Abstract
A facile approach to the synthesis of 2,3,6-trisubstituted-5,6-dihydroimidazo[2,1-b]thiazole was reported. A resin bound cyclic thiourea was formed by the treatment of a resin bound diamine with 1,1′-thiocarbonyldiimidazole, and then reacted with a α-haloketone to generate a resin bound isothiourea. HF treatment of the resin bound isothiourea resulted in the cleavage of the product and simultaneous formation of an enamine bond. This led to the formation of the 2,3,6-trisubstituted-5,6-dihydroimidazo[2,1-b]thiazole in high yield and purity.
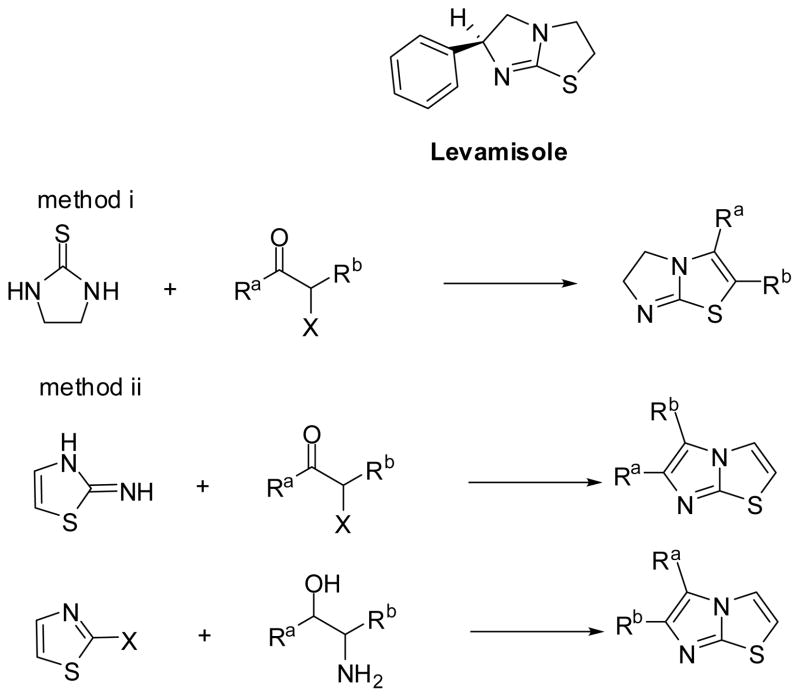
Heterocyclic compounds have been found to have multiple applications in the treatment of human diseases.1 Imidazole and thiazole are among the most common heterocycles currently utilized in medicinal science and drug discovery.2 Imidazole- and thiazole-containing structures have been found in many developed drugs, as well as in many bioactive natural products and synthetic compounds.3 From the well-known natural drugs penicillin and vitamin B, to the synthetic drugs Meloxicam, Cimetidine, Metronidazole, and Eprosartan, these important drugs are either imidazole- or thiazole-containing compounds.4 Imidazothiazole, which consists of an imidazole-ring fused with a thiazole-ring, has been reported to have excellent immunostimulating properties and anti-inflammation activities.5 Levamisole, a marketed anthelminthic and immunomodulator belongs to this class. Levamisole is also used in combination with other forms of chemotherapy for cancer treatment.6
Imidazothiazoles have generally been synthesized from starting materials that already contain a heterocyclo—either an ethylenethiourea (Scheme 1, method i) or a thiazole (Scheme 1, method ii).7 The limited accesses of commercially available starting heterocycles restrict the high throughput synthesis of multiple-substituted imidazothiazole compounds. In order to overcome these drawbacks, herein, we report a facile approach to the high throughput synthesis of 2,3,6-trisubstituted-5,6-dihydroimidazo[2,1-b]thiazole derivatives through the use of a solid-phase synthetic method.
Scheme 1.
