Abstract
Zinc (Zn) is an essential micronutrient required for over 300 different cellular processes, including DNA and protein synthesis, enzyme activity, and intracellular signaling. Cellular Zn homeostasis necessitates the compartmentalization of Zn into intracellular organelles, which is tightly regulated through the integration of Zn transporting mechanisms. The pancreas, prostate, and mammary gland are secretory tissues that have unusual Zn requirements and thus must tightly regulate Zn metabolism through integrating Zn import, sequestration, and export mechanisms. Recent findings indicate that these tissues utilize Zn for basic cellular processes but also require Zn for unique cellular needs. In addition, abundant Zn is transported into the secretory pathway and a large amount is subsequently secreted in a tightly regulated manner for unique biological processes. Expression of numerous members of the SLC30A (ZnT) and SLC39A (Zip) gene families has been documented in these tissues, yet there is limited understanding of their precise functional role in Zn metabolism or their regulation. Impairments in Zn secretion from the pancreas, prostate, and mammary gland are associated with disorders such as diabetes, infertility, and cancer, respectively. In this review, we will provide a brief summary of the specific role of Zn in each tissue and describe our current knowledge regarding how Zn metabolism is regulated. Finally, in each instance, we will reflect upon how this information shapes our current understanding of the role of Zn in these secretory tissues with respect to human health and disease.
Introduction
Zinc (Zn) is the second most abundant trace element in the human body. It is required for over 300 different cellular processes, including DNA and protein synthesis, enzyme activity, and intracellular signaling. The complexity of Zn homeostasis necessitates the compartmentalization of Zn into intracellular organelles, which is tightly regulated through the integration of Zn transporting mechanisms. Twenty-four Zn transporting proteins have been identified and studies elucidating their role in Zn homeostasis are ongoing (1, 2). Members of the SLC30A gene family (ZnT1–10) transport Zn from the cytoplasm and, with the exception of ZnT5 (3), are predicted to be structurally similar; 6 transmembrane domains and a histidine rich domain are presumed to play a key role in Zn binding (2). In contrast, members of the SLC39A gene family (Zip1–14) transport Zn into the cytoplasm (1, 4). The pancreas, prostate, and mammary gland are secretory tissues that have unique Zn requirements. These tissues accumulate abundant Zn into secretory vesicles and tightly regulate Zn secretion to provide Zn for critical biological processes. Importantly, dysregulated Zn metabolism in these tissues is implicated in disorders such as diabetes, cancer, and infertility. In this review, we will provide a brief summary of the specific role of Zn in each tissue, followed by a description of our current knowledge regarding how Zn metabolism is regulated. Finally, in each instance, we will reflect upon how this information shapes our current understanding of the role of Zn in these secretory tissues with respect to human health and disease.
Current status of knowledge
Zinc and the pancreas
The pancreas is both an endocrine and exocrine organ, contributing to the homeostasis of several aspects of digestion, including pancreatic enzyme secretion and the hormonal control of blood glucose concentration. Zn is involved in a multitude of these processes within the pancreas, including glucagon secretion, digestive enzyme activity, and insulin packaging, secretion, and signaling (Fig. 1). As a result of this extensive physiological contribution, dysregulation of Zn metabolism within the pancreas impairs a multitude of key processes, including glycemic control (5–9), and is associated with pancreatic cancer (10–12) and chronic pancreatitis (13–16).
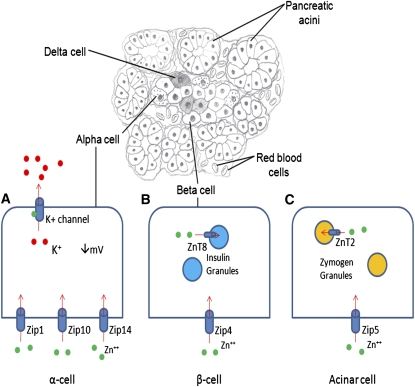
Figure 1.
