Abstract
Improved methods have recently been developed for assessing islet viability and quantity in human islet preparations for transplantation, and these measurements have proven useful for predicting transplantation outcome. The objectives of this study were to adapt these methods for use with microencapsulated islets, to verify that they provide meaningful quantitative measurements, and to test them with two model systems: (1) barium alginate and (2) barium alginate containing a 70% (w/v) perfluorocarbon (PFC) emulsion, which presents challenges to use of these assays and is of interest in its own right as a means for reducing oxygen supply limitations to encapsulated tissue. Mitochondrial function was assessed by oxygen consumption rate measurements, and the analysis of data was modified to account for the increased solubility of oxygen in the PFC-alginate capsules. Capsules were dissolved and tissue recovered for nuclei counting to measure the number of cells. Capsule volume was determined from alginate or PFC content and used to normalize measurements. After low oxygen culture for 2 days, islets in normal alginate lost substantial viable tissue and displayed necrotic cores, whereas most of the original oxygen consumption rate was recovered with PFC alginate, and little necrosis was observed. All nuclei were recovered with normal alginate, but some nuclei from nonrespiring cells were lost with PFC alginate. Biocompatibility tests revealed toxicity at the islet periphery associated with the lipid emulsion used to provide surfactants during the emulsification process. We conclude that these new assay methods can be applied to islets encapsulated in materials as complex as PFC-alginate. Measurements made with these materials revealed that enhancement of oxygen permeability of the encapsulating material with a concentrated PFC emulsion improves survival of encapsulated islets under hypoxic conditions, but reformulation of the PFC emulsion is needed to reduce toxicity.
Introduction
Allogenic transplantation of islets of Langerhans has been studied for nearly 20 years. Despite the advances made as a result of the Edmonton protocol,1 which made use of freshly isolated islet preparations, the technique is still considered experimental.2 Licensing of human islet preparations as a biological product by the U.S. Food and Drug Administration, which would facilitate reimbursement, requires that the islet products produced different ways or by different groups are safe and effective; that is, they have the same characteristics that affect clinical transplantation outcome, in particular identity, purity, viability, and potency. This requirement has not been achieved, in part because the characteristics determined by currently accepted assay methods, individually or collectively, do not correlate with transplantation outcome.2–9
Development of meaningful assays of islet properties is challenging for several reasons8: (1) Many techniques suitable for cells are not practical with islets because of their three-dimensional aggregate structure. Islets cannot be usefully dissociated into dispersed cells by agitation and incubation with serine proteases such as trypsin because from 30% to 50% of the cells are lost during dissociation.10–12 Cell damage may result from separation of the cell membrane from the extracellular matrix,13,14 and cells recovered from the dissociation process may not be representative of cells in the original islet. (2) Intact islets have nonsymmetric shapes with wide size distribution, making visual estimates inaccurate and operator dependent. (3) Human preparations have varying amounts of nonislet cell impurities. (4) Islet preparations, particularly human, are a moving target: islet volume decreases with time, and stresses that occur during pancreas retrieval, storage, islet isolation, culture, and shipment can alter properties.
Among currently accepted islet assessment techniques, staining with dithiozone followed by visual estimation with light microscopy overestimates islet volume fraction (purity) by 20%–30% and total islet volume by as much as 90%.15,16 Examination of viability by assessment of membrane integrity with vital stains such as fluorescein diacetate/propidium iodide does not reflect true viability because it may not account for cells undergoing early cell death processes, during which cells have not yet developed damage to their cell membrane.8,9,17,18 Measures of cellular energetic state, including ATP content19–24 and ATP/ADP and related ratios,25–27 have been investigated with modest success. ATP content varies enormously between islets from different species, between human islet preparations,20–22 with time in culture,20,21 and with donor age.22 The ATP/ADP ratio itself is influenced in complex ways by glucose and oxygen concentration, which in turn are coupled to oxygen consumption rate (OCR) and glucose-stimulated insulin secretion (GSIS).28–31 GSIS as an in vitro measure of potency or dose does not predict the functional capacity of human islets after transplantation.7,32,33 Highly responsive islet preparations may fail, whereas poorly responsive preparations may be capable of restoring normoglycemia after transplantation in a diabetic patient.33 These persistent findings may be associated with the stresses to which human islets are exposed, leading to extensive degranulation and/or insulin leakage from dead or dying beta cells, whereas poorly secreting but viable cells may recover when transplanted into the recipient. GSIS is also particularly sensitive to the local partial pressure of oxygen (pO2) in the beta cells,29–31 and assay procedures do not account for oxygen gradients that occur in islets.
We have recently developed improved methods for assessing islet quantity and quality in human islet preparations. Using data from election microscopy as a definitive standard, we showed that morphometric analysis with light microscopy provided accurate estimate of islet volume fraction in a preparation.15 We adapted nuclei counting methods for use with intact islets and tissues. Combination of this measurement with purity data from light microscopy provided accurate measurement of the number or volume of islet cells.16 Cell nuclei were conserved during lysis of islet tissue,16 which provided an advantage over dissociation into cells that invariably entails cell loss. Last, combination of nuclei counts and DNA measurements provided insight into the extent of recent cell damage. OCR, a direct measure of the rate of oxidative phosporylation in aerobic cells, has been suggested as a better measure of mitochondrial function than nucleotide concentrations8,9 and has been measured with various systems.34–36 We developed a stirred microchamber that permits rapid, accurate OCR measurements with a small volume of islet tissue.37 This method was used in studies with human38 and rat39 islets transplanted into immunocompromised mice. Transplantation outcome correlated with two parameters: OCR, a measure of the amount of viable tissue, and OCR/cell (which can be determined with DNA or nuclei measurements), a measure of the relative viability of the tissue. Within defined domains of the OCR–OCR/DNA parameter space, diabetes reversal occurred in all, some, or none of the animals. A model incorporating both parameters predicted transplantation outcome with sensitivity and specificity of 95% and 71% in humans38 and 93% and 94% in rats,39 respectively. OCR/DNA could be used to estimate marginal OCR (or tissue volume) required for reversing diabetes. These measurements are currently being evaluated with human transplants as part of a phase III multicenter clinical trial.9
Microencapsulation is attractive because it may reduce the extent of immunosuppression associated with transplantation of islets of Langerhans to cure diabetes and is an active area of study.40,41 Encapsulated islets are usually assessed in vivo by transplantation outcome; in vitro quantitative characterization of encapsulated islets can be even more difficult than with naked islets because of the presence of the encapsulant. When in vitro tests are used, many of the same commonly accepted techniques for naked islets are employed. These assays suffer limitations that are the same or even worse than with naked tissue. There is a need for quantitatively reliable assays that correlate with transplantation outcome and are suitable for use with encapsulated tissues. Such assays would be useful with human islet preparations in clinical studies.
The objective of this study was to adapt our new assay methods for use with microencapsulated islets, to verify that they provide meaningful quantitative measurements, and to test them with model systems so that they could eventually be relied upon for measurements with human islet preparations. As model systems, we chose two encapsulating materials: (1) barium alginate, and (2) barium alginate containing a 70% (w/v) perfluorocarbon (PFC) emulsion. Barium alginate was used because of its improved stability and mechanical properties compared to calcium alginate and because of previous success with allorejection and autoimmunity when barium alginate was employed.42 PFC alginate was chosen because it presents difficult challenges to use of the new assays. It is also of interest in its own right as a means for reducing oxygen supply limitations that restrict the packing density of encapsulated functional tissue.40 Previous reports indicated that a PFC emulsion in islet culture enhances function43 and that low concentrations of PFC emulsion in alginate have beneficial effects on encapsulated hepatic cells.44,45 Recent theoretical analysis predicted that enhancing oxygen permeability of the encapsulating matrix with a much higher concentration of PFC emulsion, for example, 70% (w/v), can increase oxygen levels surrounding islet tissue, resulting in substantial improvement of viability and function.46,47 This PFC concentration, which is much higher than used previously, posed additional challenges. First, components of the PFC emulsion might adversely affect test protocols. Second, the capsules became white, opaque spheres, and the PFC emulsion droplets might interfere with optical assays. Third, the high oxygen-carrying capacity within the dispersed capsules led to complications in data analysis of OCR measurements because of large time-dependent oxygen gradients in the capsules, which could prevent the stirred chamber from being treated as a quasisteady system. Because the distinguishing feature with these systems is the presence and nature of the encapsulating material, we made use of rat islets in the experiments.
In this study, we cultured islets encapsulated in alginate with or without PFC under controlled normal and reduced oxygen conditions. Measurements with the new assays were carried out before and after 2 days of culture. Mitochondrial function was assessed by OCR in a stirred chamber with special adaptation of data analysis for measurements with PFC-alginate. Capsules were removed, and alginate was dissolved with ethylenediamineteraacetic acid (EDTA), thereby liberating tissue for subsequent measurement of the number of cells by nuclei counting (and also for DNA measurement in normal alginate). These data allowed calculation of OCR/cell at the beginning and end of culture as well as the change in total OCR over the culture period, which reflected the change in the amount of viable tissue in the capsules. Because sampling of capsules could incur substantial experimental error, the total nuclei count and OCR of a sample was normalized by capsule volume, which was quantified by measuring alginate or PFC content. Estimates of these quantities in different samples provided insights into islet cell viability after culture relative to the initial values in the sample before culture.
