Abstract
Background
Although acupuncture-needle manipulation is an important component of acupuncture therapy, little information is currently available on whether or not specific types of needle manipulation produce different effects on the body. Bidirectional (back-and-forth) rotation is one of the most common forms of needle manipulation used in acupuncture practice.
Objectives
In this study, we hypothesized that bidirectional acupuncture needle rotation causes dose-dependent active cytoskeletal remodeling in connective tissue fibroblasts similar to that previously demonstrated with unidirectional rotation.
Interventions
Subcutaneous tissue explants from 18 mice were randomized to varying amounts of bidirectional rotation cycles (8–64) and rotation–cycle amplitude (180°–720°) ex vivo for 30 minutes, followed by tissue fixation, confocal microscopy, and measurement of fibroblast cell body cross-sectional area.
Results
As with unidirectional rotation, fibroblasts responded to bidirectional rotation with extensive cell spreading and lamellipodia formation. Bidirectional needle rotation had a significant overall effect on fibroblast cell body cross sectional area (analysis of variance, p < 0.001). The cellular response to bidirectional rotation was nonmonotonic with maximal responses occurring within specific stimulus windows with regard to cycle amplitude and cycle number.
Conclusions
These findings demonstrate that subtle differences in acupuncture-needle manipulation techniques can affect cellular responses in mouse subcutaneous connective tissue. Further studies will be needed to determine whether these connective-tissue responses are related to therapeutic effects.
INTRODUCTION
Acupuncture is performed clinically using a wide variety of treatment styles and techniques. In some cases, acupuncture needles are simply inserted and left undisturbed in the tissue for a certain amount of time, then removed. In other instances, needle insertion is followed by manipulation, usually consisting of a combination of rotational and pistoning movements. The most common form of rotational needle manipulation is bidirectional (back-and-forth) rotation, which is performed at varying speeds and amplitudes depending upon the clinical situation. Indeed, an important part of an acupuncturist’s skill is to choose an appropriate type and “dose” of needle manipulation to match the patient’s clinical presentation.
Little information is currently available on dose-related effects of needle manipulation, mainly because performing controlled studies using reproducible needle movements is difficult when the needle is manipulated manually. In previous studies, we have begun to address this issue by developing robotic needling methods allowing controlled acupuncture needling in humans and animals.1–5 We showed that, in mouse subcutaneous tissue explants, acupuncture needle rotation (unidirectional) causes winding and gathering of collagen around the needle. Within minutes of needle rotation, this pulling of collagen toward the needle induces an active cellular response in connective tissue fibroblasts up to several centimeters away from the needle.5 This cellular response consisted of an active cytoskeletal reorganization involving cell spreading that was measurable as an increase in mean fibroblast cell body-cross-sectional area. This effect required actomyosin contractility as well as Rho and Rac, two signaling molecules know to be involved in cytoskeletal regulation.5,6 Extensive evidence from cultured cell models has shown that cell shape and cytoskeletal remodeling are key components of mechanotransduction responses linking the cell’s mechanical environment to fundamental events such as gene transcription, production of the extracellular matrix, and cell-adhesion dynamics.7–11 Mechanical stimulation of fibroblasts during acupuncture-needle manipulation therefore may have important, extensive and lasting effects on connective tissue.
In our previous experiments using unidirectional needle rotation, 2 needle revolutions produced cell-spreading effects similar to those of uniaxially stretching the tissue to a load of 1.96 mN (2 g). Greater amounts of rotation, however, produced less cell spreading. This nonmonotonic response pattern was reminiscent of previously described effects of mechanical stimulation in bone in which maximal responses occurred within specific stimulus “windows.”12 The goal of the current study was to determine whether bidirectional needling shows a similar ability to stimulate connective tissue fibroblast remodeling as does unidirectional needling. This would have important clinical implications since bidirectional needling is a much more commonly used in acupuncture practice. We have used robotic acupuncture needling to examine the effect of varying amounts of bidirectional needle rotation on mouse connective tissue fibroblast morphology ex vivo. We hypothesized that both the rotation cycle amplitude and the number of rotation cycles influence the fibroblast cross-sectional area 30 minutes after needle manipulation.