Zn transport in various pancreatic cell types. (A) Localization of Zip1, Zip10, and Zip14 to pancreatic α-cells suggests that these transporters are responsible for importing Zn into the cell. Zn binds to and opens ATP dependent K(+) channels, allowing the efflux of Zn from the α-cell and inactivation of voltage dependent calcium channels, resulting in decreased glucagon secretion. (B) Zn is transported into pancreatic β-cell cells via Zip4. ZnT8 is responsible for the transport of Zn into insulin granules. Autoantibodies to ZnT8 and polymorphisms of ZnT8 are associated with the onset of DM. (C) Zip5 is responsible for the transport of Zn into pancreatic acinar cells. Zn is transported into zymogen granules by ZnT2 where it binds to and activates digestive enzymes that are subsequently secreted.
Endocrine functions
Zn modulates several aspects of pancreatic function, including glucagon secretion (17–19). Zip1, Zip10, and Zip14 are expressed in pancreatic α-cells and localization to the cell membrane suggests that one or all of these transporters are responsible for Zn import into the cell (20). In addition to regulating pancreatic cell viability (21), Zn is an intercellular signaling ion, providing an “off-switch” for glucagon release from the pancreatic α-cell during glucose deprivation (19). In this regard, pancreatic β-cells release Zn- containing insulin hexamers into intraislet periportal circulation in response to glucose. Zn dissociates from insulin and acts upon α-cells by binding to and opening ATP dependent K+ channels, which hyperpolarizes the cell and inhibits voltage dependent calcium channels. As a result, glucagon release from storage within the α-cell is inhibited (18), implicating Zn as a key regulatory component of glucagon secretion. In addition, activity of several gluconeogenic enzymes, including phosphoenolpyruvate carboxykinase and glucose 6-phosphatase, is Zn dependent (17), further implicating Zn as a key regulator of glucose metabolism.
In addition to modulating glucagon secretion, Zn plays a role in systemic glycemic control (22–25). The localization of Zip4 to the cell membrane of pancreatic β-cells suggests that Zip4 participates in Zn import into the cell (26). Insulin biosynthesis occurs within the pancreatic β-cell where proinsulin is transported to the Golgi apparatus and is subsequently incorporated into Zn rich secretory granules (27). Two Zn ions associate with 2 insulin dimers, which subsequently combine with an additional insulin dimer yielding a Zn-containing hexameric unit (28). Thus, Zn is a necessary structural component of insulin biosynthesis and is necessary for both structural and biosynthetic roles within the pancreatic β-cell. The precise contribution of Zn released into systemic circulation in the maintenance of blood glucose levels remains to be elucidated; however, Zn may function to modulate insulinemic effects on target tissues. For example, Zn modulates phosphoinositide 3′-kinase/Akt signaling pathways initiated in response to insulin in target tissues (25), illustrating a role for Zn in insulin signaling in peripheral tissues. Additionally, insulin-responsive aminopeptidase (IRAP) is a Zn dependent enzyme whose precise function is currently unknown. IRAP and glucose transporter 4 are colocalized and translocate to the cell membrane in response to insulin (29). Similar to glucose transporter 4 (23, 24), IRAP translocation to the surface of muscle cells and adipocytes is reduced in individuals with type 2 diabetes mellitus (DM)3 (22), which may provide another link between Zn metabolism and insulin signaling. However, further research is needed to understand the extent to which insulinemic signaling is Zn dependent.
Exocrine functions
Under normal dietary conditions, ∼1–2 mg/d Zn enters the digestive tract via zymogen granules secreted from pancreatic acinar cells (30). These zymogen granules contain enzymes necessary for digestion and whose activity is Zn dependent (31). Zip5 is localized to the basolateral membrane of acinar cells and appears to play a role in Zn import into the cell (26). Recently, ZnT2 (SLC30A2) has been implicated in Zn transport into zymogen granules for metallation of digestive proenzymes (32). Zn deficiency decreases ZnT2 expression and Zn concentration in zymogen granules; therefore, impairments in Zn status may modulate digestive enzyme activity and thus impair nutrient absorption. This may be of particular importance during periods of increased Zn requirements such as during pregnancy, lactation, and growth. Further studies will enhance our understanding of the impact of Zn deficiency on digestive enzyme activity and absorption.
