Abstract
Delirium is a common and serious acute neuropsychiatric syndrome with core features of inattention and global cognitive dysfunction. The etiologies of delirium are diverse and multifactorial and often reflect the pathophysiological consequences of an acute medical illness, medical complication or drug intoxication. Delirium can have a widely variable presentation, and is often missed and underdiagnosed as a result. At present, the diagnosis of delirium is clinically based and depends on the presence or absence of certain features. Management strategies for delirium are focused on prevention and symptom management. This article reviews current clinical practice in delirium in elderly individuals, including the diagnosis, treatment, outcomes and economic impact of this syndrome. Areas of future research are also discussed.
Introduction
Delirium is a common clinical syndrome characterized by inattention and acute cognitive dysfunction. The word ‘delirium’ was first used as a medical term as early as the first century AD to describe mental disorders occurring during fever or head trauma.1 A diverse range of terms has since emerged to describe delirium, including ‘acute confusional state’, ‘acute brain syndrome’, ‘acute cerebral insufficiency’ and ‘toxic–metabolic enkephalopathy’, but ‘delirium’ should still be used as the standard term for this syndrome.2 Over time, the term delirium has evolved to describe a transient, reversible syndrome that is acute and fluctuating, and which occurs in the setting of a medical condition.
Clinical experience and recent research have shown that delirium can become chronic or result in permanent sequelae. In elderly individuals, delirium can initiate or otherwise be a key component in a cascade of events that lead to a downward spiral of functional decline, loss of independence, institutionalization, and, ultimately, death. Delirium affects an estimated 14–56% of all hospitalized elderly patients. At least 20% of the 12.5 million patients over 65 years of age hospitalized each year in the US experience complications during hospitalization because of delirium.3–5
The aims of this report are to review the current clinical practice in delirium, focusing particularly on elderly individuals. The topics covered include epidemiology, clinical features, differential diagnosis, treatment, prevention and outcome. The economic impact of delirium is discussed. Potential pathological mechanisms, including evidence from neuroimaging studies, are also examined. Finally, future avenues of research are highlighted.
Epidemiology
The overall prevalence of delirium in the community is just 1–2%, but in the setting of general hospital admission this increases to 14–24%. The incidence of delirium arising during a hospital stay ranges from 6% to as high as 56%,6 and this incidence is even higher when more-specialized populations are considered, including those in postoperative, intensive-care, subacute and palliative-care settings.7–9 Postoperative delirium occurs in 15–53% of surgical patients over the age of 65 years,10 and among elderly patients admitted to an intensive care unit (ICU) the delirium incidence can reach 70–87%.11
The etiologies of delirium are diverse and multi-factorial, and they often reflect the pathophysiological consequences of an acute medical illness, drug effect or complication. Furthermore, delirium develops through a complex interaction between different risk factors (Box 1). The development of delirium frequently depends on a combination of predisposing, non modifiable factors—such as baseline dementia or serious medical illness—and precipitating, often modifiable factors—such as taking of sedative medications, infections, abnormal laboratory test results, or surgery. Among elderly patients, one of the most prominent risk factors for delirium is dementia, with two-thirds of all cases of delirium in this age-group occurring in patients with dementia. Studies have shown that delirium and dementia are both associated with decreased cerebral blood flow or metabolism,12,13 cholinergic deficiency,14 and inflammation, and these similar etiologies might explain the close relationship between these two conditions.15
Box 1 | Risk factors for delirium.
Development of delirium depends on a complex interaction of multiple risk factors. Some of these factors are modifiable and are potential targets for prevention. Among elderly patients, dementia is the most prominent risk factor, being present in up to two-thirds of all cases of delirium.
Potentially modifiable risk factors
Sensory impairment (hearing or vision)
Immobilization (catheters or restraints)
Medications (for example, sedative hypnotics, narcotics, anticholinergic drugs, corticosteroids, polypharmacy, withdrawal of alcohol or other drugs)
Acute neurological diseases (for example, acute stroke [usually right parietal], intracranial hemorrhage, meningitis, enkephalitis)
Intercurrent illness (for example, infections, iatrogenic complications, severe acute illness, anemia, dehydration, poor nutritional status, fracture or trauma, HIV infection)
Metabolic derangement
Surgery
Environment (for example, admission to an intensive care unit)
Pain
Emotional distress
Sustained sleep deprivation
Nonmodifiable risk factors
Dementia or cognitive impairment
Advancing age (>65 years)
History of delirium, stroke, neurological disease, falls or gait disorder
Multiple comorbidities
Male sex
Chronic renal or hepatic disease
Pathophysiology
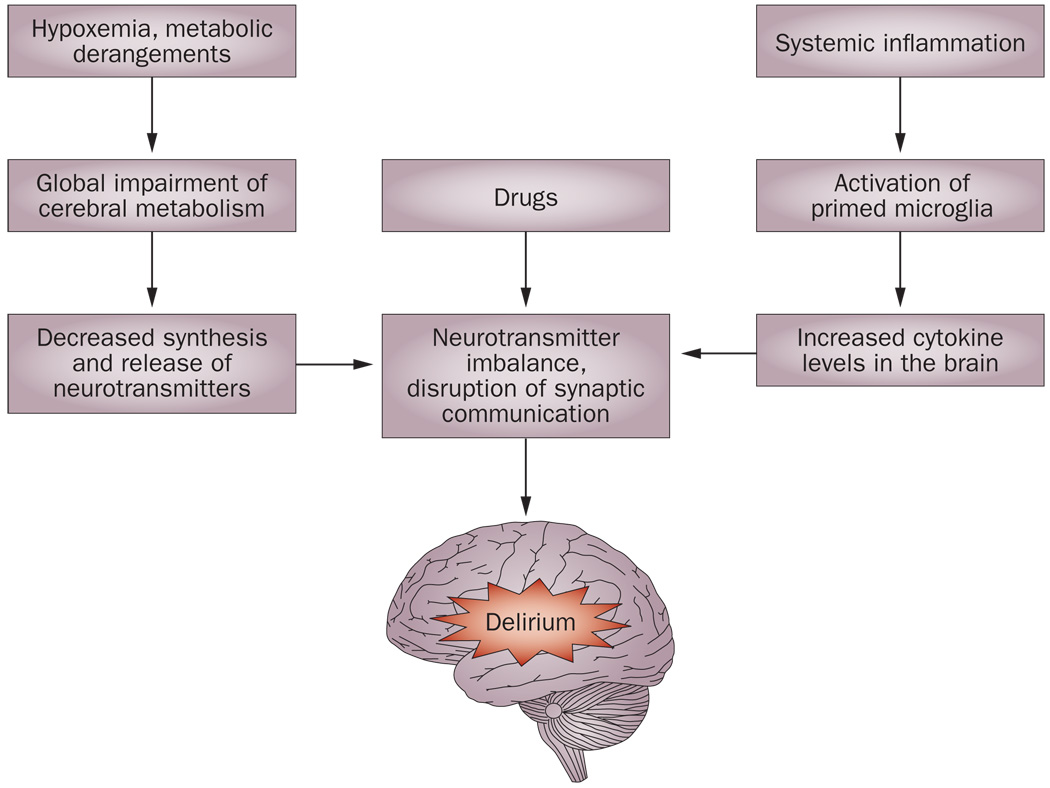
The pathophysiology of delirium is not fully understood, and the condition might arise through a variety of different pathogenic mechanisms. Current evidence suggests that drug toxicity, inflammation and acute stress responses can all contribute markedly to disruption of neurotransmission, and, ultimately, to the development of delirium (Figure 1).
Figure 1.
Relationships between various etiological factors in delirium. Systemic inflammation can be the result of systemic infection, trauma or surgery. Neurotransmitters with possible roles in delirium include acetylcholine, dopamine, 5-hydroxytryptamine, norepinephrine, glutamate and γ-aminobutyric acid.