Materials and Methods
Islets and cells
Rat islets were isolated from male Sprague-Dawley rats weighing 200–250 g using standard techniques.48 Islets were cultured in tissue culture flasks at a density of up to 30 islets/cm2 and a medium depth of 1.3 mm in RPMI 1640 supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin (Mediatech). Rat insulinoma INS-1 cells were cultured in tissue culture flasks in islet medium further supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate (Mediatech), and 50 μM 2-mercaptoethanol (Sigma-Aldrich). INS-1 cells were detached from flasks by incubation with 0.05% (w/v) trypsin in 0.52 mM EDTA (Mediatech) for 3 min at 37°C.
PFC emulsion preparation
A PFC emulsion made from perfluorodecalin (Fluoromed) and 20% (w/v) Intralipid® (Baxter) (a soybean oil emulsion composed of 20% [w/v] soybean oil, 1.2% [w/v] egg yolk phospholipids, and 2.3% [w/v] glycerin with balance water) was prepared as previously described49 except that the emulsifier adjuvant Intralipid was substituted for Liposyn II®. A 40-mL volume of Intralipid was added to the inlet reservoir of an M110Y Microfluidizer (Microfluidics) and recirculated for 2 min at 14,500 psi through the interaction chamber and cooling coil and back to the reservoir, then 11.25 mL perfluorodecalin (density 1.93 g/mL) was added dropwise over 3 min. After sitting for 5 min to cool, the coil ice bath was changed, and additional 11.25 mL perfluorodecalin was added dropwise over 3 min with recirculation, which was continued for an additional 5 min. The emulsion had a PFC content of 70% (w/v), equivalent to 36% (v/v), a calculated density of 1.34 g/mL, and contained droplets having a mean diameter of 0.4 μm measured by dynamic light scattering (Nanotrac, Microtrac, Inc.).
Microencapsulation
Microcapsules were produced using highly purified alginate (FMC BioPolymer). For normal alginate capsules, 2% (w/v) of an available supply of low viscosity high glucuronate (LVG) alginate (see Table 1) was used with INS-1 cells, 1.5% (w/v) sterile lyophilized high glucuronate (SLG100) alginate that we employed previously42 was used with islets, and 1.9% (w/v) SLM100 (sterile) was used for implantation experiments because high M alginate worked best with the coated capsules employed,50 all in 0.9% (w/v) NaCl. For PFC alginate capsules, LVG alginate with INS-1 cells, SLG100 alginate with islets, and SLM100 for implantations were dissolved into a 70% (w/v) PFC emulsion to a final concentration (based on total volume) of 1.5%, 0.5%, and 0.63% (w/v), respectively. Cells or islets were washed with calcium-free Krebs (CFK) buffer (135 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, and 25 mM HEPES, pH 7.4), resuspended in alginate (or PFC alginate) to form a tissue-alginate suspension, and microencapsulated by extrusion through a 26 gauge needle using an electrostatic droplet generator (Pronova Polymer) or a coaxial airflow droplet generator into 20 mM BaCl2 or 100 mM CaCl2. Barium alginate was used throughout except as otherwise noted. Capsules were sequentially washed in HEPES buffer (132 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, and 25 mM HEPES, pH 7.4) and Krebs buffer (133 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM HEPES, and 2.5 mM CaCl2, pH 7.4). Capsules for sterile use were made with the microfluidizer placed in a laminar flow hood. The diameter of microcapsules made with the electrostatic droplet generator, measured with 100 capsules from every batch using light microscopy with a calibrated reticule, averaged 370 to 510 μm (Table 1). Tissue concentration in the capsules was targeted at 1.5 × 107 cells/mL or 8000 islet equivalents (IE)/mL, which corresponds to 0.52 IE/500-μm-diameter capsule where an islet equivalent is the volume of a sphere with a diameter of 150 μm. In one preparation, microscopic observation of 400 capsules revealed 54% empty capsules, 33% with one islet, 10% with two islets, and 3% with three islets. PFC alginate was viscous and sticky, and its volume could not be accurately measured. A minimum volume of 0.5 mL suspension was required in the electrostatic generator. If fewer than 4000 islets were available, the concentration was lower than 8000 IE/mL.
Table 1.
Diameter of Capsules Made with Electrostatic Droplet Generator
|
Alginate |
PFC |
|
Diameter (μm) |
|||
|---|---|---|---|---|---|---|
| Concentration, % (w/v) | Type | Emulsion | Tissue | na | Mean ± SD | Range |
| 2.0 | LVG | No | INS-1 cells | 3 | 370 ± 50 | 230–460 |
| 1.5 | SLG | No | Islets | 8 | 440 ± 50 | 300–600 |
| 1.5 | LVG | Yes | INS-1 cells | 3 | 510 ± 30 | 420–640 |
| 0.5 | SLG | Yes | Islets | 3 | 500 ± 60 | 250–800 |
The number of batches, and 100 capsules were examined in each batch.
LVG, low viscosity high glucuronate; PFC, perfluorocarbon; SLG, sterile lyophilized high glucuronate.
PFC alginate capsules used to study biocompatibility were coated with poly-L-lysine (PLL) and alginate. After washing with HEPES buffer, capsules were placed in a 0.1% (w/v) PLL solution (MW 28,500[vis]; Sigma Aldrich) in CFK buffer for 10 min, washed with CFK, and then placed in 0.19% (w/v) SLM100 alginate in CFK buffer for 6 min and washed with CFK buffer and then Krebs buffer. To coat with pure alginate, after HEPES wash, capsules were placed in 0.19% (w/v) SLM100 in CFK buffer for 6 min, washed with CFK, transferred into 20 mM BaCl2 for 5 min, and washed with HEPES and then Krebs buffer.
Culture of encapsulated tissue
Capsules containing islets were cultured at 37°C with gas O2 concentrations of 20% (pO2 in humidified vessel 142 mm Hg), 3% (21 mm Hg), and 0.5% (3.5 mm Hg). For experiments at 20% and 3% O2, islets were placed in 100 cm2 silicone rubber flasks51 (PR-AY-5-0017-5; Wilson Wolf Manufacturing) in islet culture media with a depth of 2.5 mm and a surface density of <30 IE/cm2. The flasks were placed in a sealed humidified chamber that was continually flushed with 20% or 1% O2, 5% CO2, and the balance N2 and was in turn placed within an incubator at 37°C. In this configuration, oxygen was supplied by diffusion through the silicone rubber membrane at the bottom of the flask and through the layer of medium from the large (0.5 L) gas space in the sealed flask that contained 20% oxygen. When flushed with 1% O2, islets were exposed to a calculated average pO2 of 21 mm Hg,52 corresponding to culture in 3% O2. To obtain accurately known conditions at the lowest O2 level, capsules to be cultured at 0.5% O2 were placed in custom-made 24-well plates with islet culture medium depth of 10 mm and a density of 50–200 IE/cm2 in a humidified incubator (Xvivo System; Biospherix Ltd.) with gas levels controlled at 5% CO2, 0.5% O2, and the balance N2. In these plates, holes were drilled in the polystyrene bottom, and inserts with a silicone rubber bottom were placed on top of spacers in each well (PR-AY-7-0004; Wilson Wolf) to yield culture wells having a silicone rubber bottom wherein the pO2 at the membrane–medium interface was the same as in the gas phase.53
Oxygen consumption rate
Microcapsules containing islets or cells were resuspended in Dulbecco's modified Eagles medium containing 4.5 g/L glucose supplemented with 0.6 g/L L-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, and 10 mM HEPES (all from Mediatech) without serum (to avoid formation of bubbles) and without phenol red (to avoid interference with absorbance in subsequent measurement of alginate content) and placed in a nominal 200-μL stirred titanium chamber (Micro Oxygen Uptake System FO/SYSZ-P250; Instech Laboratories) maintained at 37°C and equipped with a fluorescence-based oxygen sensor (Ocean Optics), as previously described.37 The time-dependent pO2 within the chamber was recorded, and data in the region <160 mm Hg and >60 mm Hg, which yielded the highest slope of pO2 versus time, were fit to a straight line. OCR was evaluated from
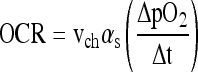 |
(1) |
where (ΔpO2/Δt) is the slope, vch is chamber volume, and αs is the average Bunsen solubility coefficient of oxygen in the entire sample in the chamber, evaluated from
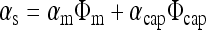 |
(2) |
where αi and Φi are the volumetric average oxygen solubility and volume fraction of the component in the sample, m refers to medium (assumed to have the properties of water), and cap refers to capsule. For PFC alginate, αcap was evaluated from
 |
(3) |
where φj is the volume fraction of the jth component in the capsule, and components are taken to be water, PFC, soybean oil, and tissue (or cells). Parameters used to calculate αs are summarized in Tables 2 and 3. Typically, 500 capsules were loaded in the chamber (range 150–1500, depending on the availability of islets and capsule culture conditions).
Table 2.
Volume Fraction of Capsule Components in Capsules Used to Calculate Physical Propertiesa
| Component | Cells+alginate | PFC emulsion | Cells+alginate+PFC |
|---|---|---|---|
| Tissue | 0.023 | 0 | 0.023 |
| Alginate | 0.02 | 0 | 0.02 |
| PFC | 0 | 0.36 | 0.36 |
| Soybean oil | 0 | 0.12 | 0.12 |
| Water | 0.957 | 0.52 | 0.477 |
Capsules contained 2.4 × 107 cells/mL when cells were encapsulated; PFC emulsion contained 70% (w/v) PFC.
PFC, perfluorocarbon.
Table 3.
