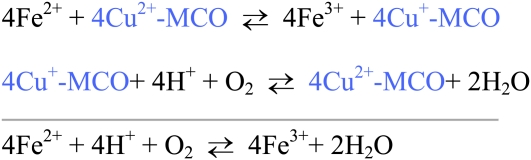Abstract
Copper is an essential trace element whose recommended intake is met by most North American diets. However, incidence of new cases of secondary copper deficiency is rising due to complications of gastric bypass surgery and high zinc exposure. Patients frequently are ataxic and anemic. Anemia of copper deficiency was first described in the 19th century, but the underlying biochemistry remains unknown. Approximately one dozen cuproenzymes have been characterized in mammals. Four of these are referred to as multicopper oxidases (MCO) due to their copper binding geometries. They have iron oxidase activity (ferroxidase). These include the hepatic secreted protein ceruloplasmin representing ∼90% of plasma copper, a splice-variant of ceruloplasmin originally characterized in brain linked by glycosylphosphatidylinositol (GPI) to membranes, an intestinal enriched MCO named hephaestin, and newly described MCO in placenta called zyklopen. Limitation in available copper appears to limit function of the MCO group exhibited as impaired iron flux due to the copper requirement of MCO for their ferroxidase activity. Dietary copper deficiency is associated with lower levels of ceruloplasmin, GPI-ceruloplasmin, and hephaestin. Limitation of copper does not appear to limit synthesis of MCO but rather their stability and turnover. However, there appears to be a disconnect between limitation in MCO function and anemia, because humans and mice missing ceruloplasmin are not anemic despite hepatic iron overload and hypoferremia. Furthermore, anemic copper-deficient mammals are not improved by iron replacement. This suggests that the anemia of copper deficiency is not caused by iron limitation but rather impairment in iron utilization.
Introduction
Copper, an essential trace metal, exists under physiological conditions as both cuprous and cupric ion. As such, copper can transfer single electrons, a useful catalytic function but potentially deleterious for generating hydroxyl radical if it interacts with hydrogen peroxide. Thus, it is no surprise that the concentration of unbound copper is exceedingly low. In fact, copper ions are driven to their final cellular destination (cuproenzymes) by an affinity driven ligand exchange process (1). This careful copper homeostasis is maintained by regulation of a Cu+ specific plasma membrane transporter (CTR1),3 and several metallochaperones [antioxidant 1 (ATOX1), copper] chaperone for superoxide dismutase (CCS), and COX 17] that escort copper to the trans-Golgi network to cytoplasmic superoxide dismutase 1 and to the mitochondrial intramembrane CuA and CuB sites of cytochrome oxidase (2–4). This intrinsic set of gene products helps ensure that only an appropriate amount of copper is absorbed and transported to target proteins that require copper for their function.
Mammals require about one dozen cuproenzymes for optimal health (Table 1). These copper-dependent oxidases participate in a diverse set of reactions affecting many physiological processes. These include pigmentation, cross-linking of collagen and elastin, removal of a reactive oxygen metabolite (superoxide), mitochondrial electron transport to support oxidative phosphorylation, amine oxidation, catecholamine biosynthesis, and neuropeptide activation. Several cuproenzymes appear to have the same function acting as a ferroxidase converting ferrous to ferric iron and are thought critical in iron efflux and homeostasis.
Table 1.
Mammalian cuproenzymes
| Common name | Putative function |
| Amine oxidase | Signal transduction, leukocyte adhesion |
| Cytochrome c oxidase | ATP production |
| Dopamine-β-monooxygenase | Norepinephrine synthesis |
| Extracellular superoxide dismutase | Superoxide scavenging |
| Lysyl oxidase | Connective tissue cross linking |
| Peptidylglycine α-amidating monoxygenase | Neuropeptide maturation |
| Superoxide dismutase 1 | Superoxide scavenging |
| Tyrosinase | Melanin production |
| MCO (ferroxidases) | |
| Ceruloplasmin | Iron mobilization |
| GPI-ceruloplasmin | Macrophage iron efflux |
| Hephaestin | Intestinal iron efflux |
| Zykopen | Placental iron efflux |
In some cases, limitations of dietary copper, genetic copper deficiency, or secondary copper deficiency lead to a phenotype correlated with altered cuproenzyme function. Examples include hypopigmentation due to limited tyrosinase and connective tissue defects due to limited lysyl oxidase. However, mechanisms for other common phenotypes of copper deficiency such as anemia remain unknown (5).
Anemia of copper deficiency
Most nutritional science students learn that the essentiality of copper for mammals was first reported by Hart et al. (6) in 1928. This landmark paper demonstrated that copper, in addition to iron, was necessary for hemoglobin production in rats. However, credit should also to be recognized for the 19th century pioneers who first described an anemia in humans called chlorosis that responded to copper supplementation. Details of this history are described elsewhere in a comprehensive review (7). Although anemia (low hemoglobin levels) remains a salient feature of copper deficiency in humans, the mechanism remains unknown despite a generous supply of hypotheses. Importantly, cases of anemia of copper deficiency are becoming more common.
