Abstract
Independent studies indicate that expression of sialylated fucosylated mucins by human carcinomas portends a poor prognosis because of enhanced metastatic spread of tumor cells, that carcinoma metastasis in mice is facilitated by formation of tumor cell complexes with blood platelets, and that metastasis can be attenuated by a background of P-selectin deficiency or by treatment with heparin. The effects of heparin are not primarily due to its anticoagulant action. Other explanations have been suggested but not proven. Here, we bring together all these unexplained and seemingly disparate observations, showing that heparin treatment attenuates tumor metastasis in mice by inhibiting P-selectin-mediated interactions of platelets with carcinoma cell-surface mucin ligands. Selective removal of tumor mucin P-selectin ligands, a single heparin dose, or a background of P-selectin deficiency each reduces tumor cell-platelet interactions in vitro and in vivo. Although each of these maneuvers reduced the in vivo interactions for only a few hours, all markedly reduce long-term organ colonization by tumor cells. Three-dimensional reconstructions by using volume-rendering software show that each situation interferes with formation of the platelet “cloak” around tumor cells while permitting an increased interaction of monocytes (macrophage precursors) with the malignant cells. Finally, we show that human P-selectin is even more sensitive to heparin than mouse P-selectin, giving significant inhibition at concentrations that are in the clinically acceptable range. We suggest that heparin therapy for metastasis prevention in humans be revisited, with these mechanistic paradigms in mind.
Hematogenous metastasis of cancer cells is a cascade of events involving many factors favoring the survival of the “fittest” tumor cells, which intravasate into the bloodstream, evade innate immune surveillance, adhere to vascular endothelial cells of distant organs, and extravasate into such tissues (1–5). After extravasation, the tumor cells settle in the parenchyma of target organs and create metastatic deposits, which are often the proximate cause of death in patients with carcinomas. Studies from the 1970s and 1980s indicate that tumor metastasis is facilitated by blood platelets, which form complexes with leukocytes and tumor cells in the vasculature and appear to contribute to the arrest of such emboli in the vasculature (reviewed in refs. 6–8). Experimental lowering of platelet counts (thrombocytopenia) before tumor cell injection diminished metastasis as well (7). Although the formation of these tumor cell–platelet aggregates is part of the current textbook description of metastasis, the mechanistic basis of this interaction has remained uncertain. Thus, the therapeutic potential of this phenomenon has not been explored in humans.
Unrelated studies from many investigators indicate that tumor metastasis in experimental animals can be inhibited by the widely used anticoagulant drug heparin (reviewed in refs. 9–12). A few clinical trials also suggested a beneficial effect of heparin in humans with cancer (13–17). However, heparin therapy can be difficult to manage on an outpatient basis and is associated with some complications. Assuming that the anticoagulant action of heparin was primarily responsible for the antimetastatic effect (18), clinical trials were then carried out by using the more easily managed anticoagulant drug warfarin, which works by a different mechanism, Vitamin K antagonism. However, most of these studies failed to show a clinical benefit (19, 20). Nevertheless, further studies continued to show an effect of heparin or low-molecular-weight heparin on reducing metastasis and prolonging survival in mice (11, 21–23). Meanwhile, other studies showed that heparin is not just an anticoagulant, but a complex set of multifunctional glycosaminoglycan molecules with many other potential biological effects (24–26). Thus, several other explanations for the heparin effect on cancer have been suggested, including modulation of growth factors, inhibition of angiogenesis via interactions with vascular endothelial growth factors, and inhibition of the heparanases thought to be required for tumor cells to invade the vascular basement membrane (10, 16, 24, 27–29). Indeed, recent studies indicate that several anionic molecules that inhibit heparanases can diminish metastasis (28, 29).
In an apparently unrelated area of research, many reports showed that carcinoma progression is associated with alterations of cell-surface glycosylation (30–34). Of note, carcinomas expressing highly sialylated or branched sugar chains and/or large amounts of mucins have a poor prognosis, because of a high rate of metastasis. In particular, the expression of sialylated fucosylated glycans like sialyl Lewisx and sialyl Lewisa correlates with a poor prognosis because of tumor progression and metastatic spread (30, 31, 33–37). It was subsequently noted that carcinoma cell-surface mucins carrying sialyl Lewisx/a can be ligands for all three members of the selectin family of cell adhesion molecules (38, 39). E-, P-, and L-selectins are vascular receptors for certain normal sialyl Lewisx/a containing mucin-type glycoproteins found on leukocytes and endothelium (40–43). Because selectins can mediate tumor cell interactions with platelets, leukocytes, and endothelium in vitro (39, 44, 45), an in vivo role for selectins in the metastatic spread of tumors has been suggested (31, 33, 34). P-selectin expressed on activated endothelium contributes to the rolling of leukocytes that can result in their arrest and extravasation. P-selectin on activated platelets can also mediate their interactions with monocytes and induce the release of inflammatory agents and tissue factor, thus enhancing platelet aggregation and thrombosis (46). Because tumor cell–platelet complexes promote metastasis (6–8, 47, 48), P-selectin seemed a logical candidate to mediate pathological interactions involving carcinoma cells with platelets, leukocytes, and endothelium. Indeed, our recent demonstration that tumor cell–platelet aggregation is diminished in P-selectin-deficient mice provided in vivo evidence for this concept (49). Furthermore, the establishment of tumor metastasis was attenuated in these mice. Meanwhile, we found that heparin is an excellent inhibitor of P-selectin binding to its natural ligands (50), presumably because it mimics these ligands, which contain negatively charged sialic acids and tyrosine sulfate residues.