MATERIALS AND METHODS
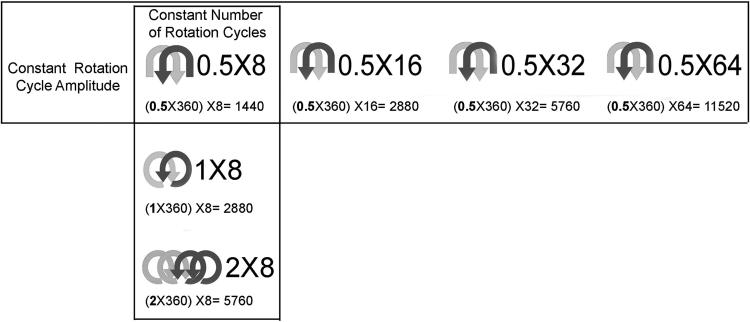
Experimental protocols were approved by the University of Vermont (Burlington, VT) Institutional Care and Use Committee. Eighteen C57Black-6 mice (19–24 g) were randomized to varying amounts of bidirectional rotation cycles (8–64) and rotation cycle amplitude (180°–720°). Three (3) mice were assigned to each condition. Rotation conditions are summarized in Figure 1. Total clockwise rotation degrees was defined as the rotation-cycle amplitude (number of clockwise revolutions per cycle × 360°) multiplied by the number of rotation cycles. Each rotation cycle contained equal amounts of clockwise and counterclockwise rotations. These rotation variables were chosen to represent a range of rotation conditions consistent with acupuncture practice. Two general groups of conditions were studied: (1) either varying the number of rotation cycles (i.e., increasingly large numbers of small rotations) or (2) the rotation cycle amplitude (i.e., small numbers of increasingly large rotations).
FIG. 1.
Summary of needle-rotation parameters. Total clockwise rotation degrees are calculated based on rotation-cycle amplitude (number of clockwise revolutions per cycle × 360°) and number of rotation cycles. Horizontal and vertical boxes indicate experimental conditions testing the effect of varying numbers of rotation cycles and rotation-cycle amplitude, respectively.
Mice were sacrificed via decapitation. Immediately after death an 8-cm × 3-cm tissue flap containing dermis, subcutaneous muscle, and subcutaneous tissue was excised from the back of each mouse. The tissue flap was placed transversely in grips and immersed in an incubation bath containing physiologic saline solution (HEPES-PSS), pH 7.4 at 37°C, containing (mM): NaCl 141.8, KCl 4.7; MgSO4 1.7; EDTA 0.39; CaCl2 2.8; HEPES 10; KH2PO4 1.2; and glucose 5.0. The tissue grips were placed vertically in a holder with the proximal grip connected to a 500 g (4.9 N) capacity load cell.13 The tissue was elongated at a rate of 1 mm/second by advancing a micrometer connected to the distal tissue grip until a preload of 0.3 g (2.9 mN) was achieved. The tissue length was fixed at this length by attaching a pair of stabilization bars, maintaining a fixed distance between proximal and distal grips. Tissue and grips together were then disconnected from the load cell, removed from the bath, and placed under a dissecting microscope. An acupuncture needle (Seirin, Japan; 0.25-mm diameter and 50 mm-length) was inserted through the middle of the subcutaneous tissue parallel to the dermis. Extreme care was used during this procedure to insert the needle without pulling on the tissue. The tissue and grips were then placed back into the incubation bath at 37°C. The needling instrument motor and needle grip were attached to the needle, and the needling instrument was placed into a clamp to maintain the needle in a fixed vertical position during rotation.
The needle was then rotated with a varying number of bidirectional needle revolutions at a constant speed (1 rev/second) and acceleration of 3 revs/sec2. After needle rotation, the motor was disconnected from the needle and the tissue was incubated for 30 minutes at 37°C. The tissue was then immersion fixed in 95% ethanol for 1 hour, after which the needlze was removed from the tissue which was then rinsed overnight in phosphate-buffered saline (PBS) at 4°C.
