Abstract
Synthetic nonionic surfactant vesicles (niosomes) are a colloidal system with closed bilayer structures, displaying distinct advantages in stability and cost compared to liposomes. In this paper, polysorbate cationic niosomes (PCNs) were developed as gene carriers. The PCNs comprised nonionic surfactants (i.e. polysorbates) and a cationic cholesterol, and were synthesized using a film hydration method. The niosomes thus prepared possessed a regular morphology, and a particle size of 100 ~ 200 nm, and a zeta potential of +30 ~ 45 mV. The PCNs showed great physical stability over the course of 4 weeks at room temperature. The binding capacity of PCNs toward oligodeoxynucleotides (ODN) was assessed by a gel retardation approach, which demonstrated that the ionic complexes were formed when +/− charge ratio reached to 4 or greater. Gene transfer study showed that the PCNs exhibited a high efficiency in mediating cellular uptake and transferred DNA expression. Based on these findings, PCNs may offer the potential to function as an effective gene delivery system.
Keywords: nonionic surfactant, synthetic vesicle, niosome, polysorbates, gene delivery
1 Introduction
Synthetic nonionic surfactant vesicles (niosomes), the closed bilayer vesicles resulting from self-assembly of non-ionic amphiphiles in aqueous media, bear similarity to liposomes in structure1. Like liposomes, they are also capable of entrapping either hydrophilic drug in the interior aqueous phase or hydrophobic drugs within their bilayer (Scheme 1). The biopharmaceutical functions of niosomes are quite like that of liposomes, such as prolonging circulation of entrapped drugs and altering their organ distribution as well as increasing metabolic stability2. Indeed, niosomes have been of great attraction due to their own intrinsic chemical properties and therefore often serve as an alternative to liposomes. At the same time, niosomes are recognized for the superiority in cost effectiveness, chemical stability and storage when compared to their relevant counterparts3. Niosomes have been widely employed in drug delivery meant to improve therapeutic efficacy of drugs (e.g. chemotherapy drugs2,4-6, peptides7-9, and protein vaccines10,11), and also been considered to be biodegradable, biocompatible and nonimmunogenic12. Nevertheless, the literature on the use of niosomes for gene delivery has a very limited amount.
Among various nonionic surfactants, sorbitan monoesters (Span®)13-15 and polysorbates (Tween®)16-19 are commonly used as additives to formulate lipid-based gene carriers. Compared to sorbitan monoesters, it is worth noting that not only are polysorbates a critical class of emulsifier and stabilizer in those reported formulations, but also play an active role in biological integration with DNA, thereby promoting gene expression16,18. In this regard, we hypothesized that polysorbate cationic niosomes (PCNs) could function as a vector for therapeutic genes in terms of the abovementioned unique properties of polysorbates. To validate our speculation, PCNs, containing Tween 20, 40, 80, or 85, were prepared by using cationic lipid (3-b-N-(N’,N’-dimethylaminoethan)-carbamoyl]cholesterol, DC-Chol) as a membrane-stabilized additive, via application of the film hydration method accompanied with sonication. The cellular uptake efficiency and gene transfer ability of these PCNs was then examined in vitro suing pEGFP-N1 plasmid as the model gene compound.
2 Materials and methods
2.1 Materials
Polysorbates surfactants (Tween® 20, 40, 80, 85) were obtained from Shanghai Chemical Reagent Co. The DC-Chol compound was synthesized according to the procedure described by Gao and Huang20. Cholesterol was obtained from Sigma-Aldrich (St. Luise, USA). A model ODN with a 15-mer random sequence was synthesized by Sangon Bio-engineering Technology Co. (Shaighai, China), and the 5′ end was labeled with carboxyfluorescein. All other reagents were of analytical grade supplied by Huadong Medical Co. (Hangzhou, China).
The COS-7 cell line and pGFP-N1 were kindly provided by Sir Run Run Shaw Hospital, Zhejiang University, and cells were cultured with Dulbecco’s Modification of Eagle’s Medium (Gibco) with 10% FBS (Hangzhou Sijiqing Bio-engineering Material Co., China).