Here we unite all of these seemingly disparate observations by demonstrating an early action of heparin on metastasis, which occurs in the vasculature, before tumor cell extravasation into the target organ. We show that heparin can block P-selectin-based platelet interactions with tumor cell-surface mucins and thereby attenuate metastasis.
Materials and Methods
Mice.
RAG2−/− immunodeficient mice in 129/J background were from Taconic, and P-selectin-deficient mice in C57BL6 background were from The Jackson Laboratory. Production and characterization of P-selectin/Rag2 double null mice (here referred to as P-sel−/−) as well as littermate controls Rag2−/− (here referred as P-sel+/+) were as described previously (49). Because the mouse strain Selptm1Bay was used, PCR screening for P-sel mutant allele was adjusted accordingly by using primers: Pselex2 -GAGTGTGATCCTGGGAGGG, and NeoPbay-GCTACCCGTGATATTGCTGA in PCR reactions at 59°C annealing temperature.
Selective Removal of Mucins from Tumor Cell Surfaces by OSGPase Treatment.
Tumor cells were incubated in Hanks' balanced salt solution (HBSS), with 50 μl of reconstituted OSGPase enzyme (Cedarlane Laboratories) for 1 h at 37°C with occasional mixing before calcein AM (Molecular Probes) labeling and injection.
In Vivo Interactions of Tumor Cells with Platelets.
Lungs were obtained for analysis at various time points after i.v. injection of tumor cells. Lung sectioning and staining were essentially as described (49). To prevent collapse, lungs were injected via the trachea with OCT/PBS 1:1 solution and frozen in OCT compound (Tissue-Tek, Sakura, Torrance, CA). Frozen lung sections were stained with anti-CD11b (Mac-1 α chain) Ab or CD41 Ab from PharMingen. CD41 Ab was either biotinylated or prelabeled with AMCA-Sulfo-NHS (Pierce). Biotinylated antibodies were detected with Streptavidin–Cy-3 (Jackson ImmunoResearch). The extent of platelet association with tumor cells was initially quantitated by evaluating sections by conventional epifluorescence. For deconvolutional microscopy analyses, stained sections were mounted in Prolong medium (Molecular Probes). Images were captured on a deconvolution microscope (Nikon) with 60× objective by using the digital camera adjusted for intensities within a linear range and obtaining digitized sections of 0.15 microns. Images were processed by using Delta Vision SoftWorxs programs (Applied Precision, Issaquah, WA) to generate deconvolved optical sections and three-dimensional reconstructions of the complexes (51). Data files representing 4.5 μm thickness of a tissue section were also used to create animated “fly-by” three-dimensional images by using NPACI Scalable Visualization tools and software developed at the San Diego Supercomputer Center (52).
Experimental Metastasis Assays.
Mice were injected with 3–4 × 105 cells of LS180 or T84 human tumor cells. Some were injected with 100 units of heparin (sodium heparin 20,000 United States Pharmacopeia (USP) units/ml (Lot no. 382173) from Fujisawa USA, Deerfield, IL), 30 min before tumor cell injection. After 6 weeks, metastasis was detected by PCR amplification of human Alu sequences as described previously (49), with the following adjustments: a template of 200 ng of DNA was used with 33 cycles.
Results and Discussion
Heparin Inhibits Tumor Cell–Platelet Interactions Mediated by Tumor Cell Surface Mucins and P-Selectin in Vitro.
Unfractionated heparin can inhibit the interaction of human P-selectin with synthetic ligands (50). To determine whether heparin can block P-selectin recognition of mucin-producing adenocarcinomas, we used recombinant soluble mouse P-selectin-IgFc chimeric molecules and tested the inhibitory effect of heparin on tumor cell adhesion. For most studies, we used the human colon adenocarcinoma cell line LS180 (ATCC CL 187), which expresses binding sites for P-selectin (39). Serial dilutions of heparin were studied in a cell adhesion assay on mouse recombinant P-selectin-coated microtiter plates. Heparin inhibited adhesion of the LS180 cells to mouse P-selectin with an IC50 value of 2.5 USP units/ml, with >90% inhibition seen at 20 USP units/ml (data not shown).
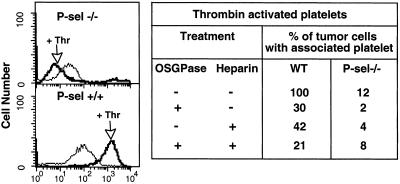
Mouse platelets can interact with tumor cells in a P-selectin-dependent fashion (38, 49). To see whether heparin can block this interaction, we mixed LS180 cells with calcein AM-labeled mouse platelets. Mouse thrombin was added to stimulate relocation of P-selectin to platelet surfaces and the tumor cells then analyzed by flow cytometry for calcein staining, indicating platelet attachment. P-selectin positive platelets (P-sel+/+) showed strong binding to tumor cells (defined as 100%), with a significant reduction in the presence of heparin (Fig. 1). Attachment of P-selectin-deficient platelets (P-sel−/−) was greatly diminished, and heparin had a small additional effect. A certain degree of thrombin-independent attachment was also consistently seen. Of potential relevance to the in vivo situation, P-selectin-dependent attachment of platelets to tumor cells could be induced even without addition of thrombin (not shown), albeit at a much slower rate than that seen in vivo.
Figure 1.
