Abstract
Therapeutic advances over the past three decades now allow most cancer patients to achieve major clinical responses. Although clinical responses can clearly decrease side effects and improve quality of life, most cancer patients still eventually relapse and die of their disease. Many cancers have now been shown to harbor cells that are phenotypically and biologically similar to normal cells with self-renewal capacity; these so-called cancer stem cells (CSC) typically constitute only a small fraction of the total tumor burden, but theoretically harbor all the self-renewal capacity. Moreover, the CSC appears to be relatively resistant to standard anticancer therapies by co-opting normal stem cells’ intrinsic defense mechanisms, such as quiescence, efflux pumps, and detoxifying enzymes. However, the clinical importance of CSC, if any, remains unproven. Nevertheless, emerging evidence suggests that initial responses in cancer represent therapeutic effectiveness against the bulk cancer cells, while the rare CSC is responsible for relapse. Better understanding of the biology of CSC, as well as reexamining both our preclinical and clinical drug development paradigms to include the CSC concept, has the potential to revolutionize the treatment of many cancers.
Keywords: Cancer stem cells, Multiple myeloma, Cancer therapy
Historical perspective
Only a minority of cells from most hematologic malignancies and solid tumors are clonogenic in vitro and in vivo. This low clonogenic potential could represent proliferative capacity exclusively restricted to a small subset of cancer cells, or alternatively all the cells within a cancer retaining the capacity to proliferate but only at a low rate. Which of these two possibilities explains the low clonogenicity of most cancers, has been debated for years. Fialkow and his colleagues first suggested that chronic myeloid leukemia (CML) arose from rare transformed hematopoietic stem cells (HSC) nearly 40 years ago, when they showed that both granulocytes and red blood cells from CML patients were derived from a common cell [1]. The first modern use of the term cancer or tumor stem cells was probably by Park et al., who found that only a minority mouse multiple myeloma cells were capable of clonogenic growth [2]. The stem cell origin of CML was confirmed more than 15 years ago when several groups utilized phenotypic characteristics of HSC to identify and isolate CML cells capable of expansion ex vivo [3]. Dick and colleagues extended these observations, showing that phenotypic primitive HSC purified from patients with both acute myeloid leukemia [4] and CML [5] would generate leukemia in vivo when injected into non-obese diabetic (NOD)/severe combined immunodeficiency (SCID) mice.
Such cells have more recently been described in many other malignancies [6, 7]. A recent consensus conference defined cancer stem cells (CSC) as tumor cells possessing the capacity for self-renewal and generating the cells that comprise the tumor bulk [7]. Although CSC have been hypothesized to arise from transformed normal cells with stem cell properties, it is very possible that the transformation process itself may be able to induce self-renewal capacity. Hence, these cells have also been designated “tumor-initiating” or “tumorigenic” cells, although the most commonly used label remains CSC [7]. The current gold standard for identification of CSC requires that they possess the ability to engraft immunodeficient mice [4, 8]. However, this is an artificial culture system that could either under or overestimate the true frequency of CSC. Differences in these in vivo culture systems may account for the widely varying frequency of melanoma CSC (ranging from one in a million, to one in four, tumor cells) that has been reported by different investigators [9].
Putative CSC have been reported to be relatively resistant to standard anticancer therapies [10-12], at least in part by co-opting normal stem cells’ intrinsic defense mechanisms such as quiescence, efflux pumps, and detoxifying enzymes [11]. The CSC concept proposes that CSC are responsible for most relapses, and must be eliminated to realize cures [6, 13]. However, currently there are no definitive data that CSC from any malignancy are in fact responsible for disease relapse or resistance. Thus, the data on CSC remain largely laboratory curiosities, leading many investigators to question the biologic and clinical relevance of these cells [14].
The paradox of response and survival
A cardinal principle of cancer therapeutics has been that evidence of a clinical response will translate into improved survival. The major advantage of using clinical response as the primary endpoint of therapeutic trials is that it is measurable over weeks to months, allowing the stepwise process of drug development to occur more rapidly and efficiently. In contrast, demonstrating a survival benefit adds significant complexity to clinical trial design, usually requiring the accrual of large patient numbers and long follow-up to provide statistical significance.