Neurotransmission
The cholinergic system has a key role in cognition and attention, and it is not surprising, therefore, that there is extensive evidence to support a role for cholinergic deficiency in delirium.14 Anticholinergic drugs can induce delirium and often contribute substantially to the delirium seen in hospitalized patients.16 Increasing acetylcholine levels by use of cholinesterase inhibitors such as physostigmine has been shown to reverse delirium associated with anticholinergic drugs.17–19 Serum anticholinergic activity, which reflects anticholinergic influences of both endogenous and exogenous drugs and their metabolites, has been shown in some studies to be increased in patients with delirium and to decline with the resolution of delirium.20–22 By contrast, other studies did not find a clear association between serum anticholinergic activity and delirium,23,24 but this might be because serum anticholinergic activity does not accurately reflect central cholinergic function. Other neurotransmitter abnormalities that are associated with delirium include elevated brain dopaminergic function, and a relative imbalance between the dopaminergic and cholinergic systems.25 The use of antiparkinsonian drugs can cause delirium, and dopamine antagonists such as haloperidol are effective at controlling the symptoms of delirium.26 The neurotransmitters glutamate, γ-aminobutyric acid, 5-hydroxytryptamine (5-HT) and norepinephrine are also hypothesized to be linked to delirium.27
Key points.
Delirium is a frequent cause and a serious complication of hospitalization and has important implications from both a functional and an economic standpoint
Delirium is potentially preventable and treatable, but major barriers, including underrecognition of the syndrome and poor understanding of the underlying pathophysiology, have hampered the development of successful therapies
Neuroimaging has identified structural changes, including cortical atrophy, ventricular dilatation and white matter lesions, to be predictors of delirium
Current evidence suggests that disruption of neurotransmission, inflammation or acute stress responses might contribute markedly to the development of delirium
Delirium is not always transient and reversible, and it can result in long-term cognitive changes
Inflammation
Increasing experimental and clinical evidence is available to suggest that trauma, infection or surgery can lead to increased production of proinflammatory cytokines,28 which might, in susceptible individuals, induce delirium.29 Peripherally secreted cytokines can provoke exaggerated responses from microglia, thereby causing severe inflammation in the brain.30 Proinflammatory cytokines can substantially affect the synthesis or release of acetylcholine, dopamine, norepinephrine and 5-HT, thereby disrupting neuronal communication,31 and they can also impart a direct neurotoxic effect.32 Furthermore, proinflammatory cytokine levels have been shown to be elevated in patients with delirium.33–35 The presence of low-grade inflammation associated with chronic neurodegenerative changes in the brains of patients with dementia might explain why these individuals are at an increased risk of delirium.
Acute stress response
High levels of cortisol associated with acute stress have been hypothesized to precipitate and/or sustain delirium.36 Steroids can cause impairment in cognitive function (steroid psychosis), although not all patients treated with high-dose steroids will develop this condition. In elderly patients, feedback regulation of cortisol might be impaired, resulting in higher levels of baseline cortisol and thereby predisposing this population to delirium. A number of studies have identified elevated levels of cortisol in patients who developed postoperative delirium.37,38 Other studies have found abnormal suppression in the dexamethasone suppression test—a result that indicates impaired cortisol regulation, leading to increased levels of cortisol—in patients with delirium.39–41 The role of cortisol in delirium merits further investigation.29
Neuronal injury
Delirium associated with direct neuronal injury can be caused by a variety of metabolic or ischemic insults to the brain. Hypoxemia, hypoglycemia and various metabolic derangements can cause energy deprivation, which leads to impaired synthesis and release of neurotransmitters, as well as impaired propagation of nerve impulses across neural networks involved in attention and cognition.36
Neuroimaging findings
Neuroimaging has contributed to our understanding of the underlying pathophysiology of delirium.42 In elderly patients with delirium attributable to various etiologies, imaging has revealed marked cortical atrophy in the prefrontal cortex, temporoparietal cortex, and fusiform and lingual gyri in the nondominant hemisphere, and atrophy of deep structures, including the thalamus and basal ganglia. Other features that are observed include ventricular dilatation, white matter changes, and basal ganglia lesions.43 These imaging changes probably reflect a state of increased vulnerability of the brain to any insult, with an increased predisposition towards the development of delirium. Another study, however, failed to uncover any significant structural differences on CT scans between patients with and those without delirium.44
To date, relatively few studies have used functional imaging to study brain changes in delirium. One prospective study of hospitalized patients with delirium of various etiologies used single-photon emission CT (SPECT) imaging, and found frontal and parietal hypoperfusion in half of the patients.12 Other studies that made use of SPECT imaging, mostly in patients with hepatic encephalopathy (a form of delirium caused by liver failure), revealed various hypoperfusion patterns, including involvement of the thalamus, basal ganglia, occipital lobes and anterior cingulate gyrus.45–47 The perfusion patterns reported were inconsistent, although some of the studies were statistically underpowered. In a single study with xenon-enhanced CT, global perfusion was decreased during delirium.13 If this finding can be replicated, it would suggest that delirium might result from brain dysfunction across multiple regions.
Rapid advances in neuroimaging technology offer the exciting prospect of applying new methods to elucidate the mechanisms of delirium. These methods include MRI with volumetric analysis, which can be useful in the estimation of the brain atrophy rate following delirium or the determination of threshold atrophy levels that predispose individuals to delirium. Diffusion tensor imaging and tractography can help to assess damage to fiber tracts that connect different areas of the brain. Arterial spin labeling perfusion measures blood flow and can be used to assess both resting brain perfusion and response to medications. MRI can also be employed to evaluate the integrity of the blood–brain barrier and its role in the development of delirium. Finally, the use of new tracers in PET and SPECT imaging should aid the imaging of cholinergic receptors and dopaminergic activity.48
Approach to patient evaluation
Clinical features
The clinical presentation of delirium is variable but can be classified broadly into three subtypes—hypoactive, hyperactive and mixed—on the basis of psychomotor behavior.49 Patients with hyperactive delirium demonstrate features of restlessness, agitation and hyper vigilance and often experience hallucinations and delusions. By contrast, patients with hypoactive delirium present with lethargy and sedation, respond slowly to questioning, and show little spontaneous movement. The hypoactive form occurs most frequently in elderly patients, and these patients are frequently overlooked or misdiagnosed as having depression or a form of dementia. Patients with mixed delirium demonstrate both hyperactive and hypoactive features. It has been suggested that each delirium subtype can result from a different pathophysiological mechanism, and that each might carry a different prognosis.
Postoperative delirium can develop on the first or second postoperative day, but the condition is often hypoactive and might, therefore, go unnoticed. Delirium can be difficult to recognize in the ICU, as standard cognitive tests of attention often cannot be used in this setting because patients are intubated and cannot answer questions verbally. However, alternative strategies are available for testing in this situation (see below).
Diagnostic criteria
The current standard for the diagnosis of delirium appears in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR®; American Psychiatric Publishing, Inc., Arlington, VA; Box 2). The diagnosis of delirium is made on the basis of clinical history, behavioral observation and cognitive assessment. The history should confirm that an acute change in baseline cognitive function has occurred. It is important to ascertain the time course of the mental status changes, as well as any history of intercurrent illnesses, medication usage (including any changes in medication and use of over-the-counter and herbal products), alcohol withdrawal, and changes in the environment. Conditions that mimic delirium (Table 1) should be excluded. Attention can easily be measured at the bedside with simple tests such as digit span or recitation of the months of the year backwards. For patients in the ICU who are unable to speak, assessment methods such as the Intensive Care Delirium Screening Checklist or the Confusion Assessment method for the ICU, described in further detail in Table 2, can be used. Patients with delirium can also demonstrate nonspecific focal findings, such as asterixis or tremor on neurological examination, although the presence of any new neurological deficit, particularly with accompanying focal neurological signs, should raise suspicion of an acute cerebrovascular event or subdural hematoma. In many elderly patients and in individuals with cognitive impairment, delirium could be the initial manifestation of a new serious disease.
Box 2 | Diagnostic criteria for delirium.
The following criteria are derived from the Diagnostic and Statistical Manual of Mental Disorders, 4th edn, text revision (DSM-IV-TR®; American Psychiatric Publishing, Inc., Arlington, VA). All four criteria (A–D) are required to confirm a diagnosis of delirium.