Diffusion Coefficient (D), Bunsen Solubility Coefficient (α), and Permeability Coefficient (αD) of Oxygen in All Pure Components and in Composite Phases of Capsules in Oxygen Consumption Rate Measurementsa
| D (cm2/s) | α (mol/mm Hg/mL) | αD (mol/cm/mm Hg/s) | Reference | |
|---|---|---|---|---|
| Water | 2.78 × 10−5 | 1.27 × 10−9 | 3.53 × 10−14 | 54 |
| PFC | 5.61 × 10−5 | 2.54 × 10−8 | 1.42 × 10−12 | 55 |
| Soybean oil | 2.13 × 10−5 | 6.84 × 10−9 | 1.46 × 10−13 | |
| Tissue | 1.24 × 10−5 | 1.00 × 10−9 | 1.24 × 10−14 | 56 |
| Alginate | 0 | 0 | 0 | 57 |
| 2% (v/v) alginate | 2.77 × 10−6 | 1.24 × 10−9 | 3.43 × 10−14 | 52 |
| Cells normal alginate | 2.73 × 10−5 | 1.23 × 10−9 | 3.36 × 10−14 | 52 |
| 70% (w/v) PFC emulsion | 9.36 × 10−6 | 1.06 × 10−8 | 9.92 × 10−14 | 52 |
| 70% (w/v) PFC emulsion in 2% (v/v) alginate | 9.08 × 10−6 | 1.06 × 10−8 | 9.63 × 10−14 | 52 |
| Cells 70% (w/v) PFC alginate | 8.83 × 10−6 | 1.06 × 10−8 | 9.36 × 10−14 | 52 |
Parameters are taken from the literature or calculated. The cell concentration in the microcapsule was 2.4 × 107 cells/mL. All calculations are based on 2% (w/v) alginate, which was the highest concentration used experimentally.
Use of Equation (1) requires that the pO2 profiles within the capsules are in quasisteady state and that pO2 differences between the medium and the capsule interior are small compared to the medium pO2. Approximate theoretical analyses developed in the Appendix show by quantitative criteria that these conditions are met for the measurements in this study.
For further assays following OCR measurements, the capsule suspension was removed and stored in a tube together with three consecutive 350-μL washes of the chamber. The capsules were allowed to settle, and 1.0 mL supernatant was removed, leaving a 250-μL suspension.
Capsule dissolution
Quantitative assays of nuclei counts, DNA, alginate, and PFC emulsion content required that the capsule first be completely dissolved by adding 800 μL of 100 mM tetrasodium EDTA (pH 8) to the 250 μL capsule suspension. Samples were incubated at 37°C for 1 h hr and vortex mixed every 30 min. Because barium binds high G alginate with a higher affinity than calcium, dissolution of barium alginate was much more difficult, and tetrasodium EDTA was used instead of sodium citrate.58,59
Nuclei counts
Nuclei were prepared from naked islets or cells as previously described.16 For cell suspensions in dissolved alginate with or without PFC, a 100-μL sample was added to an equal volume of lysis solution (0.1 M citric acid and 1%, 2%, or 10% [v/v] Triton X-100; Sigma Aldrich) and incubated for 5 min with vortex mixing three times. For islets, the dissolved sample was resuspended in 100 μL PBS before adding 100 μL of lysis buffer (0.1 M citric acid and 10% [v/v] Triton X-100) to remove EDTA, which interfered with nuclei liberation, and incubated for 15 min with vortex mixing three times. The suspensions were sheared through a 26-gauge × 3/8-in needle (Becton Dickinson) to liberate all nuclei, and 100 μL of 100 mM tetrasodium EDTA was added to prevent alginate gelling. Liberated nuclei were stained with 0.8 μM LDS 751 and 0.2 μM Sytox Orange (Invitrogen) in D-PBS and counted using a flow cytometer (Guava Personal Cell Analyzer; Guava Technologies). Nuclear membrane permeabilization by 10% Triton X-100 led to much brighter nuclei, and flow cytometer settings were adjusted accordingly.
DNA analysis
DNA concentration was quantified by fluorospectrophotometry using the CyQUANT® Cell Proliferation Assay Kit and λ DNA standard (Invitrogen), as previously described.18 Fluorescence intensity was measured with 480 nm excitation and 520 nm emission wavelengths in a plate reader (FLUOstar/POLARstar Galaxy; BMG Labtechnologies, Inc.) and was linearly related to DNA concentration. The presence of EDTA and dissolved alginate had no effect, but light scattering by the PFC emulsion droplets prevented use of this assay.
Alginate concentration
The assay for alginate was adapted from a previously described method60 using dimethyl methylene blue (Sigma Aldrich), a cationic dye that binds to polyanions such as alginate. Standard curves were prepared using known concentrations of alginate from each batch used to make capsules. To individual wells of a 96-well plate in triplicate was added 100 μL of each sample and standard and 100 μL of 40 μM or 120 μM aqueous dye solution for use with LVG and SLG100 alginate, respectively. After incubation for 15 min, the absorbance of each sample was measured at wavelengths of 520 and 650 nm using a tunable microplate reader (VERSAmax Molecular Device) and the ratio calculated. The capsule volume in the sample before dissolution was calculated from an alginate mass balance using sample volume, alginate concentration in the original capsule, and the alginate concentration measured in the sample after dissolution.
PFC emulsion concentration
The original 70% (w/v) PFC emulsion was opaque, but absorbance of a sample at any wavelength increased linearly with increasing emulsion concentration in samples diluted 200- to 1000-fold. A series of standard samples and 100 μL of each unknown sample were added to wells of a 96-well plate in triplicate. Absorbance was measured at 620 nm in a plate reader. Capsule volume was calculated using the approach described for alginate concentration.
Biocompatibility
Biocompatibility of the PFC emulsion and its components was investigated by culturing naked islets at 20% O2 and encapsulated islets at 3% or 20% O2 with perfluorodecalin and Intralipid for 2 days. Naked or encapsulated islets were fixed in 2.5% (w/v) glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) and embedded in Araldite. Thin (1 μm) sections were cut and stained with 1% (w/v) toluidine blue (Thermo Fisher Scientific).
To determine if implanted capsules elicited an immune reaction, empty capsules without islets were injected into the peritoneal cavity of Lewis rats (anesthetized with ketamine 100 mg/kg and xylazine 10 mg/kg) through a central midline 5–10 mm incision using a sterile plastic transfer pipette (Fisher Scientific). The incision was closed using 5/0 silk and the outer layers stapled. Capsules were retrieved after 2 weeks by laparotomy and peritoneal lavage, washed in PBS, fixed in buffered formalin (pH 7) for 1 h, embedded first in 2% (w/v) agar and then paraffin, sectioned, stained with hematoxylin to reveal cells on the capsule exterior, and examined by light microscopy. Cells on the capsule exterior were clearly observed only by examining stained sections due to opacity of the PFC alginate capsules. Animal experiments were approved by the Institutional Animal Care and Use Committee at Joslin Diabetes Center and the MIT Committee on Animal Care.
Statistics
Data are expressed as mean ± standard deviation. Statistical significance (p < 0.05) was determined by the Student's t-test.
Results
Assay development
Nuclei counting of encapsulated islets or cells
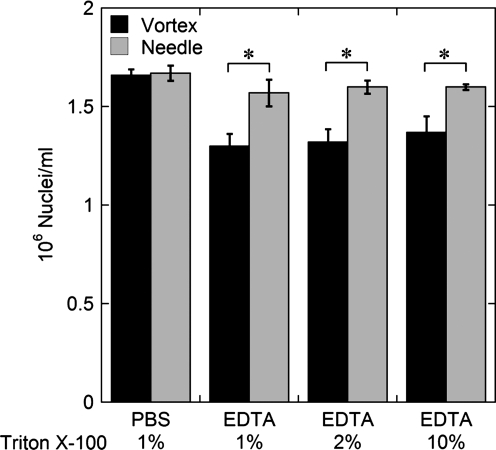
We adapted a method previously developed for measuring the number of cells in an islet preparation by nuclei counting to measurement with cells or islets encapsulated in barium alginate, with or without PFC. Nuclei counts were performed with unencapsulated INS-1 cells to investigate the effects of the presence of PFC emulsion, EDTA, and Triton X-100, and use of shearing through a 26G needle. Nuclei counts were identical to within about 1% with or without the presence of 10% (w/v) PFC emulsion; thus, PFC emulsion particles did not interfere with counting nuclei using a flow cytometer. Nuclei counts were made with INS-1 cells in either PBS with 1% (v/v) Triton X-100 in the lysis buffer or 100 mM tetrasodium EDTA (pH 8) with 1%, 2%, or 10% Triton X-100 in the lysis buffer (Fig. 1). Nuclei were liberated by vortex mixing only or vortex mixing followed by shearing through a needle. The concentration of Triton X-100 did not affect the number of nuclei counted. In the presence of EDTA, nuclei counts with vortex mixing alone were 80% ± 4.2% of the PBS control. With vortex mixing followed by shearing through a needle, nuclei counts were higher at 95% ± 2.3% of the PBS control, and the difference between measurements in PBS or EDTA was not significant.
FIG. 1.
Effect of Triton X-100, ethylenediamineteraacetic acid (EDTA), and shearing in a needle on nuclei measurements. Nuclei counts were made with INS-1 cells in either PBS with 1% (v/v) Triton X-100 in the lysis buffer or 100 mM tetrasodium EDTA (pH = 8) with 1%, 2%, or 10% Triton X-100 (*p < 0.05 two-sample Student's t-test).