Histochemical staining
Texas Red-conjugated phalloidin (Molecular Probes, Eugene, OR) (a specific stain for polymerized actin) was used to visualize subcutaneous tissue fibroblasts with confocal microscopy as previously described.14 Following an overnight rinse in PBS, three subcutaneous tissue samples (each 10 mm × 10 mm) were dissected from the tissue flap. Each subcutaneous tissue sample was placed flat on a glass slide. The sample that had included the needle was labeled “medial,” and the other samples were labeled “lateral right” and “lateral left,” respectively. The slides were stained with Texas Red conjugated phalloidin (4U/mL; Molecular Probes, Eugene OR) for 40 minutes at 4°C then counter-stained for 2 minutes with Sytox Green (Molecular Probes, Eugene, OR) nucleic acid stain or 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Invitrogen, Carlsbad, CA) nucleic-acid stain at a dilution of 1:1000 for 5 minutes, both at room temperature (RT). The samples were overlaid with glass coverslips using 50% glycerol in PBS with 1% N-propylgallate as a mounting medium.
Confocal scanning laser microscopy and morphometric analysis
Histochemically stained subcutaneous tissue samples were imaged with a Bio-Rad MRC 1024 confocal microscope (Bio-Rad Microsciences, Hercules, CA) using a 60 × oil immersion lens (N.A. 1.4) and a 568-nm laser excitation and an iris aperture of 2.7. Each sample was divided longitudinally into three roughly equal-sized areas. In each sample, one field was imaged in the center of each of these three areas for a total of nine fields per animal. Fields were chosen at low power by an individual who was blinded to the study variable (number of revolutions). Because the needle track was not always visible on the tissue slides (especially in the samples with little or no rotation), imaged fields were located in the center of each area without regard for needle position.
For each field, a stack of 20 (313 μm × 313 μm) images was acquired at a 1 μm inter-image interval. Image stacks were imported into the analysis software package Meta-Morph (version 6.0; Universal Imaging Corporation, Downington, PA) for morphometric analysis. In each stack, all cells that were in focus in the 10th optical section (middle of stack) were measured. A cell was excluded if part of its cell body perimeter was outside the image. Cells were not identified as either fibroblasts or nonfibroblasts. Instead, all cells were measured, with the assumption that fibroblasts are the overwhelmingly predominant cell population in this tissue. For each cell, a wire frame of cell-body perimeter was constructed.13 Cell-body perimeter was defined as the outline of the cell’s cytoplasm projected in the plane of the image excluding cell processes. A cell process was defined as an extension of a cell’s cytoplasm longer than 2 μm and less than 2 μm in width at any portion of its length. Processes were identified in-plane and out-of-plane by following each one through successive optical sections within the image stack. Cell-body cross-sectional area was calculated as the area delimited by the cell-body perimeter projected onto the image plane.
Statistical methods
Analysis of variance (ANOVA) was used to compare mean cell-body cross-sectional area across treatment conditions. Pairwise comparisons among means were based on Fisher’s least significant difference procedure. Statistical significance was determined based on α = 0.05. Analyses were performed using SAS statistical software (SAS Institute, Cary, NC).
RESULTS
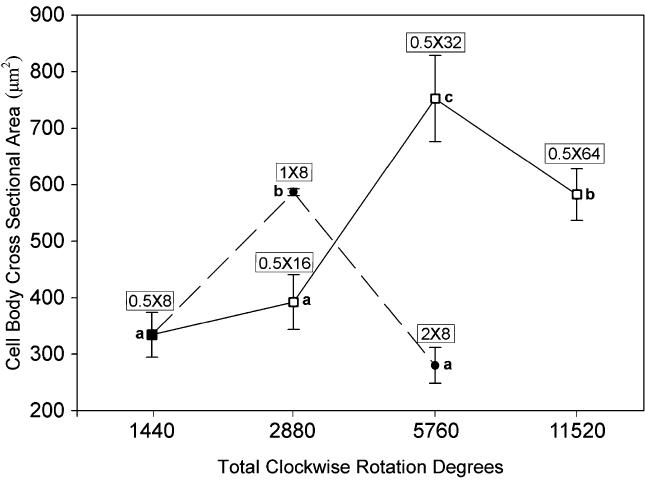
Bidirectional needle rotation had a significant overall effect on fibroblast cell-body cross-sectional area (ANOVA, p < 0.001). Fibroblasts responded nonmonotonically to both increasing rotation-cycle amplitude and increasing numbers of rotation cycles (Fig. 2). When the rotation cycle amplitude was kept constant (180°) and the number of cycles varied from 8 to 64 (Fig. 2, square symbols), fibroblast cross-sectional area peaked with 5760 total clockwise rotation degrees (32 cycles). In contrast, when the number of cycles was kept constant (8) and cycle amplitude varied from 180° to 720° (Fig. 2, closed symbols), fibroblast cross-sectional area peaked with 2880 total rotation degrees (360° cycle amplitude). Thus, fewer total rotation degrees were necessary to induce cell spreading when the cycle amplitude was increased, compared with increasing the number of cycles. Increasing the cycle amplitude also produced a narrower response window compared with increasing the number of cycles. When the cycle amplitude was increased beyond the peak response (i.e., from 360° to 720°), cells became much smaller. In contrast, when the number of rotation cycles was increased beyond the peak response (i.e., from 32 to 64), cells remained quite large. Interestingly, 5760 total rotation degrees produced both the smallest and the largest cells, depending upon the way that the rotation was administered.