General diagnostic criteria
(A) Disturbance of consciousness (that is, reduced clarity of awareness of the environment) with reduced ability to focus, sustain, or shift attention
(B) A change in cognition (such as memory deficit, disorientation, language disturbance) or the development of a perceptual disturbance that is not better accounted for by a pre-existing, established, or evolving dementia
(C) The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of the day
For delirium due to a general medical condition
(D) Evidence from the history, physical examination, or laboratory findings indicates that the disturbance is caused by the direct physiological consequences of a general medical condition
For substance intoxication delirium
(D) Evidence from the history, physical examination, or laboratory findings indicates that of either (1) the symptoms in Criteria A and B developed during substance intoxication, or (2) medication use is etiologically related to the disturbance
For substance withdrawal delirium
(D) History, physical examination, or laboratory findings indicate that the symptoms in Criteria A and B developed during, or shortly after, a withdrawal syndrome
For delirium due to multiple etiologies
(D) History, physical examination, or laboratory findings indicate that the delirium has more than one etiology (for example, more than one etiological general medical condition, a general medical condition plus substance intoxication or medication side effect)
Table 1.
Differentiating features of conditions that mimic delirium
| Feature | Condition | |||
|---|---|---|---|---|
| Delirium | Alzheimer disease | Psychotic disorders | Depression | |
| Descriptive features | Confusion and Inattention | Memory loss | Loss of contact with reality | Sadness, anhedonia |
| Onset | Acute | Insidious | Acute or slow | Slow |
| Course | Fluctuating, often worse at night |
Chronic, progressive (but stable over the course of a day) |
Chronic, with exacerbations | Single or recurrent episodes; can be chronic |
| Duration | Hours to months | Months to years | Months to years | Weeks to months |
| Consciousness | Altered | Normal | Normal | Normal |
| Attention | Impaired | Normal, except in late stages |
May be impaired | May be impaired |
| Orientation | Fluctuates | Poor | Normal | Normal |
| Speech | Incoherent | Mild errors | Normal or pressured | Normal or slow |
| Thought | Disorganized | Impoverished | Disorganized | Normal |
| Illusions and hallucinations |
Common (often visual) | Rare, except in late stages |
Common | Not usually |
| Perceptions | Altered | Altered or normal | Altered | Normal |
| Psychomotor changes | Yes | No | Yes | Yes |
| Reversibility | Usually | Rarely | Rarely | Possibly |
| EEG reading | Moderate to severe background slowing |
Normal or mild diffuse slowing |
Normal | Normal |
Table 2.
Tools for the assessment of delirium
| Tool | Description | Reference |
|---|---|---|
| CAM | Most widely used screening test for the presence of delirium; a four-item instrument based on DSM-III-R delirium criteria, requires the presence of acute onset and fluctuating course, inattention, and disorganized thinking or loss of consciousness |
Inouye et al. (1990)52 Wei et al. (2008)53 |
| CAM–ICU | Delirium is diagnosed when patients demonstrate an acute change in mental status or fluctuating changes in mental status, inattention measured with either an auditory or a visual test, and either disorganized thinking or an altered level of consciousness. Importantly, the CAM–ICU can only be administered if the patient is arousable in response to a voice without the need for physical stimulation |
Ely et al. (2001)113 Ely et al. (2001)114 |
| Drs-R98 | 16-item scale, including 13 severity items and 3 diagnostic items. Severity scores range from 0 to 39, with higher scores indicating more-severe delirium; delirium typically involves scores ≥15 points |
Trzepacz et al. (2001)115 |
| DSI | A structured interview detects the presence or absence of seven DSM-III criteria for delirium; delirium is said to be present if disorientation, perceptual disturbance or disturbance of consciousness have presented within the past 24h |
Albert et al. (1992)116 |
| MDAS | Measures delirium severity on a 10-item, four-point observer-rated scale with scores that range from 0 to 30 |
Breitbart et al. (1997)54 |
| NEECHAM Confusion Scale |
Nine scaled items divided into three subscales: subscale I, information processing (score range 0–14 points), evaluates components of cognitive status; subscale II, behavior (score range 0–10 points), evaluates observed behavior and performance ability; subscale III, performance (score range 0–16 points), assesses vital function (that is, vital signs, oxygen saturation level and urinary incontinence). Total scores can range from 0 (minimal function) to 30 (normal function). Delirium is present if the score is ≤ 24 points |
Neelon et al. (1996)117 |
| ICDSC | Bedside screening tool for delirium in the intensive care unit setting; eight-item checklist based on DSM-IV® criteria, items scored as 1 (present) or 0 (absent); a score ≥ 4 points indicates delirium |
Bergeron et al. (2001)118 |
| Cognitive Test for Delirium |
Can be used with patients unable to speak or write; assesses orientation, attention, memory, comprehension and vigilance, primarily with visual and auditory modalities. Each individual domain is scored 0–6 in two-point increments, except for comprehension, which is scored in single-point increments. Total scores range from 0 to 30, with higher scores indicating better cognitive function |
Hart et al. (1997)119 Hart et al. (1996)120 |
Abbreviations: CAM, Confusion Assessment Method; CAM–ICU, Confusion Assessment Method–Intensive Care Unit; Drs-r98, Delirium Rating Scale; DSI, Delirium Symptom Interview; DSM, Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, Arlington, VA); ICDSC, Intensive Care Delirium Screening Checklist; MDAS, Memorial Delirium Assessment Scale.
Once a diagnosis of delirium has been established, the potential cause—in particular, any life-threatening contributors—must be determined. Delirium should be considered to be a medical emergency until proven otherwise; mortality rates for patients admitted to hospital with delirium can range from 10% to 26%.50 Basic medical care, including airway protection, assessment of vital signs, and laboratory tests to exclude treatable conditions such as infections, should be administered.
Neuroimaging is performed in selected patients to exclude a focal structural abnormality, such as an acute stroke, that might mimic delirium in its presentation. However, the diagnostic yield of these scans can be quite low. In one study, for example, the risk of finding a focal lesion on neuroimaging was just 7% for patients who had no focal neurological signs, and in the presence of fever, dehydration and a history of dementia, the probability of finding a focal lesion decreased to 2%.51
Tools for evaluation
In view of the fact that cognitive impairment can be missed during routine examination, a brief cognitive assessment should be included in the physical examination of patients at risk of delirium. A standardized tool, the Confusion Assessment method (CAM), provides a brief, validated diagnostic algorithm that is currently in widespread use for the identification of delirium.52,53 The CAM algorithm relies on the presence of acute onset of symptoms and a fluctuating course, inattention, and either disorganized thinking or an altered level of consciousness. The algorithm has a sensitivity of 94–100%, a specificity of 90–95%, and high inter-rater reliability when administered by trained interviewers.52 In a recent meta-analysis in 1,071 patients, the CAM had a sensitivity of 94% and a specificity of 89%.53 The performance of the CAM might be compromised, however, if it is used without formal cognitive testing or by untrained interviewers. Once delirium is identified, the memorial Delirium Assessment Scale, a 10-item rating scale, can be used to quantify delirium severity.54 Other commonly used delirium screening and severity measures are summarized in Table 2.
Management
Prevention strategies
An estimated 30–40% of cases of delirium are preventable,7 and prevention is the most effective strategy for minimizing the occurrence of delirium and its adverse outcomes. Drugs such as benzodiazepines or anticholinergics and other known precipitants of delirium should generally be avoided. In addition, benzodiazepine or alcohol withdrawal is a common preventable cause of delirium.
