Abstract
Purpose
Biomarkers for high-grade dysplasia in patients with radiographically identified intraductal papillary mucinous neoplasms (IPMN) have not been described. We hypothesized that dysplasia in IPMN invokes an immunogenic/proinflammatory microenvironment that can be identified by cyst fluid cytokine levels.
Experimental Design
Pancreatic cyst fluid aspirates were collected at resection (2005 - 2009). Samples were grouped into low-risk (low grade (n=6) or moderate dysplasia (n=15)) and high-risk groups (high grade dysplasia (n=13) or carcinoma (n=6)). Cytokine expression was determined using a multiplex sandwich immunoassay. Differences in cytokine expression were evaluated using the Wilcoxon rank-sum test and two sample t-test. Sample classification was performed using a logistical regression adjusting for sample covariates.
Results
IL5 and IL8 concentrations were higher in the cyst fluid from patients in the high-risk group compared to the low-risk group. Interleukin 1b (IL1b) concentrations were also higher in the cyst fluid from patients with high grade dysplasia or cancer (n=19) compared to those with low or moderate grade dysplasia (n=21, 539 ± 255 pg/mL vs. 0.2 ± 0.1 pg/mL, p<0.0001). IL1b remained a significant predictor of high-risk cysts after multivariate analysis. There was no significant difference in levels of interleukins 2, 4, 10, 12, 13, TNFa, or IFN-gamma between the groups. That IL1b levels identified cysts at a high risk of malignancy was confirmed in an independent validation set.
Conclusions
Cyst fluid levels of IL1b can differentiate low from high-risk IPMN. This study introduces IL1b as a potential biomarker for validation in larger clinical studies.
Introduction
The recognition of intraductal papillary mucinous neoplasms of the pancreas (IPMN) is increasing. This is largely secondary to an increased utilization of high-quality cross-sectional abdominal imaging (1-2). Histopathologic analysis of resected IPMN specimens has revealed that these cysts contain a spectrum of dysplasia, ranging from low grade dysplasia to high grade dysplasia to invasive pancreatic cancer (3-6). Patients with highly dysplastic and invasive IPMN may present with clinical symptoms or characteristic radiographic findings, including jaundice, an associated pancreatic mass, or main pancreatic duct dilation, however, many IPMN are incidentally discovered. Once IPMN are radiographically diagnosed, there is currently no reliable way to determine the level of epithelial dysplasia or to predict the time frame of progression to high grade dysplasia or cancer (5, 7-9). High risk cysts containing high grade dysplasia or invasive cancer should be excised in suitable patients, but cysts with low grade or moderate dysplasia could potentially be followed radiographically, sparing the patient the potential morbidity and mortality of pancreatic resection. Currently, this information is only available postoperatively, and not even preoperative fine needle aspiration of the pancreatic cyst is able to confidently determine the level of cyst dysplasia.
Cell mediated and humoral immune responses have been demonstrated in multiple malignancies, including pancreatic adenocarcinoma. Furthermore, cytokine markers of the Th1 and Th2 immune response have been shown to discriminate pancreatic cancer from chronic pancreatitis or normal pancreatic tissue in both serum and pancreatic juice samples (10-11). We hypothesized that dysplasia in IPMN invokes an immunogenic/proinflammatory microenvironment that could be quantified in the cyst fluid, and allow for the identification of high-grade dysplasia or carcinoma prior to operation.
We have previously shown that cyst fluid CEA is not a marker of dysplasia in this disease, and are actively investigating other potential targets (12-13). Recent work by our group has found differential expression of mucin glycoproteins in IPMN cyst fluid, and the addition of immunologic markers may allow for the development of a highly accurate cyst fluid assay for high-grade dysplasia or invasive pancreatic cancer (14).