Effects of heparin and OSGPase treatment on P-selectin-mediated tumor–platelet interactions in vitro. Mouse platelets were isolated from citrated blood as described (44) and calcein-AM labeled. Tumor cells were detached with PBS/2 mM EDTA, washed in HBSS (Sigma), and 200,000 cells mixed in HBSS with labeled mouse platelets (5 × 107) with/without heparin (20 units/ml final) and incubated for 5 min. Tumor cells were analyzed by flow cytometry, to quantitate attachment of calcein-labeled platelets with or without preactivation with mouse thrombin (marked as Thr, 0.2 units/ml added 3 min before flow cytometry analysis). Tumor cells were also studied with or without prior mucin removal by OSGPase (see Materials and Methods). Summarized data are presented regarding the effects of heparin, OSGPase treatment, or a combination. The apparent reduction of P-selectin negative platelet interactions after thrombin is actually within the range of variation of negative results for this experiment, which was repeated several times.
To confirm that carcinoma cell surface mucins are the operational ligands for platelet P-selectin in this assay, we pretreated the tumor cells with O-sialoglycoprotease (OSGPase), an enzyme that selectively removes sialylated mucins from cell surfaces (39, 53). OSGPase treatment gave a substantial reduction of P-sel+/+ platelet adhesion to tumor cells (Fig. 1). Combining OSGPase treatment with heparin resulted in a further decrease; however, cell surface mucin removal from LS180 cells by OSGPase treatment is only partially effective (39). Taken together, the data indicate that the tumor cell–platelet interaction is P-selectin- and carcinoma mucin-dependent and can be blocked by heparin.
In Vivo P-Selectin-Mediated Tumor Cell–Platelet Interaction Is Impaired by Mucin Removal or by Heparin Treatment.
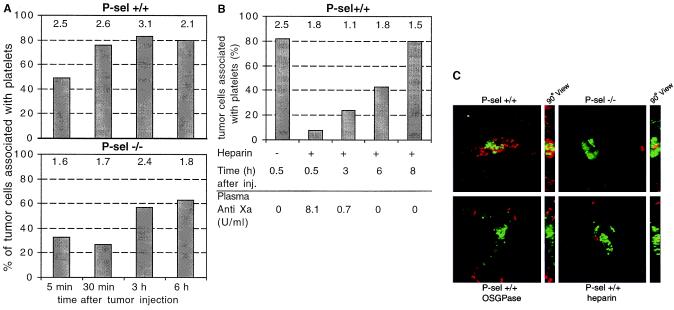
We intravenously injected fluorescently labeled LS180 cells and analyzed the tumor cells lodged in lung blood vessels. Injection into P-sel+/+ wild-type mice triggered in vivo platelet aggregation around the tumor cells as early as 5 min after injection (not shown). This observation is in line with previous studies showing the potential of tumor cells to induce platelet aggregation (6, 7). Within 30 min after injection, 80% of tumor cells lodged in lung blood vessels were coated with platelets in P-sel+/+ mice (Fig. 2A). In contrast, only 30% of tumor cells were platelet-associated at the same time point in P-sel−/− mice, and even these platelet clumps appeared less compact. The statistically significant difference in tumor cell–platelet association lasted for only 3–4 h after injection. However, the total number of tumor cells per high-power field seen in P-sel+/+ mice was always higher (by up to 50%) than in P-sel−/− mice (Fig. 2A). When Chinese Hamster Ovary cells were injected as controls, less that 5% of them were associated with platelets, and the total number of cells arrested in the lung was five times less than with tumor cells (not shown). Thus, the decreased recovery of tumor cells in the lungs of the P-sel−/− mouse indicates that P-selectin-mediated tumor cell–platelet interactions may contribute to efficient lodging of tumor cells in the lung microvasculature in vivo. To check for circulating tumor cells, we analyzed blood by flow cytometry 30 min after injection, looking for the presence of the calcein AM-labeled tumor cells. No tumor cells were detected in blood from either P-sel+/+ or P-sel−/− mice (data not shown; controls using in vitro mixing indicated that the detection level was at least 100 cells/μl). This finding suggests that the lower tumor cell–platelet association in the lungs of the P-sel −/− mouse results in a decrease in the number of arrested cells in the first-pass organ (the lung) but is not associated with a higher number of circulating tumor cells. We did not pursue the fate of the remaining cells, which may have lodged in other organs (49) or have been destroyed (membrane damage would cause release of the cytosolic calcein AM label).
Figure 2.
Effects of heparin, OSGPase treatment, and P-selectin deficiency on tumor cell–platelet interactions in vivo. Mice were intravenously injected with calcein AM-labeled LS180 cells (green fluorescence) and killed at different time points. Frozen sections of the lungs were stained with biotinylated anti-CD41 Ab for platelets (Cy3 streptavidin for red fluorescence) as described in Materials and Methods. Platelet association with tumor cells was quantitated by evaluation of sections by using conventional epifluorescence microscopy (×40 objective, evaluating 16 random fields) (A) Time dependency of tumor–platelet complex formation in P-selectin+/+ and P-selectin−/− mice. (B) Heparin effect on tumor–platelet complex formation in P-sel+/+ mice 100 units of heparin was injected intravenously 30 min before the LS180 cells. In each case, numbers above the bars represent the mean number of tumor cells seen per high-power field. Heparin in circulation was measured by an anti-Xa assay of plasma (data below the graph, kindly performed by Dzung Le, University of California, San Diego). (C) Examples from deconvolutional microscopy of typical tumor–platelet complexes observed in lung sections 30 min after LS180 injection. OSGPase indicates tumor cells treated with OSGPase before injection. Heparin indicates heparin injection 30 min before tumor cell injection.
