Abstract
Adequate nutrition during cancer plays a decisive role in several clinical outcome measures, such as treatment response, quality of life, and cost of care. However, the importance of nutrition in children and young adults with malignancies is still an underestimated topic within pediatric oncology. The importance of our work is to reinforce and indicate that malnutrition in children with cancer should not be accepted at any stage of the disease or tolerated as an inevitable process. Unique to our manuscript is the close collaboration, the exchange of knowledge and expertise between pediatric oncologists and a nutritional specialist, as well as the comprehension of the mechanisms during cancer cachexia and malnutrition. We provide a critical review of the current state of research and new knowledge related to nutritional management in childhood cancer.
Introduction
The survival of children with cancer has substantially increased over the last decades. Major reasons for this are, among others, progress in the early detection, refined multimodality treatment, and enhancements of supportive care, including prevention and efficient management of infections. Furthermore, multicenter trials guarantee a high degree of complete data and quality assurance (1). However, despite continuous progress in the field, the disease itself and associated therapies carry the burden of an array of adverse late effects (2).
The literature suggests that up to 46% of children and young adults with cancer experience malnutrition due to numerous tumor- and treatment-related factors (3–5). It is recognized that a diminished nutritional status may be a contributing factor for decreased immune function, delayed wound healing, and disturbed drug metabolism influencing prognosis (6, 7). Children with cancer are particularly vulnerable to malnutrition, because they exhibit elevated substrate needs due to the disease and its treatment. At the same time, children have increased requirements of nutrients to attain appropriate growth and neurodevelopment (8). It has been demonstrated that adequate nutrition plays a decisive role on several clinical outcome measures such as treatment response, quality of life, and cost of care (9, 10).
We provide a critical review of the current state of research and knowledge related to the nutritional management in childhood cancer.
Definition and prevalence of malnutrition in childhood cancer
Malnutrition is an unspecific term used to define an inadequate nutritional condition. It is characterized by either a deficiency or an excess of energy with measurable adverse effects on clinical outcome. Malnutrition describes the consequences of insufficient protein-energy intake. An adequate protein-energy balance is a prerequisite for age-appropriate growth and maintenance. Malnutrition also comprises circumstances of elevated energy supply resulting in overnutrition with an increase in adipose tissue.
Even though malnutrition has been defined or described in many ways, no consensus exists regarding a specific definition to identify children at risk (11, 12). The WHO recommends the weight-for-height index to assess the nutritional status of children and adolescents (13). However, it is proposed that a loss in body weight of ≥5% constitutes acute malnutrition and a height-for-age value below the 5th percentile may reflect chronic undernourishment in children (4). Ironically, many children suffering from cancer do not meet these criteria. Particularly those with large solid abdominal masses (e.g. embryonal neoplasms such as neuroblastoma, hepatoblastoma, or Wilms tumor) may present with normal weight despite severe malnutrition. Nutritional depletion may furthermore be masked in children by edema due to corticosteroid treatment. Even if no gold standard definition for undernourishment in children exists, concise definitions are needed for the institution of preventive policies.
Current information regarding the prevalence of malnutrition in childhood cancer is critically influenced by several factors: 1) different diagnostic techniques to assess the nutritional status; 2) histological type and stage of malignancy during assessment; 3) the child’s individual susceptibility toward malnutrition and anticancer regimens during classification; and finally 4) the rather nonspecific definition of malnutrition.
Thus, the frequency of undernourishment in children and adolescents with cancer is arbitrary reported as common to not existent at diagnosis. Studies report a range from 0 to 50% depending on the type of cancer (4, 9, 14). It must be stressed that body weight is not a sufficiently and adequately sensitive marker for the detection of nutritional perturbations in children with cancer. It may be affected by hydration during chemotherapy and does not identify any long-term changes in body cell mass (15). In children with adequate or excessive body weight, lean body mass loss may be concealed as fat decreases or remains unchanged while skeletal muscle is wasting. Moreover, undetectable nutritional depletion of 1 or more micronutrients due to decreased food intake, excessive enteral losses, or other factors occur in normal or overweight children (11, 14, 15).
Etiology and pathophysiology
A number of pathophysiological mechanisms contribute to the development of malnutrition and growth failure in childhood cancer. The causes are multifactorial, including: 1) complex interactions between energy and substrate metabolism; 2) hormonal and inflammatory components; and 3) alterations of metabolic compartments. These result in accelerated mobilization, oxidation of energy substrates, and loss of body proteins (16, 17).
Mechanisms during simple starvation and cancer cachexia
Anorexia is defined as the loss of the desire to eat, which frequently leads to minimized intake of nutrients. Cachexia is characterized by profound and progressive wasting of lean tissue and body fat. In prolonged fasting or starvation, weight loss occurs gradually, with a relative maintenance of lean body mass at the expense of body fat as an energy source, whereas in cancer cachexia there is an equal loss of fat and muscle (17, 18). Compensatory mechanisms during simple starvation consist of protein conservation and a decrease in energy expenditure allowing prolonged survival in the chronic fasting state (19, 20). These mechanisms might be lost or inhibited in cancer (19, 21). This most likely explains why the provision of apparently adequate calories has often been disappointing in clinical studies, suggesting a hypermetabolic state (16). In contrast to uncomplicated starvation, cachexia in humans with cancer is an advanced state of wasting marked by excess depletion of skeletal muscle mass and adipose tissue relative to total body weight (19). Metabolic and body composition changes in cancer cachexia resemble those detected in individuals with polytrauma, acute sepsis, burn injuries, and AIDS (22, 23).
Cancer and host factors mediating malnutrition
The role of cytokines.
Food intake is regulated in the ventromedial nucleus of the hypothalamus. Animal models have demonstrated that neuropeptides such as proinflammatory cytokines (IL1α, IL-1β, IL-6) released by tumor tissue, immune and stroma cells, TNFα, and INFγ in combination with other mediators affect food intake and energy expenditure, resulting in the clinical syndrome of cancer cachexia (24, 25). Cytokines are transported across the blood-brain barrier and interact with the luminal surface of brain endothelial cells to release compounds that affect appetite (26). In experimental models, many other mediators for cancer-induced anorexia have been proposed. These include leptin levels, which depend on body fat stores and are inversely related to the intensity of the inflammatory response and levels of inflammatory cytokines (24). Nevertheless, studies in humans demonstrate that leptin concentrations are not elevated in weight-losing cancer patients, suggesting that leptin is not involved in the initiation of anorexia (27, 28).
Energy deficits and metabolic abnormalities.
It is evident that energy deficits play an important role in the progress of the wasting syndrome in children with neoplastic diseases related to: 1) increased nutrient requirements; 2) energy losses caused by frequent gastrointestinal dysfunction due to to cancer therapy induced toxicity; 3) an excess utilization of energy sources as a result of aggressive multimodal cancer treatments; 4) metabolic and hormonal alterations; 5) uncontrolled pain or stress from inevitable procedures; and 6) disorders in appetite sensation or changes in taste (e.g. due to xerostomia) (3, 4, 8, 29).
A number of factors interfere with enteral nutrient ingestion, some of which are directly related to the illness (e.g. relapse) or immediate complications (e.g. infections or fever) (30). However, all these cannot exclusively explain the progression of severe wasting and energy imbalance in childhood malignancy. In simple starvation, wasting is reversible when nutritional intervention is initiated. In contrast, an increase in nutrient ingestion alone is not sufficient to prevent, reverse, or retard cancer cachexia (31, 32).
