Abstract
Nearly forty years ago the concept was proposed that lymphocytes are negatively regulated by what are now called co-inhibitory signals. Nevertheless, it is only the more recent identification of numerous co-inhibitors and their critical functions that has brought co-inhibition to the forefront of immunologic research. Although co-inhibitory signals have been considered to directly regulate conventional T cells, more recent data has indicated a convergence between co-inhibitory signals and the other major negative control mechanism in the periphery that is mediated by regulatory T cells. Furthermore, it is now clear that lymphocytes are not the sole domain of co-inhibitory signals, as cells of the innate immune system, themselves controllers of immunity, are regulated by co-inhibitors they express. Thus, in order to better understand negative regulation in the periphery and apply this knowledge to the treatment of disease, a major focus for the future should be the definition of the conditions where co-inhibition controls effector cells intrinsically versus extrinsically (via regulatory or innate cells).
Key words: tolerance, autoimmune disease, T cell, B cell, immune signaling, mechanistic model
Introduction
The question of how the adaptive immune system prevents self-reactivity continues to be at or near the top of the hierarchy of important questions in immunology, with the favored solution changing from one decade to the next. Recently regulatory T cells (Treg) have been the focus (again) of research on this question. Increasingly, however, negative regulation by receptors that work together with lymphocyte antigen-receptors to deliver ‘co-inhibitory’ signals have also taken center stage. While the rapidly increasing detailed description of co-inhibitory receptors and their intracellular signaling pathways has been reviewed elsewhere,1–7 we focus here on the relationship between co-inhibitory receptors and their functions in terms of minimal models of immune regulation (solutions to self/nonself discrimination), with a particular focus on recent studies that suggest a convergence between co-inhibitory signals and the cells that regulate the immune response (regulatory T cells and innate immune system cells).
A Brief History of Efforts to Tackle Self/Nonself Discrimination
A unique feature of the immune system is the ability to discriminate self from nonself antigens, with strong responses against many foreign antigens and tolerance to self-antigens. Many theories have been proposed to solve the problem of self/nonself discrimination.
Timing of antigen exposure.
Burnet and Fenner proposed that there is a tolerogenic window early in the ontogeny of organisms.8 Despite the elegant studies conducted by Billingham, Brent and Medawar9 that supported Burnet's theory, numerous studies also provided evidence against this view (reviewed in refs. 10 and 11). In addition, the fact that lymphocytes are generated throughout life also indicated the Burnet-Fenner theory was either incorrect or incomplete. If tolerance occurs only early in life, how do lymphocytes newly generated in an adult animal become self-tolerant? Lederberg formulated a one-signal model of lymphocyte activation12 that resolved this problem in the Burnet-Fenner theory on tolerance. He proposed that there is a tolerogenic window early in the ontogeny of each lymphocyte rather than in the organism as a whole, allowing each lymphocyte to go through self-tolerance education whether the lymphocyte was born in a neonatal or adult animal. Lederberg's 1959 model proposing that antigen exposure in immature lymphocytes is tolerogenic, is not, as recently described,13 an extension of Burnet and Fenner's idea, but instead overturned their incorrect theory that postulated tolerance was a property uniquely of the fetal or neonatal period. The emergence of the central tolerance mechanisms, primarily deletion of autoreactive T cells in the thymus,14,15 supported the Lederberg explanation that was proposed half a century ago. However, the one-signal model did not consider a need for tolerance in mature lymphocytes, that is a peripheral tolerance, a tolerance that would seem to be demanded by the presence of particular self antigens only outside the central lymphoid organs and by the capacity of lymphocytes to mutate leading to self-reactivity (e.g., somatic hypermutation).
To counter the problem of continuous lymphocyte generation and mutation in the life of lymphocytes and consequently rescue the Burnet-Fenner tolerogenic window in ontogeny, Bretscher and Cohn proposed the two-signal model of lymphocyte activation.16 According to this model, the optimal activation of T or B lymphocytes requires two signals in which the first signal arises from the engagement of the antigen with specific receptors of lymphocytes and the second signal is from the antigen specific T-helper cells (Th), which are required to complete activation of the immune response. Based on this model, absence of self-reactive helpers can enforce tolerance throughout life due to a lack of help for newly generated helpers. This latter concept opened the chicken-egg dilemma by raising the question of which cell helped the first Th cells? It also suffered from the same problem as Burnet's hypothesis, the experiments showing that there is no tolerance window defined uniquely in the fetal/neonatal period. Thus, while Lederberg explained much of self/nonself discrimination through a central tolerance mechanism, tolerance of the ‘peripheral self’ remained unresolved.
Co-stimulation, PAMPS and DAMPS.
An effort by Lafferty and Cunningham to solve the puzzle of T cell allo vs. xeno reactivity was a major step towards resolving peripheral tolerance, as it led to a revised two-signal model for lymphocyte activation.17 In this revised model, signal 2 (positive signal) or “co-stimulation” originates from antigen presenting cells (APCs) instead of Th. In both of the two-signal theories, absence of signal 2 in lymphocytes will lead to tolerance (deletion or inactivation). However, it remained unclear how co-stimulation could help discriminate self from nonself; how co-stimulation could be present with foreign but not self-antigens. Charles Janeway suggested a solution to the deficiency in Lafferty and Cunningham's theory by proposing that pattern recognition receptors of the innate immune cells influenced the expression of co-stimulation. Janeway translated from lymphocytes to the APC, the Coutinho and Moller concept of mitogen receptors binding microbial products,18 as the primary stimulus for immune responses. According to Janeway's model, the interaction of pattern recognition receptors of APC with their ligands (pathogen associated molecular patterns or PAMPS) of microbes, induced APC activation and expression of co-stimulatory molecules.19 The identification of Toll like receptors (TLR), a few years later, supported this concept.20 Presently, more than ten TLRs have been identified in mammals with their respective PAMP ligands. Despite the clear role of TLRs in regulating immune responses, Janeway's theory failed to easily explain transplant rejection and anti-viral immunity or the ability to harbor normal flora. Polly Matzinger introduced a new theory in the “danger model” to solve the issues in Janeway's proposal and left the idea of a self/nonself discrimination behind in favor of a danger no danger discrimination.21 This theory allowed for a peaceful co-existence between the immune system and our normal flora,22 unlike the self/nonself models. In the danger model, co-stimulation is induced by endogenous danger signals that arise from host cell damage. The danger model is, in a number of respects, more diverse in offering explanations for tumor immune responses, autoimmunity and transplant rejection and there is an increasing amount of evidence supporting this model.23–29 Molecules that signal danger are also now called alarmins or danger associated molecular patterns (DAMPS).
Tolerance mediated by co-inhibition.
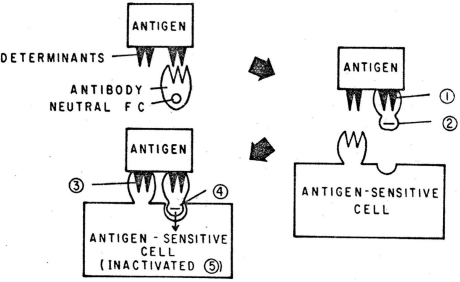
All of these minimal models of immune discrimination, be they self vs. nonself or danger vs. non-danger, give the job of tolerance inducing signals to the antigen receptor of lymphocytes. However, there is increasing evidence that tolerogenic signals are not derived from antigen receptor signals alone, and even before the concept of co-stimulation was proposed, the idea that there are co-receptors that provide inhibitory signals had been put forward. While examining the mechanisms of feedback suppression by antibody, Sinclair and Chan developed a model that explained the importance of the Fc portion of the antibody in suppression of the B cell response. Figure 1 shows the ‘Tripartite Inactivation model’ from their 1971 publication.30 Tripartite referred to the three components, antigen, antibody (a co-inhibitory ligand) and the immunologically competent cell. This, the first proposal of a receptor (in this case an Fc receptor) that works together with an activating receptor (when co-aggregated) to mediate inactivation/tolerance, was further substantiated by the identification of Fc receptors on B cells and their negative co-signaling capacity, including the identification of a critical immuno-tyrosine based inhibitory motif (ITIM) in its intracytoplasmic domain. Sinclair later proposed that these negative signals are required for tolerance in T cells as well as B cells,31 and coined the term co-inhibition for this process in further postulating that the fundamental control of self/nonself discrimination in the periphery is determined by the balance between multiple co-stimulators and co-inhibitors.32 While co-stimulation contributes to immune discrimination because it is present only with DAMPS or PAMPS (and not in ‘healthy’ self tissues), we proposed that co-inhibition contributes, at least in part, by being upregulated during prolonged antigen exposure (chronic antigen receptor signaling).32 Numerous lines of evidence now support the role of co-inhibitory molecules in self-tolerance33–35 and in control of responses during chronic antigen exposure.36–43
Figure 1.