Glycemic control
DM is the inability to regulate blood glucose levels as a result of decreased insulin secretion or action. The prevalence of DM is growing at an alarming rate (33) and has increased efforts to elucidate the causal mechanisms behind the pathogenesis. It has been determined that Zn dyshomeostasis, both systemically and in the pancreas, plays an intricate role in the pathology of both type 1 and type 2 DM (5–9, 34). Suboptimal Zn status decreases insulin secretion from the pancreas (5) and suboptimal Zn status during pregnancy and lactation decreased insulin sensitivity and increased weight gain in rat offspring (6). In addition, severe Zn deficiency induces hyperglycemia and hyperinsulinemia (7), directly implicating Zn in systemic glucose regulation. Consistent with a critical role for Zn in this process, individuals with type 1 DM often have low serum Zn concentrations (8). Whether this reflects decreased Zn absorption or tissue accumulation is currently not known. In contrast, type 1 DM is associated with an elevated serum Zn concentration (9) and elevated Zn levels in the liver and kidney (35). Further studies are clearly needed to elucidate the relationship between Zn distribution and DM.
Consistent with a role for Zn in glycemic dysregulation, Zn supplementation ameliorates some physiological symptoms of DM (34, 36). Oral administration of Zn(II)-thioallixin-N-methyl in a mouse model of type 2 DM lowered blood glucose levels (36), suggesting that increased Zn absorption helps to maintain blood glucose concentration. In addition, mice given Zn-enriched drinking water for 1 wk prior to streptozotocin administration were protected from streptozotocin induced diabetes, suggesting that adequate (or perhaps enhanced) Zn intake reduces susceptibility to DM. The mechanism of decreased susceptibility may be due to elevated metallothionein (MT) levels in the pancreas (34), because MT scavenges hydroxyl radicals and may potentially prevent the destruction of β-cells. These studies suggest a potential use for Zn therapy in the control of DM.
In addition to Zn itself playing a role in the pathogenesis of DM, defects in the Zn transporter ZnT8 (SLC30A8) may contribute to the onset of DM. Recently, ZnT8 has been implicated in the pathogenesis of both type 1 and type 2 DM (37, 38). ZnT8 is localized to insulin containing secretory vesicles within pancreatic β-cells (27, 39) and is necessary for insulin crystallization (37). Insulin granules in β-cells of ZnT8-knockout mice are immature with pale insulin “pro-granules” compared with those found in wild-type mice. In addition, ZnT8 knockout mice fed a high-fat diet are glucose intolerant (37), suggesting that ZnT8 is particularly necessary to maintain glycemic control under physiologically stressful conditions such as obesity. Aberrant ZnT8 function resulting from genetic variation in SLC30A8 is associated with increased risk of type 2 DM (40). Additionally, ZnT8 autoantibodies are present in 60–80% of new cases of type 1 DM (37). Individuals followed from birth until DM onset produced autoantibodies, the production of which increased through the time of DM onset, as early as age 2 y, (38). The combination of ZnT8 antibodies along with the detection of preexisting markers, such as glutamate decarboxylase, protein tyrosine phosphatase IA2, antibodies against insulin, and islet cytoplasmic autoantibodies has raised the detection rate of an autoimmune response related to type 1 DM to 98% at disease onset (38). Overall, ZnT8 may contribute to the pathogenesis of DM due to autoantigenic properties as well as decreased protein function, which may be exacerbated by polymorphic variance. Together, this indicates that ZnT8 dysregulation specifically may lead to impaired glycemic control from several physiological angles. Further studies are needed to understand the functional relevance and underlying trigger(s) of an autoimmune response to ZnT8.