2.2 Preparation of PCNs
Niosomes were prepared by using the film hydration method with sonication. In brief, the amphiphile mixture composed of equal molar ratios of Tween and DC-Chol (50 μmol) was dissolved in chloroform in a pear-shaped flask, and the organic solvent was dried under vacuum using a rotary evaporator. The resultant lipid film was then hydrated using 5 ml double distilled water, and the flask was rotated in a water bath at 60 °C for 20 min. The obtained PCN dispersions were then sonicated using a sonifier (200 w, 3 min) equipped with an ice-water bath. An aseptic final product for cellular studies was obtained by filtration through 0.2 μm filter membrane.
2.3 Transmission electron microscopy (TEM) study
The PCN suspension was added to a carbon-coated copper grid and left for 1 min to allow adhesion between the vesicles and the carbon substrate. Excess of the suspension was then removed with a piece of filter paper. A drop of 2% phosphotungstic acid solution was applied to the grid for negative staining, and the sample was air-dried. The morphology of PCNs was examined using a JEM-1200EX TEM apparatus (JEOL, Japan).
2.4 Measurement of particle sizes and zeta potentials
Particle-size and zeta potential of the PCNs were determined by using laser diffraction spectrometry (Malvern Zetasizer 3000HS, Malvern, U.K.).
2.5 Physical stability of niosomal vesicles
For the physical stability study, the PCN suspensions were stored in glass vials at room temperature for four weeks. Aliquots were sampled from each vial at week 0, 1, 2 and 4, for monitoring the particle size change.
2.6 Gel retardation assay
ODN/PCN complexes were prepared by simply mixing equal volumes of the PCN and ODN solutions at the +/− charge ratio of 0.5:1, 1:1, 2:1 or 4:1. Briefly, the ODN solution was rapidly pipetted into the PCN suspension under thorough mixing, and the mixture was then incubated for 10 min at room temperature to allow the formation of PCN/ODN complexes. The complexes were carefully added to an agarose gel (0.8%) at a volume representing 2 μg of ODN per well, along with varying amount of PCNs according the charge ratio described above. Free ODN was used as the control. Gel electrophoresis was carried out at a voltage of 60 V.
2.7 Cellular uptake experiment
COS-7 cells were seeded in 24-well plates and cultured in 10% FBS/DMEM medium to 90% confluence at 37 °C in a 5% CO2 humidified incubator prior to the uptake experiments. With the +/− charge ratio of 4:1, PCNs and 1 μg of fluorescein labeled ODN in equal volume were mixed and incubated for 15 min to form the complexes. The complexes were then added to cells right after replacing the culture medium with FBS-free medium. Following incubation for 4 hr, the culture medium was removed. The cells were then washed twice with PBS, collected by trypsinization, centrifuged (1200×g, 3 min), and washed twice again with PBS. The cells were then re-suspended in PBS and analyzed by flow cytometry (Beckman Coulter). The percentage of positive fluorescence cells (a) and mean of fluorescent intensity (MFI) were determined. Cellular uptake efficiencies were assessed by calculating the total fluorescent intensity (TFI) according to the equation as follows:
2.8 Cellular gene transfection experiment
Plasmid DNA of pEGFP-N1 was used as the model gene. The COS-7 cells were cultured to 80% confluence as described above. The DNA/PCNs complexes were prepared by mixing equal volumes of PCNs and DNA at a +/− charge ratio of 4:1, followed by incubation of the mixture for 15 min. The cells were exposed to the complexes at a DNA level of 2 μg per well for 4 hr in FBS-free medium, and were then further incubated for 24 hr in 10% FBS/DMEM medium. Cells were then observed using an inverted fluorescent microscope (Zeiss HBO100) to assess the gene transfection. Commercialized DOPE/DC-Chol cationic liposomes were set up as a positive control.
2.9 Statistical analysis
Each experiment was performed in triplicate, and data were expressed as mean ± SD. Statistical analyses were performed using the Student’s t-test.