In the past decade, nutritional awareness of dietary copper needs has diminished since the North American DRI were established to replace the Estimated Safe and Adequate Daily Dietary Intake guidelines. This is because the Estimated Safe and Adequate Daily Dietary Intake guidelines (∼3 mg/d) exceeded the average adult intake of copper based on NHANES data, 1.3 mg/d, compared with the current RDA, 0.9 mg/d. Extensive evaluation of copper exposure in mammals suggests an optimal intake of copper near 2.6 mg/d (8). This level is twice the current mean intake of adults and nearly 3 times the RDA. This set point is also considerably below the current upper level of 10 mg/d. Thus, one point of current interest to the greater nutrition community is to consider what are the apropos DRI for copper for optimal health and to prevent anemia.
Many feel that current dietary copper intake is adequate, because evidence of human copper deficiency is exceedingly rare. Usually, the diagnosis of copper deficiency is determined in subjects with below normal plasma copper levels. There are both genetic and environmental factors that can cause this phenotype. Genetic diseases of copper homeostasis that lead to hypocupremia include Wilson disease (mutations in ATP7B, a protein necessary for metallation of ceruloplasmin, the major plasma copper protein), Menkes disease (mutations in ATP7A, a protein necessary for copper efflux from intestine and for metallation of many secreted cuproenzymes), and aceruloplasminemia [due to loss of ceruloplasmin (CP)]. Wilson disease accounted for only 10% of the cases of human hypocupremia in a comprehensive recent review of clinical data (9). Many cases were due to secondary impact of surgery or bowel disease, but, interestingly, 23% of the causes of hypocupremia were of unknown etiology. Recently, adult onset copper deficiency leading to myeloneuropathy and anemia has become more noticeable. One cause of this clinical situation is the increase in bariatric surgery that has accompanied obesity treatment (10). Zinc induced copper deficiency has also become an important clinical concern (11). This increase in copper deficiency needs to be diagnosed properly, so ineffective and potentially harmful treatment with vitamin B-12 or iron does not happen. Treat copper deficiency with copper. Why does lack of copper result in myeloneuropathy, panocytopenia, and anemia? The answers are critical to patient health and long term care.
Current status of knowledge
Multicopper oxidases
The most widely accepted hypothesis concerning mechanisms to explain anemia of copper deficiency involves the copper-iron interaction (5). This hypothesis argues that anemia occurs because iron is not available for hemoglobin synthesis in bone marrow, because copper is necessary for iron absorption and release from stores. There are several multicopper oxidases (MCO) that could be involved.
MCO refer to a group of related proteins that contain several moles of copper per protein, usually 6. These copper atoms occupy different chemical geometries and are referred to as types 1, 2, or 3 copper sites. MCO can oxidize several substrates but physiologically are thought critical for their ferroxidase activity (Fig. 1) (Table 1).
Figure 1.
Ferroxidase activity of MCO. Ferrous iron transfers electrons to the Type 1 copper binding site and ferric iron is produced. The cuprous ion is reoxidized by losing electrons to molecular oxygen bound at the Type 2 and Type 3 copper centers. The net result of catalysis is oxidation of ferrous iron and reduction of oxygen to produce ferric iron and water. Ferric iron can then bind transferrin.
Ceruloplasmin
The first and best-characterized MCO is CP. CP was first described in the 1940s as the blue plasma amine oxidase (12). There are excellent reviews on CP structure and function that describe much of the history of this plasma copper binding protein (13). CP is a secreted glycoprotein produced mainly by liver. Osaki and Frieden (14) postulated that CP could act as a ferroxidase. This feature and the seminal studies of copper deficient swine suggested an explanation for the copper-iron interaction and anemia (15). However, the swine studies also revealed that iron supplementation could not reverse the anemia, suggesting lack of iron was not the key issue. Furthermore, others felt the function of CP was copper transport, because it represents over 90% of plasma copper. This theory was challenged when Cp−/− mice were generated without evidence of abnormal copper metabolism (16). The Cp−/−mice did demonstrate hepatic iron overload consistent with ferroxidase function, but, curiously, Cp−/− mice were not anemic (17).