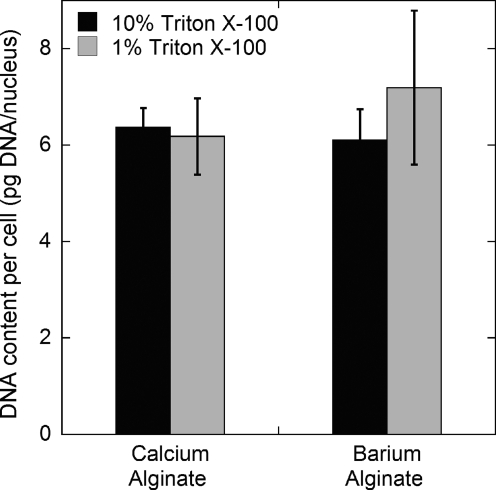
To verify that capsules containing cells were effectively dissolved and all nuclei were liberated and counted, measurements of DNA content per cell were performed after dissolution of capsules containing INS-1 cells in calcium alginate or barium alginate (Fig. 2). DNA content per cell was the same for cells liberated from calcium alginate with a lysis buffer containing 1% or 10% Triton X-100 and from barium alginate capsules with lysis buffer containing 10% Triton X-100. We chose to use a lysis buffer with 10% Triton X-100 to liberate nuclei from barium alginate capsules.
FIG. 2.
Effect of alginate encapsulation and lysis surfactant concentration on measured DNA content per cell. Nuclei counts and DNA content measurements were performed with INS-1 cells encapsulated in calcium alginate or barium alginate capsules after capsule dissolution with 100 mM tetrasodium EDTA (pH 8). The lysis buffer for liberating nuclei contained 1% (v/v) or 10% (v/v) Triton X-100. Error bars represent standard deviation for triplicate samples from the same experiment.
DNA and nuclei measurements were made with INS-1 cells, rat islets, and human islets encapsulated in barium alginate capsules. These measurements produced consistent estimates of DNA per cell that were not significantly different from values measured with unencapsulated cells and islets (Table 4).
Table 4.
DNA Content Per Cell for Naked or Encapsulated Cells and Islets
| |
DNA content (pg/cell) |
|
|---|---|---|
| Tissue | Unencapsulated | Barium alginate |
| INS-1 Cells | 6.0 ± 0.8 | 6.5 ± 0.8 |
| (n = 25) | (n = 5) | |
| Rat islets (n = 7) | 6.5 ± 0.8 | 6.1 ± 1.1 |
| Human islets 2 (n = 6) | 6.2 ± 0.8 | 7.3 ± 3.0 |
Capsule quantification
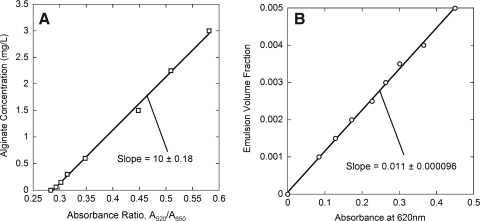
Measurements of alginate concentration and PFC emulsion volume fraction were used to generate linear standard curves (Fig. 3). PFC emulsion particles interfered with the alginate assay; thus, PFC emulsion volume fraction measurements were used with dissolved PFC alginate samples. Capsule volume in each sample was calculated from these measurements. All other measurements were normalized by the capsule volume to reduce errors associated with capsule sampling, especially with PFC alginate capsules that settled rapidly, making it difficult to obtain a representative sample from a uniform suspension. In one series of triplicate measurements with nine different PFC alginate capsule samples from the same suspension, the coefficient of variation for nuclei counting was reduced from 0.27 for the total number of nuclei to 0.12 for nuclei/mL capsule.
FIG. 3.
Example standard curves for assays of (A) alginate concentration and (B) perfluorocarbon (PFC) emulsion volume fraction. Data were fitted to a straight line yielding (A) CAlg = (−2.9 ± 0.07) + (10 ± 0.2)(A520/A650) and (B) Cemul = (1.10 ± 0.01) × 10−2 A620.
OCR measurement
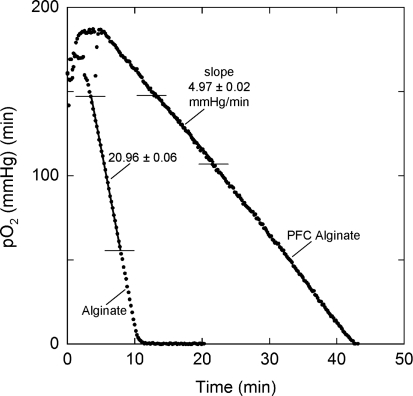
OCR/cell measurements of alginate-encapsulated INS-I cells were virtually identical to those of naked cells. The method for measuring OCR with encapsulated tissue was validated by measuring OCR/cell from the same initial batch of cells in normal and PFC alginate. Figure 4 shows data from two runs in which OCR of cells in the chamber was virtually identical. The widely different slopes reflect the difference in the average oxygen solubility in the samples, and measured OCR/cell was virtually the same in alginate and PFC alginate. In a separate series of three replicate experiments with naked INS-1 cells and with INS-1 cells encapsulated in alginate and PFC alginate, the OCR/cell was similar at 3.7 ± 0.4, 3.9 ± 0.3, and 3.3 ± 0.4 fmol/(min · cell). These data demonstrate that accurate OCR measurements can be obtained with tissue encapsulated in capsules, with or without PFC emulsion present.
FIG. 4.
Actual traces of measured pO2 versus time during oxygen consumption rate (OCR) measurement for INS-1 cells encapsulated in normal alginate or PFC alginate microcapsules. The initial increase in measured pO2 reflected the decrease in oxygen solubility as temperature increased during thermal equilibrium. A straight line was fitted to the data between the horizontal lines. Oxygen solubility for the sample in the chamber, calculated from Equations (2) and (3) and the data in Table 3, αs were 1.27 and 5.37 nmol/(mL·mm Hg) for alginate and PFC alginate, respectively. In these experiments, the number of cells per unit volume of capsule were virtually identical in the two encapsulating suspensions. The ratio of the slopes was inversely proportional to the inverse ratio of sample solubilities. At the end of each measurement, the capsules were recovered and dissolved, and the number of cells in the chamber determined by nuclei counting. OCR/cell was 4.51 and 4.59 fmol/(min·cell) for the cells in alginate and PFC alginate, respectively.
Evaluation of PFC-containing capsules
Tissue and OCR recovery
OCR measurements of samples of encapsulated islets were performed on the day of encapsulation and again after 2 days of culture under various O2 conditions. OCR and nuclei counts were normalized by capsule volume determined using measurement of alginate or PFC emulsion content. Fractional tissue recovery (the amount of tissue recovered after culture divided by the amount initially present) was calculated as the number of nuclei/mL capsule at the end of the culture period divided by the same quantity measured before culture (preculture). Fractional OCR recovery was similarly calculated using the OCR/mL capsule; it reflected the fraction of initially viable cells recovered after culture.
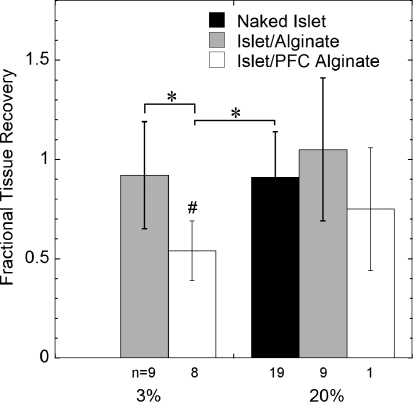
Tissue recovery averaged 0.54 ± 0.15 for islets in PFC alginate cultured for 2 days at 3% oxygen but was not significantly different from unity for normal alginate (Fig. 5). There was a similar trend at 20% (including naked islets), but the reduced tissue recovery with PFC alginate as compared to normal alginate was not statistically significant.
FIG. 5.
Tissue recovery based on nuclei counts of naked islets, islets in normal alginate capsules or islets in PFC alginate capsules cultured for 2 days at indicated oxygen levels. n is the number of experiments, each from a different rat islet preparation. Triplicate measurements were performed in each experiment (*p < 0.05 two-sample Student's t-test; #p < 0.05 for one-sample Student's t-test compared to a value of 1.0).
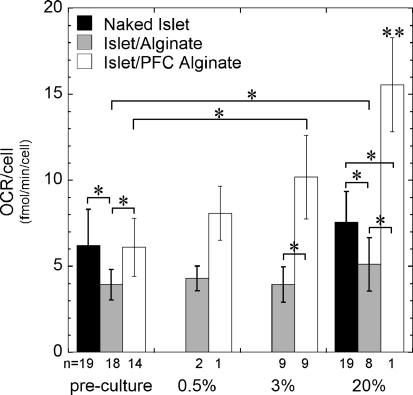
OCR recovery was 0.59 ± 0.22 in normal alginate and 0.91 ± 0.15 in PFC alginate after culture at 0.5% O2 (Fig. 6). This difference is consistent with enhanced tissue survival in PFC alginate under the most hypoxic conditions examined. OCR recovery was not significantly different from unity at 3% for islets in PFC alginate capsules and at 20% for naked islets and islets in alginate, whereas there was a significant increase with PFC alginate at 20% and a significant decrease in normal alginate at 3%.
FIG. 6.
OCR recovery of naked islets, islets in normal alginate capsules, or islets in PFC alginate capsules cultured for 2 days at indicated oxygen levels (*p < 0.05 two-sample Student's t-test; **p < 0.05 two-sample Student's t-test against the same capsule type in each of the other conditions; #p < 0.05 for one-sample Student's t-test compared to a value of 1.0).