To investigate the inhibitory potential of heparin on these in vivo platelet–tumor interactions, P sel+/+ mice were injected with 100 USP units of heparin 30 min before tumor cell injection and sections of lung analyzed at different time points (Fig. 2B). Heparin substantially reduced platelet association with tumor cells (to about 10%) in comparison to a saline-injected control (80%). Tumor cell–platelet associations in heparin-injected mice then increased with time, reaching control levels after 8 h. At this time point, there was no significant difference between heparin-treated and nontreated mice, indicating reversibility of the blocking effect of heparin. Heparin levels in mice were monitored by using plasma anti-Xa levels, showing clearance from the circulation within 5 h (Fig. 2B), which correlates with the increase of tumor cell–platelet association. The number of tumor cells per high-power view field was lower in mice receiving a heparin injection (Fig. 2B). Thus, as seen in P-selectin-deficient mice, a smaller fraction of the injected tumor cells arrested in the lung blood vessels in the presence of heparin. Selective mucin removal from tumor cells by OSGPase treatment before their i.v. injection also resulted in decreased platelet association in vivo.
All these effects were better visualized by deconvolutional microscopy of lung tissue sections stained with anti-CD41 antibody specific for platelets, to contrast with the calcein-AM labeled tumor cells (Fig. 2C). In P-sel+/+ mice, an extensive platelet decoration (red fluorescence) surrounding the tumor cells (green fluorescence) was observed (Fig. 2C). Platelet association with OSGPase-treated tumor cells was diminished (Fig. 2C), even though mucin removal was incomplete. OSGPase treatment was not toxic to the tumor cells, as shown by trypan blue exclusion and by their ability to restore cell surface mucin expression in 8 h when put back into culture (data not shown). Similar effects were seen with P-selectin deficiency or heparin treatment (Fig. 2C). Thus, the reduction of spontaneous tumor cell–platelet interactions in vivo by P-selectin deficiency can be mimicked by heparin treatment or by prior mucin removal from tumor cells.
A Single Heparin Dose Attenuates Metastasis.
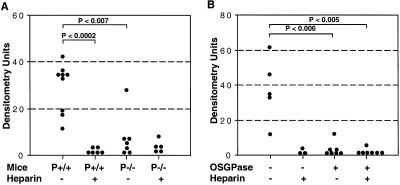
To determine the relevance of heparin inhibition of early platelet–tumor association for metastatic progression, we studied the outcome of heparin treatment in a long-term experimental metastasis model. P-sel+/+ or P-sel−/− mice were injected with or without heparin 30 min before tumor cell injection. We used a single injection of 100 USP units of heparin, which reversibly blocked the tumor cell–platelet interaction for up to 5 h (see above). Six weeks after injection, the mice were killed, and one-half of dissected lungs were used for histological analysis. Although metastatic foci were seen in P-sel+/+ mice that did not receive heparin, these were largely absent in heparin-treated animals (data not shown). To more sensitively detect metastatic tumor foci, we looked for Alu sequences from human tumor cell genomic DNA by PCR amplification (49), using lungs from noninjected mice as background controls. Although metastases were detected in all P-sel+/+ mice (Fig. 3A), heparin-treated P-sel+/+ mice showed a reduction, with four measurements not even significantly above background. As expected from our prior work (49), P-sel−/− mice also showed low organ colonization, and the addition of heparin treatment had no further effect (Fig. 3A). Thus, the early platelet–tumor interactions correlate with later metastatic progression. Furthermore, the blocking of this interaction by a single injection of heparin for ≈5 h can reduce long-term establishment of metastatic foci.
Figure 3.
Effects of Heparin, OSGPase treatment, and P-selectin deficiency on the establishment and growth of tumor metastasis. Mice were intravenously injected with 3–4 × 105 tumor cells and killed 6 weeks later. Human specific Alu-PCR was conducted on genomic DNA isolated from dissected lungs and densitometrically quantified. (A) One hundred units of heparin was injected 30 min before LS180 cell injection into P-selectin+/+ or −/− mice. (B) Cell surface mucin was removed from T84 tumor cells by OSGPase treatment, and cells were injected into P-selectin+/+ mice with or without previous heparin injection (100 units). For details, see Materials and Methods.
Prior Mucin Removal from Tumor Cells Attenuates Metastasis.
To confirm the importance of early P-selectin-based tumor cell–platelet interactions for organ colonization, we intravenously injected OSGPase-treated tumor cells and analyzed the animals 6 weeks later. Because OSGPase action was incomplete on LS-180 cells, we also used another human adenocarcinoma cell line T84 (ATCC CL248), where mucin removal was more efficient (39). Removal of cell surface mucins from T84 cells decreased metastasis to levels similar to those observed in heparin-treated mice with no OSGPase treatment (Fig. 3B). Identical experiments by using LS180 cells were performed with similar, although less dramatic, results (data not shown). We also examined mice killed 12 weeks after tumor cell injection and obtained very similar results (data not shown), indicating that the growth of metastasis was not simply delayed. Combinations of heparin and OSGPase treatment did not further diminish metastasis (Fig. 3B). Because each manipulation alone (P-selectin deficiency, heparin therapy, or OSGPase pretreatment) had a marked effect on reducing metastasis, it was hard to rule out any minor additive effect. Nevertheless, the overall results suggest that all approaches have their dominant effects on the same biological step in metastatic progression—early tumor cell–platelet interaction.