Glycosylphosphatidylinositol-linked Cp
Several independent discoveries in the late 1980s and early 1990s greatly advanced our understanding of CP and the function of MCO. First, humans lacking CP were reported in the literature (18). They had normal copper metabolism but accumulated iron in selected organs such as liver and brain. Studies in yeast discovered a MCO Cp homolog Fet3 that was necessary for high affinity iron uptake, supporting a role of MCO in iron flux (19). Building on the idea that CP must play a role in brain iron biology, Patel and David (20) reported that astrocytes contain a special form of CP anchored to the membrane by a GPI-link (GPI-CP). GPI-CP is produced as splice-variant of exons 19–20 and is 98% identical with the hepatic secreted form of CP (sCP). The N-terminal peptapeptide is different and GPI-CP contains an additional 25 amino acids (21). Recent studies in multiple organs from rats and mice have reported widespread expression of GPI-Cp at the mRNA level, with the highest levels in kidney and spleen and lower expression in liver (22). Protein expression was correlated with mRNA level in 5 organs studied. Isolated cell studies also reported GPI-Cp in Sertoli cells, leptomeningeal cells, and NK cells. The putative function of GPI-CP is to act as a ferroxidase in macrophages involved in iron efflux (5). It is estimated that the rat liver expression of sCp is 90-fold higher than GP1-Cp, whereas in spleen, mRNA expression levels of the 2 Cp variants is similar (22).
Hephaestin
Several natural mutations in mice aided in elucidating molecular details of iron absorption. One such mutant is the sla (sex-linked anemia) mouse, which has a translocation defect that prevents iron egress from the enterocyte. Vulpe et al. (23) used a genetic approach and discovered the defective gene product was a putative CP analogue with 50% identity expressed highly in intestine they named hephaestin (HEPH). HEPH is a MCO with transmembrane C-terminal domain to anchor it. HEPH contains 86 additional amino acids compared with CP. The phenotype of the sla mouse is consistent with a ferroxidase function for Heph reflected in intestinal iron retention. HEPH presumably works in conjunction with ferroportin (FPN), the iron exporter necessary to release iron from enterocytes, macrophages, and hepatocytes (24).
Zyklopen
Dietary copper deficiency during pregnancy in rats results in delivery of iron deficient pups (25, 26). This suggests a role for copper in placental iron transport. Early work in BeWo cells, a placental model system, suggested the existence of a membrane endogenous ferroxidase (27). Recent work has now described a novel MCO enriched in placenta called zyklopen (ZP) (28). ZP shares 46% identity with CP and 49% identity with HEPH. Like HEPH, ZP is predicted to be tethered to the plasma membrane by its primary sequence. ZP has all 3 types of copper centers and has in vitro ferroxidase and diamine oxidase activity. Immunohistochemical results in rodents suggest that Zp may be present in several organs, including retina, testes, kidney, and brain. However, Zp was not detected in liver or enterocytes.
Collectively, these reports reveal the presence of at least 4 distinct MCO proteins in mammals: sCP, GPI-CP, HEPH, and ZP. They are enriched in selective tissues but there is remarkable overlap. For example, in brain, GPI-Cp, Heph, and Zp all appear to be expressed. The function of these 3 MCO proteins in brain has not been established.
MCO role in iron flux
There is both nutritional and genetic evidence that MCO proteins play an important role in iron egress from cells. The most thorough data are for sCP, in part because it was discovered 5 decades before the other MCO proteins.
Nutritional evidence was provided when administration of exogenous Cp, but not an equivalent dose of copper, to copper-deficient swine resulted in iron release from liver (29). This data supported the original ferroxidase hypothesis of Osaki that ceruloplasmin was capable and necessary for ferrous iron oxidation (14, 30). There are many other comprehensive nutritional studies that have suggested a role for Cp in iron mobilization (30). However, the strongest data came from human participants lacking CP. Collectively, 3 families have been described with aceruloplasminemia (31). These participants all have iron accumulation in liver, brain, and selected other organs. Generation of Cp −/− null mice by 3 separate genetic strategies has also confirmed a massive hepatic iron overload in those mice lacking the Cp gene (17, 32, 33).
Support for a role of GPI-CP in iron flux is also suggested by genetic evidence in aceruloplasminenic humans and Cp −/− mice that both develop brain iron overload as adults (32). Others have suggested that GPI-Cp activity is necessary to maintain the iron efflux protein FPN on the cell surface (34). This indirect role for GPI-Cp would thus be mediated by FPN stability rather than ferroxidase activity directly.
The phenotype of the sla mouse, enterocyte iron accumulation, supports an iron efflux role for Heph in the GI tract. Nutritional support for Heph function is also strong. In copper-deficient rats there is a decrease in iron absorption, lower Heph expression, enterocyte iron accumulation, and reversal by dietary copper repletion (35). Similar studies in copper deficient mice also suggest a role for Heph in iron transport across the gut (36).
Data on placental iron efflux and Zp function are limited; however, as indicated earlier nutritional gestational copper deficiency results in iron deficient pups (25, 26). It is logical to assume that the MCO Zp requires adequate dietary copper for function and when copper is limiting, iron delivery to the fetus is reduced.
Impact of copper deficiency on MCO expression
Copper availability to cells may affect MCO synthesis and/or stability in addition to being required for activity. Once again, data on sCP expression is much more prevalent than for the other MCO proteins. However, a consistent pattern exists.