The OCR and nuclei content measurements were used to calculate OCR per cell (Fig. 7). OCR/cell in naked islets was 6.3 ± 1.4 fmol/(min·cell) before encapsulation, and the increase to 7.3 ± 2.0 fmol/(min·cell) after 2 days at 20% was not significant. OCR/cell decreased by about one-third immediately after encapsulation in alginate and did not change significantly after 2 days of culture under any low oxygen conditions; however, it increased slightly but significantly after culture in 20% oxygen. Conversely, OCR/cell in PFC alginate did not change after encapsulation. It was always higher than in alginate and increased significantly at 3% and 20% O2 after 2 days culture. The increased OCR/cell could have resulted from an increase in OCR/mL capsule, a decrease in nuclei/capsule, or both.
FIG. 7.
OCR/cell for naked islets, islets in normal alginate capsules, or islets in PFC alginate capsules before culture or after 2 days of culture at indicated oxygen levels (*p < 0.05 two-sample Student's t-test; **p < 0.05 two-sample Student's t-test against the same capsule type in each of the other conditions).
Biocompatibility
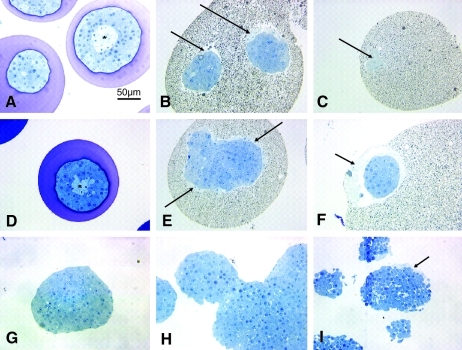
Histological sections of islets encapsulated in alginate or PFC alginate that were cultured in 3% or 20% O2 for 2 days were prepared to examine the health of the tissue after culture (Fig. 8). In Figure 8A and D, islets in normal alginate capsules showed signs of central necrosis identified by lighter toluidine blue staining at the center of the islet after culture at 20% (Fig. 8A) and 3% O2 (Fig. 8D). The islets were otherwise normal. In Figure 8B, C, E, and F, sections of islets in PFC alginate capsules revealed no actual necrosis, but there were spaces around the exterior of the islets where there was light toluidine blue staining indicative of dead cells (indicated by arrows). In all sections of islets in PFC alginate capsules, some cells in contact with the PFC alginate were dead or dying, which suggested that one or more components of the PFC emulsion was toxic to the islets.
FIG. 8.
Histological sections of naked islets and islets encapsulated in alginate and PFC alginate. (A) Normal alginate microcapsule after 2 days of culture in 20% oxygen. Asterisk (*) identifies central necrosis in the larger islet indicated by light staining at the islet center. (B, E) PFC alginate microcapsule on the day of encapsulation. Dead cells are observed at the islet exterior (arrows). (C) 70% (w/v) PFC alginate microcapsule after 2 days of culture in 20% O2. There are dead cells in a small cap of tissue at the capsule edge (arrow). (D) Normal alginate microcapsule after 2 days of culture in 3% O2. Asterisk (*) identifies central necrosis. (F) PFC alginate microcapsule after 2 days of culture in 3% oxygen. The tissue at the center of the islet appears healthy, and there are no signs of central necrosis. Dead cells surrounded the islet exterior (arrows). (G–I) Naked islets after 2 days of culture: (G) Naked islet in islet culture media; tissue appears healthy. (H) Naked islets cultured in islet culture media on top of pure perfluorodecalin; clumping occurred, but tissue appears healthy. (I) Naked islets cultured in islet culture media containing Intralipid®. Dead cells are observed at islet periphery (arrows).
To investigate further the toxicity of components of the PFC emulsion, unencapsulated islets were cultured for 2 days in standard islet culture media (Fig. 8G), at the interface between pure perfluorodecalin and islet culture media (Fig. 8H), or in islet culture media that contained Intralipid (Fig. 8I). Islets cultured in islet culture media or on perfluorodecalin were healthy with no signs of dead cells at the islet exterior. Islets cultured on top of perfluorodecallin tended to associate and form large aggregates because they had increased opportunity to interact on the liquid interface. Islets cultured in media that contained Intralipid had dead cells at the islet exterior, indicating the toxicity of Intralipid.
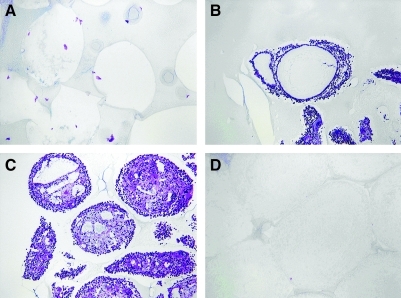
Four types of empty capsules were implanted into the peritoneal cavity of Lewis rats: (1) 1.9% (w/v) SLM100 alginate, (2) PFC alginate prepared with 0.63% (w/v) SLM100 alginate, (3) PFC alginate as in (2) coated with pure alginate, and (4) PFC alginate as in (2) coated with PLL and alginate. After 2 weeks, animals were sacrificed and capsules were recovered. No cells were detected attached to the surface of normal alginate capsules (Fig. 9A). Capsules containing PFC emulsion with no additional coatings or only a pure alginate coating were covered with many layers of cells (Fig. 9B, C), indicating that the capsule material elicited a strong immune reaction resulting from leakage of immunostimulatory components. When a coating of PLL and pure alginate was applied to capsules containing PFC emulsion, no cells were attached to the outside of the capsules (Fig. 9D). This observation suggests that the PLL and alginate restricted leakage of immunostimulatory material outside of the capsule and thereby made the capsules biocompatible.
FIG. 9.
Paraffin sections stained with hematoxylin of empty capsules implanted into the peritoneal cavity of Lewis rats and removed after for 2 weeks. (A) 1.9% (w/v) alginate; (B) 70% (w/v) PFC emulsion with 0.63% (w/v) alginate, (C) 70% (w/v) PFC emulsion with 0.63% (w/v) alginate coated with alginate, and (D) 70% (w/v) PFC emulsion with 0.63% (w/v) alginate coated with poly-L-lysine and alginate. In all cases, SLM100 alginate was used.
Discussion
In this study we examined use of several methods, notably nuclei counting and OCR measurements, with islets microencapsulated in alginate and PFC-alginate. Nuclei measurements could be performed to assess the number of cells with intact nuclei in normal alginate or PFC alginate capsules (Fig. 1). PFC emulsion particles did not interfere with nuclei measurements quantified with a flow cytometer. Modifications of the procedure previously described for naked cells and islets3,26 were necessary with encapsulated tissue. In the presence of EDTA, shearing of a cell suspension by flow through a needle was required to effectively liberate all nuclei. Nuclei measurements of encapsulated islets required removal of EDTA before incubation in the lysis buffer to liberate all nuclei in a sample.
Measurements of DNA content per cell for naked cells or islets and cells or islets encapsulated in barium alginate capsules were not significantly different (Fig. 2 and Table 4) and were comparable to expected values,16,18 thereby verifying the quantitative reliability of both nuclei counting and DNA measurement with tissue encapsulated in alginate. Data suggested that 10% (w/v) Triton X-100 in the lysis buffer resulted in effective liberation of nuclei from cells in barium alginate. PFC emulsion particles interfered with fluorescence measurement used to quantify DNA, so DNA content per cell could not be estimated to verify that nuclei counting was accurate in PFC alginate capsules. However, because lower alginate concentrations were used to make PFC alginate, which dissolved more easily than normal alginate, and PFC emulsion particles did not interfere with nuclei counting, we infer that the nuclei counting method is equally accurate for islets or cells in normal and in PFC alginate capsules.
Measurements of alginate and PFC concentration yielded linear standard curves (Fig. 3) from which capsule volume in a sample was estimated. Normalization of measurements by capsule volume eliminated errors associated with sampling capsules and thereby facilitated comparison of the total number of cells and islets in different samples from the same batch.
Measurement of OCR with encapsulated tissue using a stirred chamber was complicated by (1) steady state and transient effects associated with the presence of O2 diffusion gradients in the capsules, and (2) substantially higher oxygen solubility in PFC alginate capsules. Theoretical analyses (Appendix) demonstrated that the first issue can be neglected for measurement of OCR in small capsules of 500 μm diameter. This allowed the second issue to be handled by defining a volumetric average oxygen solubility for the entire sample in the stirred chamber. The validity of this approach was verified by the demonstration that (1) in samples containing the same number of cells in alginate and PFC alginate microcapsules, the slopes of pO2 versus time were inversely proportional to the sample solubilities (Fig. 4), and (2) the OCR/cell did not differ between measurements made with naked cells and cells encapsulated in alginate or PFC alginate.
These methods were used with islets encapsulated in barium alginate with or without 70% (w/v) PFC emulsion. After 2 days of culture, tissue recovery from islets in PFC alginate was significantly lower than in normal alginate under both normal and reduced oxygen conditions (Fig. 5). In contrast to these surprising results, a substantial fraction of the initial preculture OCR was lost under the lowest oxygen conditions (0.5%) tested with normal alginate but not with PFC alginate (Fig. 6). OCR recovery was comparable at 3% O2, and actually rose to >1.0 at 20% O2. OCR/cell in normal alginate decreased significantly after encapsulation. It remained unchanged under reduced oxygen culture and increased slightly under normal oxygen. Conversely, OCR/cell remained the same after encapsulation and increased with increasing oxygen concentration during culture.
