indicated (47°C wind-up: F1,222 = 6.20, P < .05; 48°C wind-up: F1,222 = 4.35, P < .05), Planned comparisons of the interaction effect by FAP condition showed that female participants in the FAP group demonstrated greater wind-up than male participants in the FAP group (47°C: F1,143 = 7.70, P < .05, Cohen’s d = 0,47; 48°C: F1,142 = 11.29, P < .01, Cohen’s d = 0.57). There were no differences in wind-up between men and women in the well control group. Summary data from the 48°C condition are presented in Fig. 1. Post hoc comparisons of the interaction effect by gender showed that women with FAP had greater wind-up scores than well women (47°C: F1,113 = 4.73, P < .05, Cohen’s d = 0.45: 48°C: F1,113 = 7.14, P < .01, Cohen’s d = 0.56). Well men showed higher wind-up scores than male FAP participants in the 47°C stimulus series, but this effect was not significant in the 48°C series (47°C: F1,101 = 7.17, P < .05, Cohen’s d = 0.53; 48°C: F1,101 = 1.61, NS). Mean pain ratings by group for each of the 10 stimulus trials in the 48°C condition are depicted in Fig.2.
Fig. 1.
Women with a history of pediatric functional abdominal pain (FAP) demonstrate greater temporal summation (wind-up) to heat stimuli than men in the FAP group and female/male well control subjects, No other groups differed. Data summarized are from the 48°C temperature series. The Y axis depicts mean wind-up slope for each group. Error bars show standard error of the mean. Asterisks indicate significant differences between groups at the level of P < .05.
Fig. 2.
Mean pain ratings for each of the 10 trials (depicted by the gray bars) in the 48°C temperature series. Data are presented for men and women in both the functional abdominal pain (FAP) and well groups of the study. The Y axis begins at 30 because all average pain ratings exceeded this value.
3.1.1. FAP outcome at follow-up
Of the l44 participants with a history of FAP, 54(37.5%) met Rome III criteria for a current FGID at follow-up and were considered to have ongoing FAP. Participants with ongoing FAP did not differ significantly in degree of wind-up from those with resolved FAP (47°C wind-up: ongoing FAP: mean = 0.792. SD = 1.76; resolved FAP: mean = 0.738, SD = 1.27; 48°C wind-up: ongoing FAP: mean = 1.11, SD = 2.04; resolved FAP: mean = 1.01, SD = 1.37). Neither of the FAP outcome groups differed from well control subjects in this analysis. There were no significant main effects or interactions for gender and/or FAP outcome on wind-up. The ongoing FAP group was 63% female; the resolved FAP group was 50% female.
3.1.2. Effects of stimulus temperature
A paired samples t-test indicated that women in the FAP group demonstrated a significant increase in wind-up score between the 47°C and 48°C heat stimuli conditions (t (79) = −3.59, P < .01). No other groups showed increases in wind-up score as the temperature of the heat stimuli increased (Fig. 3). However, all groups showed a significant difference between the first pain rating in the 47°C condition and the first pain rating in the 48°C condition (data shown in Fig. 4, statistics reported in Table 1). This suggests that all groups of participants were capable of perceiving a difference in temperature/intensity between the 2 series of wind-up stimuli. Within each series of heat stimuli, there were no differences between any groups in the ratings given to the first stimulus of the series, indicating no inherent perceptual differences between the groups (also shown in Fig. 4).
Fig. 3.
Women with a history of pediatric functional abdominal pain (FAP) demonstrated a significant increase in temporal summation (wind-up) between the 47°C and 48°C stimulus conditions. No other groups demonstrated this increase in wind-up based on stimulus temperature intensity. The Y axis depicts the mean wind-up slope for each group and temperature series. Error bars represent standard error of the mean. Asterisk indicates significant difference between groups at level of P < .01.
Fig. 4.