Previous studies have discussed a cancer-host rivalry for energy nutrients, suggesting tumor-induced host variances in carbohydrate, lipid, and protein metabolism (33, 34), whereas recent trials could not corroborate this competition theory indicating that there is no positive correlation between tumor size or extension and the severity of host depletion (3, 4, 8, 35). In some patients, malnutrition often occurs early in the course of the disease. Furthermore, manifestations such as alterations in appetite and substrate metabolism cannot easily be explained by a cancer-host competition for energy nutrients. This suggests that different utilization of exogenous energy substrates by host and tumor are present. In animal models, it has been demonstrated that deliberate dietary protein depletion results in temporarily diminished tumor growth rates, but also in severe host malnutrition (36, 37). In addition, in experimental models, the i.v. administration of nutrients increases tumor volume and accelerates the mitotic activity of cancer cells (38–40). It must be emphasized that neither an influence of nutritional deprivation (restricted diets) nor a direct substrate-induced stimulation and modulation of tumor-cell proliferation has ever been documented in humans (41–43). Findings from experimental parabiotic designs are difficult to transfer to the clinical setting, because animal tumor models differ in size and proliferation from human neoplasms and represent substantially different patterns of energy metabolism (36). It is important to note that nutritional strategies for adults with cancer, extrapolated from animal tumor systems, are not practical and suitable for undernourished children.
The role of energy-consuming cycles and substrate metabolism.
Protein disturbances comprise an increased whole body protein turnover, likely mediated by cytokines, a reduction in muscle protein synthesis, and an increase in hepatic protein synthesis (17). Accelerated lipolysis with increased production of glycerol and FFA contributes to the depletion of fat stores and subsequent weight loss (17). It has been suggested that the main energy source for cancer cells is the aerobic metabolism of glucose, which significantly exceeds that of normal cells producing high levels of lactic acids (44). Lactic acids are transported to the liver for renewed synthesis of glucose by the Cori cycle at a high energy cost. The use of muscle-derived proteins for gluconeogenesis contributes to an additional loss of energy and for a further increase in energy expenditure, resulting in a catabolic state (45).
The metabolic effects of cancer and chemotherapeutic agents
Changes of hormonal factors
During adaptation to uncomplicated malnutrition, there is an increase in catecholamines, glucagon, cortisol, and growth hormone levels, and a decreased insulin secretion (46). Endocrine disturbances in cancer patients present in contrast to uncomplicated malnourished humans in the form of insulin resistance and as elevated secretion of growth hormone (47, 48). The production of thyroid hormones is reduced in malnourished humans with and without cancer due to activation of the sympathetic nervous system, decreased glandular secretion, and nutritional restriction (47). Thyroid dysfunction after total body or cranio-spinal irradiation is well known (49). The physiological benefits of hormonal depression are likely the reduction of energy expenditure and the preservation of substrates in simple starvation and cancer cachexia.
Impact of energy metabolism
The metabolic turnover of a child is affected by factors such as age, gender, nutritional status, energy intake, body composition, hormones, physical activity, body and environmental temperature, and pharmacotherapy as well as by pathological conditions such as surgical stress, infections, or trauma. There is a major controversy over whether the child with cancer has aberrations in energy expenditure different from a malnourished noncancer or healthy child raised by limited energy balance studies using, among others ,different diagnostic techniques and diverse treatment strategies. Studies have reported normal (50, 51), reduced (52), and elevated energy expenditures (53) in children and adults with malignancies in contrast to control groups. Additional determinants such as cytostatic drugs, cancer type, stage, status, and the time of investigation appear to play a significant role in determining whether or not an alteration of energy expenditure is observed in cancer patients. The metabolic turnover is a function of several factors and it is not uniformly altered in patients with cancer. It has previously been proposed that sympathetic stimulation of brown adipose tissue or skeletal muscle may lead to increased energy expenditure in the cancer-bearing host in which the causes remain unclear (17, 54).
Changes in body composition
Body composition changes during normal infancy such as variations in hydration and density of body tissues are influenced by gender, age, pubertal status, genetic factors, nutrition, diseases, and physical activity (55, 56). The determination of body composition includes direct or indirect measurements of body fat, lean body mass, bone mass, and in certain instances the distribution of fat between the visceral or subcutaneous compartments. Fat tissue has relatively uniform physical properties during aging, whereas lean tissue exhibits over time decreases in water content and increases in protein and mineral composition. This leads to a decline in the ratio of extracellular to intracellular water with aging.
In children with cancer, body weight can be influenced by tumor mass and hydration, particularly during chemotherapy, masking loss of fat and skeletal muscle. The measurement of body compartments provides useful information about the nutritional status at the time of diagnosis. It identifies subsequent changes in the functional tissue during antineoplastic therapy presenting additional information to those obtained from anthropometry and subjective nutritional assessments (57, 58). Body compartments play an important role in the pathophysiology of cancer cachexia contributing, e.g., to the fatigue syndrome (59).
The distribution, absorption, metabolism, and elimination of cytostatic drugs are influenced by body composition. Antineoplastic agents have narrow therapeutic indices; consequently, minor changes in drug concentration or exposure may have a profound impact on treatment efficacy and even outcome. Previous studies have shown that the majority of children with malignancies exhibit alterations in body compartments during chemotherapy, with a reduction in lean mass and an increase in fat (57, 58). Some anticancer agents are relatively lipid insoluble and may be poorly distributed in adipose tissue (59, 60). On the other hand, for several hydrophilic drugs, the excess of fat mass is not available for compound distribution and the volume distribution adjusted to weight or surface area might significantly decrease (61). Examining body composition alterations in response to chemotherapy and radiotherapy, attention should be paid to several interactions of cancer drugs with the muscle and adipose tissues. For most cytostatics, there are limited pediatric data on the potential relationship between body composition and pharmacokinetics. Dose calculations based on true body size in obese adult patients can be 20–30% higher than the medication estimated for the same individual based on normalized or ideal body weight (62). Anticancer drugs are often applied in a reduced dose in very tall or/and overweight children. Nevertheless, 2 investigations have demonstrated that obese patients receiving diminished anticancer therapy may have an inferior outcome (63, 64). Other researchers have detected changes in drug clearance, half-life, and significant differences in the metabolism of cyclophosphamide, ifosfamide, and doxorubicin in obese cancer patients (65–68). Corticosteroids, important components of regimens for leukemias, lymphomas, and histiocytic diseases, promote fat mass, mainly via increased energy intake, induce insulin resistance and hyperinsulinemia, and may induce loss of muscle mass affecting energy balance (69). It has been reported that overweight at diagnosis is a poor prognostic indicator for children with acute myeloid leukemia and for female adolescents with acute lymphoblastic leukemia (70). It is currently being discussed whether this is an effect of disturbed chemotherapy distribution or increased drug toxicity. The timing of appearance and differences in body compartments is not clear, whereas increased fat deposition is recognized to increase the risk of developing metabolic syndrome later in life. Underweight has adverse consequences for general health (71).
Risk factors for malnutrition in children with malignancies
Risk factors concerning the type, stage, and metastatic status of cancer
Current data suggest that malnutrition is associated with the type, stage, and metastatic status of the disease as well as the toxicity of multimodal cancer therapies (10, 72). In addition to the existing classification of patients into high and low risk groups for undernourishment, risk factors for obesity induced by cancer agents have to be considered to facilitate the recognition and prevention of malnutrition at an early stage of the disease. High risk groups for starvation comprise patients with body store depletion established at diagnosis, especially children with solid tumors and neoplastic diseases with advanced stage, unfavorable biology, and localization of the tumor (30, 73). Undernourishment occurs less frequently in children diagnosed with nonmetastatic tumors or those with a favorable prognosis. The term malnutrition in childhood cancer is in the process of redefinition due to an increasingly differentiated understanding of body composition changes using new technologies. Cancer types and risk factors related to the development of malnutrition are presented in Table 1.
Table 1.