Pancreatic disease
Pancreatic cancer is a very aggressive, invasive cancer whose prognosis remains very poor with only 5% of patients living >5 y beyond the initial diagnosis (41). There is a growing body of information implicating Zn dysregulation in the pathogenesis of pancreatic cancer (10, 11) and the use of Zn therapy and Zn transporters as potential therapeutic targets (12, 42, 43). A study by Jayaraman et al. (10) demonstrated that exposure of pancreatic cancer cells to Zn leads to increased protein ubiquitination and enhanced cell death, implicating Zn as a potential therapy in the treatment of pancreatic cancer. In addition, increased expression of the Zn importer Zip4 (SLC39A4) is strongly associated with the pathology of pancreatic cancer by facilitating increased intracellular Zn accumulation (11, 12). Li et al. (11) elegantly observed overexpression of Zip4 protein in 94% of clinical pancreatic adenocarcinoma specimens compared with surrounding normal tissue and that malignant cells had significantly higher Zip4 expression compared with normal pancreatic ductal epithelial cells. In addition, forced expression of Zip4 increased cell proliferation in vitro and significantly increased pancreatic tumor volume by ~13-fold in vivo using a nude mouse model with subcutaneous xenograft. Consistent with a role for Zip4 in pancreatic cancer progression, Zip4 attenuation in a subcutaneous xenograft model of pancreatic cancer inhibited several aspects of the progression, including cell proliferation, migration, and invasion as well as tumor size and weight and increased the survival rate of nude mice with orthotopic xenografts (12). There are several intermediate signaling pathways modulated by Zip4 that have been implicated in pancreatic cancer progression. Zip4 overexpression significantly increases neuropilin-1 expression, vascular endothelial growth factor, and matrix metalloproteases, whereas Zip4 attenuation reverses these effects (43). Zhang et al. (42) found that Zip4 overexpression causes increased IL-6 transcription through cAMP response element-binding protein. Furthermore, IL-6 activates STAT3, which increases expression of cyclin D1, increasing cell proliferation and tumor progression. Overall, Zn appears to play an intricate role in the pathology of pancreatic cancer through the modulation of several signaling pathways and, along with Zip4, may prove to be a useful therapeutic target.
A role for Zn has also been implicated in chronic pancreatitis. Chronic pancreatitis results from recurring bouts of pancreatic inflammation characterized by severe upper abdominal pain, nausea, and vomiting (44). Decreased serum Zn concentrations are associated with chronic pancreatitis (13–15), which is also associated with decreased Cu/Zn superoxide dismutase and MT levels (14). It is likely that decreased MT levels limit the scavenging of hydroxyl radicals, contributing to oxidative stress. This reflects the fact that Zn is necessary to maintain adequate antioxidant status. Pancreatitis induced in an rat model decreased Zn content in the pancreas, kidney, heart, and lung and increased Zn content in the liver (16). These studies suggest that there is a significant relationship between Zn metabolism and chronic pancreatitis, although it is not clear which is the causal aspect. Taken together, Zn may prove to be a powerful addition to pancreatitis therapy. Further studies are needed to elucidate the specific mechanisms responsible for the association between Zn dyshomeostasis and chronic pancreatitis and explore the role of Zn as a therapeutic agent.
Zn and the prostate
Zn exists in very high concentrations in the healthy prostate, which is important for male fertility. First described in detail by Mawson and Fischer (45) in the 1950s, the prostate contains more Zn than any other soft tissue. In addition, the prostate secretes high Zn levels into seminal fluid, playing an important role in sperm release and motility (46, 47). The prostate is organized into distinct lobes and each lobe varies in Zn content (Fig. 2). The dorsolateral lobe in rodent prostate and the peripheral lobe in human prostate have the greatest Zn concentrations and these 2 lobes are primarily involved in the secretion of prostatic fluid (48). In its entirety, the human prostate contains >3 times more Zn than other soft tissues [∼150 μg Zn/g (1 g Zn = 0.015 mol Zn) prostate compared with ∼20–50 μg Zn/g for other organs]. Similarly, prostatic fluid contains ∼500 μg Zn/mL fluid compared with 1–2 μg Zn/mL of plasma (48). In addition to influencing sperm motility, Zn is attributed with antimicrobial functions in prostatic fluid (49) and within the prostate itself (50). Interestingly, the major function of Zn in the prostate may be to facilitate the secretion of citrate. Unlike most cells in which Zn is sequestered into vesicles and organelles, Zn in cytoplasm of the prostate cell comprises almost 35% of the total intracellular Zn content. This Zn is loosely bound to small molecular weight molecules such as citrate and is considered biologically active (48). The current dogma suggests that this bioactive Zn pool is essential for inhibiting m-aconitase, sparing citrate oxidation in the Krebs cycle, and providing high amounts of citrate for secretion into prostatic fluid [reviewed in (51)]. To maintain high cellular Zn concentration and secretion, Zn homeostasis in the prostate must be tightly regulated and only recently have possible modes of regulation for Zn homeostasis been elucidated.