Although clinical responses can clearly decrease side effects and improve quality of life, there is surprisingly little evidence that disease response is an appropriate surrogate for survival [6]. In fact, there are numerous examples in which response does not predict for an improved survival. Indolent lymphoma patients who achieved a complete remission with conventional dose therapies in the prerituximab era did not experience a survival advantage over similar patients treated with a “watch and wait” approach [15]. In multiple myeloma, neither the magnitude nor the kinetics of clinical response impact survival in some studies [16]. Even the most intensive therapy for myeloma, blood or marrow transplantation [17, 18], provided no overall survival advantage in the national intergroup trial [19] or in two recent meta-analyses [20, 21]. Similarly, significant clinical responses in pancreatic cancer [22] and prostate cancer [23] have not translated into survival benefits. Further, despite new treatments that now produce complete remissions in the majority of women with ovarian carcinoma, cures are rare [24].
CSC and cancer therapeutics: the dandelion phenomenon
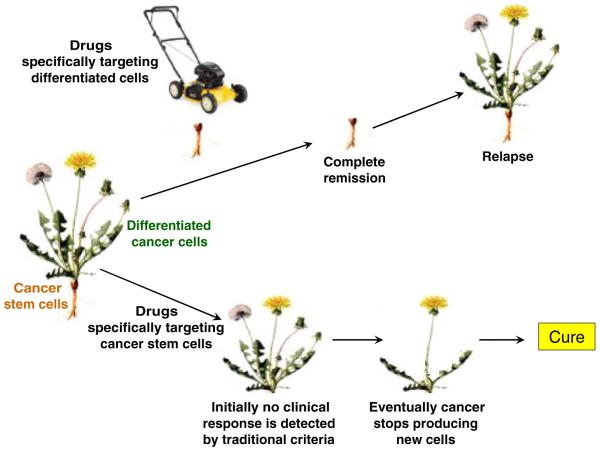
The CSC concept could explain this lack of a correlation between response and survival in many malignancies. Multiple myeloma has long been considered a malignancy of plasma cells, since they form the bulk of the tumor. However, the myeloma plasma cells actually appear to arise from a minute population of less differentiated CSC that resemble memory B cells and have the ability to self-renew, differentiate, and generate the disease in vitro [25] and in NOD/SCID mice [11]. Although not stem cells in the classic sense, memory B cells could be considered “honorary” stem cells in that they are long-lived, self-renew, and differentiate into plasma cells in order to maintain long-term immune memory. Moreover, the novel antimyeloma agents bortezomib and lenalidomide, which display significant activity against myeloma plasma cells, in vitro and clinically, exhibited little activity against myeloma CSC in vitro [11]. This pattern of activity is analogous to mowing a dandelion (Fig. 1); although this will eliminate the visible portion of the weed, the unseen root also needs to be eliminated to prevent regrowth [6, 13]. Conversely, rituximab and alemtuzumab eliminated myeloma CSC in vitro, but had no activity against myeloma plasma cells that lack the relevant target antigens (CD20 and CD52, respectively) [11]. This treatment effect mimics attacking just the root of the dandelion (Fig. 1); although this has no immediately discernible effect on the weed, over time, the weed should eventually wither and die if its root has been eliminated [6, 13].
Fig. 1.
The dandelion phenomenon. Most current anticancer therapies have been developed to target bulk tumor cell populations. The anticancer effects of such treatments are analogous to mowing dandelions; although this will eliminate the visible portion of the weeds (differentiated cancer cells responsible for the bulk of the tumor) the unseen roots (cancer stem cells) also need to be eliminated to prevent regrowth of the weeds. Equally problematic for drug development are therapies directed principally at the rare cancer stem cells. This treatment effect mimics attacking just the roots of the dandelions; since no immediately discernible effect on the weeds (differentiated cancer cells) will be seen, such potentially curative therapies could be prematurely abandoned
Evidence of clinical relevance
Although rituximab’s activity in multiple myeloma has been disappointing [26], parameters typically used to follow clinical response in myeloma (i.e., monoclonal immunoglobulin level and percentage plasma cells in the marrow) primarily measure the effect of therapies on the plasma cells. The long survival of the myeloma plasma cells could have obscured activity against the myeloma CSC responsible for maintaining the disease. Perhaps a longer duration of rituximab treatment could ultimately have demonstrated clinical responses using traditional criteria, by inhibiting new myeloma cell production for a sufficient period of time to allow terminally differentiated myeloma plasma cells to undergo spontaneous apoptosis.