Structure of Levamisole and General Routes to Synthetic Imidazothiazole
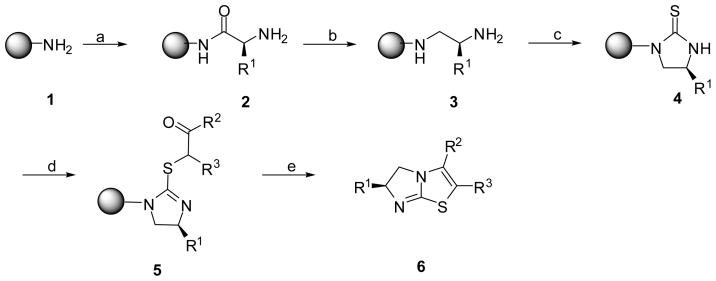
The synthesis was based on the reaction of a cyclic thiourea with an α-haloketone. The cyclic thiourea was generated through the solid-phase synthetic method described below (Scheme 2).8 Starting from MBHA resin 1, a Boc-amino acid was coupled on the resin using a standard DIC/HOBt protocol to generate the resin bound amino acid 2. After removal of the Boc group, the resin bound 2 was reduced by exhaustive borane reduction to yield the resin bound diamine 3. The resin bound diamine 3 was treated with 1,1′-thiocarbonyldiimidazole, forming the resin bound cyclic thiourea 4. An α-haloketone was then coupled to yield the resin bound isothiourea 5. The resin bound isothiourea 5 was then treated with anhydrous HF at 0 °C to release the products from the polymer support. Simultaneous ring closure was carried out by enamine formation, generating the second thiazole ring, and eventually resulting in the 2,3,6-trisubstituted-5,6-dihydroimidazo[2,1-b]thiazole derivatives 6 (Table 1, 6a–6j).
Scheme 2.
Synthesis of 2,3,6-trisubstituted-5,6-dihydroimidazo[2, 1-b]thiazole derivatives
(a) Boc-AA-OH (5 equiv), DIC (5 equiv), HOBt (5 equiv); 55% TFA/DCM. (b) BH3·THF (40 equiv), 65 °C, 72 h; piperidine, 65 °C, overnight. (c) 1,1′-thiocarbonyldiimidazole (5 equiv) in CH2Cl2, overnight. (d) α-haloketone (5 equiv) in DMF, 65 °C, 24 h. (e) anhydrous HF, 0 °C, 1.5 h.
Table 1.
Individual Products of 2,3,6-trisubstituted-5,6-dihydroimidazo[2,1-b]thiazole derivatives
| Entry | R1 | R2 | R3 | Purity% a | Yield% b |
|---|---|---|---|---|---|
| 6a | -CH3 | -C6H5(4-F) | -H | 85 | 87 |
| 6b | -CH3 | -C6H5 | -C6H5 | 98 | 85 |
| 6c | -CH3 | -C6H5 | -CH3 | 90 | 98 |
| 6d | -CH3 | 1-adamantyl | -H | 95 | 98 |
| 6e | -CH2CH(CH3)2 | 2-naphthyl | -H | 75 | 68 |
| 6f | -CH2C6H5 | -C6H5 | -CH3 | 80 | 90 |
| 6g | -CH2C6H5 | -CH3 | -CH3 | 95 | 98 |
| 6h | -CH2CH(CH3)2 | -C(CH3)3 | -H | 75 | 84 |
| 6i | -CH(CH3)2 | -C6H5 | -H | 60 | 80 |
| 6j | -CH2C6H5 | -C6H5(4-OCH3) | -H | 90 | 90 |
Purity (in %) is determinate by the peak area of HPLC at 214 nm.
Yields (in %) are based on the weight of crude product and are relative to the substitution of the resin (1.1 mmol/g).
A variety of different α-haloketones were examined under the described conditions. Generally, linear α-haloketone generated the desired product with good-to-excellent yield and purity. However attempts made with α-halocycloketones such as 2-bromocyclohexanone, 2-chlorocyclopentanone, and 2-bromo-1-indanone did not yield the desired imidazothiazole products under the current conditions. The main products obtained after HF cleavage were identical to the products obtained from resin bound 4. This indicates the resin-bound isothiourea did not form after the resin bound cyclic thiourea 4 reacted with the α-halocycloketone. This is probably caused by the stereo hindrance of both structurally rigid reactants—the resin bound cyclic thiourea and the α-halocycloketone. This problem was not be solved by increasing the reaction time or raising the temperature to 90 °C.