These data suggest that several phenomena are occurring. Although no tissue was lost during culture at 0.5% in normal alginate, the substantial loss in OCR means that a comparable fraction of cells died but their nuclei had not yet degraded after 2 days and remained largely intact. This loss in cell viability was most likely attributable to oxygen starvation, since it did not occur after culture at higher oxygen concentration. In PFC alginate at 0.5% O2, very little OCR was lost, which means that the increased permeability of the encapsulating matrix was sufficient to prevent cell death by oxygen starvation. The inferred protective effect of PFC alginate is further supported by the much more frequent observation of central necrosis with normal alginate than with PFC alginate (Fig. 8) in histological sections of cultured capsules. The substantial loss of tissue by nuclei counting suggests that the intact nuclei of damaged cells that were not respiring at the time of the initial OCR measurement were degraded by one or more components of the PFC emulsion.
A variety of biocompatibilities were encountered. Toxicity to islet cells with PFC alginate was observed as pale blue staining at the periphery of islets in histological sections (Fig. 8). In vitro culture of naked islets revealed that Intralipid was the toxic component of the PFC emulsion. Leaching of material from empty PFC alginate capsules implanted intraperitoneally in rats led to extensive coating by immune cells, which was eliminated by alginate and PLL coatings but not by alginate alone (Fig. 9).
We followed a protocol49 for preparing a concentrated PFC emulsion intended for use as a blood substitute. Intralipid, which was used as a source of emulsifying agents, is an intravenously administered parenteral lipid emulsion with a long history of clinical use for the supply of energy to compromised patients and as a source of essential fatty acids. The lipids have additional biological effects, especially on islets. Culture of islets with soybean oil or its free fatty acid constituents can induce apoptosis and reduce insulin secretion.61–64 Soybean oil could be especially deleterious at the high local concentration used in this study and may account for the limited cell death observed at the islet periphery. The high concentration of egg yolk phospholipid surfactants might also be toxic. In the previous study with a low concentration of PFC emulsion in alginate,44 the surfactant Pluronic F68 was toxic at higher concentration but not at reduced levels. In this study, the phospholipids may have also played a role in the reduced recovery of nuclei from previously damaged, nonrespiring cells at low oxygen conditions (Fig. 5) because phospholipids are the major constituents of the nuclear membrane. The source of immunostimulation from implanted cell-free PFC alginate capsules (Fig. 9) is unclear. Although the PFC emulsion and capsules were made under sterile conditions, the presence of low levels of endotoxin could have led to the presence of immune cells because hyperlipidemia after short-term Intralipid infusion enhances the inflammatory response to endotoxin.65
The data obtained in this study verify that the new assay methods can be used for meaningful quantitative measurements with islets encapsulated in materials as complex as PFC-alginate. These methods have recently been used among a battery of tests to demonstrate that small aggregates of islet cells encapsulated in alginate are superior to whole islets for enhancing oxygen supply.68 In the present study the results also demonstrate that PFC alginate has promise for improving survival of encapsulated islets but that improvements in PFC emulsion formulation are needed to minimize deleterious effects on islets.
Appendix
Validity of Equation (1) for Measuring OCR
The oxygen consumption rate N within the chamber is assumed to be related to pO2 changes within the medium (measured pm) and capsule (pcap) by
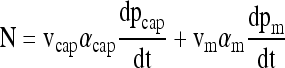 |
(4) |
where vcap and vm are the volumes of capsules and medium, respectively, and αcap and αm are the volume averaged oxygen solubility in capsules and medium, respectively. At high pO2 where OCR is constant and independent of pO2, N is related to volumetric consumption rate expressed in different forms by
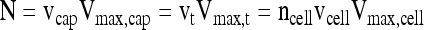 |
(5) |
where Vmax, cap, Vmax, t and Vmax, cell are the maximal consumption rates expressed per unit volume of capsule, islet tissue, or cell, vt and vcell are the total volume of total tissue in the chamber and of an individual cell, respectively, and ncell is the number of cells in the chamber.
If pcap and pm are assumed equal, Equation (4) reduces to Equations (1) and (2). These assumptions are reasonable if (1) quasisteady conditions exist within the capsules, and (2) pO2 differences within the capsule and across the boundary layer around the capsule are small compared to pm After the development of scaling analyses for a similar problem,66 the species conservation equations for oxygen diffusion in a capsule with a constant, uniformly distributed oxygen sink (e.g., encapsulated cells) is
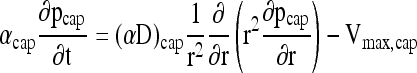 |
(6) |
By equating the transient term to the diffusion term, the time scale for relaxation of diffusion gradients within the sphere is
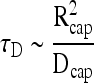 |
(7) |
Assuming that pm = pcap, Equations (2), (4), and (5) may be combined to yield
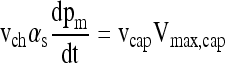 |
(8) |
from which the time scale of the experiment may be estimated as
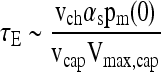 |
(9) |
The transient term in Equation (6) may be neglected if τD << τE, which leads to
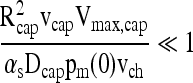 |
(10) |
This may be rewritten using Equation (5) as
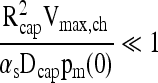 |
(11) |
where Vmax,ch = N/vch is the measured OCR per unit chamber volume.
Gradients within the capsule are small enough to be neglected if the rate of oxygen consumption is small relative to its rate of diffusion. Comparing the two terms on the right hand side of Equation (6) leads to
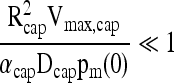 |
(12) |
which has the same form as Equation (11) but is slightly more restrictive.
The pO2 drop across the boundary layer surrounding the capsule can be obtained directly from the boundary conditions at the surface of the capsule:
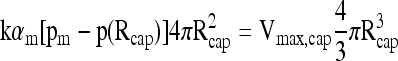 |
(13) |
The most conservative estimate for the mass transfer coefficient k corresponds to the case of a sphere of radius R in a stagnant medium,
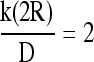 |
(14) |
so that
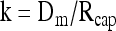 |
(15) |
and the resulting limit on the boundary layer pO2 difference Δpm = pm - p(Rcap) becomes
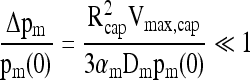 |
(16) |
which is slightly less restrictive than Equation (12).
For the case of an islet at the center of the capsule, Equation (16) remains the same, since the right side of Equation (13) remains the same when it is replaced by N. The pO2 drop within the capsule can be estimated from the boundary conditions written at the islet surface (r = RI):
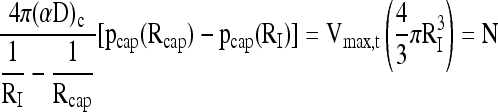 |
(17) |
where αc and Dc apply to the continuous phase of the capsule without tissue, leading to
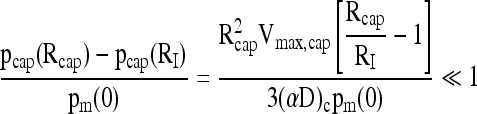 |
(18) |
To evaluate these dimensionless groups, solubilities were taken from Table 3. Effective diffusivities of heterogeneous phases were evaluated by successive application of Maxwell's equation.67
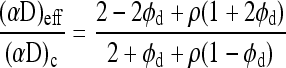 |
(19) |
where ρ = (αD)d/(αD)c, c is the continuous phase, d is the dispersed phase, and fd is the volume fraction of the dispersed phase. Additional details of the calculation are described elsewhere.52 Effective diffusivities of various combinations of materials are given in Table 3.
Estimates of dimensionless parameters for typical conditions of interest in this study are summarized in Table 5. Calculations were performed for alginate and perfluorocarbon alginate capsules of either 500 or 2000 μm diameter. In general, the criterion that each dimensionless group be substantially <1.0 for validity of Equation (1) was satisfied for 500-μm-diameter capsules but not for 2000-μm-diameter capsules.
Table 5.
Calculation of Dimensionless Groups Used to Assess Validity of Equation (1)a
| Equation (11) | Equation (12) | Equation (16) | Equation (18) | |
|---|---|---|---|---|
| 2% (v/v) Alginate 500 μm | 0.02 | 0.18 | 0.06 | 0.14 |
| 2% (v/v) Alginate 2000 μm | 0.29 | 2.96 | 0.94 | 11.91 |
| 70% (w/v) PFC 2% (v/v) Alginate 500 μm | 0.03 | 0.07 | 0.06 | 0.05 |
| 70% (w/v) PFC 2% (v/v) Alginate 2000 μm | 0.51 | 1.06 | 0.94 | 4.24 |
Parameters used for oxygen consumption rate feasibility calculations were Rcap = 0.025 cm, RI = 0.075 cm, pm(0) = 160 mm Hg, Vmax,cap = 1.6 × 10−9 mol/(cm3 · s), and vcap/vch = 0.1.
Acknowledgments
This work was supported by grants from the NIH (R01DK50657, R01-DK063108-01A1, and NCRR ICR U42 16606) and JDRF Center for Islet Transplantation at Harvard Medical School. Rat islet isolations were performed with the assistance of Jennifer Hollister-Lock, Vaja Tchipasvilli, and Vassileios Kostaras from Joslin Diabetes Center. Human islets used for DNA measurements were obtained from various Islet Cell Resource centers administered by the Administrative and Bioinformatics Coordinating Center at the City of Hope National Medical Center and supported by NCRR, NIDDK, and JDRF. Histology sections were prepared by Chris Cahill (Joslin DERC, NIH DK 36836). Biospherix provided the Xvivo System for use in these experiments.
Disclosure Statement
No competing financial interests exist.