The Hospital elder life Program (HELP)55 is an innovative strategy of hospital care for elderly patients that uses tested delirium prevention strategies to improve overall quality of hospital care. This program includes the following: maintaining orientation to surroundings; meeting needs for nutrition, fluids and sleep; promoting mobility within the limitations of physical condition; and providing visual and hearing adaptations for patients with sensory impairments. In a controlled trial that evaluated HELP, delirium developed in 9.9% of the intervention group, compared with 15.0% of the usual-care group (matched odds ratio 0.60, 95% CI 0.39–0.92). The HELP interventions can also effectively reduce the total number of episodes and days of delirium in hospitalized elderly individuals.56 Proactive geriatric consultation has been found to reduce the risk of delirium following acute hip fracture by 40%.57 Other controlled trials testing delirium interventions found that multifactorial interventions or educational strategies targeted towards health-care staff can reduce delirium rates and/or duration.56 A recent controlled trial also found that home rehabilitation after acute hospitalization in elderly individuals was associated with a lower risk of delirium, and greater patient satisfaction, when compared with the inpatient hospital setting.58
Recent studies have examined the role of pharmacological strategies in delirium prophylaxis. Haloperidol has been shown to reduce the incidence of delirium in a small group of patients who underwent surgery.59 This reduction in incidence was not confirmed statistically in a larger study,60 but haloperidol did reduce the severity and duration of delirium and length of hospital stay in some patients without causing notable adverse effects. Owing to methodological limitations and small sample sizes, these results need to be confirmed before haloperidol can be recommended for routine prophylaxis.
The few randomized, controlled clinical trials of cholinesterase inhibitors that have been performed to date have shown no benefit for these drugs in the prevention of postoperative delirium, but these studies were small and underpowered.61,62 Several case reports and one open-label study have suggested promising results with this approach,63–66 but additional randomized, controlled studies of cholinesterase inhibitors in acute medical and critical care populations, as well as the use of these drugs in combination with antipsychotics, are warranted before any definitive recommendations can be made.67 Other strategies that minimize the use of opioids or benzodiazepines through the use of alternative agents such as gabapentin68 or dexmedetomidine69 are under investigation for their capacity to reduce the incidence of delirium.
Treatment strategies
Nonpharmacological acute treatment strategies
Nonpharmacological strategies are the first-line treatments for all patients with delirium. The nonpharmaco logical approaches available include reorientation and behavioral intervention. Caregivers should use clear instructions and make frequent eye contact with patients. Sensory impairments, such as vision and hearing loss, should be minimized by use of equipment such as spectacles or hearing aids. Physical restraints should be avoided because they lead to decreased mobility, increased agitation, greater risk of injury, and prolongation of delirium. Other environmental interventions include limiting room and staff changes and providing a quiet patient-care setting, with low-level lighting at night. An environment with minimal noise allows an uninterrupted period of sleep at night and is of crucial importance in the management of delirium. Only a limited number of trials have examined the efficacy of cognitive, emotional and environmental interventions in delirium,70–74 but the use of such supportive measures has nevertheless become standard practice on the basis of clinical experience, common sense, and lack of adverse effects.75
To minimize the use of psychoactive medications, a nonpharmacological sleep protocol should be used. This protocol includes three components: first, a glass of warm milk or herbal tea; second, relaxation tapes or relaxing music; and third, back massage. This protocol has been demonstrated to be both feasible and effective, and, in one study, implementation of this strategy reduced the use of sleeping medications from 54% to 31% (P <0.002) in a hospital environment.76 This intervention strategy is part of a multicomponent prevention strategy that has been demonstrated to be effective.76,77
Pharmacological strategies
A systematic review of acute drug treatments for delirium indicated that few high-quality, randomized, controlled trials have been performed to date,67 and current clinical practice is, therefore, based largely on case series and retrospective reports.78,79 medications (Table 3) are usually reserved for patients in whom the symptoms of delirium might compromise safety or prevent necessary medical treatment (that is, those with hyperactive delirium). Some clinicians advocate the use of drugs for the treatment of hypoactive delirium, although this approach remains controversial. Given that patients with hypoactive delirium can experience distress, such treatment might be warranted. Some data indicate that treatment efficacy or even treatment choice might vary according to the delirium subtype,80 and this is an area that requires further study. A particular challenge that is inherent to drug trials in delirium is the evaluation of drug efficacy in the setting of a fluctuating course and simultaneous treatment of underlying risk factors.67
Table 3.
Pharmacological therapy for delirium
| Drug | Dose | Adverse effects | Comments |
|---|---|---|---|
| Acute therapy | |||
| Antipsychoticsa | |||
| Haloperidol | 0.5–1 mg PO or IM; can repeat every 4h (PO) or every 60 min (IM) |
Extrapyramidal syndrome, prolonged QT interval |
Randomized, controlled trials demonstrate reduction in symptom severity and duration81,82 |
| Atypical antipsychoticsa | |||
| Risperidone | 0.5 mg BID | Extrapyramidal syndrome, prolonged QT interval |
Randomized, controlled trials comparing effcacy against haloperidol showed comparable response rates82–84 |
| Olanzapine | 2.5–5 mg daily | ||
| Quetiapine | 25 mg BID | ||
| Benzodiazepinesb | |||
| Lorazepam | 0.5–1 mg PO; can repeat every 4h |
Paradoxical excitation, respiratory depression, excessive sedation, confusion |
Did not show improvement in condition; treatment limited by adverse effects81 |
| Cholinesterase inhibitorsc | |||
| Donepezil | 5 mg QD | Nausea, vomiting, diarrhea | No randomized, controlled studies have been conducted; some case studies have indicated promise63–65 |
| Prophylactic therapies (potential)c | |||
| Antipsychotics | |||
| Haloperidol | 0.5–1 mg PO or IM; can repeat every 4h (PO) or every 60 min (IM) |
Extrapyramidal syndrome, prolonged QT interval |
Use in surgical cases may reduce delirium incidence;59 needs to be confirmed in additional studies |
| Cholinesterase inhibitors | |||
| Donepezil | 5 mg QD | Nausea, vomiting, diarrhea | Prevention studies have not demonstrated efficacy61,62 |
Antipsychotics are the most widely used drugs for the treatment of delirium-related agitation but can have marked adverse effects.
Benzodiazepines should be reserved for treatment of drug withdrawal, diffuse Lewy body disease, or as second-line treatment following failure of antipsychotics.
Not currently accepted clinical therapies
Abbreviations: BID, twice daily; IM, intramuscularly; PO, per os (by mouth); QD, once daily.
The use of almost any medication to treat behavioral changes might further cloud the patient’s mental status and obscure efforts to monitor the course of the mental status change, and should, therefore, be avoided if possible. Any drug chosen to treat delirium should be initiated at the lowest starting dose for the shortest time possible. In general, neuroleptics are the preferred agents for the treatment for acute agitation. Haloperidol has been the most widely used neuroleptic in this context, and the effectiveness of this drug has been established in randomized, controlled clinical trials.81,82 This agent also has the advantage of being available in parenteral form. Haloperidol is, however, associated with a higher rate of extrapyramidal side effects and acute dystonias than are atypical antipsychotics. Some atypical antipsychotics (for example, risperidone, olanzapine and quetiapine) have been used clinically to treat agitation in patients with delirium, with controlled trials showing efficacy at least comparable to haloperidol.82–84 However, no data are available to demonstrate any verifiable advantage of one antipsychotic over another.67 Furthermore, the antipsychotics, including the atypicals and parenteral haloperidol, carry an increased risk of stroke in elderly patients with dementia and can result in prolongation of the QT interval.85
Other potential treatments for delirium include cholinesterase inhibitors (for example, donepezil), and 5-HT receptor antagonists (for example, trazodone). Several case reports and one open-label study have suggested promising results with cholinesterase inhibitors in the treatment of delirium,63–66 but additional randomized, controlled studies of these agents in acute medical and critical care populations, and of their use in combination with antipsychotics, are warranted before any definitive recommendations can be made.67 Benzodiazepines, such as lorazepam, are not recommended as first-line agents in the treatment of delirium, because they often exacerbate mental status changes and cause oversedation.