In summary, a novel strategy for the high throughput synthesis of 2,3,6-trisubstituted-5,6-dihydroimidazo[2,1-b]thiazole derivatives has been reported by the use of a solid-phase synthetic method. The resin bound cyclic thiourea was straightforwardly generated from the resin bound amino acid and was reacted with the linear α-haloketone to form the resin bound isothiourea. The HF cleavage and simultaneous formation of the enamine lead to the final products, the 2,3,6-trisubstituted-5,6-dihydroimidazo[2,1-b]thiazole derivatives. This strategy provides for the facile high throughput preparation of structurally-diverse imidazothiazole derivatives.
Acknowledgments
This work was supported by the State of Florida, Executive Officer of the Governor’s Office of Tourism, Trade and Economic Development, National Science Foundation (R.A.H. CHE 0455072), 1P41GM079590, 1P41GM081261, and U54HG03916-MLSCN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Krchnák V, Holladay MW. Chem Rev. 2002;102:61. doi: 10.1021/cr010123h. [DOI] [PubMed] [Google Scholar]; (b) Nefzi A, Ostresh JM, Houghten RA. Chem Rev. 1997;97:449. doi: 10.1021/cr960010b. [DOI] [PubMed] [Google Scholar]; (c) Cassels BK, Bermúdez I, Dajas F, Abin-Carriquiry JA, Susan W. D D T. 2005;10:1657. doi: 10.1016/S1359-6446(05)03665-2. [DOI] [PubMed] [Google Scholar]
- 2.(a) Marsilje TH, Roses JB, Calderwood EF, Stroud SG, Forsyth NE, Blackburn C, Yowe DL, Miao WY, Drabic SV, Bohane MD, Daniels JS, Li P, Wu LJ, Patane MA, Claiborne CF. Biorg Med Chem Lett. 2004;14:3721. doi: 10.1016/j.bmcl.2004.05.003. [DOI] [PubMed] [Google Scholar]; (b) Townsend LB. Chem Rev. 1967;67:533. doi: 10.1021/cr60249a002. [DOI] [PubMed] [Google Scholar]; (c) Silvestri R, Artico M, La Regina G, Di Pasquali A, De Martino G, D’Auria FD, Nencioni L, Palamara AT. J Med Chem. 2004;47:3926. doi: 10.1021/jm049856v. [DOI] [PubMed] [Google Scholar]; (d) Sanfilippo PJ, Jetter MC, Cordova R, Noe RA, Chourmouzis E, Lau CY, Wang E. J Med Chem. 1995;38:1057. doi: 10.1021/jm00007a002. [DOI] [PubMed] [Google Scholar]; (e) van Muijlwijk-Koezen JE, Timmerman H, Vollinga RC, Frijtag von Drabbe Künzel J, de Groote M, Visser S, Ijzerman AP. J Med Chem. 2001;44:749. doi: 10.1021/jm0003945. [DOI] [PubMed] [Google Scholar]
- 3.(a) De Luca L. Curr Med Chem. 2006;13:1. [PubMed] [Google Scholar]; (b) Jin Z. Nat Prod Rep. 2006;23:464. doi: 10.1039/b502166a. [DOI] [PubMed] [Google Scholar]
- 4.Eicher T, Hauptmann S. The Chemistry of Heterocycles. 2. WILEY-VCH; 2003. p. 173. [Google Scholar]
- 5.(a) Raeymaekers AHM, Roevens LFC, Van Laerhoven WJC, Van Wauwe JPF. 5527915 U S Patent. 1996; (b) Nishio K, Chiyomaru I, Anma K, Yamamoto K, Ohno H, Takayanagi N. 4556669 U S Patent. 1985; (c) Yamamoto I, Matsunari K, Nitta K, Shibata K, Takayanaqi N. 4910315 U S Patent. 1990; (d) Scribner A, Meitz S, Fisher M, Wyvratt M, Penny L, Liberatoe P, Gurnett A, Brown C, Mathew J, Thompson D, Schmatz D, Biftu T. Bioorg Med Chem Lett. 2008;18:5263. doi: 10.1016/j.bmcl.2008.08.063. [DOI] [PubMed] [Google Scholar]
- 6.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, Ungerleider JS, Emerson WA, Tormey DC, Glick JH, Veeder MH, Mailliard JA. Ann Intern Med. 1995;122:321. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 7.For method i: Debre S, Lecat-Guillet N, Pillon F, Ambroise Y. Bioorg Med Chem Lett. 2009;19:825. doi: 10.1016/j.bmcl.2008.12.008.Lecat-Guillet N, Ambroise Y. ChemMedChem. 2008:1211. doi: 10.1002/cmdc.200800052.Varma RS, Kumar D, Liesen PJ. J Chem Soc, Perkin Trans. 1998:4093.Dianov VM, Zeleev MK, Spirikhin LV. Russ J Org Chem. 2005;41:153.For method ii: Li L, Chang L, Pellet-Rostaing S, Liger F, Lemaire M, Buchet R, Wu Y. Bioorg Med Chem. 2009;17:7290. doi: 10.1016/j.bmc.2009.08.048.Birman V, Li X. Org Lett. 2006;7:1351. doi: 10.1021/ol060065s.
