Abstract
Background
Cytoplasmic fragments derived from fragile neoplastic lymphocytes are common in samples of lymph nodes collected from dogs with lymphoma. These cytoplasmic fragments interfere with accurate gating of target cells and quantification protocols used for flow cytometry because of their variable size and expression of lymphoid cell surface antigens on their membranes.
Objective
The aim of this study was to develop a method to efficiently exclude cytoplasmic fragments from flow cytometric analysis of canine lymph nodes in which lymphoma was present.
Methods
Single-cell suspensions of neoplastic cells were prepared from biopsy samples and fine-needle aspirates of lymph nodes from 23 dogs with lymphoma. Suspensions were stained using a violet laser-excitable (405 nm) membrane-permeable DNA-binding fluorescent dye (DyeCycle Violet, DCV), incubated with antibodies against CD3, CD5, CD21, CD22, and CD45, and then stained with 7-amino-actinomycin D, an argon-excitable (488 nm) membrane-impermeable DNA-binding fluorescent dye. Multi-parameter flow cytometry was used for analysis based on selective uptake and laser-activated fluorescence of these dyes.
Results
Cytoplasmic fragments, which were DCV-negative and CD45-positive, and dead cells, which were positive for 7-amino-actinomycin D, were efficiently separated from neoplastic cells.
Conclusion
Staining with DyeCycle Violet is a useful method to improve flow cytometric gating methods and quantitative analyses of lymph node samples from dogs with lymphoma.
Keywords: Canine, flow cytometry, lymphoglandular bodies
Flow cytometry is a powerful tool for separation of cells in a heterogeneous cell suspension and is used routinely to analyze suspensions of neoplastic cells in both research and clinical practice. Neoplastic lymphocytes are fragile, and mechanical disruption caused during sampling procedures, such as fine-needle aspiration or biopsy, leads to cytoplasmic fragmentation. Cytoplasmic fragments, also known as lymphoglandular bodies or Söderström bodies, are pleomorphic, ranging widely in size and appearance. These fragments may be large enough to be misinterpreted as intact cells based on light scatter properties and in addition may express lymphocyte cell surface antigens. Together, these properties can introduce artifacts to flow cytometric analyses of lymphoma samples by interfering with gating strategies and by overestimating the number of cells in the sample.
Vybrant DyeCycle Violet (DCV) is a membrane-permeable DNA-binding dye that, when bound to DNA, is excitable by laser diodes emitting light at violet wavelength frequency (395–415 nm). It was developed primarily for vital cell cycle analysis and was subsequently adapted for use in assays of side populations as a convenient alternative to Hoechst 33342, a dye that requires an ultraviolet (frequency 350 nm) or near-ultraviolet laser source for excitation.1 We predicted DCV would stain intact cells with nuclei, but not cytoplasmic fragments that do not contain DNA, allowing us to exclude cytoplasmic fragments from analysis of cells obtained from lymph nodes.
We analyzed 23 lymph node samples collected at participating veterinary hospitals with approval from the Institutional Animal Care and Use Committee or Institutional Review Board. The samples comprised 20 core biopsies and 3 fine-needle aspirates (FNA) from dogs with a diagnosis of spontaneous lymphoma, including 20 B-cell tumors and 3 T-cell tumors (Table 1). Evaluations were performed on cytologic imprints of biopsy samples or cytocentrifuged suspensions of lymph node aspirates. Preparations of single cell suspensions and immunophenotypic analyses were done as previously described2 with the additional step of staining with DCV.Briefly, cells were washed to remove platelets, treated with RBC lysing reagent (eBioscience, San Diego, CA, USA), and resuspended in RPMI medium (Gibco/BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Atlas Biologicals, Fort Collins, CO, USA), non-essential amino acids (Sigma-Aldrich, St. Louis, MO, USA), sodium pyruvate, L-glutamine, and HEPES (Mediatech Inc, Manassas, VA, USA) at 1 × 106 cells/mL. Cells were incubated for 30 minutes at 37° C in 5 μM Vybrant DyeCycle Violet (Molecular Probes Invitrogen Inc, Eugene, OR, USA). After blocking Fc receptors using canine IgG (ChromPure Dog IgG, Jackson ImmunoResearch Laboratories, Inc, West Grove, PA, USA), cells were stained on ice for 30 minutes using anti-canine CD3 conjugated to uorescein isothiocyanate (FITC), anti-canine CD5 conjugated to FITC, anti-canine CD21 conjugated to phycoerythrin (PE), anti-canine CD45 conjugated to allophycocyanin (APC), obtained from AbD Serotec (Raleigh, NC, USA), and anti-human CD22 conjugated to PE (Abcam, Cambridge, MA, USA). Each of these conjugated antibodies is routinely by our laboratory and others used to immunophenotype canine lymphoma.2 Next, 0.5 μg/mL 7-amino-actinomycin D (7-AAD; eBioscience) was added to each tube. This dye is a membrane-impermeable, DNA-binding fluorescent dye that is acquired by nucleated cells only if there has been membrane damage; thus, 7-ADD-staining can be used to exclude dead or damaged cells from analysis. Cells were analyzed using a BD LSRII flow cytometer (BD Biosciences, San Jose, CA, USA) with 405, 488, and 640 nm laser excitation. The violet laser (405 nm) was used to excite DCV, the argon laser (488 nm) to excite FITC, PE, and 7-AAD, and the red diode laser (640 nm) to excite APC. Emission of the DCV signal was detected using a 450/50 nm bandpass filter, and signal emissions of FITC, PE, 7-AAD, and APC were detected using 525/50 nm, 575/26 nm, 695/40 nm, and 660/20 nm bandpass filters, respectively. Results were analyzed using FlowJo software (Tree Star, Inc, Ashland, OR, USA).