FIG. 2.
Effect of bidirectional acupuncture-needle rotation on fibroblast cell-body cross-sectional area. Mean fibroblast cell-body cross-sectional area is plotted as a function of total clockwise-rotation degrees (see Fig. 1). N = 18 mice (3 mice per condition). Square symbols (open and closed) correspond to constant rotation amplitude (180°) and variable number of rotation cycles (8–64). Closed symbols (circles and squares) correspond to constant number of cycles (8) and variable cycle amplitude (180°–720°). Means sharing a common letter are not statistically significant (Fisher’s LSD, p < 0.05). Error bars represent standard errors. Total clockwise rotation degrees on x axis are plotted on a logarithmic scale.
DISCUSSION
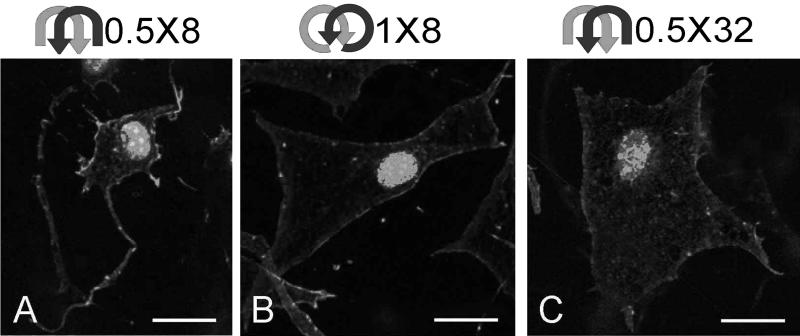
Peak responses to bidirectional acupuncture needle rotation (8 cycles of 360° amplitude and 32 cycles of 180° amplitude) yielded fibroblast morphologies similar to those previously reported with both unidirectional needle rotation and simple tissue stretch, namely, large flat cells with lamellipodia extending in multiple directions in the tissue plane but no distinct stress fibers (Fig. 3 B,C). In contrast, fibroblasts exposed to less needle rotation (8 cycles of 180° amplitude) had smaller cell bodies with more compact actin cytoskeletal structures (Fig. 3A). Together with our previous study, these results show that both unidirectional and bidirectional acupuncture needle rotation can induce an active cytoskeletal response in mouse subcutaneous tissue fibroblasts. Another similarity between unidirectional and bidirectional needle rotation was the strikingly nonmonotonic cellular response. In other words, maximal cellular responses were not simply the result of increasing the amount of needle rotation but rather were produced by specific combinations of cycle amplitude and cycle number (Fig. 4).
FIG. 3.
Effect of bidirectional acupuncture-needle rotation on fibroblast morphology. Both 8 cycles of 360° amplitude (1 × 8) and 32 cycles of 180° amplitude (0.5 × 32) resulted in extensive fibroblast spreading and lamellipodia formation (B, C). A smaller amount of rotation (8 cycles of 180° amplitude) resulted in smaller cell bodies with more compacted polymerized actin staining (A). Tissue was stained with Rhodamine-phalloidin (Molecular Probes, Eugene, OR) (a specific stain for polymerized actin) and Sytox Green (Molecular Probes, Eugene, OR) nuclear stain. Scale bars = 40 μm.