The first pain rating given for each thermal stimulus series (47°C and 48°C) significantly increased with temperature for each group in the study. This indicates that all groups were capable of discerning temperature differences between the 2 sets of heat stimuli. When comparing the groups within each temperature series, there were no differences in the first pain ratings reported. The Y axis depicts group means for first pain ratings on a scale of 0 (no pain) to 100 (worst possible pain). Error bars represent standard error of the mean. Asterisks indicate significant differences between groups at the level of P < .05.
Table 1.
Statistics for paired t-tests comparing first rating between 47°C and 48°C wind-up conditions.
| t | df | P | |
|---|---|---|---|
| FAP | |||
| Female | −4.94 | 78 | .000 |
| Male | −4.67 | 64 | .000 |
| Well | |||
| Female | −3.44 | 40 | .001 |
| Male | −3.19 | 36 | .003 |
FAP, functional abdominal pain.
3.1.3. Early termination of trials
Few participants asked to terminate wind-up trials prematurely or gave a rating of 100 before all 10 trials in each series were complete. During the 47°C series of heat stimuli, only 2 FAP and 2 well subjects terminated the sequence early, in the 48°C series, 4 FAP (3%) and 3 well (5%) subjects terminated early. The differences between these groups were not statistically significant.
3.1.4. Evidence of temporal summation
Demonstration of temporal summation was defined by the presence of positive wind-up slopes >0.1. The majority of participants in both the FAP and the well groups demonstrated positive wind-up scores in response to heat stimuli. In the 47°C heat stimulation condition, 65% of well subjects and 64% of FAP participants demonstrated wind-up. In the 48°C condition, 69% of well and 67% of FAP patients showed evidence of wind-up. Thus, both temperature series used in this study were capable of evoking at least some wind-up in most participants, although the degree of wind-up was significantly greater in FAP than Well Participants.
3.2. Heat pain threshold and tolerance
ANCOVAs indicated significant main effects for gender on both pain threshold (F1,222 = 4.81, P < .05) and tolerance (F1,222 = 38.43, P < .01), and these analyses indicated that women had lower values than men for both measures (Table 2). There were no significant main effects for FAP history or gender by FAP history interactions on threshold or tolerance.
Table 2.
Mean thermal pain threshold, pain tolerance, and STAI-T score by gender and FAP history.
| Threshold | Tolerance | STAI-T score | |
|---|---|---|---|
| Gender | |||
| Female | 41.35 ± 3.34 | 46.20 ± 1.73 | 36.52 ± 9.45 |
| Male | 42.33 ± 3.23 | 47.83 ± 2.08 | 33.90 ± 8.75 |
| P level | <.05 | <.01 | <.05 |
| Effect size | 0.25 | 0.85 | 0.29 |
| FAP history | |||
| FAP | 42.04 ± 3.20 | 47.03 ± 2.09 | 37.30 ± 8.75 |
| Well | 41.35 ± 3.51 | 46.81 ± 2.03 | 31.48 ± 8.93 |
| P level | NS | NS | <.01 |
| Effect size | - | - | 0.67 |
Values reported are means ± standard deviations. Effect size is reported as Cohen’s d.
FAP, functional abdominal pain: STAI-T, Spielberger State Trait Anxiety Inventory-Trait Form.
3.3. Anxiety
Significant main effects for both FAP history (F1,219 = 13.95, P < .01) and gender (F1,219 = 4.42, P < .05) were demonstrated on STAI-T scores (Table 2). Former pediatric FAP patients scored significantly higher on the STAI-T than well control subjects (F1,218 = 21.58, P < .01). In addition, women had higher anxiety scores than men (F1,218 = 4.46, P < .05). There was no interaction between FAP history and gender on STAI score. To determine whether anxiety contributed to the FAP history by gender interaction on wind-up scores, we re-ran this analysis with STAI-T score as a covariate. The STAI-T was not a significant covariate, and a significant FAP history by gender interaction was still obtained even after controlling for anxiety (47°C: F1,219 = 5.21, P < .05; 48°C: F1,219 = 4.86, P < .05). Thus, observed group and gender differences in anxiety did not account for the pattern of wind-up findings in primary analysis.