The origins of the concept of co-inhibition. The tripartite inactivation model30 proposed that B cells are inactivated by antibody bound to antigen via the co-aggregation of the B cell antigen receptors with a receptor for the Fc portion of antibody. The model predicted the presence of negative signaling Fc receptors on B cells and that B cells are tolerized not by antigen receptor signals but instead by the co-operative signaling of antigen and Fc receptors. Reprinted with kind permission of Springer Science and Business Media. From page 611 in: Lindahl-Kiessling K, Aim G, Hanna MG, (eds.,). Morphological and Fundamental Aspects of Immunity, pp. 609–15. New York: Plenum Press 1971.
Tolerance and regulatory T cells.
The presence of autoreactive T cells in the periphery from healthy individuals44,45 underscored the importance of peripheral tolerance, especially to control the low affinity autoreactive T cells that escape from the thymus.46 The potential outcomes of peripheral tolerance are diverse, and include clonal anergy or unresponsiveness,47–51 clonal deletion,52–54 ignorance,55–57 downregulation of T cell receptors or co-receptors58,59 and suppression by Treg cells.60 Among peripheral tolerance mechanisms, Treg cells have become of great interest due to their potential therapeutic applications in controlling autoimmunity and transplant rejection.61–64 Treg exhibit dominant peripheral tolerance mechanisms by suppressing self-reactive T cells.60 The suppressive function of Treg is mediated by negative signals to other T cells and APCs through cell contact or cytokines such as TGFβ and IL-10. The concept of Treg or suppressor T cells originated in 1970 from studies of Gershon and Konda.65 Research on these cells flourished until the discovery that there was no I–J region in major histocompatability gene complex (MHC), which had been expected to be the locus controlling Treg. Moreover, other studies14,15,48 suggested that deletion or inactivation/anergy of lymphocytes were the relevant mechanisms of immunological tolerance, which further dwindled enthusiasm for the Treg field. However, studies by Sakaguchi66 demonstrated the importance of CD4+ CD25+ suppressor cells in controlling autoimmunity, which rejuvenated enthusiasm for the potential importance of Treg in immunological tolerance. Despite the popularity of Treg studies, there have been very few efforts to incorporate them into a model of self/nonself discrimination.67–69 What are the rules that allow Treg to suppress self but not appropriate foreign antigen specific responses? The rules are far from clear at this point despite the immense amount of data exploring these cells. While Treg mediate a dominant form of tolerance where effectors cells are regulated in a cell extrinsic fashion, co-inhibition has mostly been considered a cell intrinsic recessive form of tolerance. However, recent data that we will discuss is challenging this mutually exclusive viewpoint, and suggesting that many co-inhibitory receptors are involved in both recessive and dominant tolerance.
Co-Inhibitors in Recessive and Dominant Tolerance Mechanisms
For the purposes of this discussion we will consider that dominant tolerance is an antigen specific tolerance that is dominant when lymphocytes from the tolerant animal are mixed with lymphocytes from naïve animals (the mixture acts like the tolerant cells). Conversely, recessive tolerance is manifested by a lack of tolerance when the lymphocytes are mixed. However, it should be noted that there is at least the potential for an additional dominant tolerance mechanism that would not pass the ‘mixing’ test: The upregulation of co-inhibitory ligands within tissues leading to a local dominant tolerance that is not transferable with ‘tolerant’ lymphocytes to naïve recipients.
Summarized in Figure 2 and Table 1, is a minimal model of the currently described cellular interactions, either cell intrinsic (recessive) or extrinsic (dominant/regulatory), in which co-inhibitory pathways are known or thought to be involved. Multiple co-inhibitory receptor ligand pairs are likely to be involved in each of the five pathways illustrated, each serving substantially or slightly different roles in the problem of self/nonself discrimination. Mechanism number 1 in Figure 2 and Table 1 represents the most well documented co-inhibition scenario, where co-inhibitory ligands, which can be soluble (e.g., antibody) or expressed on the surface of cells (non-T cells; APC or other tissue cells), interact with co-inhibitory receptors and generate tolerance. This tolerance is generally considered to be a recessive form of tolerance and involves recruitment of phosphatases to ITIM motifs. However, it cannot be excluded that in some cases these interactions may turn the T cell into a Treg, in which case dominant tolerance would ensue. In fact recent studies indicate that expression of PD-L1 on APC promotes generation of iTreg in a population of naïve T cells.70,71 It remains unclear how such a mechanism could function in vivo without causing a state of generalized immunosuppression. Another example of co-inhibition via mechanism 1 is the ability of HVEM on radioresistant cells to prevent T cell activation by its interaction with BTLA and/or CD160 on the responding T cells.72 PD-L1 is also expressed in non-hematopoeitic cells73–77 and may bind with PD-1 on conventional T cells (Tcon) to maintain recessive tolerance within tissues and tumors.78 Although mechanism 1 is a recessive tolerance (not mediated by Treg) acting directly on responding T cells, the inhibition of proliferation/activation of the responding T cell population could be expected to alter the ratio of effector to Treg cells, favoring the Treg. The concept that mechanism 1 is recessive is contingent upon the determining factor in responsiveness being regulation of co-inhibitor expression on the responding lymphocyte. That is, co-inhibitor levels changes while co-inhibitory ligands are a constant (not inducible) and thus do not ‘decide’ the outcome. However, the picture may become even more complex for mechanism 1 if co-inhibitory ligands are themselves also inducibly expressed in tissues, as has been seen in the setting of inflammatory cytokines and autoimmunity.76,79 In this latter case, mechanism 1 would itself seem to be an effort to establish a form of dominant tolerance locally within the tissue, a dominant tolerance that is not mediated by Treg but may nevertheless be useful.
Figure 2.
Mechanisms of co-inhibitory signaling involving dominant (Treg) versus recessive mechanims. Abbreviations, include Ci (co-inhibitor), Ci-L (co-inhibitory ligand), Cs (co-stimulator), Cs-L (co-stimulatory ligand), Tcon (conventional T cell), Treg (regulatory T cell). See also Table 1 for a description of each type of mechanism shown in 1–5 in the figure.
Table 1.
Distinct mechanisms by which co-inhibitory receptors/ligands block conventional T cell (Tcon) responses depends on the cells expressing co-inhibitors vs. co-inhibitor ligands, and may even switch their function from inhibition to stimulation
| Co-inhibitor | aCo-inhibitor ligand | Outcome | Examples; cBinding | |
| 1 | Tcon | APC or tissue | Inhibitory signals to Tcon; recessive tolerance | PD-1/PD-L1, Fas; trans |
| 2 | Tcon | Treg | Inhibitory signals to Tcon; dominant tolerance | PD-1/PD-L1, BTLA/HVEM; trans |
| 3 | Treg | APC | Reduced co-stimulation to Tcon; dominant tolerance | CTLA-4/CD80-CD86; trans |
| 4 | Tcon | bTcon | Survival signals to Tcon; prolonged responses | BTLA/CD160/HVEM; cis |
| 5 | Tcon | APC | Reduced co-stimulation to Tcon; recessive tolerance | CTLA-4/CD80-CD86; trans |
In some cases, specifically #3 and 5, ligand for the co-inhibitor is also a co-stimulatory ligand.
The co-inhibitory ligand (HVEM) is also expressed on Treg, and at high levels, although low BTLA levels on these cells likely preclude significant cis interactions.
Interactions between receptors and ligands on the same cell (cis) versus different cells (trans).
The role of co-inhibitors in the dominant tolerance mediated by Treg has only recently emerged. Treg play a key role in the immunological tolerance against self-antigens as well as foreign antigens. Treg can be divided into natural Treg (nTreg) and induced Tregs (iTreg). The development of nTreg is different from induced Treg as the former develop in the thymus whereas iTreg are induced in the periphery.80 The mechanism of suppression by Treg can be contact dependent or through cytokine dependent mechanisms.81,82 It has recently emerged that the suppressive function of Treg is mediated by co-inhibitory receptors. For example, Treg lacking PD-L1 or CTLA-4 are not good suppressors.83,84 It has been shown recently that the CTLA-4 in Treg downregulates co-stimulatory molecules CD80 and CD86,84,85 on APCs to maintain tolerance (mechanism 3 in Fig. 2 and Table 1). In contrast to CTLA-4, ligands of PD-1 and BTLA are more highly expressed by Treg than Tcon such that co-inhibitory ligands of Treg bind with their receptors on Tcon (mechanism 2 in Fig. 2 and Table 1). However, some studies have suggested an alternate possibility, that PD-1 on Treg could negatively regulate immune responses by binding with its ligand, PD-L1, on other cells.86 The mechanisms involved in this latter possibility are not clear. It was recently shown that PD-L1 was not only required for Treg functions, but also required for the development and maintenance of iTreg.71 CTLA-4 and PD-1 are not the only co-inhibitory pathways key to Treg function. Treg that lack HVEM have reduced capacity to suppress naïve wild type (WT) T cells.87 Conversely, WT Treg could not efficiently suppress BTLA-/- Tcon, which implied that Treg utilized HVEM to inhibit the effectors through BTLA87 and possibly CD160. Increasing the complexity even further, receptors involved in co-inhibition apparently can also have a positive impact on immune responses. BTLA expression and function in T cells is associated with increased T cell survival in both graft versus host disease and colitis models.72,88–90 How BTLA functions to increase survival is not yet clear. However, a recent study indicates that BTLA and HVEM can interact in cis on T cells (see mechanism 4 in Fig. 2 and Table 1) and that the cis interaction promotes survival.90 Surprisingly, it promotes survival even though the cis interaction blocks trans interaction of HVEM ligands (BTLA, CD160) with HVEM on adjacent cells, preventing HVEM signals (NFϰB activation).