FIG. 4.
Summary diagram relating the findings of this study to our previous study of unidirectional acupuncture needle rotation. Dashed box corresponds to unidirectional rotation. With both unidirectional and bidirectional rotation, fibroblast cross-sectional area increased within specific response windows (insets). Data for unidirectional rotation were published previously (see ref. 5)
Our previously proposed model for the effect of needle manipulation holds that elements of connective tissue adhere, at least weakly, to the inserted needle and, when the needle is rotated, the tissue is drawn around with it.15 After a small amount of rotation, the connective tissue tightens and effectively becomes “locked” to the needle. Further rotation causes large amounts of connective tissue to wind around the needle forming a whorl and presumably delivering a strong mechanical stimulation to the tissue. This model is conceptually straight-forward when the direction of the needle rotation is in one direction only (unidirectional). It is less immediately clear what happens during bidirectional needling when the needle is rotated a small amount in one direction and then rotated the same amount in the opposite direction. A simple interpretation of the model might suggest that no net effect would be achieved because an equal amount of winding and unwinding occurs. Previous experimental evidence, however, suggests that winding is not entirely reversed by subsequent unwinding. Repeated bidirectional winding/unwinding cycles in humans caused an incremental increase in the peak torques achieved during each cycle.1 This previous work using bidirectional needling focused on mechanical outcome measures (needle force and torque). The results of this study support this mechanism further, although it cannot be ruled out at this time that the active cellular response of fibroblasts may have been the result of cyclical tissue stretching (with no torque buildup) rather than progressive tissue winding. Because our current needling system did not allow for measurement of torque in the range of those produced in this study, further experiments using more sensitive torque sensors and comparing bidirectional rotation with cyclical tissue stretch will be needed to address this question.
Regardless of whether fibroblast spreading is caused by torque buildup or cyclical tissue stretch, the findings of this study demonstrate that subtle differences in needle-manipulation techniques can affect cellular responses differentially within connective tissue. It is, however, important to emphasize that, at this time, there is no known link between the connective-tissue response to needling and the response of the whole patient to acupuncture (i.e., clinical improvement). Possible mechanisms leading to therapeutic effects include changes in matrix composition and biomechanical properties leading to modulation of sensory afferent input from connective tissue.16 Further studies will be needed to examine these potential responses to acupuncture needling ex vivo and in vivo.
CONCLUSIONS
We conclude that, in mouse subcutaneous tissue ex vivo, bidirectional acupuncture needle rotation produced an active cytoskeletal response that was dose-dependent with respect to rotation cycle number and cycle amplitude. Future work will examine the relationship of this connective tissue response to that of other physiologic systems (e.g., nervous system) and to therapeutic effects.
ACKNOWLEDGMENTS
We thank Kirsten N. Storch, M.S., for technical assistance, and Douglas Gomez and Gilbert Gianetti of the University of Vermont Instrumentation and Modeling Facility for fabrication of the ex vivo needling system used in this study. This study was funded by the National Center for Complementary and Alternative Medicine Research Grant RO1-AT01121.
Its contents are solely the reponsibility of the authors and do not necessarily represent the offical views of the National Center for Complementary and Alternative Medicine, National Institutes of Health.
Footnotes
POTENTIAL CONFLICTS OF INTEREST Helene M. Langevin, M.D., is a partner of Stromatic, Inc., Burlington, VT, a company dedicated to the development of technology for the investigation, diagnosis, and treatment of connective tissue dysfunction.