Tumor types associated with malnutrition for pediatric oncology patients
| High risk factor for undernourishment | Moderate risk factor for undernourishment | High risk factor for fat accumulation |
| Solid tumors with advanced stages | Nonmetastatic solid tumors | Acute lymphoblastic leukemia receiving cranial irradiation |
| Wilms tumors | Uncomplicated acute lymphoblastic leukemia | Craniopharyngeoma |
| Neuroblastoma stage III and IV rhabdomyosarcoma | Advanced diseases in remission during maintenance treatment | Malignancies with large and prolonged doses of corticosteroid therapy or other drugs increasing body fat stores |
| Ewing sarcoma | Total body or abdominal or cranial irradiation | |
| Medulloblastoma | ||
| Multiple relapsed leukemia and lymphoma | ||
| Head and neck tumors | ||
| Post stem cell transplantation (graft vs. host-disease) | ||
| Diencephalic tumors |
Risk factors relating to the modalities of cancer therapies
The majority of childhood neoplasms are treated by combined modality therapy, including surgery, radiotherapy, and antineoplastic schedules commonly providing a variety of side effects, which may lead a child into a state of nutritional deprivation (73, 74). Each of these treatment modalities may produce injuries to major organ systems (liver and pancreas). Furthermore, their combination may cause a potentiation or a synergism of adverse effects. Multimodality therapies combined with the effects of the malignancy itself affect nutritional status and damage rapidly growing cells, e.g., in the gastrointestinal tract, inducing serious and undesirable symptoms. As a result of intense diarrhea, vomiting, mucositis, and systemic effects of therapy, affected children often experience a prolonged period of minimal oral intake. This contributes to fluid loss, electrolyte and trace elements imbalance, and alterations of their carrier proteins, as well as iron and vitamin deficiency that may result in acute and chronic malabsorption of micro- and macronutrients (74). Cancer survivor studies have demonstrated that treatment with alkylating agents or anthracyclines and total body irradiation are associated with underweight (75).
Consequences of starvation in childhood cancer
Morbidity and mortality
One of the first publications, almost 30 y ago, reported that nutritional depletion in humans with cancer causes debility, reduction in immunocompetence and wound healing, an increase in drug toxicity, and changes in psychological well-being (76, 77). Since that time, a wealth of research discussed the consequences of starvation. These primarily consider the impact of weight loss in adults with malignancies. It has been demonstrated that the prognosis of underweight humans with and without cancer is inferior compared with well-nourished patients (78–80). Individuals with cancer exhibiting pronounced weight loss demonstrate decreased survival rates (79), diminished response to cytostatics (79, 81), prolonged hospital stays, higher rates of readmissions (82, 83), and a reduced quality of life (84, 85).
In childhood cancer, undernourishment has a significant prognostic weight on survival rate, especially in children with solid tumors and metastatic diseases with a high risk of body store depletion (10, 85, 86). Inferior survival rates in children with newly diagnosed stage IV neuroblastoma (87), acute lymphoblastic leukemia (88), and acute myeloid leukemia (89, 90) are associated with the degree of an unfavorable weight loss (90, 91).
Long-term consequences
Children with malignancies tolerate the acute side effects of antineoplastic agents better than adults, but the growing child is more susceptible to long-term diseases that have implications for later life (92). The number of childhood cancer survivors increases every year (93). Therefore, research into the effects of long-term consequences of modern antineoplastic therapies is in the early stages, and chronic illnesses are now observed and detected in this cohort (65, 75, 94–96). Comparing the BMI of pediatric cancer survivors to the general population of similar age, it has been demonstrated that survivors of specific cancer types are more likely to be underweight (BMI ≤ 18.5 kg/m2) (75). This group includes children with soft tissue sarcoma, neuroblastoma, non-Hodgkin’s lymphoma, brain tumors, male survivors of leukemia, nonamputated females with bone cancer, Wilms tumors, and survivors of Hodgkin’s disease (75). At the same time, it has been realized that survivors of common pediatric malignancies are at risk for adult-onset diseases such as obesity, which is associated with a high risk for cardiovascular and endocrine diseases (96–98). Increased BMI (≥25 kg/m2) was found in pediatric survivors of acute lymphoblastic leukemia with an age of fewer than 4 y at diagnosis receiving cranial radiation therapy and in children with brain tumors, especially in craniopharyngeoma survivors (75, 99, 100). Obesity was analyzed in these studies by determining BMI. One may speculate that if employing other techniques to more accurately quantify body fat content, the incidence of obesity could be much higher in this cohort of survivors. Interestingly, visceral fat volume is an important determinant that has been linked to increases in the risk for cardiovascular diseases and some types of cancer (e.g. breast, colorectal cancer) (101–105) Table 2 demonstrates the short- and long-term consequences of malnutrition on pediatric cancer survivors.
Table 2.
Short- and long-term consequences of malnutrition on the pediatric cancer survivor
| Short-term consequences | Long-term consequences |
| Wasting of muscle and fat mass (3,15) | Growth impairment, reduced final height (56,75) |
| Decreased tolerance of chemotherapy (30,67) | Decreased long-term survival in several tumor types (78–80) |
| Unfavorable response to chemotherapy (29,30,76,77) | Impact on motor, cognitive, and neurodevelopmental impairment (95) |
| Treatment delays (76,77,81) | Risk for metabolic syndrome (93,96) |
| Fatigue (29,59) | Risk for secondary cancers (106–108) |
| Biochemical disturbances (anemia and hypoalbuminemia) (29) | Risk for aging (101–105) |
| Delayed recovery of normal marrow function (88–91) | Increased mortality rate (79,68) |
| Changes in body composition (75,90,91) | Retardation of skeletal maturation (109) |
| Drug dose alteration (65–68) | Abnormal bone mineral density (109) |
| Decreased quality and productivity of life (84,85) | Decreased quality of life (65,71) |
| Greater levels of psychological distress (90,91) | |
| Higher susceptibility to infections (89,90) |
Methods to detect and analyze in depth poor nutritional status in children with cancer
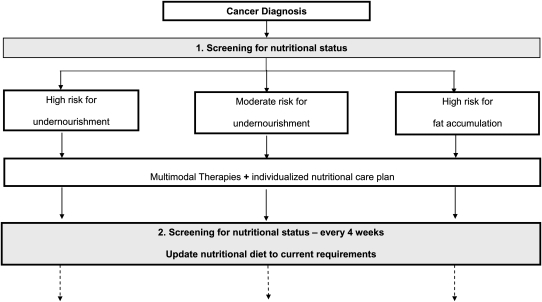
The objective is to detect children with preexisting malnutrition with a risk of substrate depletion before cancer treatment starts and worsens the nutritional status over time. Common anthropometric tests involve measurements of stature, body weight, and BMI that should be measured on admission and at follow-up points in all affected children so that trends can be identified. To ascertain the head circumference is an important aspect of nutritional evaluation, especially in the young ill child, to avoid disturbances in neurodevelopmental outcome throughout critical periods of brain growth. Currently, new noninvasive and inexpensive methodologies to longitudinally and accurately analyze changes in body compartments are successfully applied in the pediatric clinical setting (Table 3). Selected complementary biochemical markers such as plasma protein, pre-albumin, retinol-binding protein, transthyretin, and transferrin have restricted usefulness in the recognition of malnutrition in children with cancer and in critically ill children due to the fact that these variables are influenced by fewer inflammations and infections (110, 111). Chemotherapy and recurrent episodes of sepsis may deplete the body of the essential macro- and micronutrients needed for appropriate growth and metabolism, especially zinc (112). Recently developed nutritional risk scores for adults and children should be a part of the evaluation of malnutrition in patients with cancer (113–115). The determination of individualized energy expenditure provides an understanding of the precise energy and substrate requirements during cancer treatment, minimizing the risk for under- and overnutrition. Enteral analyses of substrate losses through stools for the calculation of energy needs adapting nutritional support to individual intestinal permeability are useful in children with marked intestinal complications. A close link of anthropometric, laboratory, body composition tests, and evaluation of energy intake and losses are helpful in all children with cancer to prevent and potentially treat malnutrition. These methods should be used repeatedly for surveillance and for optimal dietary support during the entire duration of the disease (Fig. 1).