T cell immunoglobulin (Ig) domain and mucin domain-3 (Tim-3), is a co-inhibitory molecule expressed by terminally differentiated Th1 T cells. The binding of Tim-3 with its ligand galectin-9 induced apoptosis of Th1 cells.91 A recent study reported that galectin-9 was expressed by Treg and proposed that it could inhibit Th1 cells by binding with Tim-3 on those cells.92 Consistent with their speculation, blocking antibodies to Tim-3 reduced the suppressive function of Treg in vitro and in vivo. Although blocking Tim-3 pathway partially restored Tcon proliferation in vitro, there was no evidence that it directly reduced the suppressive function of Treg. A previous study from the same group reported that the ligand of Tim-3 can negatively regulate alloreactive CD8+ T cells.93 Based on these findings, the interpretation that blocking Tim-3 pathway in vivo negated the suppressive function of Treg is complicated by the possibility that the treatment could have directly enhanced alloreactive CD8+ T cell responses subsequently resulting in allograft rejection.
Given the above evidence that co-inhibitors are critical in Treg function it raises the question of whether co-inhibitors actually have a critical role in recessive tolerance mechanisms. Conditional deletion of CTLA-4 only in Treg showed delayed onset of the rapid lymphoproliferative disorder and autoimmunity that occurs when there is global deletion of CTLA-4, suggesting that CTLA-4 may also regulate the Tcon intrinsically.84 More recently, this concept was supported by elegant experiments by Ise et al.94 and Jain et al.95 that demonstrated the requirement for CTLA-4 in controlling Tcon to prevent autoimmunity. Hence, the expression of CTLA-4 in T cells has a dual role. The expression of CTLA-4 in Treg serves to control aberrant activation of Tcon extrinsically, whereas CTLA-4 has intrinsic effect on Tcon to maintain tolerance. Furthermore, numerous lines of evidence showed the involvement of co-inhibitory molecules in recessive tolerance mechanisms such as deletion and anergy of T cells.96–99 Interestingly, even the well-known ability of B cell antigen presentation to tolerize naïve T cells100,101 has been found to be dependent on the co-inhibitors PD-1 and CTLA-4.102 In another recent study, the adoptive transfer of CD25−CD4+CD45RBhigh naive T cells into syngeneic Rag-/- recipients that induces colitis was shown to be accelerated in HVEM-/- Rag-/- recipients. HVEM expression on radioresistant cells reduced the disease via interactions with BTLA and/or CD160,72 indicating a non-Treg mediated tolerance through co-inhibition. Interestingly, BTLA was also required in non-T cells to reduce the disease.
Feto-maternal tolerance.
The mechanisms of feto-maternal tolerance in humans and mice have been discussed in detail elsewhere.103 Here we will focus on the role of co-inhibitory molecules in the maintenance of maternal tolerance. Aluvihare et al.104 reported the expansion of Treg in allogeneic pregnancy in mice when compared to syngeneic pregnancy. Consistent with the mouse studies, it has been demonstrated that there is also expansion of Treg in human pregnancies.105 Furthermore, adoptive transfer of Treg in an abortion prone mouse model106 prevented fetal resorption, which suggested the importance of Treg in allogeneic mating. PD-L1 is expressed by mouse75 and human placenta,107 which may serve to inhibit paternal antigen reactive T cells. Consistent with this possibility, paternal antigen specific T cells upregulated PD-1 upon encounter of cognate fetal antigen in pregnancy108 and blockade of PD-L1 pathway induced fetal resorption and reduced litter sizes.75 In contrast to this recessive tolerance action of PD-1 in pregnancy, adoptive transfer of purified Treg from WT mice but not from PD-L1-/- mice was shown to reduce the semi-allogeneic fetal resorption in PD-L1-/- mice.83 However, litter sizes were small when compared to WT females, suggesting the requirement of PD-L1 in other immune cells or tissues. Another study showed that PD-L1-/- mice had an increased percentage of antigen presenting cells, which expressed a higher level of co-stimulatory molecules,109 raising the possibility that this mechanism might have enhanced the allo-immune responses against semi-allogeneic fetuses. It therefore remains an open question as to whether co-inhibition contributes to fetal tolerance primarily via recessive or dominant83 tolerance mechanisms. The role of co-inhibitory molecules in the maintenance of maternal tolerance may involve protecting suppressive functions of Treg,103,110 induction of apoptosis in paternal antigen specific T cells,108 and a balancing of Th1/Th2 responses.75,103
Exhaustion.
T cell adaptation or ‘exhaustion’ is a property that occurs in T cells due to persistent systemic antigen exposure43,58,111–114 and chronic viral infections, respectively.36,115 Previous studies reported that exhausted anti-viral T cells expressed high levels of multiple co-inhibitory receptors,38 including CTLA-4, PD-1 and LAG-3, which leads to T cells dysfunction36,116,117 and persistent viremia. Furthermore, blocking co-inhibitory molecules induced strong immune responses by reversing the state of adaptation or exhaustion of T cells.43,117–119 Reversal of exhausted T cells by blocking co-inhibitory pathways has become an important area due to its therapeutic applications in chronic viral infections such as HIV, and blocking multiple co-inhibitors is synergistic in reversing exhaustion.38,41 While a number of studies implicate Treg in the reduced responses in chronic viral infection, there are not yet many studies addressing the question of whether co-inhibition's contribution to ‘exhaustion’ is a recessive tolerance or via Treg. Current data favors a non-Treg contribution of co-inhibition.41
While the Treg literature may have to some degree promoted a descriptive biology approach to immunology, the exhaustion literature may also have suffered this inertia. Exhaustion studies seem to be an example where evaluation of concepts is lost in the rush to generate descriptions of mechanism of an immunologic phenomenon. In providing the description of what molecules are involved in controlling exhaustion, the fact that these descriptions actually overturn (disprove) the concept of exhaustion seems to have been overlooked. The word and concept of exhaustion means to consume or tire completely, that is, the entities or resources used for positive action have been depleted. The literature showing a key role for co-inhibitors in putative “exhaustion” show the phenomenon is in fact not exhaustion, all the resources for positive action are present; it is instead an upregulation of negative regulatory pathways. As shown in Figure 3, relieving the cells of these co-inhibitory signals reveals that the cells are not exhausted and have all the resources to respond. Like the term “negative-costimulation”, an oxymoron often used to describe what is really co-inhibition, use of the term exhaustion when discussing tolerance through chronic antigen exposure misconstrues the essence of the phenomenon. While it is exciting to discover molecules that underlie the tolerance during chronic antigen exposure, as this provides new avenues for clinical treatments,120 it is not clear why there would not also be excitement in (or even recognition of) the advance that it provides for a fundamental understanding of how the immune system works; that such tolerance works not through exhausting T cells (i.e., too many positive signals exhaust resources) but through a decision to shut down T cells by employing co-inhibitory (negative) signals when positive signals become chronic. As we have argued previously,121 if chronic antigen/virus were truly exhausting T cells, then additional positive signals to T cells should have no effect or deepen the exhaustion if the exhaustion was not already complete. Instead, “exhaustion” can be rescued by providing to T cells what can only be considered additional positive (exhausting) signals.122 Given the ability of co-inhibition blockade to restore responses, the only way to maintain the concept that chronic antigen exposure leads to exhaustion would be to postulate that co-inhibition is itself acting as additional positive signals responsible for depletion of resources. Based on existing data we favor the model that co-inhibitory signals inhibit the elaboration of effector functions but do not deplete the resources needed for effector function. Chronic antigen exposure can also lead to deletion of some of the responding repertoire of T cells, and co-inhibition is also likely to be central to this process. There is no evidence that this deletion is a result of exhausting resources.
Figure 3.
Relief from co-inhibition reveals that chronic antigen exposure does not lead to exhaustion of T cells. Chronic antigen exposure leads to long-term expression of co-inhibitory receptors in conventional T cells. Two alternative outcomes of chronic antigen exposure are depicted. The conventional view is shown on top, where chronic antigen exposure (e.g., chronic LCMV infection) leads to exhaustion of T cells. Exhaustion is a loss of resources needed for differentiation to effector function. The resources (R) that are putatively depleted have not been defined but could include signaling elements, transcription factors, cytokines, ATP etc. The second possible outcome is shown at the bottom, where the T cell is not exhausted, resources within the cell are maintained but not deployed because they are held in check by co-inhibitory signals. Blocking co-inhibitory signals differentiates between these two possibilities, as co-inhibitory blockade is predicted to restore effector function in the second model (bottom) but not if chronic antigen leads to exhaustion (top). Abbreviations are as described in Figure 2.
Tumor evasion mechanisms.