Enhanced Monocyte (Macrophage Precursor) Association with Tumor Cells May Explain the Attenuation of Metastasis.
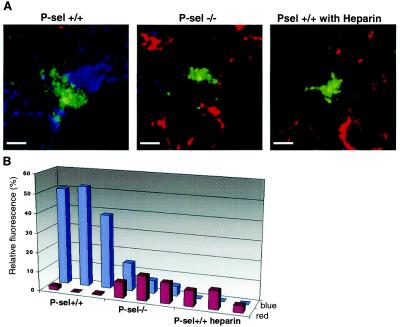
The reduction in metastases seen with various manipulations cannot be accounted for solely by the ≈50% decrease in the number of tumor cells arrested in the lungs. Thus, additional mechanisms are likely involved in the facilitation of metastasis by formation of tumor cell–platelet complexes. Possibilities include different types of molecular crosstalk between platelets and tumor cells as well as physical protection from various components of the innate immune system, including leukocytes (48). The full exploration of all these mechanisms is beyond the scope of this paper. We did study the effect of differences in platelet aggregation on the association of endogenous mouse leukocytes with tumor cells. Lung sections of mice injected with the calcein-labeled tumor cells (green fluorescence) were stained with AMCA-labeled anti-CD41 for platelets (blue fluorescence) and Cy3-labeled anti-Mac-1 for monocytes (red fluorescence) and analyzed by deconvolutional microscopy. Viewing of these tumor cell–platelet–leukocyte complexes was enhanced by a three-dimensional computer reconstruction and the subsequent generation of animated “fly-by” movies of the images that allowed us a detailed perspective view. This was done by using the volume-rendering software originally developed to reconstruct astronomical images of nebulas obtained from large telescopes [the Internet-based reader can activate the movies associated with supplemental Fig. 9A (www.pnas.org)]. A dense coat of CD41-positive platelets was observed surrounding tumor cells in wild-type P-sel+/+ lungs (supplemental Fig. 9A). In contrast, Mac-1 positive cells were present only in small numbers and were generally not in direct contact with the tumor cells (>5 microns away; supplemental Fig. 9A). Tumor cells in the lungs of P-sel −/− mice showed a more limited and looser platelet coat and a closer association (<5 microns away) of Mac-1 positive cells (supplemental Fig. 9A). When P-sel+/+ mice were given a single heparin dose before tumor cell injection, the pattern observed was similar to that in P-sel−/− mice, i.e., a tight Mac-1 positive cell association with tumor cells and loose, limited platelet coat.
Although the Mac-1 epitope is maximally expressed on monocytes/macrophages, it can also be found on some activated neutrophils and natural killer (NK) cells. We therefore checked for the presence of the latter cell types by staining with Gr-1 Ab (neutrophils) or anti-asialo-GM-1 Ab (NK cells). A few Gr-1+ neutrophils were seen in all samples, and NK cells were found in proximity to tumor cells in only 20% of instances surveyed. No major differences were seen between P-sel−/− and P-sel+/+ mice with regard to the pattern of either NK or Gr-1-positive cells. Thus, the increase in Mac-1 positive cells seen with either P-selectin deficiency or heparin injection most likely represents an increase in association of monocytes (circulating macrophage precursors) with tumor cells. To more accurately quantitate these interactions, we have also developed a new software program called nearcount (http://vis.fdsc.edu). This software was used to quantify the association of red-staining (Mac-1 positive) monocytes or blue-staining (CD41 positive) platelets with green-staining (calcein-AM positive) tumor cells in the volume surrounding a central tumor cell. The numerical data summarized in Fig. 4B present quantitations of three randomly captured images of a tumor cell from each mouse genotype. A 10-fold increase of red staining (monocytes) in association with tumor cells was documented in P-sel−/− and heparin-injected P-sel+/+ mice compared with that observed in P-sel+/+ mice (P < 0.001). Likewise, a 10- to 20-fold decrease in blue staining (platelets) in association with tumor cells was observed in P-sel−/− and heparin-injected P-sel+/+ mice when compared with control P-sel +/+ mice (P < 0.01). These data confirm the loss of platelet association with tumor cells and the increased association of monocytic cells, resulting from either P-selectin deficiency or heparin injection.
Figure 4.
Three-dimensional reconstruction of in vivo tumor cell complexes to determine the effects of P-selectin deficiency on monocyte association with tumor cells. (A) Lung sections of mice injected with calcein AM-labeled LS180 cells (green fluorescence) were obtained 30 min after injection and stained with CD-41 Ab (blue fluorescence) for platelets and Mac-1 Ab (red fluorescence) for monocytes. The figure shows typical examples of the complexes seen surrounding the tumor cells (optical section thickness 4.5 microns). The data were captured by deconvolutional microscopy and processed to generate volume views. (Bar = 2 μm.) For details, see Materials and Methods. “Fly-by” views of three-dimensional reconstructions can be viewed by activating Movies 1–3 presenting Psel+/+, Psel−/−, and Psel+/+ heparin (which are published as supplemental data on the PNAS web site, www.pnas.org). (B) nearcount software was used for quantification of fluorescence. The fluorescent signals for red (Mac-1 positive cells) and blue (platelets) were detected within a 100-μm2 area surrounding the central tumor cell (green) on 14 sections encompassing 2 μm in depth. Relative fluorescence refers to a total pixel signal from red (monocyte) or blue (platelet) channel relative to the green channel (tumor).