3 Results
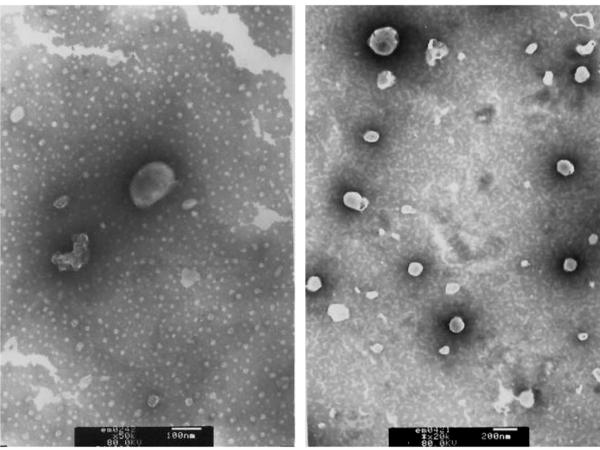
The typical TEM images of PCNs formulated by using Tween 85 and DC-Chol are shown in Fig. 1. The niosomes displayed discrete spherical shapes with distinct boundaries. The size ranged from 100 to 200 nm, in accordance with results obtained from particle sizing by laser diffraction spectrometry (Tab. 1). The average diameters of PCNs containing various Tween were slightly different, but all were less than 200 nm. Cholesterol is the most commonly membrane stabilizer, which is found both in living cells and synthetic bilayer membranes (e.g., niosomes). The well-defined vesicle shapes observed in the TEM images indicated that the strategy of substituting cholesterol with DC-Chol to achieve the bilayer membrane formation worked reasonably well, presumably due to the same parent structure. Meanwhile, with the cationic component of DC-Chol, the zeta potentials of all the niosomes were above +30 mV; a well-accepted electrostatic stabilization level21. Such high surface charges were expected to provide sufficient electrostatic repulsion between vesicles, thereby preventing aggregation. This assumption was further confirmed by the subsequent stability study.
Figure 1.
TEM images of Tween85 cationic niosomes
TABLE 1.
Particle Size and Zeta Potential of Tween Cationic Niosomes
| Tw85-nio | Tw80-nio | Tw40-nio | Tw20-nio | |
|---|---|---|---|---|
| Size/nm | 182.6 ± 7.3 | 127.5 ± 9.2 | 151.3 ± 6.7 | 159.8 ± 10.1 |
| Zeta/mV | 41 ± 1 | 45 ± 2 | 32 ± 1 | 34 ± 2 |
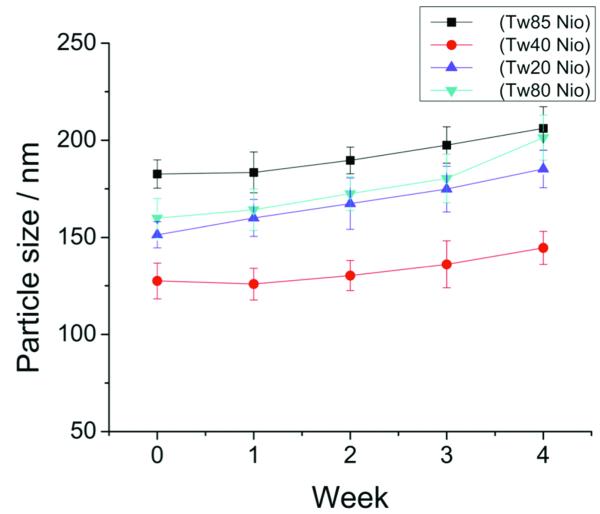
It should be noted that particle size is not only a parameter for characterization of the colloidal drug delivery systems but also a stability response indicator in that aggregation and fusion of colloidal vesicles would normally lead to increased particle size21. The more the size increase is, the less stable the niosomal system. In this regard, the stability of PCNs was investigated by monitoring the change in particle size for a period of 4 weeks. Results showed that the size of PCNs increased relatively insignificantly during the 4-week storage period (Fig. 2), indicating good physico-chemical stability of these PCNs. As noted, the nano-scale particle size and high absolute value of zeta potential are two important parameters in stabilizing this colloidal system. In addition, the hydrophilic polyoxyethylene chains of Tween on the surface of the vesicle would cover it like an umbrella, actively preventing it from fusion with other vesicles.