Tumor cells, as a mechanism of immune evasion, have exploited the property of co-inhibitory molecules that regulates immune responses against self-antigens. The anti-tumor T cell responses are limited due to the expression of co-inhibitory molecule by T cells, and co-inhibitory ligands by antigen presenting cells as well as by the tumor microenvironment. For example PD-L1 expressed by tumor cells induced T cell dysfunction, by binding with PD-1 expressed by tumor specific cytotoxic T cells.78,123 A good prognosis for cancer patients was inversely proportional to the expression of PD-L1 in tumor cells.124 Interestingly, a recent study suggests that PD-L1 sends signals directly to the tumor cells to trigger their resistance to killing, rather than PD-L1 sending co-inhibitory signals to T cells.125 Another recent study in humans demonstrated the relationship of BTLA expression in anti-tumor effector T cells and inhibition of their function.126 Treg and APCs can also block the anti-tumor T cells by direct effects and also by the production of the indoleamine 2,3-dioxygenase (IDO) enzyme. The negative association of Treg with tumor immune responses has been shown in various studies.127 Depletion of Treg using anti-CD25,128 induced tumor immune responses and other strategies that were meant to attenuate Treg function by anti-CTLA-4, anti-GITR treatment also induced strong tumor immune responses and rejection of tumors.129,130 Although the latter studies demonstrated the induction of tumor immune responses, they did not demonstrate that the treatments affected Treg directly. Indirect effects could occur through expression of the targeted molecules on other cells of the immune system. The role of CTLA-4 in Treg mediated tumor immune suppression was demonstrated by the development of a Treg-specific CTLA-4 knockout, lacking CTLA-4 only in Tregs.84 In this mouse model the tumor immune responses were enhanced. The involvement of multiple co-inhibitory pathways opens up a possibility to develop an innovative tumor immunotherapy.
Role of co-inhibitory molecules in transplantation.
Induction of transplantation tolerance to foreign antigens remains the Holy Grail for transplantation immunology. The involvement of co-inhibitory molecules in the mechanisms of peripheral tolerance has allowed immunologists to develop new strategies that promote tolerance to allogeneic tissues. Long-term acceptance of allografts was achieved in various allograft models by using CTLA-4-Ig131,132 (although this works by blocking CD28 co-stimulation), PD-L1-Ig,133,134 and anti-BTLA treatments132,135 alone or in combination with other therapies. On the other hand, blocking co-inhibitory pathways accelerated allograft rejection.136–138 It has been demonstrated that intratracheal delivery of alloantigen prolonged the survival of cardiac allografts by allowing the development of donor specific Treg.139 Blockade of the PD-1/PD-L1 pathway during the administration of alloantigen, by using either anti-PD-1 or anti-PD-L1, accelerated rejection.140 The conclusion was that PD-L1 blockade prevented the induction of Treg. However, there was no direct evidence that PD-1 or PD-L1 blockade pre vented the induction of Treg in this setting, as the adoptive transfer studies employed whole splenocytes rather than purified Treg. Tolerance to various allografts achieved by treating the animals with several regimens could be prevented by using blocking antibodies to Tim-3,141 or PD-L1,131 or CTLA-4.142 The effects could be due to enhanced proliferation and cytokine responses. Blockade of co-inhibitory molecules induced strong immune responses by favoring Th1 responses and expansion of cytotoxic T cells that lead to accelerated rejection. In terms of strategies to induce transplantation tolerance, there are at least two major approaches that could prove useful. One is the generation of biologics that act as agonists for co-inhibitory signals, with few such agents having been developed at this point. A second approach is to overexpress ligands within tissues to create an immune privileged environment for the transplant.143–146
Co-inhibition, a controller of homeostasis, antigen specific responses or both?
The engagement of a co-inhibitory receptor with its ligand could influence the homeostasis of T cells. Blockade or absence of co-inhibitory molecules induced expansion of antigen-specific reactive T cells.136,147 CTLA-4-/- mice die by 3 w of age due to a lymphoproliferative disorder, which implied the importance of CTLA-4 in T cell homeostasis. However, in an important recent study the hyperproliferative response in CTLA-4-/- T cells appear to be autoantigen driven to a large extent, and for the first time it was shown that CTLA-4 is critical in controlling T cells specific to natural autoantigens.94 It has been demonstrated that antigen independent homeostatic expansion of T cells could be negatively regulated by BTLA.148 In addition, the loss of BTLA in naïve T cells enhanced the generation of CD8+ memory T cells. Using an elegant model, Welsh and colleagues showed that PD-1 also plays a key role in controlling lymphopenia induced homeostatic proliferation of established anti-viral T cells.149 However, our recent studies have shown that PD-1 and BTLA are mainly required to control lymphopenia induced homeostatic proliferation and effector function of recent thymic emigrants (Thangavelu G, et al. unpublished). Recent thymic emigrants are a T cell population with distinct properties and are particularly important early in immune system generation and during immune reconstitution post lymphocyte depletion that occurs in some viral infections and in conditioning used for bone marrow transplantation. Interestingly, syngeneic bone marrow transplantation induced autoimmunity in sub-lethally irradiated immunodeficient animals, but not in lethally irradiated immunocompetent mice.150 It was suggested that the presence of radio-resistant Treg cells prevented the onset of disease in lethally irradiated immunocompetent mice. The onset of the disease was affected by gut flora and could be prevented by co-transfer of Treg along with the syngeneic bone marrow transplant. In addition, Treg have also been shown to play a key role in controlling lymphopenia induced homeostatic proliferation of T cells.151 Whether Treg require co-inhibitory molecules to prevent lymphopenia induced homeostatic proliferation of T cells has yet to be determined.
Interpreting experiments using antibodies targeting co-inhibitors.
The blockade of co-inhibitory pathways with monoclonal antibodies (mAb) has been an important strategy in various experimental models to test co-inhibitor function and generally has been found to increase immune responses, although in some cases the antibodies appear to be agonistic. The induction of strong immune responses could occur through releasing effector cells from co-inhibitory signals, by altering the ratio of Treg/Tcon, reducing Treg function, or favoring a particular class of response (Th1/Th2/Th17). Conversely, putative agonistic antibodies are assumed to inhibit responses by providing negative co-inhibitory signals to the cells they bind. However, in most cases there is very limited data to support the contention that the antibodies simply act by blocking or stimulating the co-inhibitory receptor. Often the evidence that a particular mAb blocks or activates a co-inhibitor is derived solely in vitro and then assumed to function similarly in vivo. However, mAb have the potential to do things in vivo that do not readily occur in vitro, such as opsonize cells leading to their destruction via various mechanisms. A recent example of this is an interesting study showing the importance of HVEM on radioresistant cells interacting with BTLA on T cells in the prevention of colitis.72 The mAb 6F7 specific to BTLA was used to show that an agonist mAb (conclusion derived from in vitro data) inhibits colitis. However, when we studied the effects of 6F7 in vivo, we found that this antibody physically depletes BTLA expressing cells.135 Although it is possible the depletion is due to induction of apoptosis triggered by BTLA signaling, this seems unlikely given that loss of BTLA expressing cells occurs in a large fraction of these cells, while only a small fraction are likely to be engaging cognate antigen (a requirement for a co-inhibitory signal). Whether an antibody is blocking, depleting or agonistic when bound to a co-inhibitory receptor (or ligand) has important implications for its use therapeutically. An agonistic anti-co-inhibitor mAb may only temporarily inactivate the relevant antigen specific cells, while depletion would be a permanent elimination of relevant clones that could only be countered by recruitment of new thymic emigrants and newly generated B cells into the peripheral repertoire.
Controlling the Controllers, Innate Immunity
While Treg cells and B cells are, a priori, the only cells with the potential to naturally generate an antigen specific dominant tolerance, they are not the only cells that can negatively control immune responses. The innate immune system can negatively regulate immune responses in an antigen nonspecific fashion, and perhaps also in a location specific fashion (e.g., tissue localized tolerogenic DCs). The function of co-inhibitory molecules in the innate immune system and their subsequent effect on tolerance vs. immunity in the adaptive immune system remains uncertain. It is important to decipher the function of co-inhibitory molecules on innate cells and how they may affect immune dysfunction. Multiple co-inhibitory receptors and receptors involved in inhibition along with their ligands such as PD-1:PD-L1/PD-L2, BTLA:HVEM, B7-H4 (B7S1), Pir-B:MHC I, and Siglec-10 (Siglec-G in mouse):CD24 are expressed or inducible on innate immune cells. These receptors and ligands are important in inhibition of the adaptive immune response by T and B cells and appear to be important for inhibition of the innate immune response as well.