4. Discussion
Results of this study suggest that young women with a history of pediatric functional abdominal pain may have long-term changes in how the central nervous system processes nociceptive information. Two mechanisms have been offered to explain the role of the central nervous system in the initiation arid maintenance of chronic pain. First, an initial injury may cause long-term changes to pain responsiveness throughout the body, leaving an individual exceptionally sensitive to mildly noxious or even non-painful stimuli [1,32]. Often, these enhanced responses to pain (central sensitization) outlast the initial source of noxious stimulation and may be an explanation for the persistence of chronic pain in the absence of pathological findings [28]. Thus, even though no injury or disease was identified in the childhood medical evaluations of FAP patients in this study, it is possible that unidentified pathophysiological mechanisms associated with FAP resulted in long-term alterations in the central processing of noxious stimuli.
A second possibility is that individuals who develop chronic pain have an innate or early predisposition toward enhanced pain responsiveness [25,26]. Thus, it is possible that inherent differences existed before the development of FAP in childhood and even made these patients more susceptible to FAP. Along these lines, studies suggest that women in general exhibit enhanced pain responsiveness relative to men [14,15,22] and exhibit greater prevalence of chronic pain conditions such as migraines, irritable bowel syndrome, and fibromyalgia [26,33,34].
Sex hormones have been associated with sensitivity to pain in women [21], and there is direct evidence that they can affect neural mechanisms underlying pain. For example, animal models have shown that estradiol will increase activity of NMDA receptors [27] which are an integral component in the temporal summation of pain [10,32]. Therefore, it is entirely plausible that reproductive hormones could mediate gender-related differences in pain processing. However, this remains to be directly tested.
Although inherent biological differences between the sexes may seem like an obvious explanation for women’s enhanced pain responses, some research has indicated that greater trait anxiety in women may account for gender differences in pain sensitivity [15,22]. Generally, anxiety has been associated with greater responses to laboratory-induced acute pain [9], and in some studies, controlling for trait anxiety eliminates gender differences in pain perception [15], In the current study, women endorsed more trait anxiety on the STAI-T than men, and did exhibit a significantly lower mean heat pain threshold and tolerance than men. However, anxiety did not account for the increased temporal summation shown by women with a pediatric history of FAP.
As reported in other studies [13–15,24], wind-up was observed in the healthy control participants, although we did not observe gender differences in this group. Reasons for the absence of gender differences in wind-up in the well control sample in the current study are not entirely clear. Given the greater prevalence of many chronic pain conditions in women, it is possible that results of some healthy population studies showing greater wind-up in women might have been influenced by undiagnosed or subclinical pain complaints in a subset of female participants. In the current study, the well sample was screened for abdominal pain and individuals who reported such conditions were not eligible for our study. Therefore, our well group may have been more free of pain complaints than some samples in previous studies.
The current study is unique in taking a prospective look at the relation of pediatric abdominal pain to long-term changes in how the central nervous system responds to painful stimuli. Importantly, our results suggest that biological mechanisms such as central sensitization may characterize functional abdominal pain—a condition that lacks significant organic pathology. Biological indicators of increased pain susceptibility in functional pain syndromes are important for 2 reasons. First, they help explain the presence of significant pain complaints among individuals in whom previous medical explanations have been elusive. However, this interpretation must be made cautiously because direct measurements of central nervous system responses to pain were not made in the current study—an important point addressed in the study limitations section later. Second, these indicators may identify populations that are at risk for the persistence of pain symptoms throughout their lifetime, allowing clinicians to target treatments and interventions to these groups. Our finding that temporal summation to heat pain is enhanced in women with a history of FAP suggests that there may be clinical similarities (enhanced central sensitization) between FAP and other chronic pain disorders (eg. fibromyalgia) [28,33].
A limitation of the current study that could be addressed in future prospective studies is that we did not determine Whether central sensitization was evident in children at the time they first presented to the gastrointestinal clinic with abdominal pain. Measuring pain psychophysics at the initial evaluation period of this study would have allowed us to investigate whether differences in temporal summation occurred early in the patients’ pain history. If differences in wind-up were not apparent at initial evaluation, this would suggest that chronicity of the pain experience might be a contributing factor to pronociceptive changes in the central nervous system, as opposed to the theory that biological or genetic predispositions may account for central sensitization.