The concept that monocytes/macrophages might help eradicate metastatic tumor cells has been well recognized (54). Recently, 2-chloroadenosine pretreatment to eliminate monocytes/macrophages was used as indirect evidence to indicate that they may be involved in elimination of metastatic tumor cells during the early phase of metastasis (55). Our data now demonstrate that in the absence of the initial protective platelet coating, monocytic cells are much better able to associate directly with tumor cells in the lung vasculature. These monocytic cells could thus eliminate the tumor cells by becoming activated locally into functional effector macrophages. These results underline the importance of “a platelet cloak” not only for assisting tumor cells to initially lodge in blood vessels, but also for protecting them from potentially cytotoxic effector cells in the vasculature (48), thus permitting subsequent metastatic progression.
Implications and Perspectives.
The importance of P-selectin–tumor mucin interactions is demonstrated for the particular tumor cell lines we have studied. However, there are reported examples of tumor cells that do not have P-selectin-binding sites, even in the context of sialyl Lewisx/a expression (39, 56). It is possible that some of these tumors use other mechanisms to interact with platelets (e.g., via integrins). There are also likely to be examples of tumors that use completely different mechanisms to succeed in the metastatic cascade. Further work is needed to define the frequency with which native human tumors actually use P-selectin-based mechanisms to succeed in metastasizing, as well as the extent to which endothelial P-selectin and/or E-selectin may contribute to the process. Also, because our goal was to study initial platelet–tumor cell interactions occurring in a time span of less than a few hours, we used an experimental metastasis assay system involving human tumors in immunodeficient mice. Given the well-known limitations of this approach, we are also currently studying the role of selectins in syngeneic and/or spontaneous metastasis models, which might better reflect the complexity of the metastatic process.
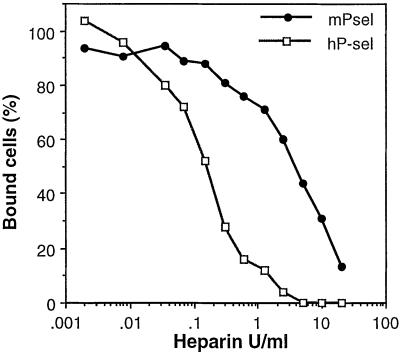
Although heparin was already known to inhibit P-selectin, a connection to the inhibition of tumor metastasis by heparin has not been previously shown. Our finding that heparin inhibits the initial platelet–tumor cell interactions in vivo is also, to our knowledge, novel, as is the fact that a single heparin dose that transiently blocks this interaction is sufficient to prevent long-term organ colonization. To address the potential clinical implications of this work in mice, we compared the effects of heparin in inhibiting tumor cell interactions with recombinant human and mouse P-selectin. Human P-selectin was even more sensitive to heparin inhibition than mouse P-selectin (Fig. 5; IC50 values 0.2 and 2.5 USP units/ml, respectively). The value for human P-selectin is within the acceptable range for the current therapeutic use of heparin in clinical settings (57, 58). Thus, we suggest that the use of heparin therapy to inhibit human carcinoma metastasis deserves to be reexamined. Of course, heparin may well have other beneficial effects in cancer via inhibition of angiogenesis or heparanases (10, 16, 21, 24, 28, 29). All previously recognized heparanase inhibitors we have tested can also inhibit tumor cell adhesion to P-selectin (unpublished data). Thus, these polyanionic compounds may have a multipotent effect on metastasis, the very first of which would be inhibition of P-selectin-mediated platelet adhesion to tumor cells. The use of more specific and selective P-selectin inhibitors (59) may dissect the relative roles of the different mechanisms of heparin action. However, it will be a long while before such molecules are available for clinical use. Meanwhile, we suggest that the failure of Vitamin K antagonists to improve cancer prognosis should be ignored and heparin therapy revisited under this new paradigm. Unlike previous studies, heparin treatment might be explored during the interval from initial visualization of a primary tumor until just after its definitive surgical removal. This could potentially prevent establishment of metastatic deposits by tumor cells that are circulating during this time period, by blocking their interaction with platelets via a P-selectin-mediated process.
Figure 5.
Heparin inhibition of human and mouse P-selectin interactions with tumor cells. Microtiter plates (Corning) were coated with 400 ng of soluble Protein A by overnight incubation at 4°C in 100 μl of 50 mM sodium carbonate/bicarbonate buffer, pH 9.5. Plates were blocked with 1% BSA in HBSS (HBSS/BSA, Sigma) for 30 min at room temperature (RT). Human P-sel-Fc (hP-sel) or mouse P-sel-Fc (mP-sel) was added to the plate at a concentration of 400 ng/well in HBSS/BSA and incubated for 3 h at RT. Plates were washed twice with HBSS/BSA. Calcein AM-labeled tumor cells LS180 were added in the presence or absence of serial dilutions of sodium heparin at concentrations ranging from 0.002 units/ml to 20 units/ml and cells incubated for 1 h at 4°C while gently rotating on an Orbital Rotor shaker (Bellco Glass, Vineland, NJ) at 70 rpm. Wells were washed twice with HBSS/BSA and once with HBSS. HBSS (85 μl), Triton X-100 (15 μl of 5%) was added, and fluorescence read after 2 min rotation on a Cytofluor II at excitation, 488 nm and emission, 508 nm. The IC50 values for hP-sel and mP-sel were 0.2 and 2.5 USP units/ml respectively.
Supplementary Material
Acknowledgments
We thank Dr. Dzung Le (University of California, San Diego) for measurement of heparin levels. This work was supported by U.S. Public Health Service Grant R01CA38701 (to A.V.), P50HL 23594 (to R. Spragg), and a fellowship of the Novartis-Jubilaums-Stiftung, Basel (to L.B.).