Materials and Methods
Between 2005 and 2009, 147 patients underwent pancreatic resection for IPMN at Memorial Sloan-Kettering Cancer Center. Patients were pre-operatively consented to an IRB tissue collection protocol and a waiver of authorization was obtained from the institutional IRB prior to data review. Pancreatic cyst fluid aspirates were collected at the time of resection from 40 of these patients. All 40 samples were included in this study. Correlative studies on pre-operative cyst fluid carcinoembryonic antigen (CEA) and fine needle aspiration (FNA) cytology were performed on a subset of these patients.
Resected specimens were immediately transported to the MSKCC tumor procurement facility in the Department of Pathology where cyst fluid was aspirated with an 18 - 21 gauge needle, divided into 500 μl aliquots, and stored at −80°C. Aspiration was performed by a surgeon, pathologist, or technician. All analyses were performed on samples with no prior freeze-thaw cycles. Samples were run neat without dilution except for 11 highly viscous samples, which were serially diluted in PBS.
IPMN were classified as gastric, intestinal, pancreatobiliary, or oncocytic, based on their histopathologic characteristics. In some cases, the cyst contained two dominant histologic subtypes, in which case they were classified as a mix of the two pathologies. The most severe degree of cellular atypia identified in the cyst determined the grade of dysplasia as being low, moderate, or high grade. Invasive carcinoma in IPMN was characterized as tubular or colloid based upon cellular morphology and mucin content. Samples were divided into low risk (low grade and moderate dysplasia) and high risk groups (high grade dysplasia and invasive carcinoma). IPMNs were distinguished from mucinous cystic neoplasms by the absence of ovarian type stroma in the cyst wall. Histopathology was independently reviewed and assessed by a single dedicated GI pathologist who was blinded to the cytokine assay results (N.K.).
Cyst fluid was assessed for cytokine expression using an ultrasensitive multiplex sandwich immunoassay (Meso Scale Discovery, Gaithersburg, Maryland). Standard curves were performed for individual cytokines and verified. All samples were run according to manufacturer’s guidelines. Six cyst fluid aspirates from serous cystadenomas were included as benign non-IPMN pancreatic cyst control specimens.
Cytokine expression levels were log2 transformed in order to improve the normality of the data. Differential expression analysis of data sets were assessed utilizing the Wilcoxon rank sum test and two sample t test. Sample classification was performed using a logistical regression adjusting for sample covariates including cyst size and duct type. Cytokine values are expressed as the mean ± SEM.
Results
Patient demographics and cyst characteristics
Cyst fluid was available from 40 patients with IPMN (13 men and 27 women). There were 19 “high risk” cysts, consisting of five with invasive carcinoma and 14 with high grade dysplasia, and 21 “low risk” cysts, consisting of 15 with moderate dysplasia and six with low grade dysplasia. Cyst size ranged from 1.2 to 23 cm with a mean diameter of 4.2 cm. There were 12 main duct lesions, 19 branch duct lesions, and nine mixed (branch and main duct) cysts. Six high risk cysts and one low risk cyst contained a solid component or nodularity, while the majority of cysts (thirty-three) were void of any concerning radiographic features.
Histopathologic subtype and dysplasia
Of the 19 high risk cysts, eight were of the intestinal subtype, seven were pancreatobiliary, two were oncocytic, and two were gastric type IPMN. All of the low risk cysts were of the gastric subtype.
Degree of cyst dysplasia and cytokine expression
Interferon gamma (IFN-g) and interleukin-4 (IL4) expression was very low (< 1 pg/mL) in the cyst fluid across all groups of IPMN and there was no correlation between these cytokine levels and the degree of cyst dysplasia. IL2, 10, 12, 13, and tumor necrosis factor alpha (TNF-a) expression was much higher than IFN and IL4 across all IPMN groups, but there was no association between the level of dysplasia and cytokine expression (Table 1).
Table 1.