Outcomes
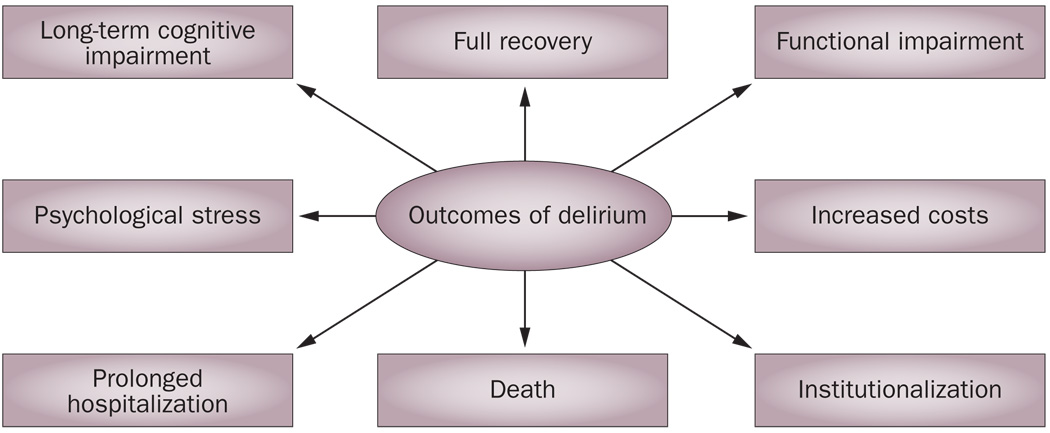
The occurrence of delirium, which can result from multiple and diverse etiologies, can contribute to poor patient outcome, irrespective of the underlying cause. The agitation and lethargy that can occur in delirium increase the risk of complications, including aspiration, pressure ulcers, pulmonary emboli, and decreased oral intake, and it has been shown that delirium is associated with inferior outcomes even after controlling for baseline patient characteristics and etiological factors.86 Also, the more severe the episode of delirium, the poorer the outcome.87 The outcomes of delirium are summarized in Figure 2.
Figure 2.
Outcomes of delirium.
Delirium has previously been characterized as an acute, severe and reversible condition. However, in some cases, symptoms endure despite treatment or resolution of the precipitating factor, resulting in persistent functional and cognitive losses.88,89 A spectrum ranging from persistent delirium88,90–94 to reversible dementia95 has been devised to characterize such cases.
Some patients never recover to their baseline level of cognitive function following an episode of delirium and demonstrate persistent functional and cognitive losses.88,89 For example, following an episode of delirium, patients can develop subjective memory complaints, show reduced performance on tests of executive functioning, attention, and processing speed, and achieve reduced scores on the mini-mental State examination.96–98 Such findings suggest that the pathological processes associated with delirium can cause direct neuronal injury, leading to persistent cognitive impairment.
Newly diagnosed dementia following a hospitalization that is complicated by delirium has also been observed,99 and some investigators have proposed that delirium has an increased likelihood of occurring in patients with incipient dementia. It has also been observed that delirium can accelerate the rate of progression of dementia.100 Outcomes for patients with dementia who develop delirium are worse than for those who do not develop this condition.88,89 In addition to showing worse cognitive function, patients with dementia who experience delirium have higher rates of hospitalization, institutionalization and death.101–103
Health-care quality and costs
Conditions such as delirium that are common, frequently iatrogenic, and linked to the care that patients receive in hospital, can be considered to be indicators of quality of health care.104 In fact, the National Quality Measures Clearinghouse™ of the Agency for Healthcare Research and Quality105 has determined the occurrence of delirium to be a marker of the quality of care and patient safety. Many aspects of hospital care, including adverse effects of medications, complications from procedures, immobilization, dehydration, poor nutrition, and sleep deprivation, are factors that can be modified to prevent the development of delirium. Delirium is an important independent determinant of hospital stay, mortality, rates of nursing home placement, and functional and cognitive decline. After adjusting for age, sex, dementia, illness severity, and baseline functional status, a higher delirium rate probably correlates with lower quality of hospital care, although variations in case mix and study populations need to be taken into consideration. Direct comparisons should be made with care, as delirium rates might also be increased in tertiary care settings that frequently offer care to patients who are particularly old and ill.10 Delirium has been identified as one of the top three conditions for which quality of care needs to improve.106
In line with observations that delirium can result in long-term clinical effects, the occurrence of the condition has important implications for health-care utilization and costs. Delirium results in increased nursing time per patient, higher per-day hospital costs, and an increased length of hospital stay.7 The resulting economic burden is substantial, with increased costs attributable to delirium estimated at US $2,500 per patient per hospitalization, totaling approximately $6.9 billion in medicare hospital expenditure (2004 figures).56,107 Further costs accrue after hospital discharge because of a greater need for long-term care or additional home health care, rehabilitation services, and informal caregiving. In a recent study looking at costs over 1 year following an episode of delirium, it was conservatively estimated that delirium is responsible for between $60,000 and $64,000 in additional health-care costs per patient with delirium per year; thus, total direct 1-year health-care costs attributable to delirium might range from $38 billion to up to $152 billion nationally.108
It is instructive to compare these figures with the estimated annual health-care costs for other conditions that affect elderly adults, including hip fracture ($7 billion),109 nonfatal falls ($19 billion),110 diabetes mellitus ($91.8 billion),111 and cardiovascular disease ($257.6 billion).112 evidently, there are limitations and difficulties in making such comparisons across conditions for which the study methodology might be different, but the fact remains that the economic burden of delirium is substantial. Given that a number of effective interventions have been developed to prevent or treat delirium, at least some of these costs might be avoidable, thereby emphasizing the need to recognize this common condition.
Conclusions and future directions
Many avenues of future research exist in the delirium field. For example, given that this condition is underrecognized and underdiagnosed, optimization of the diagnostic approach is essential, including identification of any biomarkers that could aid in the clinical diagnosis. While some markers of risk, such as dementia, have been identified, other populations might exist that are at high risk of developing delirium. It will also be important to establish whether the risk of delirium is influenced by genetic factors, cognitive and/or brain reserve, or even pre-existing brain abnormalities, such as atrophy or white matter disease.
From a pathophysiological perspective, it would be interesting to determine, in view of the association between dementia and delirium, whether the degree of amyloid pathology correlates with the risk of delirium or the likelihood of recovery from delirium. As mentioned above, the potential roles of inflammation and impaired cholinergic neurotransmission, and the interactions between these two factors, need further exploration. Also, it will be essential to determine the underlying pathophysiology in order to explain the diversity in delirium presentation, so as to advance the diagnosis and treatment of delirium.
With regard to treatment, current data support the use of antipsychotics and nonpharmacological treatment protocols. However, it will be necessary to conduct further randomized trials to evaluate other prevention and treatment strategies in multiple populations, stratified according to delirium subtype, associated comorbid dementia, or risk.
Several issues relating to outcomes also need to be clarified. For example, there is evidence for long-term effects on cognition following delirium, but how often this leads to permanent cognitive impairment, including mild cognitive impairment or dementia, is still not known. Also, it is not yet clear whether delirium leads to permanent neurological injury that can be measured with laboratory, electrophysiological or neuroimaging markers.
Delirium is a serious cause and complication of hospitalization in elderly patients and should be considered to be a medical emergency until proven otherwise. Irrespective of the specific etiology, this condition has the potential to markedly affect the overall outcome and prognosis of severely ill patients, as well as substantially increasing health-care utilization and costs. For these reasons, prevention, early recognition and effective treatment of delirium are essential.
Review criteria.
A comprehensive literature review was performed in PubMed (1990–2008), using the keyword “delirium” in combination with one other search term to review major areas including the following: “epidemiology”, “clinical features”, “pathogenesis”, “acetylcholine”, “dopamine”, “inflammation”, “neuroimaging”, “evaluation”, “treatment” and “prevention”. Only original articles in the English language were included. The Hospital Elder Life Program (HELP) website bibliography (http://www.hospitalelderlifeprogram.org), a comprehensive reference resource on delirium, was also searched for relevant articles on delirium.
Acknowledgments
The authors are supported by NIA PHS Grants K24AG000949 (SK Inouye) and K23AG031320 (TG Fong), and Grant IIRG-08-88737 (SK Inouye) from the Alzheimer’s Association. Désirée Lie, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Footnotes
Competing interests
The authors, the Journal editor H wood and the CME questions author D Lie declared no competing interests.