Abbreviations
- OSGPase
O-sialoglycoprotease
- HBSS
Hanks' balanced salt solution
- USP
United States Pharmacopeia
References
- 1.Hart I R, Liotta L A. Developments in Oncology 7: Tumor Invasion and Metastasis. The Hague: Martinus Nijhoff; 1982. [Google Scholar]
- 2.Folkman J. Cancer Metastasis Rev. 1990;9:171–174. doi: 10.1007/BF00046358. [DOI] [PubMed] [Google Scholar]
- 3.Fidler I J. Cancer Res. 1990;50:6130–6138. [PubMed] [Google Scholar]
- 4.Kerbel R S. Cancer Metastasis Rev. 1995;14:259–262. doi: 10.1007/BF00690597. [DOI] [PubMed] [Google Scholar]
- 5.Clark E A, Golub T R, Lander E S, Hynes R O. Nature (London) 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 6.Karpatkin S, Pearlstein E. Ann Intern Med. 1981;95:636–641. doi: 10.7326/0003-4819-95-5-636. [DOI] [PubMed] [Google Scholar]
- 7.Gasic G J. Cancer Metastasis Rev. 1984;3:99–114. doi: 10.1007/BF00047657. [DOI] [PubMed] [Google Scholar]
- 8.Honn K V, Tang D G, Crissman J D. Cancer Metastasis Rev. 1992;11:325–351. doi: 10.1007/BF01307186. [DOI] [PubMed] [Google Scholar]
- 9.Zacharski L R, Ornstein D L. Thromb Haemostasis. 1998;80:10–23. [PubMed] [Google Scholar]
- 10.Engelberg H. Cancer. 1999;85:257–272. doi: 10.1002/(sici)1097-0142(19990115)85:2<257::aid-cncr1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Hejna M, Raderer M, Zielinski C C. J Natl Cancer Inst. 1999;91:22–36. doi: 10.1093/jnci/91.1.22. [DOI] [PubMed] [Google Scholar]
- 12.Hettiarachchi R J K, Smorenburg S M, Ginsberg J, Levine M, Prins M H, Büller H R. Thromb Haemostasis. 1999;82:947–952. [PubMed] [Google Scholar]
- 13.Fielding L P, Hittinger R, Grace R H, Fry J S. Lancet. 1992;340:502–506. doi: 10.1016/0140-6736(92)91708-g. [DOI] [PubMed] [Google Scholar]
- 14.Lebeau B, Chastang C, Brechot J M, Capron F, Dautzenberg B, Delaisements C, Mornet M, Brun J, Hurdebourcq J P, Lemarie E. Cancer. 1994;74:38–45. doi: 10.1002/1097-0142(19940701)74:1<38::aid-cncr2820740108>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Nitti D, Wils J, Sahmoud T, Curran D, Couvreur M L, Lise M, Rauschecker H, dosSantos J G, Stremmel W, Roelofsen F. Eur J Cancer. 1997;33:1209–1215. doi: 10.1016/s0959-8049(97)00052-x. [DOI] [PubMed] [Google Scholar]
- 16.Ornstein D L, Zacharski L R. Haemostasis. 1999;29 Suppl. 1:48–60. doi: 10.1159/000054112. [DOI] [PubMed] [Google Scholar]
- 17.Smorenburg S M, Hettiarachchi R J K, Vink R, Büller H R. Thromb Haemostasis. 1999;82:1600–1604. [PubMed] [Google Scholar]
- 18.Zacharski L R. Haemostasis. 1986;16:300–320. doi: 10.1159/000215302. [DOI] [PubMed] [Google Scholar]
- 19.Zacharski L R, Henderson W G, Rickles F R, Forman W B, Cornell C J J, Forcier R J, Edwards R L, Headley E, Kim S H, O'Donnell J F. Cancer. 1984;53:2046–2052. doi: 10.1002/1097-0142(19840515)53:10<2046::aid-cncr2820531007>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 20.Zielinski CC, Hejna M. N Engl J Med. 2000;34:1991–1993. doi: 10.1056/NEJM200006293422612. [DOI] [PubMed] [Google Scholar]
- 21.Vlodavsky I, Mohsen M, Lider O, Svahn C M, Ekre H P, Vigoda M, Ishai-Michaeli R, Peretz T. Invasion Metastasis. 1994;14:290–302. [PubMed] [Google Scholar]
- 22.Lapierre F, Holme K, Lam L, Tressler R J, Storm N, Wee J, Stack R J, Castellot J, Tyrrell D J. Glycobiology. 1996;6:355–366. doi: 10.1093/glycob/6.3.355. [DOI] [PubMed] [Google Scholar]
- 23.Sciumbata T, Caretto P, Pirovano P, Pozzi P, Cremonesi P, Galimberti G, Leoni F, Marcucci F. Invasion Metastasis. 1996;16:132–143. [PubMed] [Google Scholar]
- 24.Folkman J, Weisz P B, Joullié M M, Li W W, Ewing W R. Science. 1989;243:1490–1493. doi: 10.1126/science.2467380. [DOI] [PubMed] [Google Scholar]
- 25.Salmivirta M, Lidholt K, Lindahl U. FASEB J. 1996;10:1270–1279. doi: 10.1096/fasebj.10.11.8836040. [DOI] [PubMed] [Google Scholar]
- 26.Tyrrell D J, Horne A P, Holme K R, Preuss J M, Page C P. Adv Pharmacol. 1999;46:151–208. doi: 10.1016/s1054-3589(08)60471-8. [DOI] [PubMed] [Google Scholar]