Holtzman et al. (37) developed specific antibodies that could distinguish between apo- and holo- ceruloplasmin. This allowed careful dissection of the impact of copper limitation in rats on Cp abundance. Next, they showed that copper deficiency was associated with lower plasma Cp protein, because apo-Cp was degraded faster than holo-Cp (38). They also showed that in humans there was lower total CP protein in copper-deficient infants, patients with Menkes disease, and subjects with Wilson disease (39). All 3 human conditions would limit holo-CP biosynthesis, although the mechanisms would be different. Careful later studies in rats demonstrated that Cp synthesis was not altered by dietary copper deficiency, suggesting no impact of low copper on Cp transcription (40). This work was recently reconfirmed using qRT-PCR in both copper-deficient rats and mice (41). This later work also confirmed a dramatic (60–90%) reduction in plasma sCp protein level using Western-blot technology. These studies collectively support the hypothesis that adequate liver copper is necessary to metallate apo-CP for release and that enhanced turnover of apo-CP results in lower total immunoreactive sCP following copper limitation.
Because sCP metallation depends on adequate copper delivery to liver and then to trans-Golgi, it is not surprising that genetic knockout of intestinal or liver Ctr1 in mice results in lower liver copper and lower holo-Cp in plasma (42, 43). Likewise, analogous to the prior published data on Wilson disease, mice with deletion of Atp7B also secrete mainly apo-Cp (44). sCp levels have not been evaluated in Atox1−/− mice. ATOX1 is the metallochaperone that delivers copper from CTR1 transport to the secretory pathway. Because murine Atox1 deletion is associated with increased perinatal lethality, it may be difficult to assess an effect on holo-Cp formation (45). Liver copper is reduced in Atox1 null mice.
The impact of copper limitations on GPI-Cp has recently been studied in both rats and mice (22). Data show a rapid loss of GPI-Cp protein in spleen and to a lesser extent in liver. In fact, after only 1 wk of diet, copper-deficient rat spleen GPI-Cp levels were lower by nearly 60%. The reduction in spleen and liver GPI-Cp protein was detected without change in GPI-Cp mRNA level, consistent with the theory that apo-GP1-Cp is not stable and is degraded rather than any impact of copper limitation on GPI-Cp transcription.
Work in polarized colon carcinoma cells with the copper chelator bathocuproine sulfonate (BCS) suggested the MCO HEPH protein level was greatly reduced when copper was lowered (46). This in vitro study also provided evidence that HEPH underwent proteasome mediated degradation. This seminal work was followed up by nutritional experiments in both rats and mice, confirming that steady-state levels of Heph are indeed lower following dietary copper deficiency (35, 36). In the mouse study, enterocyte Heph mRNA level was evaluated and shown not to be altered by copper deficiency, similar to other work previously discussed on sCp and GPI-Cp mRNA expression.
The 4th mammalian MCO, ZP, also seems to require adequate copper for stability. A cell culture study with BeWo cells and BCS reported lower ZP abundance with increasing BCS exposure (28). Thus, adequate copper appears necessary to form holo-MCO and stabilize protein structure and decrease turnover for all 4 mammalian MCO.
Role of MCO in the anemia of copper deficiency
There is strong evidence, as discussed earlier, that CP is a ferroxidase, that copper deficiency results in near total loss of ferroxidase activity, and that copper deficient rats exhibit high liver iron levels, low plasma iron, and anemia. However, association does not prove causation. Thus, it was shocking when the initial reports on Cp−/− mice failed to detect any alteration in hemoglobin levels despite massive hepatic iron overload (17). Furthermore, plasma iron levels were normal in these Cp−/− mice. Two other labs have generated Cp −/− mice; both reported hepatic iron overload but only in 1 mild anemia (Table 2) (32, 33). Plasma iron was reported lower in these 2 additional mouse knockout studies, further confusing the issue. Also, mice derived from the first Cp −/− experiment by Harris et al. (47) were later showed to indeed have lower serum iron although only modest anemia (Table 2). Thus, there appears to be a disconnect between loss of murine MCO function (sCp and GP1-Cp) and anemia and even consistent hypoferremia. Further support for lack of connectivity between CP and anemia of copper deficiency is shown in important human genetic data. The calculated hemoglobin concentration of 6 patients with aceruloplasminemia was 12.4 ± 1.71 g/dL, a level consistent with lack of pronounced anemia (18, 48, 49).
Table 2.
Tabular ratios of iron status data in ceruloplasmin null mice and wild-type mice (Cp −/− / Cp +/+)1
| Research group2 | 1 | 2 | 3 |
| Age at analysis, mo | 3 | 16 | 5 |
| Liver iron | 4.4 | 10.6 | 3.4 |
| Spleen iron | NS | NS | ND |
| Serum iron | 0.4 | 0.2 | 0.5 |
| Hemoglobin | 0.9 | NS | 0.8 |
Tabular ratios were calculated only when means were significantly different, NS = not significant, > 0.05, ND = not determined.