Until recently it was not known whether PD-1 could be expressed on innate immune cells; however, recent reports have found PD-1 to be expressed on dendritic cells (DCs), NKT cells and macrophages.6,152,153 Yao and colleagues found that PD-1 is inducible on splenic DCs, and upregulation of PD-1 on DCs inhibits the release of IL-12p70 and TNFα by T cells. PD-1 was also found to be inducible by lipoteichoic acid, Poly I:C, lipopolysaccharide (LPS) and peptidoglycan, but could be inhibited by IL-4 and CpG. Lack of PD-1 conferred a better innate immune response presumably by permitting the release of proinflammatory cytokines during Listeria monocytogenes (LM) infection.152 Furthermore, PD-1 on macrophages can play a role in the innate immune response to bacteria during sepsis. Blood monocytes from septic mice and patients, along with peritoneal macrophages in mice, express increased levels of PD-1, and this increase is associated with cellular dysfunction and characteristic morphological changes in these cells.153 However, PD-1-/- mice are protected from sepsis. It has been established that PD-L1 is a molecule that triggers a negative signal to T cells and is expressed on a wide range of cells including hematopoietic and nonhematopoietic cells.6 Negative regulation of T-cell proliferation may either be through interaction with PD-1 or B7-1,154–157 and PD-L1-/- mice have been shown to have enhanced CD4 and CD8 T-cell proliferation.154 While the requirement of PD-L1 to regulate T-cell responses has been established, PD-L1 has also been reported to be necessary on T cells for proper DC maturation, which in turn appeared necessary for proper T cell responses.158 Together these data paint a seemingly contradictory function of the PD-1 pathway in innate cells, inhibiting or enhancing their function, and will require further studies to elucidate the specific conditions that determine the outcome.
PD-L2 is a second ligand for PD-1, however, its expression is limited to DCs, macrophages and B1 B cells.6,159 Recently, a naturally occurring IgM antibody in humans was found to be capable of binding and potentially cross-linking PD-L2. Cross-linking of PD-L2 on immature DCs increases antigen uptake and presentation of MHC/peptide complexes and increases their ability to stimulate T-cell responses.160,161 Survival of DCs is enhanced when PD-L2 is cross-linked along with increased IL-12p70 production suggesting a Th1 polarized response.160,162 Release of cytokines such as IFNγ, TNFα and IL-10 in addition to IL-12p70 has been reported from PD-L2 cross-linking.161,163 In vivo adoptive transfer of DCs treated with the PD-L2 cross-linking antibody in a mouse model of inflammatory airway disease can prevent disease when compared to untreated DCs.163 Signaling of PD-L2 in DCs appears to be possible as PD-L2 cross-linking alters the cytokines produced by DCs, although the potential signaling pathway is not known, and given the very short intracellular domain in PD-L2, associated signaling adaptors may be involved. PD-L2 has clearly been demonstrated to have important in vivo functions in the setting of oral tolerance and airway hypersensitivity.164,165 Immature DCs are known to be poor stimulators of T cells and the expression of PD-L1 and PD-L2 may contribute to immature DCs favoring inhibition of T-cell responses.157 In addition, since PD-L1 can be induced on macrophages by LPS and IFNγ and PD-L2 can be induced by IL-4, the expression of these molecules by DCs may be influenced by Th1 and Th2 cells, respectively.
B and T lymphocyte attenuator (BTLA) and its ligand, HVEM, is another co-inhibitory pathway and both receptor and ligand are expressed on myeloid cells. In addition to its interaction with BTLA, HVEM interacts with another receptor named LIGHT.1 Innate cells from BTLA-/- and HVEM-/- mice secrete increased amounts of proinflammatory cytokines and are more resistant to Listeriosis.166 In contrast, LIGHT does not appear contribute to this resistance. Differences in bacterial clearance are seen as early as the first day post-infection with Listeria monocytogenes (LM), suggesting that the innate immune system is involved. Signaling from HVEM to BTLA potentially suppresses the innate immune response to prevent septic shock and cytokine storms; however, it is not clear whether the BTLA signaling occurs on innate cells or other cells/tissues.166 Consistent with this possibility, Kim et al. found that the presence of T cells may be necessary to negatively regulate the innate immune system and prevent cytokine storms,167 although the role of co-inhibitors were not examined. As well, the BTLA-HVEM pathway may also contribute to DC homeostasis within the lymphoid tissue by acting as an inhibitory checkpoint and contributing to the restriction of DC proliferation and accumulation. Both HVEM and BTLA-deficient mice have an increase in DCs within the spleen, particularly the CD8α− subsets. Mice that are LIGHT-deficient have normal DC subsets suggesting that the HVEM and BTLA is the pathway regulating DC proliferation.168 Since all conventional DCs express HVEM and BTLA it is possible that these cells are capable of both delivering and receiving an inhibitory signal.168
B7-H4 is another co-inhibitory molecule that regulates T-cell activation. B7-H4 was found to be expressed on a subset of tumor macrophages that can suppress T-cell responses.169 In addition to its role in macrophage function, B7-H4-/- mice display augmented neutrophil responses. These mice are resistant to LM infection and have increased numbers of neutrophils.170 Another receptor involved in innate cell inhibition is the Pir-B system, balancing the activating Pir-A receptor. Pir-B is an immunoglobulin-like receptor that provides a negative signal upon interaction with its ligand, MHC I. The Pir-B receptor is widely expressed on B cells, mast cells, dendritic cells, macrophages and neutrophils.171 This pathway, like the B7-H4 pathway, affects neutrophils as well as macrophages. When these cells are deficient in Pir-B there is an inability to inhibit integrin signaling and activation involved in adhesion. Pir-B-/- neutrophils exhibit enhanced respiratory burst and secondary granule release along with hyperadhesion, and Pir-B-/- macrophages are hyperadhesive and undergo rapid spreading due to an inability to inhibit activation.172 The recognition of MHC I by innate immune cells expressing Pir-B seems to be essential in preventing or dampening activation, however, DC maturation is impaired in Pir-B-/- mice, even with the addition of anti-CD40, which normally causes maturation of the DC along with increased levels of MHC II, CD80 and CD86.173 Whether these differences in function of Pir-B are controlled by the cell type or other factors has not been determined. There is little to no increase of these markers in Pir-B-deficient DCs and IL-12 production is impaired, which leads to defective regulation of T-cell activation and skews towards a Th2 response. Although it is not known why Pir-B is required for DC maturation it does not appear to alter the function of immature DCs since antigen uptake is comparable to that of WT DCs.173
Another potentially important co-inhibitory pathway in innate immunity is found in the Siglec family of receptors that bind sialic acid. These receptors contain an ITIM, but their role in the immune system is not completely understood.174 It has recently been discovered that the interaction of CD24 and Siglec-10 can detect DAMPs and inhibit an immune response, but do not respond to pathogen-associated molecular patterns PAMPs.24 This pathway can detect high mobility group box 1, heat shock protein 70 and heat shock protein 90 which are released following tissue damage and correspond to both cytoplasmic and nuclear DAMPs. While these data support the concept of a mechanism by which the innate immune system can distinguish between pathogens and tissue damage to direct the necessary immune response,24 it is not yet clear why it is necessary to have differential control of responses to DAMPS versus PAMPS.
Conclusions
The data we have reviewed here present a strong case that co-inhibitory receptor ligand pathways are central to both recessive and dominant tolerance mechanisms, and that their control of innate immunity is a promising area for future research. The complexity of their interactions, including the cell types that express the receptors/ligands and other issues related to the context in which these signals are perceived can alter the outcome. Receptors predominantly contributing negative co-inhibitory signals may under some conditions positively regulate responses. It therefore becomes difficult to neatly categorize receptors based simply on their structural relationships or predominant functions. The precise role of co-inhibition in recessive vs. dominant tolerance needs to be more fully defined. Most experiments investigating co-inhibitors in dominant Treg function suffer from the flaws common to experimental systems evaluating Treg. The Treg studied usually do not have a defined antigen specificity.94,175 In systems where tolerance depends on a particular receptor/pathway (e.g., a co-inhibitor) the loss of tolerance by depleting Treg does not by itself prove the receptor/pathway works via Treg. In addition, dominant tolerance is tested by studying the response to an antigen of naïve cells that have been mixed with cells that are putatively tolerant via a Treg mechanism. The control for such experiments has almost universally been the addition of non-Treg (e.g., CD25− cells) to the na≤ve population to show that the non-Treg do not inhibit the response. However, if one is to demonstrate a true dominant tolerance preventing immune responses specifically to self, the required control is instead the addition of a control population of T cells that is tolerant via a recessive mechanism (e.g., tolerant by deletion of T cells with the appropriate specificity). Without this control, the dominant Treg tolerance demonstrated might simply be a non-specific cellular competition that raises the threshold for naïve T-cell activation. Nevertheless, such a non-specific suppression could be important for inhibiting low affinity anti-self cells, buffering against homeostatic activation, and allowing recessive tolerance mechanisms to take hold.98,175 Defining the role of co-inhibitors in these processes should provide important insights into the evolutionary solution for self/nonself discrimination and new avenues of immune intervention in disease.
Acknowledgements
We thank Dawne Colwell for artwork. This work was supported by studentships from the Muttart Diabetes Research and Training Center and Alberta Diabetes Institute to G.T. and from the Canadian Institutes of Health Research to C.S.