Mean Cytokine Concentration in IPMN Cyst Fluid (pg/mL)
| IL1b* | IL2 | IL4 | IL5 | IL8 | IL10 | IL12 | IL13 | TNFa | IFNg | |
|---|---|---|---|---|---|---|---|---|---|---|
| High Risk (n=19) | 539 | 1.8 | <0.1 | 0.2 | 8088 | 2.5 | 2.2 | 3 | 31.6 | 0.4 |
| Low Risk (n=21) | 0.2 | 0.2 | <0.1 | <0.1 | 2758 | 0.6 | 0.6 | 0.4 | 2.2 | <0.1 |
| Serous cystadenoma (n=6) | 0.1 | 1.8 | <0.1 | 6.7 | 6576 | 2.1 | 0.2 | 1.3 | 3.4 | <0.1 |
p<0.0001
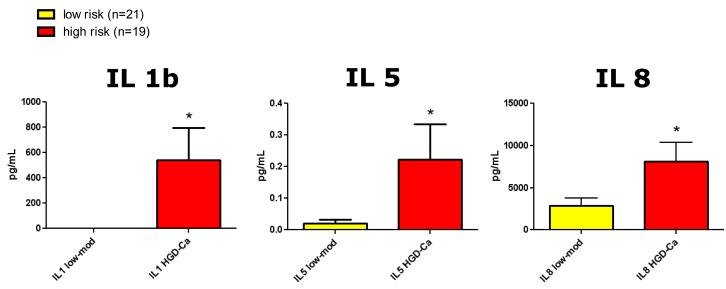
In a univariate analysis, IL1b, IL5, and IL8 had higher levels of expression in the presence of high grade dysplasia or invasive carcinoma. High risk cysts (n=19) had increased expression of IL1b (539 ± 255 vs. 0.2 ± 0.1 pg/mL, p<0.0001) and IL5 (0.2 ± 0.5 vs. 0.02 ± 0.05 ng/mL, p=0.03) compared to low risk cysts (n=21). IL8 levels were the highest of all cytokines measured and were different between high and low risk cysts (8089 ± 2288 vs. 2758 ± 847 pg/mL, p=0.03), though this difference did not maintain significance when the sample variance was normalized (Wilcoxon rank sum test, p=0.07) (Figure 1). IL1b levels measured in non-mucinous serous cystadenomas were barely detectable (0.1 ± 0.1 pg/mL, n=6).
Figure 1.
Levels of interleukin-1b (IL1b), IL5, and IL8 were increased in high risk cysts (high grade dysplasia (HGD) or invasive cancer (Ca), n=19) compared to low risk cysts (low grade dysplasia (low) or moderate dysplasia (mod), n=21), *p<0.05. In a multivariate predictive analysis model, only IL1b remained a significant predictor of dysplasia (p=0.0003).
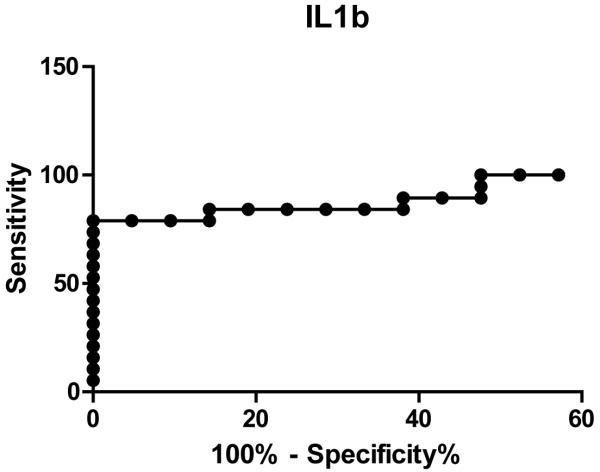
Multivariate analysis of all tested cytokines revealed that IL1b levels alone predicted high from low risk cysts, and remained a significant predictor on logistical regression when corrected for IPMN type (main or side branch) and cyst size (p=0.0003). The receiver operating characteristic curve for IL1b had an area under the curve of 0.92 (p<0.0001). At a cutoff of 1.26pg/mL, IL1b levels had a sensitivity of 79.0% (54.4-93.9%) and specificity of 95.2% (76.2-99.9%) to distinguish low from high risk cysts (Figure 2).
Figure 2.