Table 1.
Percentages of DCV-negative particles in 7-AAD-negative populations in lymph node samples from dogs with lymphoma.
| Dog # | Sample type | Tumor cell size* | Phenotype | DCV-negative particles (%) |
|---|---|---|---|---|
| 1 | Biopsy | Large | B-cell | 14.4 |
| 2 | FNA | Large | B-cell | 20.0 |
| 3 | Biopsy | Large | B-cell | 11.8 |
| 4 | Biopsy | Intermediate | T-cell | 5.5 |
| 5 | Biopsy | Intermediate | B-cell | 6.0 |
| 6 | Biopsy | Intermediate | B-cell | 4.0 |
| 7 | Biopsy | Large | B-cell | 9.7 |
| 8 | Biopsy | Large | B-cell | 13.5 |
| 9 | Biopsy | Intermediate | T-cell | 5.0 |
| 10 | Biopsy | Large | B-cell | 23.8 |
| 11 | Biopsy | Large | B-cell | 4.8 |
| 12 | Biopsy | Large | B-cell | 3.0 |
| 13 | Biopsy | Large | B-cell | 10.4 |
| 14 | FNA | Large | T-cell | 2.4 |
| 15 | FNA | Large | B-cell | 12.6 |
| 16 | Biopsy | Large | B-cell | 5.5 |
| 17 | Biopsy | Large | B-cell | 3.0 |
| 18 | Biopsy | Large | B-cell | 11.6 |
| 19 | Biopsy | Large | B-cell | 10.1 |
| 20 | Biopsy | Large | B-cell | 2.0 |
| 21 | Biopsy | Large | B-cell | 7.4 |
| 22 | Biopsy | Large | B-cell | 10.3 |
| 23 | Biopsy | Large | B-cell | 7.1 |
DCV indicates DyeCycle Violet; 7-AAD, 7-amino-actinomycin D.
Tumor cell size was based on the microscopic appearance of the malignant cells in cytologic preparations and on their forward side scatter as determined by flow cytometry.
Lymph node samples were stained with Wright-Giemsa and analyzed microscopically. Abundant cytoplasmic fragments were observed both on the imprint slides from the 20 biopsy samples and the cytocentrifuged preparations from the 3 FNA samples. The size of the cytoplasmic fragments varied widely and ranged from smaller than the diameter of an RBC (< 7 μm) to > 1.5 RBC diameters (~10–12 μm; Figure 1). Thus, gating using light scatter properties (forward scatter and right angle side scatter) was not likely to eliminate these interfering cell fragments during the analysis of flow cytometric data from these samples. Beyond their identification, live cells can be required for other applications, such as sorting of enriched tumor subpopulations and tumor-infiltrating lymphocytes. Thus, we evaluated the cytotoxicity of DCV and Hoechst dyes on cells isolated from lymph nodes of dogs with lymphoma. Hoechst 33342 (Sigma-Aldrich) was extremely toxic, resulting in poorly viable cells that showed increased uptake of 7-AAD (data not shown). In contrast, toxicity attributable to DCV using the protocol described here was negligible (Figure 2A).
Figure 1.