Table 3.
ethods to detect and analyze nutritional status and alterations in the pediatric oncology population
| Methods | Screening | Comments |
| Body weight | Daily, weekly | Alone; no information of nutritional status |
| Height/length | Daily, weekly | Alone; no information of nutritional status |
| Head circumference | Monthly | Especially in infants under 3 y; brain development |
| BMI | Biannually | Body weight (kg) divided by height in meters squared; inaccurate |
| Body composition tools | Monthly | |
| Bioelectrical impedance | Rapid, easy, inexpensive, portable but sensitive to hydration and temperature | |
| Dual X-ray absorptiometry | Accurate, but exposure to radiation | |
| Isotope dilution method | Expensive | |
| Energy expenditure | Weekly, monthly | Determine noninvasive resting energy expenditure. |
| Indirect calorimetry | Provide understanding of energy and substrate requirements | |
| Stool analysis | Weekly, monthly | Analyses of carbohydrates and protein stool losses. |
| Determination of energy losses using bomb-calorimetry |
Figure 1.
A screening schedule for nutritional status after diagnosis, including a classification in risk groups for undernutrition and fat accumulation in children undergoing cancer therapies is demonstrated. We strongly recommend an adaptation of substrate intake according to current requirements.
Nutritional support for children with cancer
Criteria for nutritional interventions
At the present time, no universally agreed specific substrate requirements, criteria for, timing of, and duration of nutritional interventions exist in pediatric oncology. There is an ample array of dissimilar approaches with limited efficacy for prevention and early treatment of growth failure in children with cancer (8, 116, 117). Several nutritional recommendations are based on ideal body weight, BMI, and estimated energy needs without considering changes in body compartments to capture muscle wasting and body mass depletion. Weight loss is a rather poor predictor of undernourishment that reflects past rather than current nutritional status. The primary objectives of nutritional interventions in pediatric oncology should be: the maintenance of body stores as close to the ideal as possible, minimization of wasting, promotion of appropriate growth development, and providing a good quality of life.
Nutrition strategies are indicated in all affected children, beginning with the diagnosis of cancer to prevent and/or restore abnormalities in growth development before nutritional and general status are severely compromised. These should be integrated into cancer treatment protocols starting directly after admission independent of the initial body weight to establish the essential role of adequate nutrition in the mind of the child and parents. The assessment of the nutritional status is indispensable to stratify the child into nutritional risk groups in view of actual nutritional condition and the extent of the disease, considering psychological and socioeconomic aspects as well as the prescribed multimodal procedures for each tumor type. According to these factors, an individualized nutritional care plan may be initiated. Absolutely desirable is the multidisciplinary management including close communication and collaboration between the child, family, and the medical team to determine together the expectations for dietary support as well as to develop further objectives. The expertise of a medical specialist in pediatric oncology nutrition is useful under these circumstances. We recommend monthly evaluation to test the efficacy of the implemented nutritional tools using the methods described above.
Nutritional strategies (parenteral and enteral nutrition)
It is without doubt that the enteral route is the preferred and safest way for the provision of nutrition in any child with an undamaged and functional gastrointestinal tract, preventing intestinal atrophy, toxicity, and complications of i.v. infusion (118). Depending on gastric tolerance and pretenses of the child depending on family participation, an individualized nutritional schedule should be prepared. The selection of further means of support depends on the capability of oral intake, gut stability, absorption rate, and the possibility of expected adverse effects induced by cytostatics (e.g. severe mucositis). Contraindications to enteral feeding in pediatric oncology are similar to those in other diseases or metabolic disorders such as intestinal obstruction, permanent vomiting, or acute hemorrhage (119). If oral or any kind of enteral tube feeding is not possible, parenteral nutrition is indicated without delay. A latency of 3–7 d, as suggested by some reports (120), to initiate parenteral energy supply could be unfavorable in children who have on preadmission evidence of protein-energy depletion or/and a history of lower food intake. The aim of providing partial parenteral support is to meet nutrient requirements until the child tolerates oral intake or tube feeding. Complete parenteral nutrition support should be reserved for short periods in children with failure of enteral absorption and no response to dietary supplement. However, emaciation should be anticipated early and induce parenteral nutrition. The literature offers few clinical trials studying the optimal timing and efficacy of supplemental parenteral nutrition as well as the benefit and outcome of children with malignancies (121, 122). Most studies have been conducted in adults, presenting controversial results and recommendations in the use of total parenteral nutrition for patients undergoing hematopoietic stem cell transplantation (123, 124). The potential complications of parenteral nutrition include risks for infection, metabolic disorders, hepatotoxicity, and reduction of oral intake (119). Exogenous glutamine application appears to play a role in diminishing the detrimental effects of anticancer drugs on the gastrointestinal mucosa. Furthermore carnitine supplementation has been suggested to improve the cancer fatigue syndrome by influencing nutritional and immunological parameters (125, 126).
Recently, the management of cancer cachexia has been supplemented by new therapeutic strategies that may help increase lean mass or treat the cytokine-induced catabolism of skeletal muscle by distinguishing specific mediators of muscle atrophy and the identification of sensitive biomarkers of protein degradation (127). An interest for the improvement of agents that promote muscle anabolism has recently been verbalized (127). Several agents are under clinical investigation and some of them are in clinical trials for adult patients (127, 128). Currently available therapeutic approaches are megestrol acetate, a synthetic derivate of progesterone; anabolic steroids, such as testosterone derivatives; and the peptide hormones ghrelin and ghrelin agonists (127–129). The use of these agents is critical in children concerning the associated side effects on a growing and immature organism. However, there are currently no drugs approved for the prevention or treatment of cancer cachexia.
Methods of enteral nutritional support (tube feeding and percutaneous endoscopic gastrostomy)
Algorithms for nutritional strategies in children and adults with malignancies have been proposed in the literature with the objective to treat rather than to anticipate weight loss (8, 116, 120, 130, 131). The application of nutritional protocols may be useful in advance for selecting those children who are at high risk for malnutrition during antineoplastic therapies. Energy requirements may be based on published pediatric nutritional guidelines (117–119). Specific and scientifically based recommendations for children with cancer are not yet available. High-energy protein formulas and liquid supplements are offered to increase energy density in pediatric patients, but with less success and poor tolerance because of taste and smell perception. A balanced diet with sufficient proteins and high energy levels is required to prevent an extreme overload of carbohydrate and fat consumption, as seen in many children with cancer. Children with painful sequel (e.g. severe mucositis or vomiting) may tolerate gastric or jejunal tube feeding better than oral (e.g. via nasogastric tube) when the nasopharynx may be bypassed. A percutaneous endoscopic gastrostomy (PEG) is a successfully used method with high acceptance by oncologists, children, and parents demonstrating somatic improvements and reduced family frustration due to eating problems (132, 133). The placement of a PEG tube is indicated when oral ingestion is not sufficient to cover the daily energy needs. Table 4 demonstrates nutritional strategies.
Table 4.
Nutritional strategies for children with cancer
| Nutritional strategies | Indications | |
| Enteral route | In all patients with functional gastrointestinal tract Meeting >95–100% of estimated energy needs | |
| Tube feeding (nasogastric) | Inability to ingest full energy requirements (>90%) through oral diet for 3–5 d | |
| Severe mucositis <3 d | ||
| PEG jejunostomy | Inability to meet full energy needs through tube diet for 3–5 d | |
| Severe vomiting for 3–5 d | ||
| Weight loss despite tube feeding | ||
| Parenteral nutrition | Altered gastrointestinal absorption for 3–5 d | |
| Severe vomiting and diarrhea | ||
| Severe pancreatitis | ||
| Intestinal manifestation of graft vs. host disease | ||
| Paralytic ileus |
Conclusions
The importance of nutrition in children and young adults with cancer is an underestimated topic within pediatric oncology. There are new, inexpensive, and noninvasive techniques for the evaluation of the nutritional status providing evidence for the quality of nutritional interventions for children with a high risk of undernutrition and a tendency for body fat accumulation. Malnutrition in children with cancer should not be accepted at any stage of the disease or tolerated as an inevitable process. Nutritional strategies should be considered and integrated as a fundamental feature of pediatric oncology with the same diligence as one does for other supportive care measures to prevent chronic illness and adverse late effects caused by malnutrition in this population.