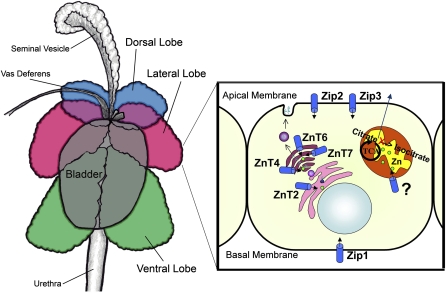
Figure 2.
Zn transporter expression in the secreting epithelium of the rat prostate. Lobes of the rat prostate are illustrated on the left. On the right, a cartoon of an epithelial cell of the lateral lobe illustrates our current understanding of Zn transporter localization and Zn function. Zip2 and Zip3 are localized to the apical membrane and Zip1 is localized to the basal membrane; all function to import Zn into the cell. ZnT4, ZnT6, and ZnT7 are localized to the Golgi apparatus and ZnT2 is localized to the ER; these transporters likely function to maintain the secretory pathway. There is no known transporter for the mitochondria where high Zn levels inhibit the oxidation of citrate, providing a source for secretion.
Normal prostate Zn biology
Such substantial Zn accumulation for optimal function likely requires integration of multiple Zn importing processes. To date, 3 Zip proteins (Zip1, Zip2, and Zip3) have been described in the prostate (52–54). Zip1 is primarily found on the basolateral membrane of epithelial cells of the peripheral zone, (although some Zip1 is detected on the apical membrane) (52) and is thought to be responsible for Zn uptake from circulation. Iguchi et al. (53) documented Zip2 expression in the lateral prostate of the rat, which positively correlates with Zn concentration in this lobe and is positively regulated by testosterone. Zip2 and Zip3 are localized to the apical membrane of prostate epithelium in humans (54) and are implicated in maintaining cellular Zn status by importing Zn from prostatic secretions. These 3 Zn transporters offer us candidate regulatory molecules responsible for Zn accumulation, although the contribution of further Zip proteins has not been ruled out.
Once acquired by the prostate cell, Zn is compartmentalized for numerous functions. Expression of 6 ZnT proteins has been described in the prostate, and their expression and cellular localization are lobe dependent, likely reflecting the differential need for lobe-specific functions. Evidence suggests that changes in Zn requirements occur in the prostate during sexual development. Expression of ZnT1 is greatly reduced at sexual maturity (55), potentially reflecting a role for Zn accumulation in the prostate for optimal cellular proliferation. In addition, ZnT2 expression increases in the anterior lobe but remains constant in the other lobes during sexual maturation (55). Although the dorsolateral prostate is the primary site of Zn secretion, the anterior and ventral prostate produce secretions of their own; the anterior prostate produces many of the same proteins as the dorsolateral prostate such as probasin, experimental auto-immune prostatitis antigen 2, and IgG-binding protein-like protein (56). In all lobes, ZnT2 staining pattern is consistent with endoplasmic reticulum (ER) localization (55), suggesting that while ZnT2 imports Zn into the ER, there may be lobe specific Zn requirements for optimal function. In addition, several other Zn transporters known to import Zn into the secretory compartment have been detected in the prostate. ZnT4, ZnT5, ZnT6, and ZnT7 are expressed throughout the prostate and localization is consistent with a role for Zn import into the ER and/or Golgi apparatus (55), presumably to provide Zn directly for secretion (57–59) or for Zn specific proteins that function within the secretory pathway as has been proposed for the mammary gland (60). Surprisingly little is known regarding the mechanism(s) responsible for Zn secretion from the prostate considering the high Zn required to provide for optimal sperm viability.
Prostate mitochondria also accumulate a large amount of Zn and do so in a lobe specific manner (∼1 μg Zn/mg protein in lateral prostate compared with ∼0.1 μg Zn/mg protein in the ventral or dorsal lobe) (61). Additionally, prostate mitochondria have a higher Zn content compared with other cell types such as hepatocytes (∼0.05 μg Zn/mg protein). A specific role for Zn in prostate mitochondria may be to prevent citrate oxidation through the inhibition of m-aconitase activity, thus expanding the citrate pool. Similarly, we hypothesize that modulation of mitochondria Zn pools may serve to regulate cell metabolism in other secretory tissues such as the mammary gland (60). A distinct gap in our knowledge is understanding how Zn is accumulated by prostate mitochondria, because there are currently no known Zn transporters localized to this organelle. Understanding the role, regulation, and consequences of subsequent dysregulation of Zn metabolism in the prostate may offer insight into the cause and treatment of prostate disease. Future studies need to determine the role and regulation of Zip proteins expressed in prostate epithelial cells to help understand how and why this unique cell type accumulates and utilizes Zn.