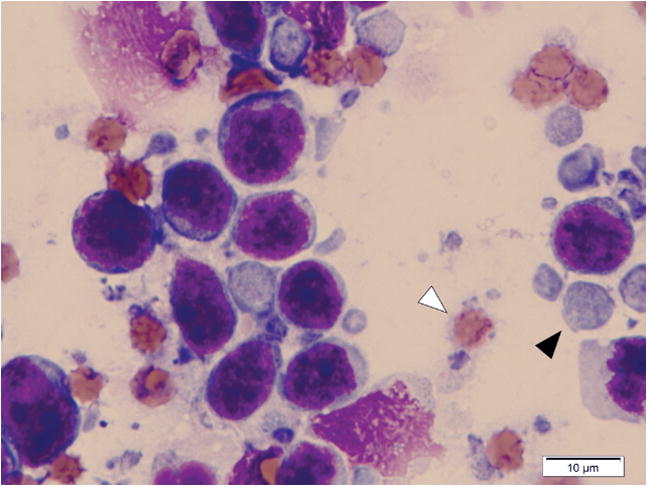
Cytoplasmic fragments in a lymph node imprint from a dog with high-grade large B-cell lymphoma. Note a cytoplasmic fragment (black arrowhead) and an RBC (white arrowhead). Wright-Giemsa.
Figure 2.
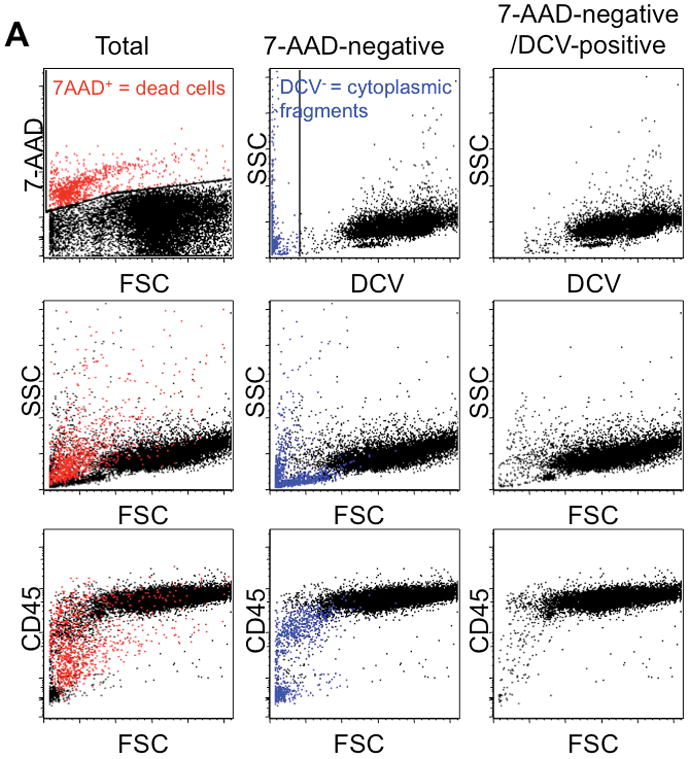
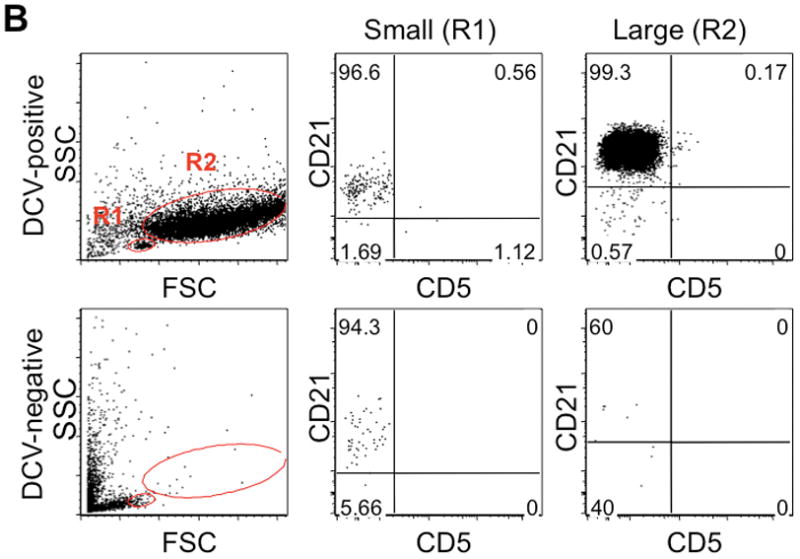
Detection of cytoplasmic fragments by multi-parameter flow cytometry. (A) Stepwise exclusion of dead cells and anucleate cells from the analyzed population in a representative sample of large B-cell lymphoma. Each column represents the same population displaying different parameters; the middle row of each column shows 2-dimensional light scatter dot plots (forward scatter [FSC] vs. right angle side scatter [SSC]), and the bottom row shows 2-dimensional dot plots of FSC vs. CD45 expression. Left column: “total” population after RBC lysis and prior to exclusion of 7-AAD-positive events, ie, dead cells (red), with gating for 7-AAD shown in the top panel. Middle column: 7-AAD-negative events, ie, live cells and particles. Right column: 7-AAD-negative/DCV-positive events, ie, live cells, with gating to exclude DCV-negative particles (middle column, blue) shown in the top row (compare middle and right columns). (B) Left column: small (R1) and large (R2) cells were gated using forward scatter as a surrogate measure for cell size in both the DCV-positive (top row) and DCV-negative (bottom row) populations. DCV-negative evens mostly segregated with small lymphocytes. R1 (middle column) and R2 (right column) populations were each analyzed for expression of B-cell (CD21) and T-cell (CD5) commitment antigens, shown as 2-dimensional dot plots. Expression of CD21 by DCV-negative particles (cytoplasmic fragments) is similar to that of small and large DCV-positive B-cells.
Use of 7-AAD and DCV allowed exclusion of dead cells and of residual platelets, RBCs, and cytoplasmic fragments, respectively, from these samples, resulting in data sets that were gated on both small and large subpopulations based on light scatter properties with less interference from sample artifacts. Data from one dog (#13) with B-cell lymphoma were representative (Figure 2). Following hemolysis and prior to exclusion of 7-AAD-positive events and anucleate particles, the “total” population was obtained (Figure 2A, left column). Similar to propidium iodide, the membrane-impermeant, DNA-binding, and fluorescent properties of 7- AAD allow exclusion of dead or dying cells with permeable cell membranes by gating out