Receiver operating characteristic curve for IL1b shows an area under the curve of 0.92 (p<0.0001). At levels greater than 1.26 pg/mL, IL1b had 95% specificity and 79% sensitivity for predicting a high risk cyst (high grade dysplasia or invasive cancer) with a likelihood ratio (LR) of 16.6.
Cyst fluid from an additional 15 patients were analyzed for IL1b levels in a blinded fashion. IL1b levels differentiated high risk from low risk cysts (343 ± 298 pg/mL, n=8 vs. 0.15 ± 0.09 pg/mL, n=7; p=0.05). Based on the previously determined ROC calculated value of 1.26 pg/mL to distinguish high risk from low risk cysts, IL1b levels had a positive predictive value of 71% for correctly identifying high risk cysts with high grade dysplasia or invasive cancer, and a negative predictive value of 75% for correctly identifying low risk cysts that contained low or moderate dysplasia.
Cyst Fluid Fine Needle Aspiration Cytology and CEA
Preoperative fine needle aspirates were available from the cysts of 29 patients: 14 high risk patients and 15 low risk patients (Table 2). Cytology was read as suspicious or atypical in six of 15 low risk and 10 of 14 high risk samples. The findings of suspicious or atypical cells on FNA did not differentiate the level of cyst dysplasia or determine high from low risk cysts (p=0.14). Cytology was read as negative in six of 15 low risk and three of 14 high risk samples; and was unsatisfactory or non-diagnostic in three of 15 low risk samples and one of 14 high risk samples.
Table 2.
Fine Needle Aspiration Cytology in IPMN
| Low risk | High risk | |
|---|---|---|
| Carcinomatous, Suspicious, or Atypical cells | 6 | 10 |
| Negative for malignancy | 6 | 3 |
| Unsatisfactory or Non-diagnostic sample | 3 | 1 |
|
| ||
| Total | 15 | 14 |
Eight of the ten cysts with branch duct pathology, no nodularity or solid component, and larger than 3 cm had FNA cytology available for analysis. Six cysts were branch duct lesions (two high risk lesions and four low risk) and four cysts were mixed branch + main duct lesions(one high risk lesion and three low risk). The corresponding cytology and IL1b levels are displayed in Table 3.
Table 3.
IL1b and FNA Cytology in Branch Duct IPMN >3cm Without Nodularity
| Low risk | High risk | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| IPMN Duct type | N/A | Non-Diagnostic | Negative | Suspicious | N/A | Non-Diagnostic | Negative | Suspicious | |
| Branch Duct | FNA cytology | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 2 |
| IL 1b levels (pg/mL) | 0, 0 | 0 | 0 | - | - | - | - | 2715, 16.9 | |
|
| |||||||||
| Branch + Main duct | FNA cytology | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| IL 1b levels (pg/mL) | 0.3 | 0.02 | 0 | - | - | - | 275 | - | |
Fine needle aspirate cyst fluid CEA values were available for 15 low risk lesions and nine high risk lesions. CEA values did not differentiate between low and high risk cysts (4423 ± 2523 pg/mL vs. 3079 ± 2910 pg/mL, p=0.12) whereas, IL1b levels did differentiate the groups (0.15 ± 0.09 pg/mL vs. 343 ± 298, p=0.0007). There was no correlation between cyst fluid CEA levels and IL1b levels in high risk lesions (p=0.61) or in low risk lesions (p=0.91), and the combination of IL1b and CEA levels together did not distinguish high risk from low risk cysts (p=NS).
Discussion
The management of IPMN has evolved in the last decade due a better understanding of the disease process and an increase in patients followed with small (<3 cm) incidentally discovered branch duct IPMN. Current treatment recommendations are based primarily on radiographic findings . Approximately 50% of lesions involving the main pancreatic duct harbor high-grade dysplasia or microinvasive disease and resection is generally recommended (15-16). Branch duct lesions (cystic dilation without dilation of the main pancreatic duct) rarely contain invasive cancer, and are benign in > 95% of IPMN less than 3 cm in diameter and without a solid component. These branch duct lesions can be followed radiographically in selected patients (17-18). Many patients, however, present without a dilated main duct, solid component, or nodularity, or present with a large branch duct lesion. The recommendations for treatment in these patients is controversial. In our data set, many of the lesions were radiographically equivocal and without a solid component or nodularity. Identification of markers highly predictive of dysplasia would spare patients with low risk lesions the morbidity/mortality of pancreatectomy and allow patients with high risk lesions to undergo resection prior to the development of invasive cancer.