Further clinical and basic research programs are needed to establish guidelines analyzing the efficacy and impact of nutritional interventions, especially in children with cancer. Despite the well-documented need for adequate nutrition in long-term outcome in children with cancer (9, 10, 29, 89), there are no applicable management strategies or pharmacotherapeutic options available at this time to successfully prevent or treat undernourishment and its associated disorders in this population. One of the main objectives in this field is the early detection of children with preexisting malnutrition and a high risk of substrate depletion before cancer therapies start employing standardized methodologies. These methods should analyze nutritional status (3–5, 11, 15, 64, 94, 106), energy intake (8, 10, 14, 30), and energy expenditure (29, 51) as recently proposed. The literature offers few randomized prospective clinical trials that explore short- and long-term consequences of nutritional support (69, 95–99) and the special needs of pediatric cancer patients (71, 73, 89). Additional research is required to analyze the impact of nutrition on morbidity, mortality, and quality of life and to prevent chronic illnesses for pediatric cancer survivors. Because cancer malnourishment is not unique to underweight children (96–98), an assessment of body composition is important for the characterization of the severity of the condition. Furthermore, the effects of malnutrition can be surveyed. In adults with cancer, various agents are in development, including medications that inhibit the mediators (inflammatory cytokines) and tumor-induced inflammation that occur during cancer cachexia (17, 18). Current treatment options, including drugs (127–129) or anabolic agents, for cancer malnutrition in pediatric oncologic patients are limited due to adverse side effects in a growing and immature child.
Acknowledgments
J.B. was responsible for contact; J.B. and M.C.F. designed and conceived the study; J.B. and M.C.F. wrote the manuscript; and H.J., J.B., and M.C.F. critically discussed and corrected the study. All authors read and approved the final version of the paper.
Footnotes
Author disclosures: J. Bauer, J. Jürgens, and M. C. Frühwald, no conflicts of interest.
Literature Cited
- 1.Creutzig U, Zimmermann M, Hannemann J, Kraemer I, Herold G, Henze G. Quality management within the competence network of paediatric oncology and haematology. Klin Padiatr. 2003;215:338–40 [DOI] [PubMed] [Google Scholar]
- 2.Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M, Hammond S, Yasui Y, Inskip PD. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2356–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pietsch JB, Ford C. Children with cancer: measurements of nutritional status at diagnosis. Nutr Clin Pract. 2000;15:185–8 [Google Scholar]
- 4.Smith DE, Stevens MC, Booth IW. Malnutrition at diagnosis of malignancy in childhood: common but mostly missed. Eur J Pediatr. 1991;150:318–22 [DOI] [PubMed] [Google Scholar]
- 5.Sala A, Puncher P, Barr RD. Children, cancer, and nutrition: a dynamic triangle in review. Cancer. 2004;100:677–87 [DOI] [PubMed] [Google Scholar]
- 6.Bosaeus I, Daneryd P, Svanberg E, Lundholm K. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. Int J Cancer. 2001;93:380–3 [DOI] [PubMed] [Google Scholar]
- 7.Tisdale MJ. Cancer cachexia: metabolic alterations and clinical manifestations. Nutrition. 1997;13:1–7 [DOI] [PubMed] [Google Scholar]
- 8.Han-Markey T. Nutritional considerations in pediatric oncology. Semin Oncol Nurs. 2000;16:146–51 [DOI] [PubMed] [Google Scholar]
- 9.Van Eys J. Malnutrition in children with cancer: incidence and consequences. Cancer. 1979;43:2030–5 [DOI] [PubMed] [Google Scholar]
- 10.Rickard KA, Grosfeld JL, Coates TD, Weetman R, Baehner RL. Advances in nutrition care of children with neoplastic diseases: a review of treatment, research, and application. J Am Diet Assoc. 1986;86:1666–76 [PubMed] [Google Scholar]
- 11.Brennan BMD, Thomas AG. Nutritional status in children with acute leukemia. J Pediatr Gastroenterol Nutr. 1997;25:248–51 [DOI] [PubMed] [Google Scholar]
- 12.Wright JA, Ashenburg CA, Whitaker RC. Comparison of methods the categorize undernutrition in children. J Pediatr. 1994;124:944–6 [DOI] [PubMed] [Google Scholar]
- 13.Butte NE, Garza C, de Onis M. Evaluation of the feasibility of international growth standards for school-aged children and adolescents. Food Nutr Bull. 2006;27 No 4, Suppl 2:169–74 [DOI] [PubMed] [Google Scholar]
- 14.Reilly JJ, Ventham JC, Newell J, Aitchison T, Wallace WH, Gibson BE. Risk factors for excess weight gain in children treated for acute lymphoblastic leukaemia. Int J Obes Relat Metab Disord. 2000;24:1537–41 [DOI] [PubMed] [Google Scholar]
- 15.White M, Davies P, Murphy A. Validation of percent body fat indicators in pediatric oncology nutrition assessment. J Pediatr Hematol Oncol. 2008;30:124–9 [DOI] [PubMed] [Google Scholar]
- 16.Skipworth RJ, Stewart GD, Dejong CH, Preston T, Fearon KC. Pathophysiology of cancer cachexia: much more than host-tumour interaction? Clin Nutr. 2007;26:667–76 [DOI] [PubMed] [Google Scholar]
- 17.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410 [DOI] [PubMed] [Google Scholar]
- 18.Tisdale MJ. Cancer cachexia. Curr Opin Gastroenterol. 2010;26:146–51 [DOI] [PubMed] [Google Scholar]
- 19.Elia M. Hunger disease. Clin Nutr. 2000;19:379–86 [DOI] [PubMed] [Google Scholar]
- 20.Lelbach A, Muzes G, Feher J. Current perspectives of catabolic mediators of cancer cachexia. Med Sci Monit. 2007;13:RA168–73 [PubMed] [Google Scholar]
- 21.Korbonits M, Blaine D, Elia M, Powell-Tuck J. Metabolic and hormonal changes during the refeeding period of prolonged fasting. Eur J Endocrinol. 2007;157:157–66 [DOI] [PubMed] [Google Scholar]
- 22.Plank LD, Hill GL. Energy balance in critical illness. Proc Nutr Soc. 2003;62:545–52 [DOI] [PubMed] [Google Scholar]
- 23.Moreno S, Miralles C, Negredo E, Domingo P, Estrada V, Gutiérrez F, Lozano F, Martínez E. Disorders of body fat distribution in HIV-1-infected patients. AIDS Rev. 2009;11:126–34 [PubMed] [Google Scholar]
- 24.Argilés JM, Busquets S, García-Martínez C, López-Soriano FJ. Mediators involved in the cancer anorexia-cachexia syndrome: past, present, and future. Nutrition. 2005;21:977–85 [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum M, Leibel RL, Hirsch J. Obesity. N Engl J Med. 1997;337:396–407 [DOI] [PubMed] [Google Scholar]
- 26.Banks WA. Anorectic effects of circulating cytokines: role of the vascular blood-brain barrier. Nutrition. 2001;17:434–7 [DOI] [PubMed] [Google Scholar]
- 27.Sato T, Laviano A, Meguid MM, Rossi-Fanelli F. Plasma leptin, insulin and free tryptophan contribute to cytokine-induced anorexia. Adv Exp Med Biol. 2003;527:233–9 [DOI] [PubMed] [Google Scholar]
- 28.Ramos EJ, Suzuki S, Marks D, Inui A, Asakawa A, Meguid MM. Cancer anorexia-cachexia syndrome: cytokines and neuropeptides. Curr Opin Clin Nutr Metab Care. 2004;7:427–34 [DOI] [PubMed] [Google Scholar]