positively stained cells (Figure 2A, top left panel). Although most dead cells in this representative sample were small, having relatively low forward scatter, and were of low granularity and complexity, having low right angle side scatter, dead cells with light scatter properties comparable to those of live cells remained (Figure 2A, middle left panel). Dead cells also could not be excluded based only on expression of surface antigens such as CD45 (Figure 2A, bottom left panel).
Events negative for 7-AAD included live cells with intact, functional cell membranes and cells or membrane-bound particles with no DNA (Figure 2A, middle column) that also failed to accumulate the DCV dye (DCV-negative events, marked in blue). Most of the 7-AAD-negative/DCV-negative events were small with low granularity and complexity (Figure 2A, middle column, middle panel). Nevertheless, there were DCV-negative events with light scatter properties similar to those of small and large live cells, and almost half the DCV-negative events expressed CD45 (Figure 2A, middle column, bottom panel), indicating that DCV-negative events were not exclusively CD45-negative anucleate blood cells (platelets and RBCs), but included CD45-positive cytoplasmic fragments.
Events that were 7-AAD-negative/DCV-positive included only live, nucleated cells (Figure 2A, right column). Definition of small and large subpopulations within the sample became clearer after exclusion of 7-AAD-positive/DCV-negative events (Figure 2A, right column, middle and bottom panels). DCV-negative events ranged from 2.0 to 23.8% of the total events analyzed (Table 1). Thus, addition of DCV to staining and analysis protocols effectively eliminated residual platelets, RBCs, and cytoplasmic fragments (Figure 2A, middle and right columns).
Exclusion of DCV-negative events had an impact on immunophenotyping and separation of cell populations based on size (Figure 2B). Many DCV-negative events segregated with small lymphocytes (R1) and, in fact, masked the boundaries for live cells in R1. Thus, exclusion of DCV-negative events was essential to define small and large cell gates. Cytoplasmic fragments showed robust expression of CD21, comparable to that seen in both small and large DCV-positive B-cell populations. Generally, DCV-negative particles accounted for a greater proportion of events in R1 than in the large cell population (R2). The mean ± SD percent of DCV-negative events in R1 was 7.1 ± 6.1 (range 0.2–22.9%) compared with 1.1 ± 2.8% (range 0–12.5%) in R2.