Dietary copper deficient models also contribute to our knowledge of the role of MCO in anemia. Both copper deficient rats and mice on the same dietary protocols exhibit lower hemoglobin compared with copper adequate controls (50). Plasma from both copper deficient mice and rats contain nondetectable sCp activity measured with o-dianisidine and nondetectable ferroxidase activity using Western membrane assay (41). However, plasma iron was only lower in copper-deficient rats but not mice (41, 50). How can mice without detectable ferroxidase maintain normal plasma iron? No plasma ferroxidase was detected in copper deficient mice or Cp−/− mice in 1 study using a Western-blot approach (41). Recent work from another group reported that mice may have an additional plasma ferroxidase (51). Early work on plasma ferroxidase in rodents and humans following separation of proteins by gel permeation chromatography detected ferroxidase activity assessed by holo-transferrin assay in the void volume and coincident with Cp amine oxidase activity in rats and humans but not mice (52). Clearly, additional research will be necessary to clarify this apparent difference between rodents. It should be noted, however, that some investigators have detected lower plasma iron in mice following dietary copper deficiency (36). This is similar to the mixed outcomes on serum iron in Cp −/− mice.
Perhaps as puzzling as the serum iron and hemoglobin data is the fact that spleen iron is not elevated in Cp−/− mice despite evidence of massive hepatic iron overload (Table 2). Splenic macrophages play an important role in recycling hemoglobin iron from phagocytized erythrocytes during the daily iron cycle. If GPI-Cp functions as a ferroxidase in concert with Fpn, why is there no hyperaccumulation of iron in spleen when GPI-Cp is absent? In 1-y-old Cp −/− mice there was an augmentation in spleen iron (17). However, the same mice at 3 mo did not reveal any spleen iron augmentation (Table 2). Recent studies in copper deficient rats reported markedly lower than normal levels of spleen GPI-Cp protein (22). Interestingly, the same study reported lower, rather than higher, spleen nonheme iron levels, challenging the role of GPI-Cp in iron efflux from splenic macrophages. Further, it was reported by others that when challenged by phlebotomy, Cp −/− mice had no impairment in releasing splenic iron stores (47). The ability of copper deficient rat spleen to release iron in the absence of GPI-Cp activity may be due in part to the augmentation in splenic Fpn recently shown in 2 models of copper deficiency (53). This augmentation in spleen Fpn protein in the presence of diminished GPI-Cp is likely because copper deficient rats exhibit very low levels of hepcidin, the liver hormone that can trigger Fpn degradation. Thus, low hepcidin and high Fpn may help explain why splenic iron is not trapped in copper deficient rats with diminished GPI-Cp.
There is clearly a copper dependent factor besides sCP and GPI-Cp that is necessary to prevent anemia when copper is limiting. Evidence to support this statement comes from several genetic models. Despite the absence of holo-Cp in tissue specific Ctr1 intestinal knockout mice and a massive liver iron overload due to induced peripheral copper deficiency, no anemia was detected (43). Hemoglobin in the intestinal Ctr1 −/− mice was 74 ± 18 g/L compared with 93 ± 23 in wild-type mice. The reduction in sCp, augmentation in liver iron, and degree of cardiac hypertrophy were more severe than dietary copper deficient mice that exhibit frank anemia (50). Brindled mice, a genetic model of Menkes disease, die midway through lactation of severe copper deficiency, but they are not anemic, although they have greatly diminished Cp activity (54). Brindled mice do develop anemia if their mothers are offered a copper deficient diet, emphasizing the role of dietary copper in expression of anemia (55).
There is also strong data that challenges the popular thought that limitation in Heph function is associated with anemia of copper deficiency. Copper deficiency in mice was associated with intestinal iron elevation, lower Heph levels, and anemia (36). However, others, using a similar dietary approach, have shown that anemic copper-deficient mice, despite a modest increase in intestinal iron concentration, have unaltered whole body iron content (50). This challenges the idea that limitation in the function of Heph can explain anemia of copper deficiency. Data in copper deficient rats also suggested a function for Heph in explaining anemia. Copper deficient rats had impaired iron absorption, increased intestinal iron, low Heph, and anemia (35). However, when iron was administered by injection to these copper deficient anemic rats to bypass the gut blockade, animals remained anemic (56). This outcome was similar to that described earlier for copper deficient swine (15). Iron injection compared with copper injection also failed to elevate hemoglobin in older copper deficient mice (57).
It should be mentioned, however, that iron injection can rescue the anemia of neonatal copper deficiency in both mice and rats (58, 59). This seems likely, because neonates born to and nursed by copper deficient dams are truly iron deficient. They derive less iron from placental transport, presumably because the function of Zp is limited. Fetal liver iron and whole body pup iron is lower in copper deficient rats (25, 26). Sucking pups are also deprived of iron, because milk iron is lower in copper deficient mouse and rat dams (26, 60, 61). Puzzling, however, is the fact that placental and mammary tissues from copper deficient dams show no evidence of iron retention as one might predict if a MCO was limiting (25, 26).