References
- 1.Chadwick J, Mann MN. The Medical Works of Hippocrates. Oxford: Blackwell; 1950. [Google Scholar]
- 2.Morandi A, et al. Understanding international differences in terminology for delirium and other types of acute brain dysfunction in critically ill patients. Intensive Care Med. 2008;34:1907–1915. doi: 10.1007/s00134-008-1177-6. [DOI] [PubMed] [Google Scholar]
- 3.Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement. Geriatr. Cogn. Disord. 1999;10:393–400. doi: 10.1159/000017177. [DOI] [PubMed] [Google Scholar]
- 4.Inouye SK. Delirium in hospitalized older patients: recognition and risk factors. J. Geriatr. Psychiatry Neurol. 1998;11:118–125. doi: 10.1177/089198879801100302. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services. CMS statistics. Washington, DC: Centers for Medicare and Medicaid Services; 2004. (publication no. 03445) [Google Scholar]
- 6.Inouye SK. Delirium in hospitalized older patients. Clin. Geriatr. Med. 1998;14:745–764. [PubMed] [Google Scholar]
- 7.Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35:350–364. doi: 10.1093/ageing/afl005. [DOI] [PubMed] [Google Scholar]
- 8.Bruce AJ, Ritchie CW, Blizard R, Lai R, Raven P. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int. Psychogeriatr. 2007;19:197–214. doi: 10.1017/S104161020600425X. [DOI] [PubMed] [Google Scholar]
- 9.Girard TD, Ely EW. Delirium in the critically ill patient. Handb. Clin. Neurol. 2008;90:39–56. doi: 10.1016/S0072-9752(07)01703-4. [DOI] [PubMed] [Google Scholar]
- 10.Inouye SK. Delirium in older persons. N. Engl. J. Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 11.Pisani MA, McNicoll L, Inouye SK. Cognitive impairment in the intensive care unit. Clin. Chest Med. 2003;24:727–737. doi: 10.1016/s0272-5231(03)00092-3. [DOI] [PubMed] [Google Scholar]
- 12.Fong TG, et al. Cerebral perfusion changes in older delirious patients using 99mTc HMPAO SPECT. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1294–1299. doi: 10.1093/gerona/61.12.1294. [DOI] [PubMed] [Google Scholar]
- 13.Yokota H, Ogawa S, Kurokawa A, Yamamoto Y. Regional cerebral blood flow in delirium patients. Psychiatry Clin. Neurosci. 2003;57:337–339. doi: 10.1046/j.1440-1819.2003.01126.x. [DOI] [PubMed] [Google Scholar]
- 14.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:764–772. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eikelenboom P, Hoogendijk WJ. Do delirium and Alzheimer’s dementia share specific pathogenetic mechanisms? Dement. Geriatr. Cogn. Disord. 1999;10:319–324. doi: 10.1159/000017162. [DOI] [PubMed] [Google Scholar]
- 16.Han L, et al. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch. Intern. Med. 2001;161:1099–1105. doi: 10.1001/archinte.161.8.1099. [DOI] [PubMed] [Google Scholar]
- 17.Blitt CD, Petty WC. Reversal of lorazepam delirium by physostigmine. Anesth. Analg. 1975;54:607–608. doi: 10.1213/00000539-197509000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Mendelson G. Pheniramine aminosalicylate overdosage. Reversal of delirium and choreiform movements with tacrine treatment. Arch. Neurol. 1977;34:313. doi: 10.1001/archneur.1977.00500170067014. [DOI] [PubMed] [Google Scholar]
- 19.Schuster P, Gabriel E, Kufferle B, Strobl G, Karobath M. Reversal by physostigmine of clozapine-induced delirium. Clin. Toxicol. 1977;10:437–441. doi: 10.3109/15563657709046281. [DOI] [PubMed] [Google Scholar]
- 20.Flacker JM, et al. The association of serum anticholinergic activity with delirium in elderly medical patients. Am. J. Geriatr. Psychiatry. 1998;6:31–41. [PubMed] [Google Scholar]
- 21.Mach JR, et al. Serum anticholinergic activity in hospitalized older persons with delirium: a preliminary study. J. Am. Geriatr. Soc. 1995;43:491–495. doi: 10.1111/j.1532-5415.1995.tb06094.x. [DOI] [PubMed] [Google Scholar]
- 22.Mussi C, Ferrari R, Ascari S, Salvioli G. Importance of serum anticholinergic activity in the assessment of elderly patients with delirium. J. Geriatr. Psychiatry Neurol. 1999;12:82–86. doi: 10.1177/089198879901200208. [DOI] [PubMed] [Google Scholar]
- 23.Flacker JM, Lipsitz LA. Serum anticholinergic activity changes with acute illness in elderly medical patients. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:M12–M16. doi: 10.1093/gerona/54.1.m12. [DOI] [PubMed] [Google Scholar]
- 24.Thomas C, et al. Serum anticholinergic activity and cerebral cholinergic dysfunction: an EEG study in frail elderly with and without delirium. BMC Neurosci. 2008;9:86. doi: 10.1186/1471-2202-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin. Clin. Neuropsychiatry. 2000;5:132–148. doi: 10.153/SCNP00500132. [DOI] [PubMed] [Google Scholar]
- 26.Young BK, Camicioli R, Ganzini L. Neuropsychiatric adverse effects of antiparkinsonian drugs. Characteristics, evaluation and treatment. Drugs Aging. 1997;10:367–383. doi: 10.2165/00002512-199710050-00005. [DOI] [PubMed] [Google Scholar]
- 27.Gaudreau JD, Gagnon P. Psychotogenic drugs and delirium pathogenesis: the central role of the thalamus. Med. Hypotheses. 2005;64:471–475. doi: 10.1016/j.mehy.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph JL, et al. Chemokines are associated with delirium after cardiac surgery. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:184–189. doi: 10.1093/gerona/63.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J. Psychosom. Res. 2008;65:229–238. doi: 10.1016/j.jpsychores.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J. Leukoc. Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn AJ. Effects of cytokines and infections on brain neurochemistry. Clin. Neurosci. Res. 2006;6:52–68. doi: 10.1016/j.cnr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eikelenboom P, Hoogendijk WJ, Jonker C, van Tilburg W. Immunological mechanisms and the spectrum of psychiatric syndromes in Alzheimer’s disease. J. Psychiatr. Res. 2002;36:269–280. doi: 10.1016/s0022-3956(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 33.de Rooij SE, van Munster BC, Korevaar JC, Levi M. Cytokines and acute phase response in delirium. J. Psychosom. Res. 2007;62:521–525. doi: 10.1016/j.jpsychores.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham C, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol. Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Munster BC, et al. Time-course of cytokines during delirium in elderly patients with hip fractures. J. Am. Geriatr. Soc. 2008;56:1704–1709. doi: 10.1111/j.1532-5415.2008.01851.x. [DOI] [PubMed] [Google Scholar]
- 36.Trzepacz PT, van der Mast R. The Neuropathophysiology of Delirium. Oxford: Oxford University Press; 2002. [Google Scholar]
- 37.Kudoh A, Takase H, Katagai H, Takazawa T. Postoperative interleukin-6 and cortisol concentrations in elderly patients with postoperative confusion. Neuroimmunomodulation. 2005;12:60–66. doi: 10.1159/000082365. [DOI] [PubMed] [Google Scholar]
- 38.Mcintosh TK, et al. Beta-endorphin, cortisol and postoperative delirium: a preliminary report. Psychoneuroendocrinology. 1985;10:303–313. doi: 10.1016/0306-4530(85)90007-1. [DOI] [PubMed] [Google Scholar]