We believe this application will be most important for investigation of minor small cell populations in cancer, such as cancer stem cells3 and non-neoplastic cells, including tumor-infiltrating T- and B-cells, which have received considerable attention as important modulators that may influence patient outcome.4–6 Such investigations may be especially critical when non-neoplastic cells and tumor cells share immunophenotypic properties, as occurs with infiltration of non-malignant B-cells in follicular lymphoma.7 Microparticles in leukemia/lymphoma have been detected by flow cytometry based on differences in size compared with tumor cells8; however, as we show here, the pleomorphic nature of cytoplasmic fragments makes exclusion of these particles from cell populations obtained from lymph nodes difficult using cell size or other light scatter properties as the sole discriminators. As DCV-staining is independent of cell size or cell type, this method also can be applied to other tumors where cells undergo blebbing and fragmentation, as well as to normal tissue samples when cell fragments interfere with flow cytometric analysis.
In summary, DCV staining effectively excluded unwanted contaminants, such as platelets, RBCs, and, most importantly, cytoplasmic fragments from lymph node samples for flow cytometric analysis. The DCV staining protocol added only 30 minutes to routine staining methods. In addition, the violet laser diodes necessary to excite DCV are becoming a common fixture on contemporary flow cytometers, such as the LSRII and FACSAria (BD Biosciences), available in many core facilities. Previous generation cytometers still in wide use (ie, FACSCalibur, BD Biosciences), however, are not equipped with violet laser diodes. We conclude that DCV staining should be included in protocols for quantitative flow cytometric analysis of lymph node samples from animals with lymphoma. This step will not only improve accuracy when it is necessary to enumerate subpopulations in low frequency, but will increase efficiency during sorting runs, especially when sorts target rare cells, such as hematopoietic stem cells or tumor-infiltrating lymphocytes, that co-localize with smaller cells in the sample.
Acknowledgments
The authors wish to acknowledge the assistance of staff from the Flow Cytometry Core Facility of the Masonic Cancer Center, University of Minnesota and thank Dr. Leslie Sharkey, Department of Veterinary Clinical Sciences, College of Veterinary Medicine, University of Minnesota, for critical review of the manuscript and helpful discussions. This work was supported in part by grant P30 CA77598 from the National Cancer Institute, National Institutes of Health, by grants 615a and 1113 from the AKC Canine Health Foundation, and by philanthropic funds directed to the Minnesota Medical Foundation and the University of Minnesota Foundation.
Footnotes
Disclosure: The authors have indicated they have no affiliations or financial involvement with any organization or entity with a financial interest in, or in financial competition with, the subject matter or materials discussed in this paper.
References
- 1.Telford WG, Bradford J, Godfrey W, et al. Side population analysis using a violet-excited cell-permeable DNA binding dye. Stem Cells. 2007;25:1029–1036. doi: 10.1634/stemcells.2006-0567. [DOI] [PubMed] [Google Scholar]
- 2.Jubala CM, Wojcieszyn JW, Valli VE, et al. CD20 expression in normal canine B cells and in canine non-Hodgkin lymphoma. Vet Pathol. 2005;42:468–476. doi: 10.1354/vp.42-4-468. [DOI] [PubMed] [Google Scholar]
- 3.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;19:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 4.Alvaro T, Lejeune M, Salvadó MT, et al. Immunohistochemical patterns of reactive microenvironment are associated with clinicobiologic behavior in follicular lymphoma patients. J Clin Oncol. 2006;24:5350–5357. doi: 10.1200/JCO.2006.06.4766. [DOI] [PubMed] [Google Scholar]
- 5.Lee NR, Song EK, Jang KY, et al. Prognostic impact of tumor infiltrating FOXP3 positive regulatory T cells in diffuse large B-cell lymphoma at diagnosis. Leuk Lymphoma. 2008;49:247–256. doi: 10.1080/10428190701824536. [DOI] [PubMed] [Google Scholar]
- 6.Pretscher D, Distel LV, Grabenbauer GG, et al. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292. doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irish JM, Czerwinski DK, Nolan GP, Levy R. Altered B-cell receptor signaling kinetics distinguish human follicular lymphoma B cells from tumor-infiltrating nonmalignant B cells. Blood. 2006;108:3135–42. doi: 10.1182/blood-2006-02-003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savaşan S, Büyükavci M, Buck S, et al. Leukaemia/lymphoma cell microparticles in childhood mature B cell neoplasms. J Clin Pathol. 2004;57:651–653. doi: 10.1136/jcp.2003.011643. [DOI] [PMC free article] [PubMed] [Google Scholar]