Figure 2.
Particle size change during 4-week storage
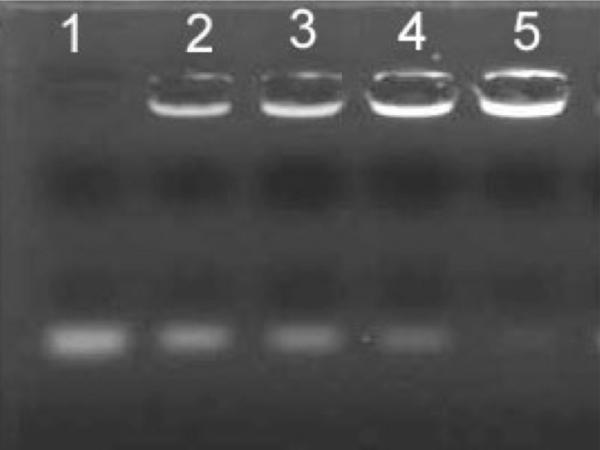
The formation of the self-assemble complexes of ODN/PCNs was examined using agarose gel electrophoresis. The ODN-binding capacity of PCNs was depicted by a specific charge ratio (+/−) at which ODN gel migration was retarded. As shown in Fig 3, the migration band of ODN gradually decreased along with the elevating charge of PCNs. When the charge ratio (+/−) was 4 or greater, ODN was completely retarded, indicating that a solid complex was formed. The ODN, as low as 0.08 μg, was able to be detected in this gel electrophoresis system. Thus, any invisible band of free ODN can be calculated as 1ess than 0.08 μg, i.e., the retarded ODN greater than 1.92 μg (taken 2 μg of total ODN into account). This means that the binding efficiency of ODN with PCNs is > 96%. Such an ODN-binding capacity (i.e. the charge ratio of 4:1) was used for the preparation of ODN/PCN complexes.
Figure 3.
Agarose gel electrophoresis of Tween 85 niosome/ODN . lane, free OND; lane 2-5, Niosome/ODN with +/− ratio of 0.5:1, 1:1, 2:1 and 4:1 respectively With +/− ratio of 2:1 (Lane 4), slightly strap of OND was visible, while at lane 5 a complete retardation of ODN was performed with +/− charge ratio of 4:1 or above.
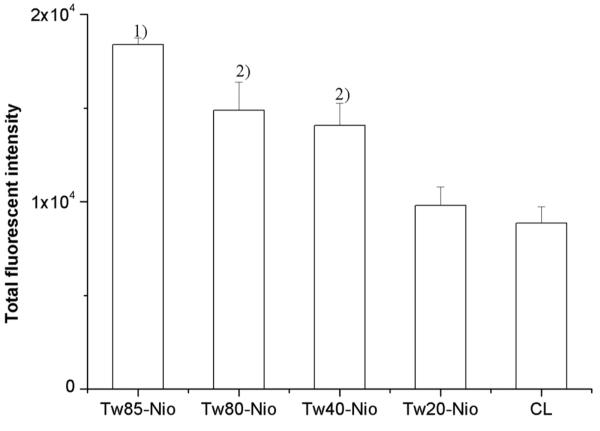
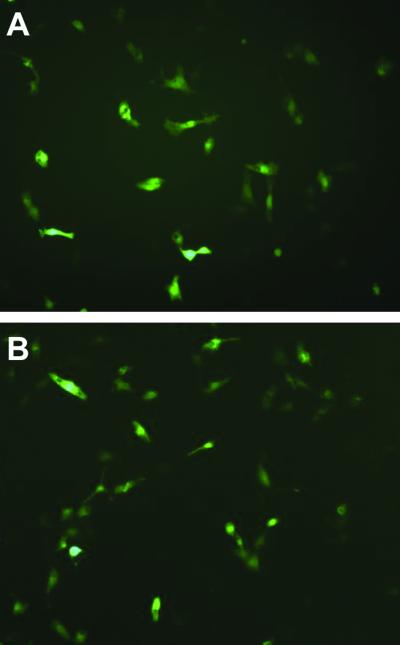
The cellular uptake study showed that PCNs achieved a varying degree of enhancement of cellular uptake compared to the cationic liposomes (soybean phospholipids/DC-Chol, 1:1, molar ratio) in the COS-7 cell line. The cellular uptake efficiency of the control naked fluorescent ODN was very low: at a background level. Significant elevations were seen in Tween 85-, 80-, and 40-niosomes (p < 0.05) (Fig. 4). This indicated that, besides DC-Chol, Tween also acted importantly on mediating ODN cellular uptake. The enhancement was in a pattern corresponding to the decreasing hydrophilic/lipophilic balance (HLB) values of these tested Tween surfactants. Since the highest cellular uptake efficiency was seen in Tween-85 niosomes (p < 0.05), the plausibility of mediating gene transfection was investigated by using the plasmid pEGFP-N, which encodes a red-shifted variant of wild-type green fluorescent protein (GFP). Adequate GFP expression was found under an inverted fluorescent microscope, which displayed a level comparable to the control DOPE/DC-Chol liposomes (Fig. 5). This revealed the high gene transfection capacity of Tween-85 cationic niosomes.