- 29.Bechard LJ, Adiv OE, Jaksic T, Duggan C. Nutritional supportive care. : Pizzo PA, Poplack DG, Principles and practice of pediatric oncology. 5th ed Philadelphia: Williams & Wilkins; 2006. p. 1330–47 [Google Scholar]
- 30.Ward E, Hopkins M, Arbuckle L, Williams N, Forsythe L, Bujkiewicz S, Pizer B, Estlin E, Picton S. Nutritional problems in children treated for medulloblastoma: implications for enteral nutrition support. Pediatr Blood Cancer. 2009;53:570–5 [DOI] [PubMed] [Google Scholar]
- 31.Ovesen L, Allingstrup L, Hannibal J, Mortensen EL, Hansen OP. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: a prospective, randomized study. J Clin Oncol. 1993;11:2043–9 [DOI] [PubMed] [Google Scholar]
- 32.Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo ME. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck. 2005;27:659–68 [DOI] [PubMed] [Google Scholar]
- 33.Morrison SD. Control of food intake in cancer cachexia: a challenge and a tool. Physiol Behav. 1976;17:705–14 [DOI] [PubMed] [Google Scholar]
- 34.Costa G. Cachexia, the metabolic component of neoplastic diseases. Cancer Res. 1977;37:2327–35 [PubMed] [Google Scholar]
- 35.Pietilä S, Mäkipernaa A, Sievänen H, Koivisto AM, Wigren T, Lenko HL. Obesity and metabolic changes are common in young childhood brain tumor survivors. Pediatr Blood Cancer. 2009;52:853–9 [DOI] [PubMed] [Google Scholar]
- 36.Torosian MH. Stimulation of tumor growth by nutrition support. JPEN J Parenter Enteral Nutr. 1992;16 Suppl 6:S72–5 [DOI] [PubMed] [Google Scholar]
- 37.Daly JM, Copeland EM, Dudrick SJ. Effects of intravenous nutrition on tumor growth and host immunocompetence in malnourished animals. Surgery. 1978;84:655–8 [PubMed] [Google Scholar]
- 38.Edén E, Lindmark L, Karlberg I, Koivisto AM, Wigren T, Lenko HL. Role of whole-body lipids and nitrogen as limiting factors for survival in tumor-bearing mice with anorexia and cachexia. Cancer Res. 1983;43:3707–11 [PubMed] [Google Scholar]
- 39.Kokal WA, Chan W, Banks WL, Jr, Lawrence W., Jr The efficacy of total parenteral nutrition in malnourished tumor-bearing rats. Cancer. 1985;55:1271–5 [DOI] [PubMed] [Google Scholar]
- 40.Sauer LA, Nagel WO, Dauchy RT, Miceli LA, Austin JE. Stimulation of tumor growth in adult rats in vivo during an acute fast. Cancer Res. 1986;46:3469–75 [PubMed] [Google Scholar]
- 41.Bozzetti F, Arends J, Lundholm K, Micklewright A, Zurcher G, Muscaritoli M. ESPEN. ESPEN guidelines on parenteral nutrition: non-surgical oncology. Clin Nutr. 2009;28:445–54 [DOI] [PubMed] [Google Scholar]
- 42.Buzby GP, Mullen JL, Stein TP, Miller EE, Hobbs CL, Rosato EF. Host-tumor interaction and nutrient supply. Cancer. 1980;45:2940–8 [DOI] [PubMed] [Google Scholar]
- 43.Nitenberg G, Raynard B. Nutritional support of the cancer patient: issues and dilemmas. Crit Rev Oncol Hematol. 2000;34:137–68 [DOI] [PubMed] [Google Scholar]
- 44.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–71 [DOI] [PubMed] [Google Scholar]
- 45.Roh MS, Ekman L, Jeevanandum M, Brennan MF. Gluconeogenesis in tumor-influenced hepatocytes. Surgery. 1984;96:427–34 [PubMed] [Google Scholar]
- 46.Love AH. Metabolic response to malnutrition: its relevance to enteral feeding. Gut. 1986;27 Suppl 1:9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danforth E, Jr, Burger AG. The impact of nutrition on thyroid hormone physiology and action. Annu Rev Nutr. 1989;9:201–27 [DOI] [PubMed] [Google Scholar]
- 48.Heber D, Byerley LO, Tchekmedyian NS. Hormonal and metabolic abnormalities in the malnourished cancer patient: effects on host-tumor interaction. JPEN J Parenter Enteral Nutr. 1992;16 Suppl 6:S60–4 [DOI] [PubMed] [Google Scholar]
- 49.Shalet SM, Clayton PE, Price DA. Growth and pituitary function in children treated for brain tumours or acute lymphoblastic leukaemia. Horm Res. 1988;30:53–61 [DOI] [PubMed] [Google Scholar]
- 50.Bond SA, Han AM, Wootton SA, Kohler JA. Energy intake and basal metabolic rate during maintenance chemotherapy. Arch Dis Child. 1992;67:229–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green GJ, Weitzman SS, Pencharz PB. Resting energy expenditure in children newly diagnosed with stage IV neuroblastoma. Pediatr Res. 2008;63:332–6 [DOI] [PubMed] [Google Scholar]
- 52.Knox LS, Crosby LO, Feurer ID, Buzby GP, Miller CL, Mullen JL. Energy expenditure in malnourished cancer patients. Ann Surg. 1983;197:152–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shellock FG, Riedinger MS, Fishbein MC. Brown adipose tissue in cancer patients: possible cause of cancer-induced cachexia. J Cancer Res Clin Oncol. 1986;111:82–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology-drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–67 [DOI] [PubMed] [Google Scholar]
- 55.Bartelink IH, Rademaker CM, Schobben AF, van den Anker JN. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet. 2006;45:1077–97 [DOI] [PubMed] [Google Scholar]
- 56.Warner JT. Reliability of indices of weight and height in assessment of nutritional state in children. Lancet. 2000;356:1703–4 [DOI] [PubMed] [Google Scholar]
- 57.McMillan DC, Sattar N, Talwar D, Schobben AF, van den Anker JN. Changes in micronutrient concentrations following anti-inflammatory treatment in patients with gastrointestinal cancer. Nutrition. 2000;16:425–8 [DOI] [PubMed] [Google Scholar]
- 58.Evans WJ, Lambert CP. Physiological basis of fatigue. Am J Phys Med Rehabil. 2007;86 Suppl 1:29–46 [DOI] [PubMed] [Google Scholar]
- 59.Servaes P, Verhagen S, Schreuder HW, Veth RP, Bleijenberg G. Fatigue after treatment for malignant and benign bone and soft tissue tumors. J Pain Symptom Manage. 2003;26:1113–22 [DOI] [PubMed] [Google Scholar]
- 60.Santos CA, Boullata JI. An approach to evaluating drug-nutrient interactions. Pharmacotherapy. 2005;25:1789–800 [DOI] [PubMed] [Google Scholar]
- 61.Canal P, Chatelut E, Guichard S. Practical treatment guide for dose individualisation in cancer chemotherapy. Drugs. 1998;56:1019–38 [DOI] [PubMed] [Google Scholar]
- 62.Colleoni M, Li S, Gelber RD, Price KN, Moore TB, Bomgaars LR, Ellis KJ, Renbarger J, Berg SL. Relation between chemotherapy dose, oestrogen receptor expression, and body-mass index. Lancet. 2005;366:1108–10 [DOI] [PubMed] [Google Scholar]
- 63.Rosner GL, Hargis JB, Hollis DR, Budman DR, Weiss RB, Henderson IC, Schilsky RL. Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: results from cancer and leukemia group B study 8541. J Clin Oncol. 1996;14:3000–8 [DOI] [PubMed] [Google Scholar]
- 64.Meacham LR, Gurney JG, Mertens AC, Ness KK, Sklar CA, Robison LL, Oeffinger KC. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer. 2005;103:1730–9 [DOI] [PubMed] [Google Scholar]
- 65.Lind MJ, Margison JM, Cerny T, Thatcher N, Wilkinson PM. Prolongation of ifosfamide elimination half-life in obese patients due to altered drug distribution. Cancer Chemother Pharmacol. 1989;25:139–42 [DOI] [PubMed] [Google Scholar]
- 66.Rodvold KA, Rushing DA, Tewksbury DA. Doxorubicin clearance in the obese. J Clin Oncol. 1988;6:1321–7 [DOI] [PubMed] [Google Scholar]
- 67.Thompson PA, Rosner GL, Matthay KK, Thatcher N, Wilkinson PM. Impact of body composition on pharmacokinetics of doxorubicin in children: a Glaser Pediatric Research Network study. Cancer Chemother Pharmacol. 2009;64:243–51 [DOI] [PubMed] [Google Scholar]