References
- 1.Shapiro A.M.J. Lakey J.R.T. Ryan E.A. Korbutt G. Toth E. Warnock G. Kneteman N. Rajotte R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Weber D.J. FDA regulation of allogenic islets as a biological product. Cell Biochem Biophys. 2004;40(3 Suppl):19. doi: 10.1385/cbb:40:3:19. [DOI] [PubMed] [Google Scholar]
- 3.FDA Biological Response Modifiers Advisory Committee Summary Minutes, Meeting no. 26. Mar 20–21, 2000. www.fda.gov/ohrms/dockets/ac/00 minutes/3604m1_minutes.pdf www.fda.gov/ohrms/dockets/ac/00 minutes/3604m1_minutes.pdf
- 4.Weber D.J. MacFarland R.D. Irony I. Selected Food and Drug Administration review issues for regulation of allogenic islets of Langerhans as somatic cell therapy. Transplantation. 2002;74:1816. doi: 10.1097/00007890-200212270-00034. [DOI] [PubMed] [Google Scholar]
- 5.FDA Biological Response Modifier Advisory Committee Summary Minutes, Meeting no, 36. Oct 9–10, 2003. www.fda.gov/ohrms/dockets/ac/cber03.html#BiologicalResponseModifiers www.fda.gov/ohrms/dockets/ac/cber03.html#BiologicalResponseModifiers
- 6.Eckhard E. Brandhorst D. Winter D. Jaeger C. Jahr H. Bretzel R.G. Brendel M.D. The role of current product release criteria for identification of human islet preparations suitable for clinical transplantation. Transplant Proc. 2004;36:1528. doi: 10.1016/j.transproceed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Bertuzzi F. Ricordi C. Prediction of clinical outcome in islet allotransplantation. Diabetes Care. 2007;30:410. doi: 10.2337/dc06-1233. [DOI] [PubMed] [Google Scholar]
- 8.Colton C.K. Papas K.K. Pisania A. Rappel M.J. Powers D.E. O'Neil J.J. Omer A. Weir G.C. Bonner-Weir S. Characterization of islet preparations. In: Halberstadt C, editor; Emerich D.F., editor. Cellular Transplantation: From Laboratory to Clinic. New York: Elsevier, Inc.; 2007. pp. 85–135. [Google Scholar]
- 9.Papas K.K. Suzynski T.M. Colton C.K. Islet assessment for transplantation. Curr Opin Organ Transplant. 2009;14:674. doi: 10.1097/MOT.0b013e328332a489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pipeleers D.G. Pipeleers-Marichal M.L. Hannarert J.C. Berghmnas L.M. Int'Veld P.A. Rozing J. Van De Winkel M. Gepts W. Transplantation of purified islet cells in diabetic rats. I. Standarization of islet grafts. Diabetes. 1991;40:908. doi: 10.2337/diab.40.7.908. [DOI] [PubMed] [Google Scholar]
- 11.Pipeleers D.G. Pipeleers-Marichal M.L. A method of the purification of single A-cell, B-cell and D-cell and for the isolation of coupled cells from isolated rat islets. Diabetologia. 1981;20:654. doi: 10.1007/BF00257436. [DOI] [PubMed] [Google Scholar]
- 12.Weir G.C. Halban P.A. Meda P. Wolheim C.B. Orci L. Renold A.E. Dispersed adult rat pancreatic islet cells in culture: A, B, and D cell function. Metabolism. 1984;33:447. doi: 10.1016/0026-0495(84)90146-x. [DOI] [PubMed] [Google Scholar]
- 13.Ricordi C. Scharp D.W. Lacy P.E. Reversal in nude mice after transplantation of fresh and 7 days cultured (24°C) human pancreatic islets. Transplantation. 1988;45:994. doi: 10.1097/00007890-198805000-00035. [DOI] [PubMed] [Google Scholar]
- 14.Smets F.N. Chen Y. Wang L.J. Soriano E. Loss of anchorage triggers apoptosis (anoikis) in primary mouse hepatocytes. Mol Gen Metab. 2002;75:344. doi: 10.1016/S1096-7192(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 15.Pisania A. Weir G. O'Neil J. Omer A. Tchipashvili V. Lei J. Colton C. Bonner-Weir S. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab Invest. 2010;90:1661. doi: 10.1038/labinvest.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisania A. Papas K.K. Powers D.E. Rappel M.J. Omer A. Bonner-Weir S. Weir G.C. Colton C.K. Enumeration of islets by nuclei counting and light microscopic analysis. Lab Invest. 2009;90:1676. doi: 10.1038/labinvest.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnett M.J. McGhee-Wilson D. Shapiro A.M.J. Lakey J.R.T. Variation in human islet viability based on different membrane integrity stains. Cell Transplant. 2004;13:481. doi: 10.3727/000000004783983701. [DOI] [PubMed] [Google Scholar]
- 18.Pisania A. Department of Chemical Engineering. Massachusetts Institute of Technology; Cambridge, MA: 2007. Development of quantitative methods of quality assessment of islets of Langerhans [Ph.D. Thesis] [Google Scholar]
- 19.Brandhorst D. Brandhorst H. Hering B.J. Eckhard T. Jahr H. Federlin K. Bretzel R.G. ATP content of isolated islets: indication for species-dependent vulnerability for cell-mediated graft rejection? Transplant Proc. 1997;29:2058. doi: 10.1016/s0041-1345(97)00229-7. [DOI] [PubMed] [Google Scholar]
- 20.Brandhorst D. Brandhorst H. Hering B.J. Federlin K. Bretzel R.G. The intracellular ATP content of fresh and cultured human islets isolated from different donors. Transplant Proc. 1997;29:1979. doi: 10.1016/s0041-1345(97)00192-9. [DOI] [PubMed] [Google Scholar]
- 21.Brandhorst D. Brandhorst H. Hering B.J. Federlin K. Bretzel R.G. Large variability of the intracellular ATP content of human islets isolated from different donors. J Mol Med. 1999;77:93. doi: 10.1007/s001090050310. [DOI] [PubMed] [Google Scholar]
- 22.Ihm S. Matsumoto I. Sawada T. Nakano M. Zhang H.J. Ansite J.D. Sutherland D.E.R. Hering B.J. Effect of donor age on function of isolated human islets. Diabetes. 2006;55:1361. doi: 10.2337/db05-1333. [DOI] [PubMed] [Google Scholar]
- 23.Suszynski T.M. Wildey G.M. Falde E.J. Cline G.W. Maynard K.S. Ko N. Stiris J. Naji A. Hering B.J. Papas K.K. The ATP/DNA ratio is a better indicator of islet cell viability than the ADP/ATP ratio. Transplant Proc. 2008;40:346. doi: 10.1016/j.transproceed.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J.H. Park S.G. Lee H.N. Lee Y.Y. Park H.S. Kim H. Yu J.E. Kim S.H. Park C. Ha J. Kim S.J. Park K.S. ATP measurement predicts porcine islet transplantation outcome in nude mice. Transplantation. 2009;87:166. doi: 10.1097/TP.0b013e318191e925. [DOI] [PubMed] [Google Scholar]
- 25.Ishii S. Sato Y. Terashima M. Saito T. Suzuki S. Murakami S. Gotoh M. A novel method for determination of ATP, ADP, and AMP contents of a single pancreatic islet before transplantation. Transplant Proc. 2004;36:1191. doi: 10.1016/j.transproceed.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 26.Goto M. Holgersson J. Kumagai-Braesch M. Korsgren O. The ADP/ATP ratio: a novel predictive assay for quality assessment of isolated pancreatic islets. Am J Transplant. 2006;6:2483. doi: 10.1111/j.1600-6143.2006.01474.x. [DOI] [PubMed] [Google Scholar]
- 27.Ihm S. Matsumoto I. Zhang H.J. Ansite J.D. Hering B.J. Effect of short-term culture on functional and stress-related parameters in isolated human islets. Transplant Intern. 2008;8:207. doi: 10.1111/j.1432-2277.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- 28.Sweet I.R. Cook D.L. DeJulio E. Wallen A.R. Khalil G. Callis J. Reems J. Regulation of ATP/ADP in pancreatic islets. Diabetes. 2004;53:401. doi: 10.2337/diabetes.53.2.401. [DOI] [PubMed] [Google Scholar]
- 29.Ohta M. Nelson D. Nelson J. Meglasson M.D. Erecińska M. Oxygen and temperature dependence of stimulated insulin secretion in isolated rat islets of langerhans. J Biol Chem. 1990;265:17525. [PubMed] [Google Scholar]
- 30.Dionne K.E. Colton C.K. Yarmush M.L. A microperifusion system with environmental control for studying insulin secretion by pancreatic tissue. Biotechnol Prog. 1991;7:359. doi: 10.1021/bp00010a011. [DOI] [PubMed] [Google Scholar]
- 31.Dionne K.E. Colton C.K. Yarmush M.L. Effect of hypoxia on insulin secretion by isolated rat and canine islets of langerhans. Diabetes. 1993;42:12. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 32.Gerling I.C. Kotb M. Fraga D. Sabek O. Gaber A.O. No correlation between in vitro and vivo function of human islets. Transplant Proc. 1998;30:587. doi: 10.1016/s0041-1345(97)01417-6. [DOI] [PubMed] [Google Scholar]
- 33.Street C.N. Lakey J.R.T. Shapiro A.M.J. Imes S. Rajotte R.V. Ryan E.A. Lyon J.G. Kin T. Avila J. Tsujimura T. Korbutt G.S. Islet graft assessment in the Edmonton protocol: implications for predicting long-term clinical outcome. Diabetes. 2004;53:3107. doi: 10.2337/diabetes.53.12.3107. [DOI] [PubMed] [Google Scholar]