- 8.General procedure for the synthesis of 5,6- dihydroimidazo[2,1-b]thiazole derivatives. 100 mg MBHA resin was sealed within a polypropylene mesh packet. Reactions were carried out in polypropylene bottles. A solution of N-Boc-amino acid (5 equiv, 0.10 M in DMF), HOBt (5 equiv, 0.10 M in DMF) and DIC (5 equiv, 0.10 M in DMF) were added to the reaction vessel. The reaction was shaken at room temperature for 2 h, followed by washing with DMF (3 times). Upon removal of the Boc- group with 55% TFA in DCM at room temperature for 30 min, the resin was washed and neutralized with 5% DIEA in DCM. After washing with DCM (2 times), DMF (1 time), DCM (2 times) and air dried, the resin bound amino acids were reduced with borane in THF at 65 °C for 72 h, followed by treatment with piperidine at 65 °C overnight. The resin bound diamines were then reacted with 1,1′-thiocarbonyldiimidazole (5 equiv, 0.10 M in DCM) overnight. After washing with DCM, α-bromoketone (5 equiv, 0.10 M in DMF) was added to the reaction vessel. The reaction was performed at 65 °C for 24 h. The resin packet was then washed with DMF (3 times), DCM (3 times) and MeOH (3 times). The cleavage of the product was carried out by the treatment with anhydrous HF at 0 °C for 90 min, followed by nitrogen gas flow to remove the HF. The product was extracted by 95% acetic acid. After lyophilization, the product of 2,3,6-trisubstituted-5,6-dihydroimidazo[2,1-b]thiazole was obtained. The product was characterized by LC-MS under ESI conditions and NMR spectroscopy. For representative product 6b: separation yield: 63%; HPLC column condition: CH3CN(+ 0.05% formic acid) in H2O (+ 0.05% formic acid), 5–95% in 6 min; column: luna C18, 5 μm, 50 × 4.60 mm, detection 254 nm, tR = 2.41 min; ESI-MS (m/z): 293.1 [M+H]; 1H NMR (500 MHz, CDCl3) δ: 0.93 (d, 3H, J = 6 Hz), 3.83 (dd, 1H, J = 3.3, 12 Hz), 4.39 (dd, 1H, J = 9.5, 12 Hz), 4.42–4.44 (m, 1H), 7.06–7.08 (m, 2H), 7.16–7.18 (m, 3H), 7.31–7.33 (m, 2H), 7.38–7.42 (m, 3H). 13C NMR (250 MHz, CDCl3) δ: 19.9, 56.5, 63.6, 127.8, 128.3, 128.9, 129.4, 129.5, 129.8. For compound 6g: separation yield: 72%; HPLC: tR = 2.07 min; ESI-MS (m/z): 245.0 [M+H]; 1H NMR (500 MHz, CDCl3) δ: 2.00 (s, 3H), 2.07 (s, 3H), 2.87 (dd, 1H, J = 9.2, 13.6 Hz), 3.06 (dd, 1H, J = 4.3, 13.6 Hz), 3.97 (dd, 1H, J = 3.9, 12.8 Hz), 4.11 (dd, 1H, J = 9.3, 12.8 Hz), 4.41–4.46 (m, 1H), 7.17 (d, 2H, J = 7.1 Hz), 7.27–7.36 (m, 3H).