Another limitation of this study is that the serial subtraction stressor experienced before the thermal pain task could have potentially exaggerated wind-up differences between groups, or in contrast, might have limited group differences by evoking stress-related increases in wind-up in both groups. We do not believe that either of these are the case, however, because (1) we were able to detect a very specific interaction between gender and FAP history, and (2) we did not see much maximal responding indicative of ceiling effects that would mask the detection of differences between groups.
An additional limitation of the current study is that data from temporal summation procedures in human subjects can only be used to make inferences about what is occurring in the central nervous system—direct measurement of wind-up at the neuronal level was not attempted [17]. Also, it is possible that the enhanced pain responsiveness we observed was caused by central nervous system—mediated processes other than central sensitization. For example, reduced descending pain modulation also can enhance pain responses in patients with chronic pain [4,12,19,25,33].
Our findings raise questions regarding whether the role of central sensitization in FAP is different in male than in female subjects. Whether this is the case remains to be determined in future work. Regardless, central sensitization is likely to be only one among multiple mechanisms contributing to the disorder. This study provides evidence that young women with a childhood history of functional abdominal pain may have a long-term vulnerability to pain that is associated with enhanced responses of the central nervous system to pain stimuli. Identifying subgroups of patients that are particularly vulnerable may enable clinicians to target these groups for early interventions that may improve outcomes in these patients. Also, understanding how pain history can alter nociceptive processing in individuals with supposed functional chronic pain disorders can help validate the legitimacy of these complaints for both patients and their doctors, and facilitate enhanced coping with the long-term consequences of functional pain.
Acknowledgements
The authors thank Sara Rippel, M.D., for reviewing medical evaluations of participants for study eligibility. This research was supported by Award Number R01 HD23264 (L. S. Walker) from the National Institute on Child Health and Development and does not necessarily represent the official views of the National Institute on Child Health and Development or the National Institutes of Health. Support also was provided by National Institute of Neurological Disorders and Stroke Award Number R01 NS046694 (S. Bruehl), National Institute of Mental Health Award T32MH075883 through the National Institutes of Health Roadmap for Medical Research (C.M. Dengler-Crish), the Vanderbilt Kennedy Center (P30 HD15052), the Vanderbilt Digestive Disease Research Center (DK058404), and the Vanderbilt General Clinical Research Center (M01 RR-00095).
Footnotes
The authors have no conflicts of interest to disclose
References
- 1.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 2.Apley J, Hale B. Children with recurrent abdominal pain: how do they grow up? Br Med J. 1973;3:7–9. doi: 10.1136/bmj.3.5870.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baber KF, Anderson J, Puzanovova M, Walker LS. Rome II versus Rome III classification of functional gastrointestinal disorders in pediatric chronic abdominal pain. J Pediatr Gastroenterol Nutr. 2008;47:299–302. doi: 10.1097/MPG.0b013e31816c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruehl S, Chung OY, Diedrich L, Diedrich A, Robertson D. The relationship between resting blood pressure and acute pain sensitivity: effects of chronic pain and alpha-2 adrenergic blockade. J Behavior Med. 2008;31:71–80. doi: 10.1007/s10865-007-9133-4. [DOI] [PubMed] [Google Scholar]
- 5.Burns JW, Bruehl S, Chung OY, Magid E, Chont M, Goodlad JK, Gilliam W, Matsuura J, Somar K. Endogenous opioids may buffer effects of anger arousal on sensitivity to subsequent pain. Pain. 2009;146:276–282. doi: 10.1016/j.pain.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chitkara DK, Rawat DJ, Talley NJ. The epidemiology of childhood recurrent abdominal pain in Western countries: a systematic review. Am J Gastroenterol. 2005;100:1868–1875. doi: 10.1111/j.1572-0241.2005.41893.x. [DOI] [PubMed] [Google Scholar]