Pancreatic cyst fluid biochemistry has been useful in distinguishing serous from mucinous lesions. An elevated cyst fluid CEA greater than 192ng/dl has approximately 80% accuracy for the diagnosis of mucinous cysts, but the degree of CEA elevation has not been found to correlate with the degree of dysplasia (12-13, 19). That cyst fluid CEA levels do not correlate with the presence or absence of malignancy was confirmed in the large scale prospective multicenter PANDA trial (20). Therefore, for patients with IPMN and equivocal radiographic findings, the present or future risk of malignant cyst transformation cannot be determined by CEA levels. This was again confirmed in the current study where cyst fluid CEA levels were elevated in the cysts, corresponding with diagnosis of an IPMN, but did not correlate with the level of cyst dysplasia. Cyst fluid CEA levels alone, or in combination with IL1b, did not identify high risk lesions in this subset, whereas IL1b levels alone did confidently identify high risk lesions (p=0.0007).
Most IPMN show variable amounts of dysplasia throughout the cyst, therefore preoperative sampling of IPMN by FNA has not accurately reflected the degree of dysplasia in the cyst as a whole. Since the sample represents only a small representation of the lesion and does not provide tissue architecture, cyst FNA cytology is most often described to contain non-diagnostic, normal, atypical, or suspicious cells, and pathologists cannot comment on the level of dysplasia (N. K./D. K.). We, and others, have shown that even these descriptions are often incorrect and lead to the wrong clinical conclusion, with a sensitivity of only 28% for correctly identifying premalignant or malignant mucinous pancreatic lesions (8). As a result, when cyst fluid is aspirated at our institution, it is no longer clinical practice to submit that sample for cytology due to confusing and often unreliable findings. When we examined FNAs from the current patient samples, suspicious or atypical cells were found in 40% of low risk cysts and 71% of high risk cysts, and cytology did not differentiate the level of cyst dysplasia (p=0.14). Furthermore, suspicious or atypical cells were identified by the pathologist as possibly representing a mucinous cystic neoplasm and could not differentiate between low grade dysplasia and high grade dysplasia. In addition, 21% of high risk cysts containing high grade dysplasia or invasive cancer on final pathology were FNA cytology negative preoperatively for evidence of cyst atypia or malignancy, which reinforces the need for a better marker of dysplasia than cyst fluid cytology. Furthermore, in a subset analysis of branch and branch+main duct lesions > 3 cm in size without a solid component or mural nodule, two branch and branch+main duct cysts had negative FNA cytology. The IL1b levels in the fluid of both of these cysts was 0 pg/mL and both were low risk lesions. However, there was one branch+main duct lesion > 3cm in size without a solid component or mural nodule that had negative FNA cytology and an IL1b level of 275 pg/mL. This was in a high risk lesion. It is exactly in these instances that a biomarker of dysplasia would be very clinically useful, and in this case IL1b levels in the cyst fluid could identify a false negative FNA cytology result.