REFERENCES
- 1.Langevin HM, Churchill DL, Fox JR, et al. Biomechanical response to acupuncture needling in humans. J Appl Physiol. 2001;91:2471–2478. doi: 10.1152/jappl.2001.91.6.2471. [DOI] [PubMed] [Google Scholar]
- 2.Langevin HM, Yandow JA. Relationship of acupuncture points and meridians to connective tissue planes. Anat Rec. 2002;269:257–265. doi: 10.1002/ar.10185. [DOI] [PubMed] [Google Scholar]
- 3.Langevin HM, Churchill DL, Wu J, et al. Evidence of connective tissue involvement in acupuncture. FASEB J. 2002;16:872–874. doi: 10.1096/fj.01-0925fje. [DOI] [PubMed] [Google Scholar]
- 4.Langevin HM, Konofagou EE, Badger GJ, et al. Tissue displacements during acupuncture using ultrasound elastography techniques. Ultrasound Med Biol. 2004;30:1173–1183. doi: 10.1016/j.ultrasmedbio.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Langevin HM, Bouffard NA, Badger GJ, et al. Subcutaneous tissue fibroblast cytoskeletal remodeling induced by acupuncture: Evidence for a mechanotransduction-based mechanism. J Cell Physiol. 2006;207:767–774. doi: 10.1002/jcp.20623. [DOI] [PubMed] [Google Scholar]
- 6.Langevin HM, Storch KN, Cipolla MJ, et al. Fibroblast spreading induced by connective tissue stretch involves intracellular redistribution of alpha- and beta-actin. Histochem Cell Biol. 2006;125:487–495. doi: 10.1007/s00418-005-0138-1. [DOI] [PubMed] [Google Scholar]
- 7.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 8.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 9.Katsumi A, Orr AW, Tzima E, et al. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 10.Chiquet M, Renedo AS, Huber F, et al. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;22:73–80. doi: 10.1016/s0945-053x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 11.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 12.Brand RA, Stanford CM. How connective tissues temporally process mechanical stimuli. Med Hypotheses. 1994;42:99–104. doi: 10.1016/0306-9877(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 13.Langevin HM, Bouffard NA, Badger GJ, et al. Dynamic fibroblast cytoskeletal response to subcutaneous tissue stretch ex vivo and in vivo. Am J Physiol Cell Physiol. 2005;288:C747–756. doi: 10.1152/ajpcell.00420.2004. [DOI] [PubMed] [Google Scholar]
- 14.Langevin HM, Cornbrooks CJ, Taatjes DJ. Fibroblasts form a body-wide cellular network. Histochem Cell Biol. 2004;122:7–15. doi: 10.1007/s00418-004-0667-z. [DOI] [PubMed] [Google Scholar]
- 15.Langevin HM, Churchill DL, Cipolla MJ. Mechanical signaling through connective tissue: A mechanism for the therapeutic effect of acupuncture. FASEB J. 2001;15:2275–2282. doi: 10.1096/fj.01-0015hyp. [DOI] [PubMed] [Google Scholar]
- 16.Langevin HM, Sherman KJ. Pathophysiological model for chronic low back pain integrating connective tissue and nervous system mechanisms. Med Hypotheses. 2007;68:74–80. doi: 10.1016/j.mehy.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 1.Paley CA, Bennett MI, Johnson MI. Acupuncture for Cancer-induced Bone Pain? Evidence-based Complementary and Alternative Medicine. 2010 doi: 10.1093/ecam/neq020. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Julias Margaret, Buettner Helen M., Shreiber David I. Varying Assay Geometry to Emulate Connective Tissue Planes in an In Vitro Model of Acupuncture Needling. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2010 doi: 10.1002/ar.21308. n/a-n/a. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin Mike. The fascia of the limbs and back - a review. Journal of Anatomy. 2009;214(1):1–18. doi: 10.1111/j.1469-7580.2008.01011.x. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Napadow Vitaly, Ahn Andrew, Longhurst John, Lao Lixing, Stener-Victorin Elisabet, Harris Richard, Langevin Helene M. The Status and Future of Acupuncture Mechanism ResearchThe Status and Future of Acupuncture Mechanism Research. The Journal of Alternative and Complementary Medicine. 2008;14(7):861–869. doi: 10.1089/acm.2008.SAR-3. [Abstract] [PDF] [PDF Plus] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouffard Nicole A., Cutroneo Kenneth R., Badger Gary J., White Sheryl L., Buttolph Thomas R., Ehrlich H. Paul, Stevens-Tuttle Debbie, Langevin Helene M. Tissue stretch decreases soluble TGF-β1 and type-1 procollagen in mouse subcutaneous connective tissue: Evidence from ex vivo and in vivo models. Journal of Cellular Physiology. 2008;214(2):389–395. doi: 10.1002/jcp.21209. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]