Figure 4.
The cellular uptake of OND mediated by Tween cationic niosomes. Each experiment was performed in triplicate and the values were expressed as mean ± S.D. Statistical analyses were performed using Student’s t-test. Tw85 niosomes was with the highest efficiency among the Tween cationic niosomes. The result showed that higher hydrophilic the Tween was, the less cellular uptake efficient. Note: 1) vs other three groups, p<0.05; 2) vs CL groups, p<0.05.
Figure 5.
Green Fluorescent Protein expression in COS-7 cell line with Tw85 cationic niosomes (A) and DOPE/DC-Chol liposomes (B) mediated pEGFP-N1 gene transfection.
4 Discussion
Niosomes composed of Tween and membrane additives (e.g., cholesterol22, fatty alcohols23) have been investigated for drug delivery. However, the preparation of PCN and its application gene delivery have not been reported yet. A series of surfactants, including Tween 85, 80, 40 and 20, were selected to formulate the cationic niosomes in this study. Tween are one of the most commonly used non-ionic surfactants in pharmaceutical industry. An attractive property in Tween structure is the hydrophilic polyoxyethylene chains (Fig. 6), which have been demonstrated to possess a functional interaction with DNA, and used as a gene transfer helper. For example, Tween 80 was reported to have an advantage in preventing the large formation of gene/lipid complexes over other non-ionic surfactants like Span, and this activity may associate with its hydrophilic polyoxyethylene head16,18. Essentially, all Tween surfactants possess the common hydrophilic polyoxyethylene head (i.e., 20 POE groups and a sorbitan ring), but only varying in their hydrophobic tails, and thus different kinds of Tween compounds presumably possess a function in facilitating gene transfer. Our results showed that there were enhancements in efficiency of ODN cellular uptake mediated by all PCNs, compared to the cationic liposomes.
Figure 6.
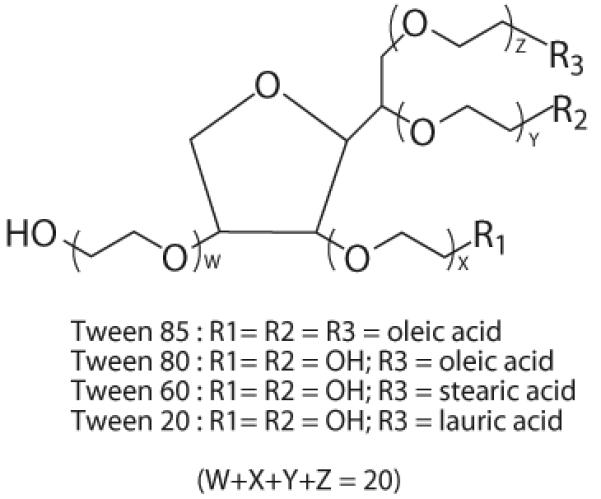
Chemical structure of a series of Tween
It was interesting that cellular uptake efficiency increased in correspondence with the lower HLB value of Tween. The Tween 85 cationic niosomes, with a significant enhancement, displayed the highest efficiency among all the tested formulations. Although the precise mechanism is yet not well understood, some factors such as hydrophobicity, electrostatic interaction and amphiphilic structure are believed to be involved in the process of cellular uptake mediated by non-virus vectors24. Among these four surfactant compounds, Tween 85 displays the unique structure in which there are 3 hydrophobic chains with 18 carbon atoms, with the lowest HLB of 11, and it thereby acquires some organic solubility. Solubilization and perturbation of membrane lipid layers by nonionic surfactants have been revealed to be closely related to their hydrophobicity, indicated by HLB values; for example, increasing membrane fluidity was caused by Tween surfactants in an order of Tween 85 > Tween 80 > Tween 60 > Tween 40 > Tween 2025. Therefore, the longer hydrophobic chains of Tween 85 may favor cellular uptake via a stronger interaction with membrane lipids.