Conclusions
Further research is necessary to set the safe and optimal intake of copper in humans. Copper is necessary for the catalytic activity of about one dozen mammalian proteins that affect many physiological systems. One consequence of dietary copper deficiency or impaired copper absorption is anemia. Current hypotheses supporting the connection between anemia and the ferroxidase activity of MCO proteins are likely not correct. Total loss of sCP and GPI-CP does not lead to anemia. Reduced Heph function and expression results in intestinal iron retention, but iron injection does not reverse anemia. Copper deficient anemic rats have low serum iron, but in mice, serum iron is often normal and anemia is more severe (50). Thus, as suggested in another recent review, further studies on the copper dependent factors in bone marrow seem prudent (5). Anemia seems to result not from a failure to deliver adequate iron but in a failure to utilize it properly.
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Footnotes
Supported in part by NIH grant HD039708.
Author disclosures: J. R. Prohaska, no conflicts of interest.
Abbreviations used: ATOX1, antioxidant 1; BCS, bathocuproine sulfonate; CCS, copper chaperone for superoxide dismutase; CP, ceruloplasmin; CTR1, copper transporter 1; FPN, ferroportin; GPI-CP, glycosylphosphatidylinositol-ceruloplasmin; HEPH, hephaestin; MCO, multicopper oxidase; sCP, secreted form of ceruloplasmin; ZP, zyklopen.
Literature Cited
- 1.Banci L, Bertini I, Ciofi-Baffoni S, Kozyreva T, Zovo K, Palumaa P. Affinity gradients drive copper to cellular destinations. Nature. 2010;465:645–8 [DOI] [PubMed] [Google Scholar]
- 2.Lutsenko S. Human copper homeostasis: a network of interconnected pathways. Curr Opin Chem Biol. 2010;14:211–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prohaska JR. Role of copper transporters in copper homeostasis. Am J Clin Nutr. 2008;88:S826–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson NJ, Winge DR. Copper metallochaperones. Annu Rev Biochem. 2010;79:537–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins JF, Prohaska JR, Knutson MD. Metabolic crossroads of iron and copper. Nutr Rev. 2010;68:133–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hart EB, Steenbock H, Waddell J, Elvehjem CA. Iron in nutrition. VII. Copper as a supplement to iron for hemoglobin building in the rat. J Biol Chem. 1928;77:797–812 [PubMed] [Google Scholar]
- 7.Fox PL. The copper-iron chronicles: the story of an intimate relationship. Biometals. 2003;16:9–40 [DOI] [PubMed] [Google Scholar]
- 8.Chambers A, Krewski D, Birkett N, Plunkett L, Hertzberg R, Danzeisen R, Aggett PJ, Starr TB, Baker S, et al. An exposure-response curve for copper excess and deficiency. J Toxicol Environ Health B Crit Rev. 2010;13:546–78 [DOI] [PubMed] [Google Scholar]
- 9.Kumar N, Butz JA, Burritt MF. Clinical significance of the laboratory determination of low serum copper in adults. Clin Chem Lab Med. 2007;45:1402–10 [DOI] [PubMed] [Google Scholar]
- 10.Griffith DP, Liff DA, Ziegler TR, Esper GJ, Winton EF. Acquired copper deficiency: a potentially serious and preventable complication following gastric bypass surgery. Obesity (Silver Spring). 2009;17:827–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedera P, Peltier A, Fink JK, Wilcock S, London Z, Brewer GJ. Myelopolyneuropathy and pancytopenia due to copper deficiency and high zinc levels of unknown origin II. The denture cream is a primary source of excessive zinc. Neurotoxicology. 2009;30:996–9 [DOI] [PubMed] [Google Scholar]
- 12.Holmberg CG, Laurell CB. Investigations in serum copper; nature of serum copper and its relation to the iron-binding protein in human serum. Acta Chem Scand. 1947;1:944–50 [DOI] [PubMed] [Google Scholar]
- 13.Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr. 2002;22:439–58 [DOI] [PubMed] [Google Scholar]
- 14.Osaki S, Johnson DA, Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966;241:2746–51 [PubMed] [Google Scholar]
- 15.Lee GR, Nacht S, Lukens JN, Cartwright GE. Iron metabolism in copper-deficient swine. J Clin Invest. 1968;47:2058–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer LA, Durley AP, Prohaska JR, Harris ZL. Copper transport and metabolism are normal in aceruloplasminemic mice. J Biol Chem. 2001;276:36857–61 [DOI] [PubMed] [Google Scholar]