With this background, our group initiated a clinical trial targeting the putative myeloma CSC with rituximab [27]. In this trial, high doses of cyclophosphamide as used in myeloablative conditioning for stem cell transplantation were also given to debulk myeloma plasma cells. HSC are protected from the toxic effects of high-dose cyclophosphamide because of their high expression of aldehyde dehydrogenase, the major mechanism of the drug’s inactivation [25]. Accordingly, high doses of cyclophosphamide do not require autologous HSC rescue, and the possibility of circulating myeloma CSC [11] reinfusion. Although all patients quickly recovered normal blood counts and there were no unexpected toxicities, only three of the 21 patients entered on the trial remain progression-free beyond 2 years. Importantly, however, although the number of myeloma CSC after high-dose cyclophosphamide did not correlate with outcome, there was a strong and significant association between myeloma CSC numbers in patients after treatment with rituximab and progression-free survival [27]. Moreover, rituximab could be detected on the surface of circulating myeloma CSC from patients who progressed, suggesting that the therapeutic concept was valid but that rituximab was unable to kill the myeloma CSC. Residual breast tumor cell populations persisting after conventional treatment have recently been shown to be enriched for phenotypic breast CSC [28]. These data from myeloma and breast cancer patients suggest for the first time clinical relevance for CSC.
Eliminating CSC
Currently, much of the search for novel anticancer therapies has focused on oncogenes that are specific for (e.g., BCR-ABL in CML), or overexpressed in (e.g., c-myc or bcl-2), selected cancers. However, attacking cancer-specific targets has met with variable success, and many of the most effective anticancer therapies, such as rituximab in lymphomas, high-dose cytotoxic therapy, or the allogeneic graft-versus-tumor effect, show limited or even no tumor selectivity. Targeting a cancer-specific pathway could fail for several reasons. It is likely that many cancers have already acquired multiple oncogenic mutations, even at initial diagnosis, capable of driving tumor growth; in such cases, targeting only one oncogene might be expected to generate limited activity. Further, even when the initiating oncogenic event is targeted as with imatinib in CML, inherent properties of stem cells may make the target inaccessible or irrelevant. For example, stem cells’ quiescence and high expression of ABC transporters may limit a drug’s access to a target; moreover, inhibiting an oncogene would not be expected to eradicate cells that are already long-lived in the absence of the oncogene [13, 29].
Properties shared with normal stem cells not only appear to be responsible for CSC resistance to many anticancer agents [11], they may also lead to the development of novel therapies active across many malignancies. In fact, prospective targets shared with normal stem cells may have particularly strong anticancer potential since their conserved expression suggests a critical function retained by the CSC. Several signaling pathways that are important for the generation and maintenance of normal stem cells during embryonic development, (e.g., Notch, Wnt, and Hedgehog) [30] and/or postnatally (e.g., telomerase [31]) also appear to be important for the growth of many cancers. Preliminary data suggest that inhibition of these pathways, even when they are not mutated or overexpressed, may produce potent antitumor activity across a range of malignancies, possibly because of the key roles these pathways play in stem cell maintenance and growth [32].
While toxicity from lack of tumor-specificity is an obvious concern for shared stem cell targets, there are several potential differences between normal and CSC that may provide a therapeutic ratio for shared targets. Normal stem cells have normal cell cycle checkpoints that are likely to protect them from cellular damage or crisis. The stage of differentiation at which cancers arise may also provide a therapeutic ratio for approaches targeting cancer. Although many cancers may arise from tissue stem cells, they may not be the most primitive tissue stem cells as exemplified by CML which appears to arise from myeloid or low-quality stem cells [3]. Accordingly, if a therapy equally eliminated both CML stem cells and their normal counter-parts, the existence of more primitive normal HSC should replenish the normal progenitor pool [29].
Another example of a shared stem cell target potentially providing tumor selectivity is telomerase, where differences between CSC and their normal counterparts in the interplay of telomere length and telomerase may provide a therapeutic ratio. Normal stem cells require telomerase to prevent telomere shortening leading to replicative senescence. However, even in the absence of telomerase, normal stem cells can maintain replicative capacity for some period of time because of their relatively long telomeres. Accordingly, telomerase knockout mice show a phenotype only after four to six generations [33]. In addition, the major cause of death in dyskeratosis congenita, a congenital disease that results from loss of function mutations in telomerase components, is bone marrow failure, but this usually does not manifest until the second or third decade of life [34]. In contrast, uninterrupted telomerase activity may be absolutely required for the maintenance and growth of most malignancies, in order to stabilize the short telomeres that have been hypothesized to characterize CSC [35]. In fact, crossing telomerase knock-out mice with INK4a−/− [36] or APCmin [37] mice predisposed to cancer, significantly lowered the development of cancers in these mice. Thus, the differential in telomere length between normal (long) and cancer (short) stem cells could provide telomerase inhibition selectivity toward cancer.