We are interested in identifying biomarkers of malignancy in these cysts and have recently evaluated cyst fluid mucins for their ability to differentiate low risk and high risk IPMN. In the current study we have explored further the utility of cyst fluid for measuring markers of dysplasia. We found that cyst fluid, which can be obtained preoperatively by endoscopic ultrasound fine needle aspiration, also contains measurable levels of cytokines reflective of a Th1 and Th2 immune response. Specifically, IL1b levels correlated with the degree of cyst dysplasia, and were highly predictive of high-risk lesions. Using a highly sensitive immunoassay, lesions with IL1b concentrations greater than 0.98 pg/mL were 8.3 times more likely to be high risk cysts. At 1.26 pg/mL IL1b levels had a high sensitivity and specificity with a likelihood ratio of 17× to distinguish low from high risk cysts. Low risk lesions contained essentially undetectable concentrations of IL1b (<1.4 pg/mL) and high risk lesions expressed IL1b with a mean concentration of 539 pg/mL. We also prospectively collected cyst fluid samples to analyze for IL1b levels in a validation set, and IL1b did differentiate high risk from low risk cysts, confirming our initial observations. Based on the previously determined ROC calculated value of 1.26 pg/mL to distinguish high risk from low risk cysts, IL1b levels had a PPV of 71% for correctly identifying high risk cysts with HGD or invasive cancer, and a NPV of 75% for correctly identifying low risk cysts that contained low or moderate dysplasia. At the current time, there is no reliable marker in IPMN cyst fluid to aid the surgeon in clinical decision making, or to inform the patient that they may have a high risk IPMN that should be resected, therefore to identify a single test with a validated sensitivity and PPV >70% is very promising and will encourage validation studies in a larger set of patients. As these samples are difficult to obtain in large numbers with correlative surgical pathology, this is an ongoing multi-institutional effort.
IL1 is synthesized primarily by monocytic phagocytes that are stimulated in immunogenic and pro-inflammatory environments. Whereas IL1a is mostly intracellular or membrane bound, IL1b is secreted into the extracellular space, which makes it an appropriate cytokine to measure in pancreatic cyst fluid (21). Plasma concentrations of IL1b are usually undetectable in the absence of inflammation, severe autoimmune disease, or organ rejection, therefore, levels in simple serous cysts should also be very low (22-23). These data are consistent with our finding of near undetectable levels of IL1b in the cyst fluid of low grade IPMN and serous cystadenomas. The presence of IL1b in dysplastic cysts may reflect an inflammatory microenvironment in the cyst.
Dysplasia is known to arise in a background of chronic inflammation. IL1 mediated inflammation has been shown to be associated with the pathogenesis of colon cancer, where tissue concentrations of IL1 are increased in chronic inflammatory bowel disease, and in gastric cancer, where IL1b production is upregulated in chronic gastritis, initiating the inflammatory response and exacerbating mucosal damage that leads to neoplasia (24-27). Similar to the cancers associated with inflammatory bowel disease and gastritis, chronic inflammation in pancreatic IPMN may lead to severe dysplasia and be reflected in IL1b cyst fluid levels. IL1b expression in tumor specimens has also been found to be a determinant of cancer risk and survival in many other neoplasms including ovarian, cervical, and breast cancer, and is a mediator of pancreatic cancer cell invasion (21, 25, 28-31).
Many cancers present tumor antigens for recognition by host immune cells, and the antitumor immune response can be measured through cytokine levels in the tumors. Cell-mediated and humoral immune responses occur through Th1 and Th2 CD4+ T lymphocytes and their activity can be measured through quantification of the cytokines IL1, IL2, IL4, IL5, IL8, IL10, IL12, IL13, IFN-γ, and TNF-α (11). IL 1b is a key mediator of the inflammatory response, and is central to cell proliferation, differentiation, and apoptosis. IL1b signaling also activates CD4 T cells in an antigen dependent fashion, a cascade that may be triggered when low grade IPMN transform into highly dysplastic IPMN (32). IL 5 is produced by Th2 cells and mast cells. Among other functions, it stimulates B cell growth and increases immunoglobulin secretion. Increased serum levels of IL5 have been found in patients with pancreatic adenocarcinoma compared to chronic pancreatitis, and this finding supports a systemic Th2 response in patients with pancreas cancer (11). However, the absolute levels of IL5 expressed in this study were too low to be clinically relevant, and many of the samples, both high and low risk, contained concentrations below the detectable limits of our assay. IL8 is a major mediator of the inflammatory response and functions as a chemoattractant and an angiogenic factor. IL8 has been shown to be overexpressed in the duodenal juice of patients with pancreatic carcinoma and here was found in high concentrations in high risk IPMN (10). However, the large variance in concentrations and low sample size limit its utility as a biomarker, and it was not found to be a significant factor on multivariate predictive analysis. The increased IL8 levels observed in high risk IPMN may, however, be explained by IL1 stimulated production of IL8. Furthermore, cyst concentrations of IL5 and IL8 were not significantly lower in serous cystadenomas compared to high risk IPMN, as they were in the case of IL1b.