- 34.Sweet I.R. Gilbert M. Jensen R. Sabek O. Fraga D.W. Gaber A.O. Reems J. Glucose stimulation of cytochrome C reduction and oxygen consumption as assessment of human islet quality. Transplantation. 2005;80:1003. doi: 10.1097/01.tp.0000178381.35014.37. [DOI] [PubMed] [Google Scholar]
- 35.Wang W. Upshaw L. Strong D.M. Robertson R.P. Reems J. Increased oxygen consumption rates in response to high glucose detected by a novel oxygen biosensor system in non-human primate and human islets. J Endocrinol. 2005;185:445. doi: 10.1677/joe.1.06092. [DOI] [PubMed] [Google Scholar]
- 36.Goto M. Abe H. Ito-Sasaki T. Goto M. Inagaki A. Ogawa N. Fujimori K. Kurokawa Y. Matsue T. Satomi S. A novel predictive method for assessing the quality of isolated pancreatic islets using scanning electrochemical microscopy. Transplant Proc. 2009;41:311. doi: 10.1016/j.transproceed.2008.10.075. [DOI] [PubMed] [Google Scholar]
- 37.Papas K.K. Pisania A. Wu H. Weir G.C. Colton C.K. A stirred microchamber for oxygen consumption rater measurements with pancreatic islets. Biotechnol Bioeng. 2007;98:1071. doi: 10.1002/bit.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papas K.K. Colton C.K. Nelson R.A. Rozak P.R. Avgoustiniatos E.S. Scott W.E., 3rd Wildey G.M. Pisania A. Weir G.C. Hering B.J. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007;7:707. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papas K.K. Colton C.K. Qipo A. Wu H. Nelson R.A. Hering B.J. Weir G.C. Koulmanda M. Prediction of marginal mass required for successful islet transplantation. J Invest Surg. 2010;23:28. doi: 10.3109/08941930903410825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colton C.K. Implantable biohybrid artificial organs. Cell Transplant. 1995;4:415. doi: 10.1177/096368979500400413. [DOI] [PubMed] [Google Scholar]
- 41.De Vos P. Faas M.M. Strand B. Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27:5603. doi: 10.1016/j.biomaterials.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Duvivier-Kali V.F. Omer A. Parent R.J. O'Neil J.J. Weir G.C. Complete protection of islets against allorejection and autoimmunity by a simple barium-alginate membrane. Diabetes. 2001;50:1698. doi: 10.2337/diabetes.50.8.1698. [DOI] [PubMed] [Google Scholar]
- 43.Zekorn T. Siebers U. Bretzel R.G. Heller S. Meder U. Ruttkay H. Zimmerman U. Federlin K. Impact of the perfluorochemical FC43 on function of isolated islets: a preliminary report. Horm Metab Res. 1991;23:302. doi: 10.1055/s-2007-1003682. [DOI] [PubMed] [Google Scholar]
- 44.Khattak S.F. Chin K.-S. Bhatia S.R. Roberts S.C. Enhancing oxygen tension and cellular function in alginate cell encapsulation devices through the use of perfluorocarbons. Biotechnol Bioeng. 2007;96:156. doi: 10.1002/bit.21151. [DOI] [PubMed] [Google Scholar]
- 45.Chin K. Khattak S.F. Bhatia S.R. Roberts S.C. Hydrogel-perfluorocarbon composite scaffold promotes oxygen transport to immobilized cells. Biotechnol Prog. 2008;24:358. doi: 10.1021/bp070160f. [DOI] [PubMed] [Google Scholar]
- 46.Johnson A.S. Fisher R.J. Weir G.C. Colton C.K. Oxygen consumption and diffusion in assemblages of respiring spheres: performance enhancement of a bioartificial pancreas. Chem Eng Sci. 2009;64:4470. [Google Scholar]
- 47.Lewis A.S. Fisher R.J. Weir G.C. Colton C.K. Improving oxygen supply to encapsulated cells and islets. In: Halle J.P., editor; De Vos P., editor; Rosenberg L., editor. The Bioartificial Pancreas and Other Biohybrid Therapies 2008. Kerala, India: Transworld Research Network, Research Signpost; 2009. pp. 205–241. [Google Scholar]
- 48.Gotoh M. Maki T. Kiyoizumi T. Satomi S. Monaco A.P. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985;40:437. doi: 10.1097/00007890-198510000-00018. [DOI] [PubMed] [Google Scholar]
- 49.Schweighardt F.K. Kayhart C.R. Concentrated stable fluorochemical aqueous emulsions containing triglycerides. US Pat. 1990;4:895–876. [Google Scholar]
- 50.Omer A. Duvivier-Kali V. Fernandes J. Tchipashvili V. Colton C.K. Weir G.C. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation. 2005;79:52. doi: 10.1097/01.tp.0000149340.37865.46. [DOI] [PubMed] [Google Scholar]
- 51.Papas K.K. Avgoustiniatos E.S. Tempelman L.A. Weir G.C. Colton C.K. Pisania A. Rappel M.J. Friberg A.S. Bauer A.C. Hering B.J. High-density culture of human islets on top of silicone rubber membranes. Transplant Proc. 2005;37:3412. doi: 10.1016/j.transproceed.2005.09.086. [DOI] [PubMed] [Google Scholar]
- 52.Lewis A.S. Department of Chemical Engineering. Massachusetts Institute of Technology; Cambridge, MA: 2008. Eliminating oxygen supply limitations for transplanted microencapsulated islets in the treatment of type 1 diabetes [Ph.D. Thesis] [Google Scholar]
- 53.Powers D.E. Millman J.R. Rappel M.J. Colton C.K. Accurate control of oxygen level in cells during culture on silicone rubber membranes with application to stem cell differentiation. Biotech Progress. 2010;26:805. doi: 10.1002/btpr.359. [DOI] [PubMed] [Google Scholar]
- 54.Avgoustiniatos E.S. Colton C.K. Effect of external oxygen mass transfer resistance on viability of immunoisolated tissue. Ann NY Acad Sci. 1997;83:1145. doi: 10.1111/j.1749-6632.1997.tb52192.x. [DOI] [PubMed] [Google Scholar]
- 55.Tham M.K. Walker R.D. Modell J.H. Diffusion coefficients of O2, N2 and CO2 in fluorinated ethers. J Chem Eng Data. 1973;18:411. [Google Scholar]
- 56.Bailey A.E. In: Bailey's Industrial Oil and Fat Products. 4th. Swern D., editor. Vol. 1. New York: Wiley; 1979. [Google Scholar]
- 57.Fillion B. Morsi B.I. Gas-liquid mass-transfer and hydrodynamic parameters in a soybean oil hydrogenation process under industrial conditions. Ind Eng Chem Res. 2000;39:2157. [Google Scholar]
- 58.Lim F. Sun A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 59.Morch Y.A. Donati I. Strand B.L. Skjak-Braek G. Effect of Ca2+, Ba2+ and Sr2+ on alginate microbeads. Biomacromolecules. 2006;7:1471. doi: 10.1021/bm060010d. [DOI] [PubMed] [Google Scholar]
- 60.Halle J.-P. Landry D. Fournier A. Beaudry M. Leblond F.A. Method for the quantification of alginate in microcapsules. Cell Transplant. 1993;2:429. doi: 10.1177/096368979300200511. [DOI] [PubMed] [Google Scholar]
- 61.Shimabukuro M. Zhou Y.-T. Levi M. Unger R.H. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kharroubi I. Ladriere L. Cardozo A.K. Dogusan Z. Cnop M. Eizirik D.L. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappab and endoplasmic reticulum stress. Endocrinology. 2004;145:5087. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 63.Oprescu A.I. Bikopoulos G. Naassan A. Allister E.M. Tang C. Park E. Uchino H. Lewis G.F. Fantus I.G. Rozakis-Adcock M. Wheeler M.B. Giacca A. Free fatty- acid-induced reduction in glucose-stimulated insulin secretion—evidence for a role of oxidative stress in vitro and in vivo. Diabetes. 2007;56:2927. doi: 10.2337/db07-0075. [DOI] [PubMed] [Google Scholar]
- 64.Nunes E. Peixoto F. Louro T. Sena C.M. Santos M.S. Matafome P. Moreira P.I. Seica R. Soybean oil treatment impairs glucose-stimulated insulin secretion and changes fatty acid composition of normal and diabetic islets. Acta Diabetol. 2007;44:121. doi: 10.1007/s00592-007-0252-8. [DOI] [PubMed] [Google Scholar]
- 65.Krogh-Madsen R. Plomgaard P. Akerstrom T. Møller K. Schmitz O. Pedersen B.K. Effect of short-term intralipid infusion on the immune response during low-dose endotoxemia in humans. Am J Physiol Endocrinol Metab. 2008;294:E371. doi: 10.1152/ajpendo.00507.2007. [DOI] [PubMed] [Google Scholar]
- 66.Avgoustiniatos E.S. Dionne K.E. Wilson D.F. Yarmush M.L. Colton C.K. Measurements of effective diffusion coefficient of oxygen in pancreatic islets. Ind Eng Chem Res. 2007;46:6157. [Google Scholar]
- 67.Maxwell J.C. A Treatise on Electricity and Magnetism. Vol. 1. London, UK: Clarendon Press; 1881. p. 440. [Google Scholar]
- 68.O'Sullivan E.S. Johnson A.S. Omer A. Hollister-Lock J. Bonner-Weir S. Colton C.K. Weir G.C. Islet cell aggregates are superior to islets for transplantation in microcapsules. Diabetologia. 2010;53:937. doi: 10.1007/s00125-009-1653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]