Footnotes
Previously published online: www.landesbioscience.com/journals/selfnonself/article/11548
References
- 1.Murphy KM, Nelson CA, Sedy JR. Balancing costimulation and inhibition with BTLA and HVEM. Nat Rev Immunol. 2006;6:671–681. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 2.Cady CT, Rice JS, Ott VL, Cambier JC. Regulation of hematopoietic cell function by inhibitory immunoglobulin G receptors and their inositol lipid phosphatase effectors. Immunol Rev. 2008;224:44–57. doi: 10.1111/j.1600-065X.2008.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 4.Daeron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224:11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 5.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 6.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnet FM, Fenner F. The production of Antibodies. Melbourne: Macmillan and Company Limited; 1949. [Google Scholar]
- 9.Billingham RE, Brent L, Medawar PB. ‘Actively acquired tolerance’ of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 10.Anderson CC. On the sorting of the repertoire: an analysis of Cohn's challenge to integrity (Dembic), Round 2. Scand J Immunol. 2009;70:321–325. doi: 10.1111/j.1365-3083.2009.02288.x. [DOI] [PubMed] [Google Scholar]
- 11.Chan WF, Perez-Diez A, Razavy H, Anderson CC. The ability of natural tolerance to be applied to allogeneic tissue: determinants and limits. Biol Direct. 2007;2:10. doi: 10.1186/1745-6150-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lederberg J. Genes and antibodies. Science. 1959;129:1649–1653. doi: 10.1126/science.129.3364.1649. [DOI] [PubMed] [Google Scholar]
- 13.Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. J Exp Med. 2009;206:1245–1252. doi: 10.1084/jem.20090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 15.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+ 8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 16.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 17.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 18.Coutinho A, Moller G. The self-nonself discrimination: a one-signal mechanism. Scand J Immunol. 1975;4:99–102. doi: 10.1111/j.1365-3083.1975.tb02605.x. [DOI] [PubMed] [Google Scholar]
- 19.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 20.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 21.Matzinger P. Tolerance, danger and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 22.Anderson CC, Matzinger P. Danger: the view from the bottom of the cliff. Semin Immunol. 2000;12:231–238. doi: 10.1006/smim.2000.0236. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 24.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hreggvidsdottir HS, Ostberg T, Wahamaa H, Schierbeck H, Aveberger AC, Klevenvall L, et al. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86:655–662. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- 26.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peter ME. ROS eliminate danger. Immunity. 2008;29:1–2. doi: 10.1016/j.immuni.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 30.Sinclair NRS, Chan PL. Regulation of the immune response IV. The role of the Fc-fragment in feedback inhibition by antibody. Adv Exp Med Biol. 1971;12:609–615. [Google Scholar]
- 31.Sinclair NR. How many signals are enough? Cell Immunol. 1990;130:204–212. doi: 10.1016/0008-8749(90)90174-p. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair NR, Anderson CC. Co-stimulation and co-inhibition: equal partners in regulation. Scand J Immunol. 1996;43:597–603. doi: 10.1046/j.1365-3083.1996.d01-267.x. [DOI] [PubMed] [Google Scholar]
- 33.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 35.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 36.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 37.Blackburn SD, Crawford A, Shin H, Polley A, Freeman GJ, Wherry EJ. Tissue specific differences in PD-1 and PD-L1 expression during chronic viral infection: Implications for CD8 T cell exhaustion. J Virol. 2009 doi: 10.1128/JVI.01579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, et al. Antiviral Intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 40.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radziewicz H, Dunham RM, Grakoui A. PD-1 tempers Tregs in chronic HCV infection. J Clin Invest. 2009;119:450–453. doi: 10.1172/JCI38661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivas MN, Weatherly K, Hazzan M, Vokaer B, Dremier S, Gaudray F, et al. Reviving function in CD4+ T cells adapted to persistent systemic antigen. J Immunol. 2009;183:4284–4291. doi: 10.4049/jimmunol.0901408. [DOI] [PubMed] [Google Scholar]
- 44.Liblau R, Tournier-Lasserve E, Maciazek J, Dumas G, Siffert O, Hashim G, et al. T cell response to myelin basic protein epitopes in multiple sclerosis patients and healthy subjects. Eur J Immunol. 1991;21:1391–1395. doi: 10.1002/eji.1830210610. [DOI] [PubMed] [Google Scholar]
- 45.Sun JB, Olsson T, Wang WZ, Xiao BG, Kostulas V, Fredrikson S, et al. Autoreactive T and B cells responding to myelin proteolipid protein in multiple sclerosis and controls. Eur J Immunol. 1991;21:1461–1468. doi: 10.1002/eji.1830210620. [DOI] [PubMed] [Google Scholar]
- 46.Liu GY, Fairchild PJ, Smith RM, Prowle JR, Kioussis D, Wraith DC. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407–415. doi: 10.1016/1074-7613(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 47.Burkly LC, Lo D, Kanagawa O, Brinster RL, Flavell RA. Clonal anergy of I-E-tolerant T cells in transgenic mice with pancreatic expression of MHC class II I-E. Cold Spring Harb Symp Quant Biol. 1989;54:815–820. doi: 10.1101/sqb.1989.054.01.095. [DOI] [PubMed] [Google Scholar]
- 48.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rammensee HG, Kroschewski R, Frangoulis B. Clonal anergy induced in mature Vbeta6+ T lymphocytes on immunizing Mls-1b mice with Mls-1a expressing cells. Nature. 1989;339:541–544. doi: 10.1038/339541a0. [DOI] [PubMed] [Google Scholar]
- 50.Cambier JC, Getahun A. B cell activation versus anergy; the antigen receptor as a molecular switch. Immunol Lett. 2010;128:6–7. doi: 10.1016/j.imlet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Getahun A, O'Neill SK, Cambier JC. Establishing anergy as a bona fide in vivo mechanism of B cell tolerance. J Immunol. 2009;183:5439–5441. doi: 10.4049/jimmunol.0990088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones LA, Chin LT, Longo DL, Kruisbeek AM. Peripheral clonal elimination of functional T cells. Science. 1990;250:1726–1729. doi: 10.1126/science.2125368. [DOI] [PubMed] [Google Scholar]
- 53.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 54.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 55.Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 56.Starzl TE, Zinkernagel RM. Transplantation tolerance from a historical perspective. Nat Rev Immunol. 2001;1:233–239. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zinkernagel RM, Ehl S, Aichele P, Oehen S, Kundig T, Hengartner H. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol Rev. 1997;156:199–209. doi: 10.1111/j.1600-065x.1997.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 58.Chan WF, Razavy H, Anderson CC. Differential susceptibility of allogeneic targets to indirect CD4 immunity generates split tolerance. J Immunol. 2008;181:4603–4612. doi: 10.4049/jimmunol.181.7.4603. [DOI] [PubMed] [Google Scholar]
- 59.Schonrich G, Kalinke U, Momburg F, Malissen M, Schmitt-Verhulst AM, Malissen B, et al. Downregulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991;65:293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- 60.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Long E, Wood KJ. Regulatory T cells in transplantation: transferring mouse studies to the clinic. Transplantation. 2009;88:1050–1056. doi: 10.1097/TP.0b013e3181bb7913. [DOI] [PubMed] [Google Scholar]
- 62.Wright GP, Notley CA, Xue SA, Bendle GM, Holler A, Schumacher TN, et al. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci USA. 2009;106:19078–19083. doi: 10.1073/pnas.0907396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jankowska-Gan E, Sollinger HW, Pirsch JD, Cai J, Pascual J, Haynes LD, et al. Successful reduction of immunosuppression in older renal transplant recipients who exhibit donor-specific regulation. Transplantation. 2009;88:533–541. doi: 10.1097/TP.0b013e3181b0f92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Molitor-Dart ML, Andrassy J, Kwun J, Kayaoglu HA, Roenneburg DA, Haynes LD, et al. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol. 2007;179:6749–6761. doi: 10.4049/jimmunol.179.10.6749. [DOI] [PubMed] [Google Scholar]
- 65.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–737. [PMC free article] [PubMed] [Google Scholar]