Endosome escape is known as one of the crucial barriers for intracellular gene delivery. Tween was believed to bear a fusogenic property similar to DOPE, which is one of the most commonly used helper-lipids in facilitating the DNA-escape from the endosome to the cytosol by promoting a lamellar-to-inverted hexagonal phase transition of cationic liposome-DNA complexes16,26 A Tween surfactant with high HLB can be solubilized in water by hydration of the polyoxyethylene groups. As for Tween 85, however, bulky hydrophobic chain regions make the polyoxyethylene groups less accessible and thereby less soluble. A plausible explanation for the fusogenic function of Tween 85 in DNA transfer is that Tween 85 tends toward a transition to reverted phase in a lipophilic environment (e.g., inside the membrane) due to its hydrophobic bulk. Additionally, the destabilization membrane effect of Tween 85 could also play a key role on gene intracellular transport, for example, disturbing endosome membrane.
Cholesterol is the most general membrane additive in niosomal systems, in which cholesterol plays an important role in abolishing the gel-to-liquid phase transition and enhancing the physico-chemical colloidal stability, thereby facilitating the formation of bilayer vesicles. In our previous studies we have demonstrated the success of substituting cholesterol with DC-Chol for preparing cationic niosomes, and potential of utilizing sorbitan monoester (Span) cationic niosomes as gene carriers27,28. This cationic cholesterol derivative, DC-Chol, has also been shown to be capable of acting as a membrane stabilizer in the presented PCN systems. Given the cationic property of DC-Chol, its function in the PCNs is not only to condense the delivered gene, but also to stabilize the niosomal system because of the creation of an electrostatic repellent effect by addition of charged additives to the bilayer3.
Cationic niosomes are biodegradable and biocompatible drug careers. In our previous study, Span/Dc-Chol cationic niosomes displayed low cytotoxicity, remaining viability of above 80% on COS-7 cells with a concentration up to 120 μM28. We thus have good reason to believe PCNs also possess low toxicity because of the similar compositions. Further, Tween is commonly considered to be more biocompatible than Span; for example, Tween 80 is often employed as additives in clinically used injectable formulations.
5 Conclusion
The promise of developing cationic niosomes as gene delivery carriers was attractive. Compared with cationic liposomes, cationic niosomes possess great superiority in chemical stability and cost-effectiveness. PCNs were only composed of Tween surfactant and DC-Chol. The cationic cholesterol, DC-Chol, not only helps with building the niosomal bilayer structure but also offers positive charge to both bind nucleic acid drugs and facilitate the access to the negative cellular membrane, and it thereby improves intracellular transfer (e.g., escape from endosomes). Tween surfactants, owing to their unique structure, play an important role in PCNs. PCNs showed great advantage on the aspects of stability and high efficiency of gene transfer as well as the biocompatibility. Tween 85/DC-Chol niosomes specifically showed the highest efficiency and the potential to be developed as a non-viral gene carrier.