- 17.Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA. 1999;96:10812–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyajima H, Nishimura Y, Mizoguchi K, Sakamoto M, Shimizu T, Honda N. Familial apoceruloplasmin deficiency associated with blepharospasm and retinal degeneration. Neurology. 1987;37:761–7 [DOI] [PubMed] [Google Scholar]
- 19.Askwith C, Eide D, Van Ho A, Bernard PS, Li L, Davis-Kaplan S, Sipe DM, Kaplan J. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell. 1994;76:403–10 [DOI] [PubMed] [Google Scholar]
- 20.Patel BN, David S. A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J Biol Chem. 1997;272:20185–90 [DOI] [PubMed] [Google Scholar]
- 21.Patel BN, Dunn RJ, David S. Alternative RNA splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. J Biol Chem. 2000;275:4305–10 [DOI] [PubMed] [Google Scholar]
- 22.Mostad E, Prohaska JR. Glycosylphosphatidylinositol-linked ceruloplasmin is expressed in multiple rodent organs and is lower following dietary copper deficiency. Exp Biol Med. 2011; in press [DOI] [PubMed] [Google Scholar]
- 23.Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21:195–9 [DOI] [PubMed] [Google Scholar]
- 24.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200 [DOI] [PubMed] [Google Scholar]
- 25.Andersen HS, Gambling L, Holtrop G, McArdle HJ. Effect of dietary copper deficiency on iron metabolism in the pregnant rat. Br J Nutr. 2007;97:239–46 [DOI] [PubMed] [Google Scholar]
- 26.Pyatskowit JW, Prohaska JR. Multiple mechanisms account for lower plasma iron in young copper deficient rats. Biometals. 2008;21:343–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danzeisen R, Ponnambalam S, Lea RG, Page K, Gambling L, McArdle HJ. The effect of ceruloplasmin on iron release from placental (BeWo) cells; evidence for an endogenous Cu oxidase. Placenta. 2000;21:805–12 [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Attieh ZK, Syed BA, Kuo YM, Stevens V, Fuqua BK, Andersen HS, Naylor CE, Evans RW, et al. Identification of zyklopen, a new member of the vertebrate multicopper ferroxidase family, and characterization in rodents and human cells. J Nutr. 2010;140:1728–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ragan HA, Nacht S, Lee GR, Bishop CR, Cartwright GE. Effect of ceruloplasmin on plasma iron in copper-deficient swine. Am J Physiol. 1969;217:1320–3 [DOI] [PubMed] [Google Scholar]
- 30.Evans JL, Abraham PA. Anemia, iron storage and ceruloplasmin in copper nutrition in the growing rat. J Nutr. 1973;103:196–201 [DOI] [PubMed] [Google Scholar]
- 31.Harris ZL, Klomp LW, Gitlin JD. Aceruloplasminemia: an inherited neurodegenerative disease with impairment of iron homeostasis. Am J Clin Nutr. 1998;67:S972–7 [DOI] [PubMed] [Google Scholar]
- 32.Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien JP, David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci. 2002;22:6578–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto K, Yoshida K, Miyagoe Y, Ishikawa A, Hanaoka K, Nomoto S, Kaneko K, Ikeda S, Takeda S. Quantitative evaluation of expression of iron-metabolism genes in ceruloplasmin-deficient mice. Biochim Biophys Acta. 2002;1588:195–202 [DOI] [PubMed] [Google Scholar]
- 34.De Domenico I, Ward DM, di Patti MC, Jeong SY, David S, Musci G, Kaplan J. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. EMBO J. 2007;26:2823–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves PG, Demars LC, Johnson WT, Lukaski HC. Dietary copper deficiency reduces iron absorption and duodenal enterocyte hephaestin protein in male and female rats. J Nutr. 2005;135:92–8 [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Huang G, Su T, Gao H, Attieh ZK, McKie AT, Anderson GJ, Vulpe CD. Decreased hephaestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J Nutr. 2006;136:1236–41 [DOI] [PubMed] [Google Scholar]
- 37.Holtzman NA, Gaumnitz BM. Identification of an apoceruloplasmin-like substance in the plasma of copper-deficient rats. J Biol Chem. 1970;245:2350–3 [PubMed] [Google Scholar]
- 38.Holtzman NA, Gaumnitz BM. Studies on the rate of release and turnover of ceruloplasmin and apoceruloplasmin in rat plasma. J Biol Chem. 1970;245:2354–8 [PubMed] [Google Scholar]
- 39.Matsuda I, Pearson T, Holtzman NA. Determination of apoceruloplasmin by radioimmunoassay in nutritional copper deficiency, Menkes’ kinky hair syndrome, Wilson's disease, and umbilical cord blood. Pediatr Res. 1974;8:821–4 [DOI] [PubMed] [Google Scholar]