- 39.Robertsson B, et al. Hyperactivity in the hypothalamic-pituitary-adrenal axis in demented patients with delirium. Int. Clin. Psychopharmacol. 2001;16:39–47. doi: 10.1097/00004850-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 40.O’Keeffe ST, Devlin JG. Delirium and the dexamethasone suppression test in the elderly. Neuropsychobiology. 1994;30:153–156. doi: 10.1159/000119154. [DOI] [PubMed] [Google Scholar]
- 41.McKeith IG. Clinical use of the DST in a psychogeriatric population. Br. J. Psychiatry. 1984;145:389–393. doi: 10.1192/bjp.145.4.389. [DOI] [PubMed] [Google Scholar]
- 42.Soiza RL, et al. Neuroimaging studies of delirium: a systematic review. J. Psychosom. Res. 2008;65:239–248. doi: 10.1016/j.jpsychores.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Burns A, Gallagley A, Byrne J. Delirium. J. Neurol. Neurosurg. Psychiatry. 2004;75:362–367. doi: 10.1136/jnnp.2003.023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kishi Y, Iwasaki Y, Takezawa K, Kurosawa H, Endo S. Delirium in critical care unit patients admitted through an emergency room. Gen. Hosp. Psychiatry. 1995;17:371–379. doi: 10.1016/0163-8343(95)00056-w. [DOI] [PubMed] [Google Scholar]
- 45.Jalan R, et al. Oral amino acid load mimicking hemoglobin results in reduced regional cerebral perfusion and deterioration in memory tests in patients with cirrhosis of the liver. Metab. Brain Dis. 2003;18:37–49. doi: 10.1023/a:1021978618745. [DOI] [PubMed] [Google Scholar]
- 46.Strauss GI, et al. Regional cerebral blood flow during mechanical hyperventilation in patients with fulminant hepatic failure. Hepatology. 1999;30:1368–1373. doi: 10.1002/hep.510300608. [DOI] [PubMed] [Google Scholar]
- 47.Yazgan Y, et al. Value of regional cerebral blood flow in the evaluation of chronic liver disease and subclinical hepatic encephalopathy. J. Gastroenterol. Hepatol. 2003;18:1162–1167. doi: 10.1046/j.1440-1746.2003.03141.x. [DOI] [PubMed] [Google Scholar]
- 48.Alsop DC, et al. The role of neuroimaging in elucidating delirium pathophysiology. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1287–1293. doi: 10.1093/gerona/61.12.1287. [DOI] [PubMed] [Google Scholar]
- 49.Lipowski ZJ. Transient cognitive disorders (delirium, acute confusional states) in the elderly. Am. J. Psychiatry. 1983;140:1426–1436. doi: 10.1176/ajp.140.11.1426. [DOI] [PubMed] [Google Scholar]
- 50.McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch. Intern. Med. 2002;162:457–463. doi: 10.1001/archinte.162.4.457. [DOI] [PubMed] [Google Scholar]
- 51.Hufschmidt A, Shabarin V. Diagnostic yield of cerebral imaging in patients with acute confusion. Acta Neurol. Scand. 2008;118:245–250. doi: 10.1111/j.1600-0404.2008.01006.x. [DOI] [PubMed] [Google Scholar]
- 52.Inouye SK, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann. Intern. Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 53.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J. Am. Geriatr. Soc. 2008;56:823–830. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Breitbart W, et al. The Memorial Delirium Assessment scale. J. Pain Symptom Manage. 1997;13:128–137. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 55.The Hospital elder Life Program (HELP) http://www.hospitalelderlifeprogram.org.
- 56.Inouye SK, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N. Engl. J. Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 57.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J. Am. Geriatr. Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 58.Caplan GA, Coconis J, Board N, Sayers A, Woods J. Does home treatment affect delirium? A randomised controlled trial of rehabilitation of elderly and care at home or usual treatment (The REACH-OUT trial) Age Ageing. 2006;35:53–60. doi: 10.1093/ageing/afi206. [DOI] [PubMed] [Google Scholar]
- 59.Kaneko T, et al. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta. Med. 1999;42:179–184. [Google Scholar]
- 60.Kalisvaart KJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J. Am. Geriatr. Soc. 2005;53:1658–1666. doi: 10.1111/j.1532-5415.2005.53503.x. [DOI] [PubMed] [Google Scholar]
- 61.Liptzin B, Laki A, Garb JL, Fingeroth R, Krushell R. Donepezil in the prevention and treatment of post-surgical delirium. Am. J. Geriatr. Psychiatry. 2005;13:1100–1106. doi: 10.1176/appi.ajgp.13.12.1100. [DOI] [PubMed] [Google Scholar]
- 62.Sampson EL, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int. J. Geriatr. Psychiatry. 2007;22:343–349. doi: 10.1002/gps.1679. [DOI] [PubMed] [Google Scholar]
- 63.Noyan MA, Elbi H, Aksu H. Donepezil for anticholinergic drug intoxication: a case report. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:885–887. doi: 10.1016/s0278-5846(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 64.Slatkin N, Rhiner M. Treatment of opioid-induced delirium with acetylcholinesterase inhibitors: a case report. J. Pain Symptom Manage. 2004;27:268–273. doi: 10.1016/j.jpainsymman.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Wengel SP, Roccaforte WH, Burke WJ. Donepezil improves symptoms of delirium in dementia: implications for future research. J. Geriatr. Psychiatry Neurol. 1998;11:159–161. doi: 10.1177/089198879801100308. [DOI] [PubMed] [Google Scholar]
- 66.Gleason OC. Donepezil for postoperative delirium. Psychosomatics. 2003;44:437–438. doi: 10.1176/appi.psy.44.5.437. [DOI] [PubMed] [Google Scholar]
- 67.Bourne RS, Tahir TA, Borthwick M, Sampson EL. Drug treatment of delirium: past, present and future. J. Psychosom. Res. 2008;65:273–282. doi: 10.1016/j.jpsychores.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 68.Leung JM, et al. Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients. Neurology. 2006;67:1251–1253. doi: 10.1212/01.wnl.0000233831.87781.a9. [DOI] [PubMed] [Google Scholar]
- 69.Levanen J, Makela ML, Scheinin H. Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology. 1995;82:1117–1125. doi: 10.1097/00000542-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 70.Budd S, Brown W. Effect of a reorientation technique on postcardiotomy delirium. Nurs. Res. 1974;23:341–348. [PubMed] [Google Scholar]
- 71.Cole M, et al. Systematic intervention for elderly inpatients with delirium: a randomized trial. Can. Med. Assoc. J. 1994;151:965–970. [PMC free article] [PubMed] [Google Scholar]
- 72.Lazarus H, Hagens J. Prevention of psychosis following open-heart surgery. Am. J. Psychiatry. 1968;124:1190–1195. doi: 10.1176/ajp.124.9.1190. [DOI] [PubMed] [Google Scholar]
- 73.Meagher D, O’Hanlon D, O’Mahony E, Casey P. The use of environmental strategies and psychotropic medication in the management of delirium. Br. J. Psychiatry. 1996;168:512–515. doi: 10.1192/bjp.168.4.512. [DOI] [PubMed] [Google Scholar]
- 74.Williams M, Campbell E, Raynor W, Mlynarczyk S, ward S. Reducing acute confusional states in elderly patients with hip fractures. Res. Nurs. Health. 1985;8:329–337. doi: 10.1002/nur.4770080405. [DOI] [PubMed] [Google Scholar]
- 75.American Psychiatric Association. Treatment of Patients with Delirium Practice Guideline. http://www.psych.org/psych_pract/treatg/pg/prac_guide.cfm.