- 66.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 67.Cohn M. What roles do regulatory T cells play in the control of the adaptive immune response? Int Immunol. 2008;20:1107–1118. doi: 10.1093/intimm/dxn088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Modigliani Y, Bandeira A, Coutinho A. A model for developmentally acquired thymus-dependent tolerance to central and peripheral antigens. Immunol Rev. 1996;149:155–220. doi: 10.1111/j.1600-065x.1996.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 69.Dembic Z. Beginning of the end of (understanding) the immune response. Scand J Immunol. 2008;68:381–382. doi: 10.1111/j.1365-3083.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinberg MW, Turovskaya O, Shaikh RB, Kim G, McCole DF, Pfeffer K, et al. A crucial role for HVEM and BTLA in preventing intestinal inflammation. J Exp Med. 2008;205:1463–1476. doi: 10.1084/jem.20071160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 74.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005;202:231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, et al. Regulation of PD-1, PD-L1 and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 77.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, et al. Endothelial expression of PD-L1 and PD-L2 downregulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 78.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 79.Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169:3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 80.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 81.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Habicht A, Dada S, Jurewicz M, Fife BT, Yagita H, Azuma M, et al. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol. 2007;179:5211–5219. doi: 10.4049/jimmunol.179.8.5211. [DOI] [PubMed] [Google Scholar]
- 84.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt EM, Wang CJ, Ryan GA, Clough LE, Qureshi OS, Goodall M, et al. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J Immunol. 2009;182:274–282. doi: 10.4049/jimmunol.182.1.274. [DOI] [PubMed] [Google Scholar]
- 86.Kitazawa Y, Fujino M, Wang Q, Kimura H, Azuma M, Kubo M, et al. Involvement of the programmed death-1/programmed death-1 ligand pathway in CD4+CD25+ regulatory T-cell activity to suppress alloimmune responses. Transplantation. 2007;83:774–782. doi: 10.1097/01.tp.0000256293.90270.e8. [DOI] [PubMed] [Google Scholar]
- 87.Tao R, Wang L, Murphy KM, Fraser CC, Hancock WW. Regulatory T cell expression of herpesvirus entry mediator suppresses the function of B and T lymphocyte attenuator-positive effector T cells. J Immunol. 2008;180:6649–6655. doi: 10.4049/jimmunol.180.10.6649. [DOI] [PubMed] [Google Scholar]
- 88.Hurchla MA, Sedy JR, Murphy KM. Unexpected role of B and T lymphocyte attenuator in sustaining cell survival during chronic allostimulation. J Immunol. 2007;178:6073–6082. doi: 10.4049/jimmunol.178.10.6073. [DOI] [PubMed] [Google Scholar]
- 89.Cheung TC, Steinberg MW, Oborne LM, Macauley MG, Fukuyama S, Sanjo H, et al. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci USA. 2009;106:6244–6249. doi: 10.1073/pnas.0902115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheung TC, Oborne LM, Steinberg MW, Macauley MG, Fukuyama S, Sanjo H, et al. T cell intrinsic heterodimeric complexes between HVEM and BTLA determine receptivity to the surrounding microenvironment. J Immunol. 2009;183:7286–7296. doi: 10.4049/jimmunol.0902490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 92.Wang F, Wan L, Zhang C, Zheng X, Li J, Chen ZK. Tim-3-Galectin-9 pathway involves the suppression induced by CD4+CD25+ regulatory T cells. Immunobiology. 2009;214:342–349. doi: 10.1016/j.imbio.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 93.Wang F, He W, Zhou H, Yuan J, Wu K, Xu L, et al. The Tim-3 ligand galectin-9 negatively regulates CD8+ alloreactive T cell and prolongs survival of skin graft. Cell Immunol. 2007;250:68–74. doi: 10.1016/j.cellimm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 94.Ise W, Kohyama M, Nutsch KM, Lee HM, Suri A, Unanue ER, et al. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat Immunol. 2010;11:129–135. doi: 10.1038/ni.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jain N, Nguyen H, Chambers C, Kang J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci USA. 2010;107:1524–1528. doi: 10.1073/pnas.0910341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chikuma S, Terawaki S, Hayashi T, Nabeshima R, Yoshida T, Shibayama S, et al. PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J Immunol. 2009;182:6682–6689. doi: 10.4049/jimmunol.0900080. [DOI] [PubMed] [Google Scholar]
- 97.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 98.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 99.Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 101.Lassila O, Vainio O, Matzinger P. Can B cells turn on virgin T cells? Nature. 1988;334:253–255. doi: 10.1038/334253a0. [DOI] [PubMed] [Google Scholar]
- 102.Frommer F, Heinen TJ, Wunderlich FT, Yogev N, Buch T, Roers A, et al. Tolerance without clonal expansion: self-antigen-expressing B cells program self-reactive T cells for future deletion. J Immunol. 2008;181:5748–5759. doi: 10.4049/jimmunol.181.8.5748. [DOI] [PubMed] [Google Scholar]
- 103.Guleria I, Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol. 2007;178:3345–3351. doi: 10.4049/jimmunol.178.6.3345. [DOI] [PubMed] [Google Scholar]
- 104.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 105.Heikkinen J, Mottonen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol. 2004;136:373–378. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zenclussen AC. CD4(+)CD25+ T regulatory cells in murine pregnancy. J Reprod Immunol. 2005;65:101–110. doi: 10.1016/j.jri.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 107.Petroff MG. Immune interactions at the maternal-fetal interface. J Reprod Immunol. 2005;68:1–13. doi: 10.1016/j.jri.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 108.Taglauer ES, Yankee TM, Petroff MG. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J Reprod Immunol. 2009;80:12–21. doi: 10.1016/j.jri.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang W, Carper K, Malone F, Latchman Y, Perkins J, Fu Y, et al. PD-L1/PD-1 signal deficiency promotes allogeneic immune responses and accelerates heart allograft rejection. Transplantation. 2008;86:836–844. doi: 10.1097/TP.0b013e3181861932. [DOI] [PubMed] [Google Scholar]
- 110.Wafula PO, Teles A, Schumacher A, Pohl K, Yagita H, Volk HD, et al. PD-1 but not CTLA-4 blockage abrogates the protective effect of regulatory T cells in a pregnancy murine model. Am J Reprod Immunol. 2009;62:283–292. doi: 10.1111/j.1600-0897.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 111.Choi S, Schwartz RH. Molecular mechanisms for adaptive tolerance and other T cell anergy models. Semin Immunol. 2007;19:140–152. doi: 10.1016/j.smim.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inobe M, Schwartz RH. CTLA-4 engagement acts as a brake on CD4+ T cell proliferation and cytokine production but is not required for tuning T cell reactivity in adaptive tolerance. J Immunol. 2004;173:7239–7248. doi: 10.4049/jimmunol.173.12.7239. [DOI] [PubMed] [Google Scholar]
- 113.Singh NJ, Schwartz RH. The strength of persistent antigenic stimulation modulates adaptive tolerance in peripheral CD4+ T cells. J Exp Med. 2003;198:1107–1117. doi: 10.1084/jem.20030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tanchot C, Barber DL, Chiodetti L, Schwartz RH. Adaptive tolerance of CD4+ T cells in vivo: multiple thresholds in response to a constant level of antigen presentation. J Immunol. 2001;167:2030–2039. doi: 10.4049/jimmunol.167.4.2030. [DOI] [PubMed] [Google Scholar]
- 115.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 117.Kaufmann DE, Walker BD. Programmed death-1 as a factor in immune exhaustion and activation in HIV infection. Curr Opin HIV AIDS. 2008;3:362–367. doi: 10.1097/COH.0b013e3282f9ae8b. [DOI] [PubMed] [Google Scholar]
- 118.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen HR, Pyle KJ, et al. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol. 2009;182:6659–6669. doi: 10.4049/jimmunol.0804211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Breton G, Yassine-Diab B, Cohn L, Boulassel MR, Routy JP, Sekaly RP, et al. siRNA knockdown of PD-L1 and PD-L2 in monocyte-derived dendritic cells only modestly improves proliferative responses to Gag by CD8(+) T cells from HIV-1-infected individuals. J Clin Immunol. 2009;29:637–645. doi: 10.1007/s10875-009-9313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Anderson CC. Time, space and contextual models of the immunity tolerance decision: bridging the geographical divide of Zinkernagel and Hengartner's ‘Credo 2004’. Scand J Immunol. 2006;63:249–256. doi: 10.1111/j.1365-3083.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- 122.Ehl S, Hombach J, Aichele P, Rulicke T, Odermatt B, Hengartner H, et al. Viral and bacterial infections interfere with peripheral tolerance induction and activate CD8+ T cells to cause immunopathology. J Exp Med. 1998;187:763–774. doi: 10.1084/jem.187.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114:1528–1536. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 124.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 125.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Derre L, Rivals JP, Jandus C, Pastor S, Rimoldi D, Romero P, et al. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120:157–167. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 128.Tanaka H, Tanaka J, Kjaergaard J, Shu S. Depletion of CD4+ CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J Immunother. 2002;25:207–217. doi: 10.1097/00002371-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 129.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 131.Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179:5204–5210. doi: 10.4049/jimmunol.179.8.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Truong W, Plester JC, Hancock WW, Kaye J, Merani S, Murphy KM, et al. Negative and positive co-signaling with anti-BTLA (PJ196) and CTLA4Ig prolongs islet allograft survival. Transplantation. 2007;84:1368–1372. doi: 10.1097/01.tp.0000289995.70390.20. [DOI] [PubMed] [Google Scholar]