- 40.Gitlin JD, Schroeder JJ, Lee-Ambrose LM, Cousins RJ. Mechanisms of caeruloplasmin biosynthesis in normal and copper-deficient rats. Biochem J. 1992;282:835–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broderius M, Mostad E, Wendroth K, Prohaska JR. Levels of plasma ceruloplasmin protein are markedly lower following dietary copper deficiency in rodents. Comp Biochem Physiol C Toxicol Pharmacol. 2010;151:473–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim H, Son HY, Bailey SM, Lee J. Deletion of hepatic Ctr1 reveals its function in copper acquisition and compensatory mechanisms for copper homeostasis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G356–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006;4:235–44 [DOI] [PubMed] [Google Scholar]
- 44.Huster D, Finegold MJ, Morgan CT, Burkhead JL, Nixon R, Vanderwerf SM, Gilliam CT, Lutsenko S. Consequences of copper accumulation in the livers of the Atp7b−/− (Wilson disease gene) knockout mice. Am J Pathol. 2006;168:423–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamza I, Faisst A, Prohaska J, Chen J, Gruss P, Gitlin JD. The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis. Proc Natl Acad Sci USA. 2001;98:6848–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nittis T, Gitlin JD. Role of copper in the proteosome-mediated degradation of the multicopper oxidase hephaestin. J Biol Chem. 2004;279:25696–702 [DOI] [PubMed] [Google Scholar]
- 47.Cherukuri S, Potla R, Sarkar J, Nurko S, Harris ZL, Fox PL. Unexpected role of ceruloplasmin in intestinal iron absorption. Cell Metab. 2005;2:309–19 [DOI] [PubMed] [Google Scholar]
- 48.Logan JI, Harveyson KB, Wisdom GB, Hughes AE, Archbold GP. Hereditary caeruloplasmin deficiency, dementia and diabetes mellitus. QJM. 1994;87:663–70 [PubMed] [Google Scholar]
- 49.Morita H, Ikeda S, Yamamoto K, Morita S, Yoshida K, Nomoto S, Kato M, Yanagisawa N. Hereditary ceruloplasmin deficiency with hemosiderosis: a clinicopathological study of a Japanese family. Ann Neurol. 1995;37:646–56 [DOI] [PubMed] [Google Scholar]
- 50.Pyatskowit JW, Prohaska JR. Copper deficient rats and mice both develop anemia but only rats have lower plasma and brain iron levels. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:316–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gray LW, Kidane TZ, Nguyen A, Akagi S, Petrasek K, Chu YL, Cabrera A, Kantardjieff K, Mason AZ, et al. Copper proteins and ferroxidases in human plasma and that of wild-type and ceruloplasmin knockout mice. Biochem J. 2009;419:237–45 [DOI] [PubMed] [Google Scholar]
- 52.Prohaska JR. Comparison between dietary and genetic copper deficiency in mice: copper-dependent anemia. Nutr Res. 1981;1:159–67 [Google Scholar]
- 53.Jenkitkasemwong S, Broderius M, Nam H, Prohaska JR, Knutson MD. Anemic copper-deficient rats, but not mice, display low hepcidin expression and high ferroportin levels. J Nutr. 2010;140:723–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prohaska JR. Changes in tissue growth, concentrations of copper, iron, cytochrome oxidase and superoxide dismutase subsequent to dietary or genetic copper deficiency in mice. J Nutr. 1983;113:2048–58 [DOI] [PubMed] [Google Scholar]
- 55.Prohaska JR. Effect of dietary copper deficiency on heterozygous female brindled mice. Nutr Res. 1988;8:1079–84 [Google Scholar]
- 56.Reeves PG, DeMars LC. Signs of iron deficiency in copper-deficient rats are not affected by iron supplements administered by diet or by injection. J Nutr Biochem. 2006;17:635–42 [DOI] [PubMed] [Google Scholar]
- 57.Prohaska J, Bailey W, Cox D. Failure of iron injection to reserve copper-dependent anemia in mice. : Mills CF, Bremner I, Chesters JK, Trace elements in man and animals. Farnham Royal, Slough (UK): Commonwealth Agricultural Bureaux; 1985. p. 27–32 [Google Scholar]
- 58.Prohaska JR. Repletion of copper-deficient mice and brindled mice with copper or iron. J Nutr. 1984;114:422–30 [DOI] [PubMed] [Google Scholar]
- 59.Pyatskowit JW, Prohaska JR. Iron injection restores brain iron and hemoglobin deficits in perinatal copper-deficient rats. J Nutr. 2008;138:1880–6 [DOI] [PubMed] [Google Scholar]
- 60.Cohen NL, Keen CL, Hurley LS, Lonnerdal B. Determinants of copper-deficiency anemia in rats. J Nutr. 1985;115:710–25 [DOI] [PubMed] [Google Scholar]
- 61.Prohaska JR. Effect of diet on milk copper and iron content of normal and heterozygous brindled mice. Nutr Res. 1989;9:353–6 [Google Scholar]