- 76.McDowell JA, Mion LC, Lydon TJ, Inouye SK. A nonpharmacologic sleep protocol for hospitalized older patients. J. Am. Geriatr. Soc. 1998;46:700–705. doi: 10.1111/j.1532-5415.1998.tb03803.x. [DOI] [PubMed] [Google Scholar]
- 77.Inouye SK, Bogardus ST, Jr, Williams CS, Leo-summers L, Agostini JV. The role of adherence on the effectiveness of nonpharmacologic interventions: evidence from the delirium prevention trial. Arch. Intern. Med. 2003;163:958–964. doi: 10.1001/archinte.163.8.958. [DOI] [PubMed] [Google Scholar]
- 78.Lonergan E, Britton AM, Luxenberg J, Wyller T Antipsychotics for delirium. Cochrane Database of systematic reviews, 2007. 2007;(issue 2) doi: 10.1002/14651858.CD005594.pub2. Art. No.: CD005594. CD005594.pub2. [DOI] [PubMed] [Google Scholar]
- 79.Seitz DP, Gill SS, van Zyl LT. Antipsychotics in the treatment of delirium: a systematic review. J. Clin. Psychiatry. 2007;68:11–21. doi: 10.4088/jcp.v68n0102. [DOI] [PubMed] [Google Scholar]
- 80.Platt MM, et al. Efficacy of neuroleptics for hypoactive delirium. J. Neuropsychiatry Clin. Neurosci. 1994;6:66–67. doi: 10.1176/jnp.6.1.66. [DOI] [PubMed] [Google Scholar]
- 81.Breitbart W, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am. J. Psychiatry. 1996;153:231–237. doi: 10.1176/ajp.153.2.231. [DOI] [PubMed] [Google Scholar]
- 82.Hu H, Deng W, Yang H, Liu Y. Olanzapine and haloperidol for senile delirium: a randomized controlled observation. Chin. J. Clin. Rehab. 2006;10:188–190. [Google Scholar]
- 83.Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45:297–301. doi: 10.1016/S0033-3182(04)70170-X. [DOI] [PubMed] [Google Scholar]
- 84.Kim JY, et al. Antipsychotics and dopamine transporter gene polymorphisms in delirium patients. Psychiatry Clin. Neurosci. 2005;59:183–188. doi: 10.1111/j.1440-1819.2005.01355.x. [DOI] [PubMed] [Google Scholar]
- 85.US FDA Medwatch: Haloperidol (marketed as Haldol, Haldol decanoate, and Haldol lactate) http://www.fda.gov/medwatch/safety/2007/safety07.htm#Haloperidol.
- 86.Inouye S, Marcantonio E. Delirium. In: Growdon J, Rossor M, editors. The Dementias. Philadelphia: Butterworth-Heinemann Elsevier; 2007. pp. 285–312. [Google Scholar]
- 87.Marcantonio E, Ta T, Duthie E, Resnick NM. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J. Am. Geriatr. Soc. 2002;50:850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 88.Levkoff SE, et al. Delirium. The occurrence and persistence of symptoms among elderly hospitalized patients. Arch. Intern. Med. 1992;152:334–340. doi: 10.1001/archinte.152.2.334. [DOI] [PubMed] [Google Scholar]
- 89.Murray AM, et al. Acute delirium and functional decline in the hospitalized elderly patient. J. Gerontol. 1993;48:M181–M186. doi: 10.1093/geronj/48.5.m181. [DOI] [PubMed] [Google Scholar]
- 90.Cole M, McCusker J, Dendukuri N, Han L. The prognostic significance of subsyndromal delirium in elderly medical inpatients. J. Am. Geriatr. Soc. 2003;51:754–760. doi: 10.1046/j.1365-2389.2003.51255.x. [DOI] [PubMed] [Google Scholar]
- 91.Levkoff SE, Marcantonio ER. Delirium: a major diagnostic and therapeutic challenge for clinicians caring for the elderly. Compr. Ther. 1994;20:550–557. [PubMed] [Google Scholar]
- 92.Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J. Am. Geriatr. Soc. 2000;48:618–624. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 93.McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: a prospective study. J. Gen. Intern. Med. 2003;18:696–704. doi: 10.1046/j.1525-1497.2003.20602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rockwood K. The occurrence and duration of symptoms in elderly patients with delirium. J. Gerontol. 1993;48:M162–M166. doi: 10.1093/geronj/48.4.m162. [DOI] [PubMed] [Google Scholar]
- 95.Clarfield AM. The reversible dementias: do they reverse? Ann. Intern. Med. 1988;109:476–486. doi: 10.7326/0003-4819-109-6-476. [DOI] [PubMed] [Google Scholar]
- 96.Fann JR, Alfano CM, Roth-Roemer S, Katon WJ, Syrjala KL. Impact of delirium on cognition, distress, and health-related quality of life after hematopoietic stem-cell transplantation. J. Clin. Oncol. 2007;25:1223–1231. doi: 10.1200/JCO.2006.07.9079. [DOI] [PubMed] [Google Scholar]
- 97.Katz IR, et al. Validating the diagnosis of delirium and evaluating its association with deterioration over a one-year period. Am. J. Geriatr. Psychiatry. 2001;9:148–159. [PubMed] [Google Scholar]
- 98.McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165:575–583. [PMC free article] [PubMed] [Google Scholar]
- 99.Rahkonen T, Luukkainen-Markkula R, Paanila S, Sivenius J, Sulkava R. Delirium episode as a sign of undetected dementia among community dwelling elderly subjects: a 2 year follow up study. J. Neurol. Neurosurg. Psychiatry. 2000;69:519–521. doi: 10.1136/jnnp.69.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fong TG, et al. Delirium accelerates cognitive decline in Alzheimer’s disease. Neurology. doi: 10.1212/WNL.0b013e3181a4129a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baker FM, Wiley C, Kokmen E, Chandra V, Schoenberg BS. Delirium episodes during the course of clinically diagnosed Alzheimer’s disease. J. Natl Med. Assoc. 1999;91:625–630. [PMC free article] [PubMed] [Google Scholar]
- 102.Fick D, Foreman M. Consequences of not recognizing delirium superimposed on dementia in hospitalized elderly individuals. J. Gerontol. Nurs. 2000;26:30–40. doi: 10.3928/0098-9134-20000101-09. [DOI] [PubMed] [Google Scholar]
- 103.Rockwood K, et al. The risk of dementia and death after delirium. Age Ageing. 1999;28:551–556. doi: 10.1093/ageing/28.6.551. [DOI] [PubMed] [Google Scholar]
- 104.Williamson JW. Formulating priorities for quality assurance activity. Description of a method and its application. JAMA. 1978;239:631–637. [PubMed] [Google Scholar]
- 105. [accessed 13 February 2009];National Quality Measures Clearinghouse™ of the Agency for Healthcare research and Quality. doi: 10.1111/j.1945-1474.2004.tb00503.x. http://www.qualitymeasures.ahrq.gov/ [DOI] [PubMed]
- 106.Sloss EM, et al. Selecting target conditions for quality of care improvement in vulnerable older adults. J. Am. Geriatr. Soc. 2000;48:363–369. doi: 10.1111/j.1532-5415.2000.tb04691.x. [DOI] [PubMed] [Google Scholar]
- 107.Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care. Am. J. Med. 1999;106:565–573. doi: 10.1016/s0002-9343(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 108.Leslie DL, Marcantonio SR, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch. Intern. Med. 2008;168:27–32. doi: 10.1001/archinternmed.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haentjens P, Lamraski G, Boonen S. Costs and consequences of hip fracture occurrence in old age: an economic perspective. Disabil. Rehabil. 2005;27:1129–1141. doi: 10.1080/09638280500055529. [DOI] [PubMed] [Google Scholar]
- 110.Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj. Prev. 2006;12:290–295. doi: 10.1136/ip.2005.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the Us in 2002. Diabetes Care. 2003;26:917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 112.Thom T, et al. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 113.Ely EW, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM–ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 114.Ely EW, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM–ICU) Crit. Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 115.Trzepacz PT, et al. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J. Neuropsychiatry Clin. Neurosci. 2001;13:229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 116.Albert MS, et al. The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. J. Geriatr. Psychiatry Neurol. 1992;5:14–21. doi: 10.1177/002383099200500103. [DOI] [PubMed] [Google Scholar]
- 117.Neelon VJ, Champagne MT, Carlson JR, Funk SG. The NEECHAM Confusion Scale: construction, validation, and clinical testing. Nurs. Res. 1996;45:324–330. doi: 10.1097/00006199-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 118.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 119.Hart RP, Best AM, Sessler CN, Levenson JL. Abbreviated cognitive test for delirium. J. Psychosom. Res. 1997;43:417–423. doi: 10.1016/s0022-3999(97)00140-2. [DOI] [PubMed] [Google Scholar]
- 120.Hart RP, et al. Validation of a cognitive test for delirium in medical ICU patients. Psychosomatics. 1996;37:533–546. doi: 10.1016/S0033-3182(96)71517-7. [DOI] [PubMed] [Google Scholar]