- 27.Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, et al. Nat Med. 1999;5:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 28.Miao H Q, Elkin M, Aingorn E, Ishai-Michaeli R, Stein C A, Vlodavsky I. Int J Cancer. 1999;83:424–431. doi: 10.1002/(sici)1097-0215(19991029)83:3<424::aid-ijc20>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 29.Parish C R, Freeman C, Brown K J, Francis D J, Cowden W B. Cancer Res. 1999;59:3433–3441. [PubMed] [Google Scholar]
- 30.Dennis J W, Laferte S. Cancer Metastasis Rev. 1987;5:185–204. doi: 10.1007/BF00046998. [DOI] [PubMed] [Google Scholar]
- 31.Hakomori S. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 32.Kim Y S, Gum J, Brockhausen I. Glycoconj J. 1996;13:693–707. doi: 10.1007/BF00702333. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y J, Varki A. Glycoconj J. 1997;14:569–576. doi: 10.1023/a:1018580324971. [DOI] [PubMed] [Google Scholar]
- 34.Kannagi R. Glycoconj J. 1997;14:577–584. doi: 10.1023/a:1018532409041. [DOI] [PubMed] [Google Scholar]
- 35.Hoff S D, Matsushita Y, Ota D M, Cleary K R, Yamori T, Hakomori S, Irimura T. Cancer Res. 1989;49:6883–6888. [PubMed] [Google Scholar]
- 36.Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R. Cancer Res. 1993;53:354–361. [PubMed] [Google Scholar]
- 37.Nakamori S, Kameyama M, Imaoka S, Furukawa H, Ishikawa O, Sasaki Y, Kabuto T, Iwanaga T, Matsushita Y, Irimura T. Cancer Res. 1993;53:3632–3637. [PubMed] [Google Scholar]
- 38.Stone J P, Wagner D D. J Clin Invest. 1993;92:804–813. doi: 10.1172/JCI116654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mannori G, Crottet P, Cecconi O, Hanasaki K, Aruffo A, Nelson R M, Varki A, Bevilacqua M P. Cancer Res. 1995;55:4425–4431. [PubMed] [Google Scholar]
- 40.Varki A. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kansas G S. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 42.Varki A. J Clin Invest. 1997;99:158–162. doi: 10.1172/JCI119142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEver R P. Glycoconj J. 1997;14:585–591. doi: 10.1023/a:1018584425879. [DOI] [PubMed] [Google Scholar]
- 44.Kim Y J, Borsig L, Han H L, Varki N M, Varki A. Am J Pathol. 1999;155:461–472. doi: 10.1016/S0002-9440(10)65142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Izumi Y, Taniuchi Y, Tsuji T, Smith C W, Nakamori S, Fidler I J, Irimura T. Exp Cell Res. 1995;216:215–221. doi: 10.1006/excr.1995.1027. [DOI] [PubMed] [Google Scholar]
- 46.Celi A, Pellegrini G, Lorenzet R, De Blasi A, Ready N, Furie B C, Furie B. Proc Natl Acad Sci USA. 1994;91:8767–8771. doi: 10.1073/pnas.91.19.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka N G, Tohgo A, Ogawa H. Inv Metastasis. 1986;6:209–224. [PubMed] [Google Scholar]
- 48.Nieswandt B, Hafner M, Echtenacher B, Männel D N. Cancer Res. 1999;59:1295–1300. [PubMed] [Google Scholar]
- 49.Kim Y J, Borsig L, Varki N M, Varki A. Proc Natl Acad Sci USA. 1998;95:9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koenig A, Norgard-Sumnicht K E, Linhardt R, Varki A. J Clin Invest. 1998;101:877–889. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agard D A, Hiraoka Y, Shaw P, Sedat J W. Methods Cell Biol. 1989;30:353–377. doi: 10.1016/s0091-679x(08)60986-3. [DOI] [PubMed] [Google Scholar]
- 52.Nadeau D R. Proc. IEEE: Visualization and Graphics Symposium 2000. 2000. [Google Scholar]
- 53.Sutherland D R, Abdullah K M, Cyopick P, Mellors A. J Immunol. 1992;148:1458–1464. [PubMed] [Google Scholar]
- 54.Fidler I J, Schroit A J. Biochim Biophys Acta. 1988;948:151–173. doi: 10.1016/0304-419x(88)90009-1. [DOI] [PubMed] [Google Scholar]
- 55.Kikkawa H, Imafuku H, Tsukada H, Oku N. FEBS Lett. 2000;467:211–216. doi: 10.1016/s0014-5793(00)01144-3. [DOI] [PubMed] [Google Scholar]
- 56.Handa K, White T, Ito K, Hang F, Wang S, Hakomori S. Int J Oncol. 1995;6:773–781. doi: 10.3892/ijo.6.4.773. [DOI] [PubMed] [Google Scholar]
- 57.Ginsberg J S. N Engl J Med. 1996;335:1816–1828. doi: 10.1056/NEJM199612123352407. [DOI] [PubMed] [Google Scholar]
- 58.Hirsh J. Semin Thromb Hemostasis. 1996;22 Suppl. 2:7–12. [PubMed] [Google Scholar]
- 59.Epperson T R, Patel K D, McEver R P, Cummings R D. J Biol Chem. 2000;275:7839–7853. doi: 10.1074/jbc.275.11.7839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.