Supplementary Material
Scheme 1. Self-assembling of niosomes
6 Acknowledgments
This work was supported in part by NIH R01 Grants CA114612 and NS066945. This work was also partially sponsored by Grant R31-2008-000-10103-01 from the WCU project of South Korea. Victor C. Yang is currently a Participating Faculty in the Department of Molecular Medicine and Biopharmaceutical Sciences, Seoul National University, South Korea. Dr. Yongzhuo Huang is the recipient of the Hundred-talent Program of the Chinese Academy of Sciences.
References
- 1.Huang YZ, Yu FQ, Liang WQ. Niosomal delivery system for macromolecular drugs. In: Fanun M, editor. Colloids in drug delivery. CRC Press; Baco Raton: 2010. pp. 355–364. [Google Scholar]
- 2.Uchegbu IF, Double JA, Turton JA, Florence AT. Distribution, metabolism and tumoricidal activity of doxorubicin administered in sorbitan monostearate (Span 60) niosomes in the mouse. Pharm Res. 1995;12(7):1019–1024. doi: 10.1023/a:1016210515134. [DOI] [PubMed] [Google Scholar]
- 3.Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172(1-2):33–70. [Google Scholar]
- 4.Bayindir ZS, Yuksel N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J Pharm Sci. 2010;99(4):2049–2060. doi: 10.1002/jps.21944. [DOI] [PubMed] [Google Scholar]
- 5.Paolino D, Cosco D, Muzzalupo R, Trapasso E, Picci N, Fresta M. Innovative bola-surfactant niosomes as topical delivery systems of 5-fluorouracil for the treatment of skin cancer. Int J Pharm. 2008;353(1-2):233–242. doi: 10.1016/j.ijpharm.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Hong M, Zhu S, Jiang Y, Tang G, Pei Y. Efficient tumor targeting of hydroxycamptothecin loaded PEGylated niosomes modified with transferrin. J Control Release. 2009;133(2):96–102. doi: 10.1016/j.jconrel.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Varshosaz J, Pardakhty A, Hajhashemi VI, Najafabadi AR. Development and physical characterization of sorbitan monoester niosomes for insulin oral delivery. Drug Deliv. 2003;10(4):251–262. doi: 10.1080/drd_10_4_251. [DOI] [PubMed] [Google Scholar]
- 8.Ning M, Guo Y, Pan H, Yu H, Gu Z. Niosomes with sorbitan monoester as a carrier for vaginal delivery of insulin: studies in rats. Drug Deliv. 2005;12(6):399–407. doi: 10.1080/10717540590968891. [DOI] [PubMed] [Google Scholar]
- 9.Pardakhty A, Varshosaz J, Rouholamini A. In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int J Pharm. 2007;328(2):130–141. doi: 10.1016/j.ijpharm.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Chattaraj SC, Das SK. Physicochemical Characterization of Influenza Viral Vaccine Loaded Surfactant Vesicles. Drug Delivery. 2003;10(2):73–77. doi: 10.1080/713840363. [DOI] [PubMed] [Google Scholar]
- 11.Vangala A, Kirby D, Rosenkrands I, Agger EM, Andersen P, Perrie Y. A comparative study of cationic liposome and niosome-based adjuvant systems for protein subunit vaccines: characterisation, environmental scanning electron microscopy and immunisation studies in mice. J Pharm Pharmacol. 2006;58(6):787–799. doi: 10.1211/jpp.58.6.0009. [DOI] [PubMed] [Google Scholar]
- 12.Carafa M, Santucci E, Lucania G. Lidocaine-loaded non-ionic surfactant vesicles: characterization and in vitro permeation studies. Int J Pharm. 2002;231(1):21–32. doi: 10.1016/s0378-5173(01)00828-6. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Yang J, Huang L, Liu D. Effect of non-ionic surfactants on the formation of DNA/emulsion complexes and emulsion-mediated gene transfer. Pharm Res. 1996;13(11):1642–1646. doi: 10.1023/a:1016480421204. [DOI] [PubMed] [Google Scholar]