- 133.Gao W, Demirci G, Strom TB, Li XC. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation. 2003;76:994–999. doi: 10.1097/01.TP.0000085010.39567.FB. [DOI] [PubMed] [Google Scholar]
- 134.Ozkaynak E, Wang L, Goodearl A, McDonald K, Qin S, O'Keefe T, et al. Programmed death-1 targeting can promote allograft survival. J Immunol. 2002;169:6546–6553. doi: 10.4049/jimmunol.169.11.6546. [DOI] [PubMed] [Google Scholar]
- 135.Truong W, Hancock WW, Plester JC, Merani S, Rayner DC, Thangavelu G, et al. BTLA targeting modulates lymphocyte phenotype, function and numbers and attenuates disease in nonobese diabetic mice. J Leukoc Biol. 2009;86:41–51. doi: 10.1189/jlb.1107753. [DOI] [PubMed] [Google Scholar]
- 136.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:6648–6656. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 137.Sandner SE, Clarkson MR, Salama AD, Sanchez-Fueyo A, Domenig C, Habicht A, et al. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol. 2005;174:3408–3415. doi: 10.4049/jimmunol.174.6.3408. [DOI] [PubMed] [Google Scholar]
- 138.Sho M, Yamada A, Najafian N, Salama AD, Harada H, Sandner SE, et al. Physiological mechanisms of regulating alloimmunity: cytokines, CTLA-4, CD25+ cells, and the alloreactive T cell clone size. J Immunol. 2002;169:3744–3751. doi: 10.4049/jimmunol.169.7.3744. [DOI] [PubMed] [Google Scholar]
- 139.Shirasugi N, Ikeda Y, Akiyama Y, Matsumoto K, Hamano K, Esato K, et al. Induction of hyporesponsiveness to fully allogeneic cardiac grafts by intratracheal delivery of alloantigen. Transplantation. 2001;71:561–564. doi: 10.1097/00007890-200102270-00012. [DOI] [PubMed] [Google Scholar]
- 140.Aramaki O, Shirasugi N, Takayama T, Shimazu M, Kitajima M, Ikeda Y, et al. Programmed death-1-programmed death-L1 interaction is essential for induction of regulatory cells by intratracheal delivery of alloantigen. Transplantation. 2004;77:6–12. doi: 10.1097/01.TP.0000108637.65091.4B. [DOI] [PubMed] [Google Scholar]
- 141.Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 142.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 143.Dudler J, Li J, Pagnotta M, Pascual M, von Segesser LK, Vassalli G. Gene transfer of programmed death ligand-1.Ig prolongs cardiac allograft survival. Transplantation. 2006;82:1733–1737. doi: 10.1097/01.tp.0000250757.69384.79. [DOI] [PubMed] [Google Scholar]
- 144.Kawamoto K, Tanemura M, Ito T, Deguchi T, Machida T, Nishida T, et al. Prolonged survival of pig islets xenograft by adenovirus-mediated expression of either the membrane-bound human FasL or the human decoy Fas antigen gene. Xenotransplantation. 2008;15:333–343. doi: 10.1111/j.1399-3089.2008.00490.x. [DOI] [PubMed] [Google Scholar]
- 145.Plege A, Borns K, Baars W, Schwinzer R. Suppression of human T-cell activation and expansion of regulatory T cells by pig cells overexpressing PD-ligands. Transplantation. 2009;87:975–982. doi: 10.1097/TP.0b013e31819c85e8. [DOI] [PubMed] [Google Scholar]
- 146.Wen X, Zhu H, Li L, Li Y, Wang M, Liu J, et al. Transplantation of NIT-1 cells expressing pD-L1 for treatment of streptozotocin-induced diabetes. Transplantation. 2008;86:1596–1602. doi: 10.1097/TP.0b013e31818c6e64. [DOI] [PubMed] [Google Scholar]
- 147.Koehn BH, Ford ML, Ferrer IR, Borom K, Gangappa S, Kirk AD, et al. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J Immunol. 2008;181:5313–5322. doi: 10.4049/jimmunol.181.8.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Krieg C, Boyman O, Fu YX, Kaye J. B and T lymphocyte attenuator regulates CD8+ T cell-intrinsic homeostasis and memory cell generation. Nat Immunol. 2007;8:162–171. doi: 10.1038/ni1418. [DOI] [PubMed] [Google Scholar]
- 149.Lin SJ, Peacock CD, Bahl K, Welsh RM. Programmed death-1 (PD-1) defines a transient and dysfunctional oligoclonal T cell population in acute homeostatic proliferation. J Exp Med. 2007;204:2321–2333. doi: 10.1084/jem.20062150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Benard A, Ceredig R, Rolink AG. Regulatory T cells control autoimmunity following syngeneic bone marrow transplantation. Eur J Immunol. 2006;36:2324–2335. doi: 10.1002/eji.200636434. [DOI] [PubMed] [Google Scholar]
- 151.Winstead CJ, Fraser JM, Khoruts A. Regulatory CD4+CD25+Foxp3+ T cells selectively inhibit the spontaneous form of lymphopenia-induced proliferation of naive T cells. J Immunol. 2008;180:7305–7317. doi: 10.4049/jimmunol.180.11.7305. [DOI] [PubMed] [Google Scholar]
- 152.Yao S, Wang S, Zhu Y, Luo L, Zhu G, Flies S, et al. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009;113:5811–5818. doi: 10.1182/blood-2009-02-203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci USA. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schreiner B, Bailey SL, Shin T, Chen L, Miller SD. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur J Immunol. 2008;38:2706–2717. doi: 10.1002/eji.200838137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen C, Qu QX, Huang JA, Zhu YB, Ge Y, Wang Q, et al. Expression of programmed-death receptor ligands 1 and 2 may contribute to the poor stimulatory potential of murine immature dendritic cells. Immunobiology. 2007;212:159–165. doi: 10.1016/j.imbio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 158.Talay O, Shen C-H, Chen L, Chen J. B7-H1 (PD-L1) on T cells is required for T-cell-mediated conditioning of dendritic cell maturation. Proc Natl Acad Sci USA. 2009;106:2741–2746. doi: 10.1073/pnas.0813367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol. 2007;37:2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- 160.Nguyen LT, Radhakrishnan S, Ciric B, Tamada K, Shin T, Pardoll DM, et al. Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells. J Exp Med. 2002;196:1393–1398. doi: 10.1084/jem.20021466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 161.Radhakrishnan S, Nguyen LT, Ciric B, Ure DR, Zhou B, Tamada K, et al. Naturally occurring human IgM antibody that binds B7-DC and potentiates T cell stimulation by dendritic cells. J Immunol. 2003;170:1830–1838. doi: 10.4049/jimmunol.170.4.1830. [DOI] [PubMed] [Google Scholar]
- 162.Radhakrishnan S, Nguyen LT, Ciric B, Van Keulen VP, Pease LR. B7-DC/PD-L2 cross-linking induces NFkappaB-dependent protection of dendritic cells from cell death. J Immunol. 2007;178:1426–1432. doi: 10.4049/jimmunol.178.3.1426. [DOI] [PubMed] [Google Scholar]
- 163.Radhakrishnan S, Iijima K, Kobayashi T, Kita H, Pease LR. Dendritic cells activated by cross-linking B7-DC (PD-L2) block inflammatory airway disease. J Allergy Clin Immunol. 2005;116:668–674. doi: 10.1016/j.jaci.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 164.Matsumoto K, Inoue H, Nakano T, Tsuda M, Yoshiura Y, Fukuyama S, et al. B7-DC regulates asthmatic response by an IFNgamma-dependent mechanism. J Immunol. 2004;172:2530–2541. doi: 10.4049/jimmunol.172.4.2530. [DOI] [PubMed] [Google Scholar]
- 165.Zhang Y, Chung Y, Bishop C, Daugherty B, Chute H, Holst P, et al. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci USA. 2006;103:11695–11700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sun Y, Brown NK, Ruddy MJ, Miller ML, Lee Y, Wang Y, et al. B and T lymphocyte attenuator tempers early infection immunity. J Immunol. 2009;183:1946–1951. doi: 10.4049/jimmunol.0801866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, et al. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.De Trez C, Schneider K, Potter K, Droin N, Fulton J, Norris PS, et al. The inhibitory HVEM-BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J Immunol. 2008;180:238–248. doi: 10.4049/jimmunol.180.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhu G, Augustine MM, Azuma T, Luo L, Yao S, Anand S, et al. B7-H4-deficient mice display augmented neutrophil-mediated innate immunity. Blood. 2009;113:1759–1767. doi: 10.1182/blood-2008-01-133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Takai T. Paired immunoglobulin-like receptors and their MHC class I recognition. Immunology. 2005;115:433–440. doi: 10.1111/j.1365-2567.2005.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pereira S, Zhang H, Takai T, Lowell CA. The inhibitory receptor PIR-B negatively regulates neutrophil and macrophage integrin signaling. J Immunol. 2004;173:5757–5765. doi: 10.4049/jimmunol.173.9.5757. [DOI] [PubMed] [Google Scholar]
- 173.Ujike A, Takeda K, Nakamura A, Ebihara S, Akiyama K, Takai T. Impaired dendritic cell maturation and increased T(H)2 responses in PIR-B(-/-) mice. Nat Immunol. 2002;3:542–548. doi: 10.1038/ni801. [DOI] [PubMed] [Google Scholar]
- 174.Crocker PR. Siglecs in innate immunity. Curr Opin Pharmacol. 2005;5:431–437. doi: 10.1016/j.coph.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 175.Schildknech A, Brauer S, Brenner C, Lahl K, Schild H, Sparwasser T, et al. FoxP3+ regulatory T cells essentially contribute to peripheral CD8+ T-cell tolerance induced by steady-state dendritic cells. Proc Natl Acad Sci USA. 2010;107:199–203. doi: 10.1073/pnas.0910620107. [DOI] [PMC free article] [PubMed] [Google Scholar]