The rarity of many CSC (often <1% of the total tumor cells) has both limited their study and potentially masked CSC responses to treatment. Any therapeutic impact on the rare CSC may be imperceptible, hidden by the bulk of differentiated cells (Fig. 1). Current detection methods are insufficient to resolve differences in the size of the minute CSC population with treatment. Hence, as discussed above, therapies which are effective against only the differentiated cells (but inactive against CSC) may appear overly promising, while highly active agents against CSC may appear falsely ineffective if they have little impact on the differentiated leukemic bulk.
Because of the difficulty assessing the effects of therapies on the rare CSC responsible for relapse, the development of such approaches requires new clinical paradigms and methodologies [6]. We believe these new paradigms should rely heavily on preclinical modeling, employ nontraditional measures of clinical response as trial endpoints, and utilize novel preclinical assays to evaluate the fate of CSC. Preclinical studies should assess the effects of therapies on both CSC and differentiated cancer cell populations. Using suitable preclinical models, it may be possible to develop a detailed understanding of the mechanisms of action of new treatments, as well as strategies for optimizing activity; this could potentially allow a fully developed new approach to be taken directly from the “bench to the bedside”. However, effective preclinical models for CSC may ease the task of clinical trial development, but will not eliminate the need for new clinical paradigms. Evaluating the efficacy of treatments against CSC should be possible by utilizing these treatments after debulking the differentiated cells that constitute the majority of the tumor. In cancers where clinical debulking is successful (i.e., complete remissions are common but transient), studying therapies after induction of remission should permit using duration of remission as a measure of activity against the CSC. The fate of CSC could also be assessed as correlative laboratory endpoints using newly developed preclinical assays.
Conclusions
Initial responses in cancer represent therapeutic effectiveness against the cancer cells making up the bulk of the tumor; emerging data suggest that resistant CSC are often responsible for relapse. Since many currently active treatments have been developed to target the cancer cell bulk, they may have little activity against biologically distinct CSC. Moreover, traditional response criteria measure tumor bulk and may not reflect changes populations of rare cancer stem cells [6]. Standard response parameters may not only potentially overestimate the effect of therapy on the minute population of stem cells, but may also underestimate it. Therapy, selectively directed at CSC will not immediately eliminate the differentiated tumor cells; such therapy therefore might be prematurely abandoned if clinical activity is judged solely by standard response criteria that reflect the effects of treatment on the bulk of the cancer. Thus, it is likely that improving the results of cancer therapy requires identification and better understanding the biology of CSC, as well as reexamining both our preclinical and clinical drug development paradigms to include the CSC concept.
Footnotes
The authors report no conflicts of interest
References
- 1.Fialkow PJ, Gartler SM, Yoshida A. Clonal origin of chronic myelocytic leukemia in man. Proc Natl Acad Sci USA. 1967;58:1468–1471. doi: 10.1073/pnas.58.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park CH, Bergsagel DE, McCulloch EA. Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst. 1971;46:411–422. [PubMed] [Google Scholar]
- 3.Bedi A, Zehnbauer BA, Collector MI, Barber JP, Zicha MS, Sharkis SJ, Jones RJ. BCR-ABL gene rearrangement and expression of primitive hematopoietic progenitors in chronic myeloid leukemia. Blood. 1993;81:2898–2902. [PubMed] [Google Scholar]
- 4.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang TC-CJ, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 5.Sirard C, Lapidot T, Vormoor J, Cashman JD, Doedens M, Murdoch B, Jamal N, Messner H, Addey L, Minden M, et al. Normal and leukemic SCID-repopulating cells (SRC) coexist in the bone marrow and peripheral blood from CML patients in chronic phase, whereas leukemic SRC are detected in blast crisis. Blood. 1996;87:1539–1548. [PubMed] [Google Scholar]