In conclusion, high risk IPMN were associated with elevated levels of cytokines reflective of a Th1 and Th2 immunologic response. The presence of IL1b in particular was highly predictive of malignancy, even when factors such as main pancreatic duct involvement and cyst size were controlled for. This study has identified and validated a marker of IPMN dysplasia for further validation studies, and suggests that cyst fluid IL1b levels may inform surgeons to more appropriately select patients for operative resection.
Statement of Translational Relevance.
Intraductal papillary mucinous neoplasms (IPMN) are premalignant and malignant pancreatic tumors that are being increasingly diagnosed due to improved cross-sectional imaging techniques. Once IPMN are diagnosed, there is no reliable way to predict the level of epithelial dysplasia or the time frame of progression to pancreatic cancer. High-risk cysts containing high-grade dysplasia or invasive cancer should be excised in suitable patients, but cysts with low-grade or moderate dysplasia could potentially be followed, sparing the patient the potential morbidity/mortality of pancreatic resection. We have discovered and validated a marker of IPMN dysplasia in the cyst fluid, that can be sampled pre-operatively, and that identified high-risks cysts with high sensitivity and specificity. This study has identified IL1b for further validation studies that may allow surgeons to more appropriately select patients for operative resection and allow for early detection of pancreatic cancer.
Acknowledgments
Grant Support: Peter J. Allen. NCI EDRN U01 CA08496
References
- 1.Fernández-Del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL, et al. Incidental pancreatic cysts: Clinicopathologic characteristics and comparison with symptomatic patients. Archives of Surgery. 2003;138:427–434. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: Depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–553. doi: 10.1148/radiol.2232010815. [DOI] [PubMed] [Google Scholar]
- 3.Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: Delineation of an “intestinal” pathway of carcinogenesis in the pancreas. American Journal of Surgical Pathology. 2004;28:839–848. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Ban S, Naitoh Y, Mino-Kenudson M, Sakurai T, Kuroda M, Koyama I, et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am J Surg Pathol. 2006;30:1561–1569. doi: 10.1097/01.pas.0000213305.98187.d4. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa T, Klöppel G, Adsay N Volkan, Albores-Saavedra J, Fukushima N, Horii A, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: A consensus study. Virchows Archiv. 2005;447:794–799. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 6.Lüttges J, Feyerabend B, Buchelt T, Pacena M, Klöppel G. The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. American Journal of Surgical Pathology. 2002;26:466–471. doi: 10.1097/00000478-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. American Journal of Surgical Pathology. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 8.Maker AV, Lee LS, Raut CP, Clancy TE, Swanson RS. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann Surg Oncol. 2008;15:3187–3192. doi: 10.1245/s10434-008-0110-0. [DOI] [PubMed] [Google Scholar]
- 9.Takaori K. Current understanding of precursors to pancreatic cancer. J Hepatobiliary Pancreat Surg. 2007;14:217–223. doi: 10.1007/s00534-006-1165-6. [DOI] [PubMed] [Google Scholar]
- 10.Noh KW, Pungpapong S, Wallace MB, Woodward TA, Raimondo M. Do cytokine concentrations in pancreatic juice predict the presence of pancreatic diseases? Clin Gastroenterol Hepatol. 2006;4:782–789. doi: 10.1016/j.cgh.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 11.Seicean A, Popa D, Mocan T, Cristea V, Neagoe I. Th1 and Th2 profiles in patients with pancreatic cancer compared with chronic pancreatitis. Pancreas. 2009;38:594–595. doi: 10.1097/MPA.0b013e31819313d0. [DOI] [PubMed] [Google Scholar]