- 68.Reilly JJ, Brougham M, Montgomery C, Richardson F, Kelly A, Gibson BE. Effect of glucocorticoid therapy on energy intake in children treated for acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2001;86:3742–5 [DOI] [PubMed] [Google Scholar]
- 69.Lange BJ, Gerbing RB, Feusner J, Skolnik J, Sacks N, Smith FO, Alonzo TA. Mortality in overweight and underweight children with acute myeloid leukemia. JAMA. 2005;293:203–11 [DOI] [PubMed] [Google Scholar]
- 70.Barker DJ, Osmond C, Kajantie E, Eriksson JG. Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann Hum Biol. 2009;36:445–58 [DOI] [PubMed] [Google Scholar]
- 71.Mauer AM, Burgess JB, Donaldson SS, Rickard KA, Stallings VA, van Eys J, Winick M. Special nutritional needs of children with malignancies: a review. JPEN J Parenter Enteral Nutr. 1990;14:315–24 [DOI] [PubMed] [Google Scholar]
- 72.Coates TD, Rickard KA, Grosfeld JL, Weetman RM. Nutritional support of children with neoplastic diseases. Surg Clin North Am. 1986;66:1197–212 [DOI] [PubMed] [Google Scholar]
- 73.Barron MA, Pencharz PB. Nutritional issues in infants with cancer. Pediatr Blood Cancer. 2007;49 Suppl 7:1093–6 [DOI] [PubMed] [Google Scholar]
- 74.Donaldson SS. Effect of nutritional status on response to therapy. Cancer Res. 1982;42 Suppl 2:S754–5 [PubMed] [Google Scholar]
- 75.Costa G, Donaldson SS. Current concepts in cancer: effects of cancer and cancer treatment on the nutrition of the host. N Engl J Med. 1979;300:1471–4 [DOI] [PubMed] [Google Scholar]
- 76.Schein PS, Macdonald JS, Waters C, Haidak D. Nutritional complications of cancer and its treatment. Semin Oncol. 1975;2:337–47 [PubMed] [Google Scholar]
- 77.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–7 [DOI] [PubMed] [Google Scholar]
- 78.Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, Smith IE, O'Brien ME. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004;90:1905–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schnadig ID, Fromme EK, Loprinzi CL, Sloan JA, Mori M, Li H, Beer TM. Patient-physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer. 2008;113:2205–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Fiore F, Lecleire S, Rigal O, Galais MP, Ben Soussan E, David I, Paillot B, Jacob JH, Michel P. Predictive factors of survival in patients treated with definitive chemoradiotherapy for squamous cell esophageal carcinoma. World J Gastroenterol. 2006;12:4185–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goiburu ME, Goiburu MM, Bianco H, Díaz JR, Alderete F, Palacios MC, Cabral V, Escobar D, López R, et al. The impact of malnutrition on morbidity, mortality and length of hospital stay in trauma patients. Nutr Hosp. 2006;21:604–10 [PubMed] [Google Scholar]
- 82.Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22:235–9 [DOI] [PubMed] [Google Scholar]
- 83.Marín Caro MM, Laviano A, Pichard C. Nutritional intervention and quality of life in adult oncology patients. Clin Nutr. 2007;26:289–301 [DOI] [PubMed] [Google Scholar]
- 84.Petruson KM, Silander EM, Hammerlid EB. Quality of life as predictor of weight loss in patients with head and neck cancer. Head Neck. 2005;27:302–10 [DOI] [PubMed] [Google Scholar]
- 85.Donaldson SS, Wesley MN, DeWys WD, Suskind RM, Jaffe N, vanEys J. A study of the nutritional status of pediatric cancer patients. Am J Dis Child. 1981;135:1107–12 [DOI] [PubMed] [Google Scholar]
- 86.Rickard KA, Detamore CM, Coates TD, Grosfeld JL, Weetman RM, White NM, Provisor AJ, Boxer LA, Loghmani ES, et al. Effect of nutrition staging on treatment delays and outcome in Stage IV neuroblastoma. Cancer. 1983;52:587–98 [DOI] [PubMed] [Google Scholar]
- 87.Viana MB, Murao M, Ramos G, Oliveira HM, de Carvalho RI, de Bastos M, Colosimo EA, Silvestrini WS. Malnutrition as a prognostic factor in lymphoblastic leukaemia: a multivariate analysis. Arch Dis Child. 1994;71:304–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lobato Mendiz#x00E1bal E, Ruiz-Arg#x00FCelles GJ. Leukemia and malnutrition. III. Effect of chemotherapeutic treatment on the nutritional state and its repercussion on the therapeutic response of patients with acute lymphoblastic leukemia with standard risk. Sangre. 1990;35:189–195 [PubMed] [Google Scholar]
- 89.Lobato-Mendizábal E, López-Martínez B, Ruiz-Argüelles GJ. A critical review of the prognostic value of the nutritional status at diagnosis in the outcome of therapy of children with acute lymphoblastic leukemia. Rev Invest Clin. 2003;55:31–5 [PubMed] [Google Scholar]
- 90.Marín-López A, Lobato-Mendizabal E, Ruiz-Argüelles GJ. Malnutrition is an adverse prognostic factor in the response to treatment and survival of patients with acute lymphoblastic leukemia at the usual risk. Gac Med Mex. 1991;127:125–31 [PubMed] [Google Scholar]
- 91.Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M, Smith FO, Reaman GH. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pieper S, Ranft A, Braun-Munzinger G, Jurgens H, Paulussen M, Dirksen U. Ewing's tumors over the age of 40: a retrospective analysis of 47 patients treated according to the International Clinical Trials EICESS 92 and EURO-E.W.I.N.G. 99. Onkologie. 2008;31:657–63 [DOI] [PubMed] [Google Scholar]
- 93.Gurney JG, Ness KK, Sibley SD, O'Leary M, Dengel DR, Lee JM, Youngren NM, Glasser SP, Baker KS. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–12 [DOI] [PubMed] [Google Scholar]
- 94.Murphy AJ, White M, Davies PS. Body composition of children with cancer. Am J Clin Nutr. 2010;92:55–60 [DOI] [PubMed] [Google Scholar]
- 95.Diller L, Chow EJ, Gurney JG, Hudson MM, Kadin-Lottick NS, Kawashima TI, Leisenring WM, Meacham LR, Mertens AC, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27:2339–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meacham LR, Sklar CA, Li S, Liu Q, Gimpel N, Yasui Y, Whitton JA, Stovall M, Robison LL, et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Arch Intern Med. 2009;169:1381–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nysom K, Holm K, Michaelsen KF, Hertz H, Müller J, Mølgaard C. Degree of fatness after treatment of malignant lymphoma in childhood. Med Pediatr Oncol. 2003;40:239–423 [DOI] [PubMed] [Google Scholar]
- 98.Müller HL, Emser A, Faldum A, Bruhnken G, Etavard-Gorris N, Gebhardt U, Oeverink R, Kolb R, Sörensen N. Longitudinal study on growth and body mass index before and after diagnosis of childhood craniopharyngioma. J Clin Endocrinol Metab. 2004;89:3298–305 [DOI] [PubMed] [Google Scholar]
- 99.Oeffinger KC, Mertens AC, Sklar CA, Yasui Y, Fears T, Stovall M, Vik TA, Inskip PD, Robison LL, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:1359–65 [DOI] [PubMed] [Google Scholar]
- 100.Marques MD, Santos RD, Parga JR, Rocha-Filho JA, Quaglia LA, Miname MH, Avila LF. Relation between visceral fat and coronary artery disease evaluated by multidetector computed tomography. Atherosclerosis. 2010;209:481–6 [DOI] [PubMed] [Google Scholar]