- 6.Huff CA, Matsui W, Smith BD, Jones RJ. The paradox of response and survival in cancer therapeutics. Blood. 2006;107:431–434. doi: 10.1182/blood-2005-06-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 9.Refaeli Y, Bhoumik A, Roop DR, Ronai ZA. Melanoma-initiating cells: a compass needed. EMBO Rep. 2009 doi: 10.1038/embor.2009.184. doi:10.1038/embor.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 11.Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, McNiece I, Lin L, Ambinder RF, Peacock C, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68:190–197. doi: 10.1158/0008-5472.CAN-07-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 13.Jones RJ, Matsui WH, Smith BD. Cancer stem cells: are we missing the target? J Natl Cancer Inst. 2004;96:583–585. doi: 10.1093/jnci/djh095. [DOI] [PubMed] [Google Scholar]
- 14.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 15.Horning SJ. Natural history of and therapy for the indolent non-Hodgkin’s lymphomas. Semin Oncol. 1993;20:75–88. [PubMed] [Google Scholar]
- 16.Durie BG, Jacobson J, Barlogie B, Crowley J. Magnitude of response with myeloma frontline therapy does not predict outcome: importance of time to progression in southwest oncology group chemotherapy trials. J Clin Oncol. 2004;22:1857–1863. doi: 10.1200/JCO.2004.05.111. [DOI] [PubMed] [Google Scholar]
- 17.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med New. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 18.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 19.Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, McCoy J, Dakhil SR, Lanier KS, Chapman RA, et al. Standard chemotherapy compared with high-dose chemo-radiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24:929–936. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 20.Levy V, Katsahian S, Fermand JP, Mary JY, Chevret S. A meta-analysis on data from 575 patients with multiple myeloma randomly assigned to either high-dose therapy or conventional therapy. Medicine (Baltimore) 2005;84:250–259. doi: 10.1097/01.md.0000173272.71949.a1. [DOI] [PubMed] [Google Scholar]
- 21.Koreth J, Cutler CS, Djulbegovic B, Behl R, Schlossman RL, Munshi NC, Richardson PG, Anderson KC, Soiffer RJ, Alyea EP., III High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007;13:183–196. doi: 10.1016/j.bbmt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Rocha Lima CM, Green MR, Rotche R, Miller WH, Jr, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G, Miller LL. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–3783. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 23.Trump D, Lau YK. Chemotherapy of prostate cancer: present and future. Curr Urol Rep. 2003;4:229–232. doi: 10.1007/s11934-003-0074-3. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 25.Matsui WH, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, Smith BD, Civin CI, Jones RJ. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treon SP, Pilarski LM, Belch AR, Kelliher A, Preffer FI, Shima Y, Mitsiades CS, Mitsiades NS, Szczepek AJ, Ellman L, et al. CD20-directed serotherapy in patients with multiple myeloma: biologic considerations and therapeutic applications. J Immun-other. 2002;25:72–81. doi: 10.1097/00002371-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Huff CA, Wang Q, Rogers K, Jung M, Borrello IM, Jones RJ, Matsui W. Correlation of clonogenic cancer stem cell growth with clinical outcomes in multiple myeloma (MM) patients undergoing treatment with high dose cyclophosphamide and rituximab; Proc AACR Late Breaking Abstract: LB:87; 2008. [Google Scholar]
- 28.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angstreich GR, Matsui W, Huff CA, Vala MS, Barber J, Hawkins AL, Griffin CA, Smith BD, Jones RJ. Effects of imatinib and interferon on primitive chronic myeloid leukaemia progenitors. Br J Haematol. 2005;130:373–381. doi: 10.1111/j.1365-2141.2005.05606.x. [DOI] [PubMed] [Google Scholar]
- 30.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 31.Harrington L. Does the reservoir for self-renewal stem from the ends? Oncogene. 2004;23:7283–7289. doi: 10.1038/sj.onc.1207948. [DOI] [PubMed] [Google Scholar]
- 32.Peacock CD, Wang Q, Gesell GS, Corcoran-Schwarz IM, Jones E, Kim J, Devereux WL, Rhodes JT, Huff CA, Beachy PA, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci USA. 2007;104:4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D, Greider CW. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Dokal I, Vulliamy T. Dyskeratosis congenita: its link to telomerase and aplastic anaemia. Blood Rev. 2003;17:217–225. doi: 10.1016/s0268-960x(03)00020-1. [DOI] [PubMed] [Google Scholar]
- 35.Ju Z, Rudolph KL. Telomeres and telomerase in cancer stem cells. Eur J Cancer. 2006;42:1197–1203. doi: 10.1016/j.ejca.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg RA, Chin L, Femino A, Lee KH, Gottlieb GJ, Singer RH, Greider CW, DePinho RA. Short dysfunctional telomeres impair tumorigenesis in the INK4a(delta2/3) cancer-prone mouse. Cell. 1999;97:515–525. doi: 10.1016/s0092-8674(00)80761-8. [DOI] [PubMed] [Google Scholar]
- 37.Rudolph KL, Millard M, Bosenberg MW, DePinho RA. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet. 2001;28:155–159. doi: 10.1038/88871. [DOI] [PubMed] [Google Scholar]