- 7.Chong PS, Cros DP. Technology literature review; quantitative sensory testing. Muscle Nerve. 2004;29:734–747. doi: 10.1002/mus.20053. [DOI] [PubMed] [Google Scholar]
- 8.Chung OY, Bruehl S, Diedrich L, Diedrich A. The impact of blood pressure and baroreflex sensitivity on wind-up. Anesth Analg. 2008;107:1018–1025. doi: 10.1213/ane.0b013e31817f8dfe. [DOI] [PubMed] [Google Scholar]
- 9.Cornwall A, Donderi DC. The effect of experimentally induced anxiety on the experience of pressure pain. Pain. 1988;35:105–113. doi: 10.1016/0304-3959(88)90282-5. [DOI] [PubMed] [Google Scholar]
- 10.Davies SN, Lodge D. Evidence for involvement of N-methylaspartate receptors in ’wind-up’ of class 2 neurones in the dorsal horn of the rat. Brain Rese. 1987;424:402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- 11.Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE, editors. Rome III: the functional gastrointestinal disorders. 3rd ed. McLean, VA: 2006. [Google Scholar]
- 12.Filatova E, Latysheva N, Kurenkov A. Evidence of persistent central sensitization in chronic headaches: a multi-method study. J Headache Pain. 2008;9:295–300. doi: 10.1007/s10194-008-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fillingim RB, Edwards RR. Is self-reported childhood abuse history associated with pain perception among healthy young women and men? Clin J Pain. 2005;21:387–397. doi: 10.1097/01.ajp.0000149801.46864.39. [DOI] [PubMed] [Google Scholar]
- 14.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 15.Jones A, Zachariae R, Arendt-Nielsen L. Dispositional anxiety and the experience of pain: gender-specific effects. Eur J Pain. 2003;7:387–395. doi: 10.1016/S1090-3801(02)00139-8. [DOI] [PubMed] [Google Scholar]
- 16.Konijnenberg AY, de Graeff-Meeder ER, van der Hoeven J, Kimpen JL, Buitelaar JK, Ulterwaal CS. Psychiatric morbidity in children with medically-unexplained chronic pain: diagnosis from the pediatrician’s perspective. Pediatrics. 2006;117:889–897. doi: 10.1542/peds.2005-0109. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen J, Arendt-Nielsen L. The importance of stimulus configuration for temporal summation of first and second pain to repeated heat stimuli. Eur J Pain. 1998;2:329–341. doi: 10.1016/s1090-3801(98)90031-3. [DOI] [PubMed] [Google Scholar]
- 18.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 19.Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain. 2009;144:16–19. doi: 10.1016/j.pain.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley JL, III, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–235. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- 22.Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain. 2004;5:77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 24.Staud R, Bovee CE, Robinson ME, Price DD. Cutaneous C-fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain. 2008;139:315–323. doi: 10.1016/j.pain.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8:893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 27.Tang B, Ji Y, Traub RJ. Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain. 2008;137:540–549. doi: 10.1016/j.pain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- 29.Walker LS, Baber KF, Garber J, Smith CA. A typology of pain coping strategies in pediatric patients with chronic abdominal pain. Pain. 2008;137:266–275. doi: 10.1016/j.pain.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker LS, Smith CA, Garber J, Claar RL. Testing a model of pain appraisal and copingin children with chronic abdominal pain. Health Psychol. 2005;24:364–374. doi: 10.1037/0278-6133.24.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker LS, Smith CA, Garber J, Van Slyke DA, Claar RL. The relation of daily stressors to somatic and emotional symptoms in children with recurrent abdominal pain. J Consult Clin Psychol. 2001;69:85–91. [PMC free article] [PubMed] [Google Scholar]
- 32.Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci USA. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yunus M. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Zohsel K, Hohmeister J, Oelkers-Ax R, Flor H, Hermann C. Quantitative sensory testing in children with migraine: preliminary evidence for enhanced sensitivity to painful stimuli especially in girls. Pain. 2006;123:10–18. doi: 10.1016/j.pain.2005.12.015. [DOI] [PubMed] [Google Scholar]