- 101.Demerath EW, Reed D, Rogers N, Sun SS, Lee M, Choh AC, Couch W, Czerwinski SA, Chumlea WC, et al. Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levels. Am J Clin Nutr. 2008;88:1263–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamaji T, Iwasaki M, Sasazuki S, Kurahashi N, Mutoh M, Yamamoto S, Suzuki M, Moriyama N, Wakabayashi K, et al. Visceral fat volume and the prevalence of colorectal adenoma. Am J Epidemiol. 2009;170:1502–11 [DOI] [PubMed] [Google Scholar]
- 103.Kang HW, Kim D, Kim HJ, Kim CH, Kim YS, Park MJ, Kim JS, Cho SH, Sung MW, et al. Visceral obesity and insulin resistance as risk factors for colorectal adenoma: a cross-sectional, case-control study. Am J Gastroenterol. 2010;105:178–87 [DOI] [PubMed] [Google Scholar]
- 104.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38 [DOI] [PubMed] [Google Scholar]
- 105.Suárez-Santamaría M, Santolaria F, Pérez-Ramírez A, Alemán-Valls MR, Martínez-Riera A, González-Reimers E, de la Vega MJ, Milena A. Prognostic value of inflammatory markers (notably cytokines and procalcitonin), nutritional assessment, and organ function in patients with sepsis. Eur Cytokine Netw. 2010;21:19–26 [DOI] [PubMed] [Google Scholar]
- 106.Armstrong GT, Stovall M, Robison LL. Long-Term effects of radiation exposure among adult survivors of childhood cancer: results from the childhood cancer survivor study. Radiat Res. 2010;174:840–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nagarajan R, Kamruzzaman A, Ness KK, Marchese VG, Sklar C, Mertens A, Yasui Y, Robison LL, Marina N. Twenty years of follow-up of survivors of childhood osteosarcoma: a report from the childhood cancer survivor study. Cancer. Epub. 2010. Oct 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilson CL, Cohn RJ, Johnston KA, Ashton LJ. Late mortality and second cancers in an Australian cohort of childhood cancer survivors. Med J Aust. 2010;193:258–61 [DOI] [PubMed] [Google Scholar]
- 109.Glasser DB, Duane K, Lane JM, Healey JH, Caparros-Sison B. The effect of chemotherapy on growth in the skeletally immature individual. Clin Orthop Relat Res. 1991;262:93–100 [PubMed] [Google Scholar]
- 110.Brennan BM. Sensitive measures of the nutritional status of children with cancer in hospital and in the field. Int J Cancer Suppl. 1998;11:10–3 [PubMed] [Google Scholar]
- 111.Russell ST, Siren PM, Siren MJ, Tisdale MJ. The role of zinc in the anti-tumour and anti-cachectic activity of D-myo-inositol 1,2,6-triphosphate. Br J Cancer. 2010;102:833–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reilly JJ, Wilson J, Durnin JV. Determination of body composition from skinfold thickness: a validation study. Arch Dis Child. 1995;73:305–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwegler I, von Holzen A, Gutzwiller JP, Schlumpf R, Mühlebach S, Stanga Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg. 2010;97:92–7 [DOI] [PubMed] [Google Scholar]
- 114.Sermet-Gaudelus I, Poisson-Salomon AS, Colomb V, Brusset MC, Mosser F, Berrier F, Ricour C. Simple pediatric nutritional risk score to identify children at risk of malnutrition. Am J Clin Nutr. 2000;72:64–70 [DOI] [PubMed] [Google Scholar]
- 115.Rogers PC, Melnick SJ, Ladas EJ, Halton J, Baillargeon J, Sacks N. Children's Oncology Group (COG) Nutrition Committee. Pediatr Blood Cancer. 2008;50 Suppl 2:447–50 [DOI] [PubMed] [Google Scholar]
- 116.Mehta NM, Compher C. A.S.P.E.N. Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support of the critically ill child. JPEN J Parenter Enteral Nutr. 2009;33:260–76 [DOI] [PubMed] [Google Scholar]
- 117.Fusch C, Bauer K, Böhles HJ, Jochum F, Koletzko B, Krawinkel M, Krohn K, Mühlebach S. Working group for developing the guidelines for parenteral nutrition of The German Society for Nutritional Medicine. Neonatology/paediatrics: guidelines on parenteral nutrition. Ger Med Sci. 2009;18;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duggan C. Nutritional assessment in sick or hospitalized children. : Hendricks L, Duggan C, Manual pediatric nutrition. 4th ed Hamilton: BC Decker; 2005. p. 239–51 [Google Scholar]
- 119.Arends J, Zuercher G, Dossett A, Fietkau R, Hug M, Schmid I, Shang E, Zander A. Working group for developing the guidelines for parenteral nutrition of The German Association for Nutritional Medicine. Non-surgical oncology: guidelines on parenteral nutrition. Ger Med Sci. 2009;7:1–14 [Google Scholar]
- 120.Uderzo C, Rovelli A, Bonomi M, Fomia L, Fomia L, Pirovano L, Masera G. Total parenteral nutrition and nutritional assessment and leukaemic children undergoing bone marrow transplantation. Eur J Cancer. 1991;27:758–62 [DOI] [PubMed] [Google Scholar]
- 121.Forchielli ML, Azzi N, Cadranel S, Paolucci G. Total parenteral nutrition in bone marrow transplant: what is the appropriate energy level? Oncology. 2003;64:7–13 [DOI] [PubMed] [Google Scholar]
- 122.Weisdorf S, Hofland C, Sharp HL, Teasley K, Schissel K, McGlave PB, Ramsay N, Kersey J. Total parenteral nutrition in bone marrow transplantation: a clinical evaluation. J Pediatr Gastroenterol Nutr. 1984;3:95–100 [DOI] [PubMed] [Google Scholar]
- 123.Murray SM, Pindoria S. Nutrition support for bone marrow transplant patients. Cochrane Database Syst Rev. 2009;21:CD002920. [DOI] [PubMed] [Google Scholar]
- 124.Kuhn KS, Muscaritoli M, Wischmeyer P, Stehle P. Glutamine as indispensable nutrient in oncology: experimental and clinical evidence. Eur J Nutr. 2010;49:197–210 [DOI] [PubMed] [Google Scholar]
- 125.Hockenberry MJ, Hooke MC, Gregurich M, McCarthy K. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J Pediatr Hematol Oncol. 2009;31:664–9 [DOI] [PubMed] [Google Scholar]
- 126.Huhmann MB, August DA. Review of American Society for Parenteral and Enteral Nutrition (ASPEN) clinical guidelines for nutrition support in cancer patients: nutrition screening and assessment. Nutr Clin Pract. 2008;23:182–8 [DOI] [PubMed] [Google Scholar]
- 127.August DA, Huhmann MB. American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. 2009;33:472–500 [DOI] [PubMed] [Google Scholar]
- 128.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:S1123–7 [DOI] [PubMed] [Google Scholar]
- 129.Gullett NP, Hebbar G, Ziegler TR. Update on clinical trials of growth factors and anabolic steroids in cachexia and wasting. Am J Clin Nutr. 2010;91:S1143–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jones L, Watling RM, Wilkins S, Pizer B. Nutritional support in children and young people with cancer undergoing chemotherapy. Cochrane Database Syst Rev. 2010;7:CD003298. [DOI] [PubMed] [Google Scholar]
- 131.Avitsland TL, Kristensen C, Emblem R, Veenstra M, Mala T, Bjørnland K. Percutaneous endoscopic gastrostomy in children: a safe technique with major symptom relief and high parental satisfaction. J Pediatr Gastroenterol Nutr. 2006;43:624–8 [DOI] [PubMed] [Google Scholar]
- 132.Skolin I, Hernell O, Larsson MV, Wahlgren C, Wahlin YB. Percutaneous endoscopic gastrostomy in children with malignant disease. J Pediatr Oncol Nurs. 2002;19:154–63 [DOI] [PubMed] [Google Scholar]
- 133.Pedersen AM, Kok K, Petersen G, Nielsen OH, Michaelsen KF, Schmiegelow K. Percutaneous endoscopic gastrostomy in children with cancer. Acta Paediatr. 1999;88:849–52 [DOI] [PubMed] [Google Scholar]