- 14.Ohama Y, Heike Y, Sugahara T, Sakata K, Yoshimura N, Hisaeda Y, Hosokawa M, Takashima S, Kato K. Gene transfection into HeLa cells by vesicles containing cationic peptide lipid. Biosci Biotechnol Biochem. 2005;69(8):1453–1458. doi: 10.1271/bbb.69.1453. [DOI] [PubMed] [Google Scholar]
- 15.Huang YZ, Gao JQ, Chen JL, Liang WQ. Cationic liposomes modified with non-ionic surfactants as effective non-viral carrier for gene transfer. Colloids Surf B Biointerfaces. 2006;49(2):158–164. doi: 10.1016/j.colsurfb.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Yang J, Huang L, Liu D. New cationic lipid formulations for gene transfer. Pharm Res. 1996;13(12):1856–1860. doi: 10.1023/a:1016041326636. [DOI] [PubMed] [Google Scholar]
- 17.Kim TW, Chung H, Kwon IC, Sung HC, Jeong SY. Optimization of lipid composition in cationic emulsion as in vitro and in vivo transfection agents. Pharm Res. 2001;18(1):54–60. doi: 10.1023/a:1011074610100. [DOI] [PubMed] [Google Scholar]
- 18.Choi WJ, Kim JK, Choi SH, Park JS, Ahn WS, Kim CK. Low toxicity of cationic lipid-based emulsion for gene transfer. Biomaterials. 2004;25(27):5893–5903. doi: 10.1016/j.biomaterials.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Kang HS, Jin SJ, Myung CS, Hwang SJ, Park JS. Delivery of interleukin-18 gene to lung cancer cells using cationic emulsion. J Drug Target. 2009;17(1):19–28. doi: 10.1080/10611860802438710. [DOI] [PubMed] [Google Scholar]
- 20.Gao X, Huang L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem Biophys Res Commun. 1991;179(1):280–285. doi: 10.1016/0006-291x(91)91366-k. [DOI] [PubMed] [Google Scholar]
- 21.Heurtault B, Saulnier P, Pech B, Proust JE, Benoit JP. Physico-chemical stability of colloidal lipid particles. Biomaterials. 2003;24(23):4283–4300. doi: 10.1016/s0142-9612(03)00331-4. [DOI] [PubMed] [Google Scholar]
- 22.Abdelbary G, El-Gendy N. Niosome-encapsulated gentamicin for ophthalmic controlled delivery. AAPS PharmSciTech. 2008;9(3):740–747. doi: 10.1208/s12249-008-9105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devaraj GN, Parakh SR, Devraj R, Apte SS, Rao BR, Rambhau D. Release studies on niosomes containing fatty alcohols as bilayer stabilizers instead of cholesterol. J Colloid Interface Sci. 2002;251(2):360–365. doi: 10.1006/jcis.2002.8399. [DOI] [PubMed] [Google Scholar]
- 24.Fujii G. To fuse or not to fuse: the effects of electrostatic interactions, hydrophobic forces, and structural amphiphilicity on protein-mediated membrane destabilization. Adv Drug Deliv Rev. 1999;38(3):257–277. doi: 10.1016/s0169-409x(99)00032-0. [DOI] [PubMed] [Google Scholar]
- 25.Koga K, Murakami M, Kawashima S. Contribution of hydrophobicity of nonionic detergents to membrane lipid fluidity and disopyramide uptake by rat intestinal brush-border membrane vesicles. Biol Pharm Bull. 1997;20(6):674–679. doi: 10.1248/bpb.20.674. [DOI] [PubMed] [Google Scholar]
- 26.Koltover I, Salditt T, Radler JO, Safinya CR. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science. 1998;281(5373):78–81. doi: 10.1126/science.281.5373.78. [DOI] [PubMed] [Google Scholar]
- 27.Huang YZ, Chen JL, Chen XJ, Gao JQ, Liang WQ. PEGylated synthetic surfactant vesicles (Niosomes): novel carriers for oligonucleotides. J Mater Sci Mater Med. 2008;19(2):607–614. doi: 10.1007/s10856-007-3193-4. [DOI] [PubMed] [Google Scholar]
- 28.Huang YZ, Han G, Wang H, Liang WQ. Cationic niosomes as gene carriers: preparation and cellular uptake in vitro. Pharmazie. 2005;60(6):473–474. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scheme 1. Self-assembling of niosomes