- 12.Belletrutti PJ, DiMaio CJ, Nagula S, Schattner MA, Markowitz AJ, Allen PJ, et al. Pancreatic cyst fluid CEA concentration >1000 ng/mL is not an indicator of malignancy. Gastroenterology. 2010;138:S549. [Google Scholar]
- 13.Nagula S, Kennedy TJ, Schattner MA, Brennan MF, Gerdes H, Markowitz AJ, et al. Performance characteristics of cyst fluid CEA analysis for the diagnosis of mucinous cysts of the pancreas. Gastroenterology. 2009;136:A148. doi: 10.1007/s11605-010-1281-0. [DOI] [PubMed] [Google Scholar]
- 14.Maker AV, Katabi N, Gonen M, DeMatteo RP, D’Angelica MI, Fong Y, et al. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas (IPMN) Annals of Surgical Oncology. 2010 doi: 10.1245/s10434-010-1225-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–408. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen PJ, D’Angelica M, Gonen M, Jaques DP, Coit DG, Jarnagin WR, et al. A selective approach to the resection of cystic lesions of the pancreas: Results from 539 consecutive patients. Annals of Surgery. 2006;244:572–579. doi: 10.1097/01.sla.0000237652.84466.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 19.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, et al. Diagnosis of Pancreatic Cystic Neoplasms: A Report of the Cooperative Pancreatic Cyst Study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095–1102. doi: 10.1016/j.gie.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 21.Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 22.Cannon JG, Friedberg JS, Gelfand JA, Tompkins RG, Burke JF, Dinarello CA. Circulating interleukin-1 beta and tumor necrosis factor-alpha concentrations after burn injury in humans. Crit Care Med. 1992;20:1414–1419. doi: 10.1097/00003246-199210000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Eastgate JA, Symons JA, Wood NC, Grinlinton FM, di Giovine FS, Duff GW. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988;2:706–709. doi: 10.1016/s0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- 24.Basso D, Scrigner M, Toma A, Navaglia F, Di Mario F, Rugge M, et al. Helicobacter pylori infection enhances mucosal interleukin-1 beta, interleukin-6, and the soluble receptor of interleukin-2. Int J Clin Lab Res. 1996;26:207–210. doi: 10.1007/BF02592984. [DOI] [PubMed] [Google Scholar]
- 25.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 26.Jung HC, Kim JM, Song IS, Kim CY. Helicobacter pylori induces an array of pro-inflammatory cytokines in human gastric epithelial cells: quantification of mRNA for interleukin-8, -1 alpha/beta, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1 and tumour necrosis factor-alpha. J Gastroenterol Hepatol. 1997;12:473–480. doi: 10.1111/j.1440-1746.1997.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 27.Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- 28.Goode EL, Maurer MJ, Sellers TA, Phelan CM, Kalli KR, Fridley BL, et al. Inherited determinants of ovarian cancer survival. Clin Cancer Res. 2010;16:995–1007. doi: 10.1158/1078-0432.CCR-09-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Zhai X, Jin G, Hu Z, Wang S, Wang X, et al. Functional variants in the promoter of interleukin-1beta are associated with an increased risk of breast cancer: a case-control analysis in a Chinese population. Int J Cancer. 2006;118:2554–2558. doi: 10.1002/ijc.21652. [DOI] [PubMed] [Google Scholar]
- 30.Qian N, Chen X, Han S, Qiang F, Jin G, Zhou X, et al. Circulating IL-1beta levels, polymorphisms of IL-1B, and risk of cervical cancer in Chinese women. J Cancer Res Clin Oncol. 2010;136:709–716. doi: 10.1007/s00432-009-0710-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greco E, Basso D, Fogar P, Mazza S, Navaglia F, Zambon CF, et al. Pancreatic cancer cells invasiveness is mainly affected by interleukin-1beta not by transforming growth factor-beta1. Int J Biol Markers. 2005;20:235–241. doi: 10.1177/172460080502000406. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]