Aging
Aging is associated with low Zn levels in the prostate and prostate fluid, which is associated with decreased fertility in humans (62). Iguchi et al. (63) also showed similar age related differences in prostate Zn concentration in the ventral prostate of aged rats. Somewhat counterintuitively, this is associated with increased expression of ZnT2, a Zn responsive (32), key Zn transporter in the prostate, suggesting Zn independent mechanisms of transcriptional control as has been observed in the mammary gland (64). Curiously, little is known regarding changes in Zn transporter expression in the prostate of aged animals and whether this tissue may experience a diminished capacity to accumulate or secrete Zn or modulate specific Zn responsive functions. In light of our lack of understanding of the relationship among age, low prostate Zn content, and dysregulated function, it is important to explore what may be causing this dysregulation in Zn homeostasis and evaluate its specific role in age-related decreased fertility.
Prostate disease
Low prostate Zn content is also associated with prostatic disease. The major prostate diseases that affect older men are benign prostatic hyperplasia and prostate carcinoma. Some studies have documented reduced Zn content in prostate (65) and prostatic secretions (66) in men with prostate disease compared with healthy men. It is not fully understood whether low Zn content is a cause or consequence of prostate cancer, but recent data suggest it may in fact be a cause and is undoubtedly a critical factor in malignancy (67–69). Understanding the exact role Zn plays in the transformation of prostate cells will help to identify mechanisms of dysregulation, and understanding these mechanisms in relation to aging may help to develop therapeutic tools or perhaps preventative measures. Zn may play a key role in the prevention of prostatic disease by ameliorating oxidative stress, which can subsequently result in DNA damage, increasing the risk of mutation and malignant transformation. As discussed above, there is a relationship among advanced age, decreased prostate Zn content, and increased oxidative stress (70). In fact, dietary Zn deficiency has been associated with increased DNA damage in the prostate during oxidative stress (71). Specifically, Zn deficient prostate cells have greater DNA damage and altered expression of genes associated with this damage, indicating that marginal Zn intake may sensitize the prostate to oxidative damage (71, 72). As oxidative stress increases, so does the cellular Zn requirement for protective mechanisms, thus perpetuating the harmful effects of Zn deficiency.
In addition, Zn inhibits citrate oxidation in the mitochondria of prostate cells (67). Low Zn accumulation in the prostate will therefore allow excessive citrate oxidation and ATP production, which will in turn increase oxidative stress in the mitochondria and may in turn exacerbate DNA damage. Increased ATP production coupled with increased risk of DNA damage creates the perfect opportunity for malignant transformation. Many associations between low prostate Zn and prostatic disease exist, but direct evidence of the function of Zn in preventing oxidative stress and DNA damage in the prostate is still needed. Franklin and Costello (69) examined the relationship between Zn and prostate malignancy and implicated Zn dysregulation in malignant transformation of prostate cells. In normal tissues, high mitochondria Zn levels inhibit m-aconitase activity, sparing citrate from oxidation in the Krebs cycle, thus making it available for secretion into prostatic fluid (69). Cancerous prostate tissue accumulates and secretes less citrate than normal tissue, which is associated with low prostate Zn levels. It is thought that the low Zn levels permit citrate oxidation, producing more ATP and providing an energy source for excess proliferation and transformation. Zn also imparts antitumorigenic effects by inducing apoptosis; Zn induces Bax expression, which initiates mitochondrial apoptosis by allowing the release of cytochrome c to initiate the caspase cascade (68). Suppressed expression of Zn importers such as Zip1, Zip2, and Zip3 (52, 54) is associated with prostate cancer and suggests a possible reason for low Zn levels seen in the malignant prostate. The cause, however, of reduced Zip expression in malignant prostate cells is yet to be determined. Great strides have been made in understanding the interplay of Zn and malignant transformation in the prostate, but more information is needed to fully understand Zn homeostasis in normal tissue and the dysregulation that may lead to malignancy. If we consider this information along with the decreased expression of key Zn importers and cell Zn content in cancerous prostate tissue, we see the highlighted importance of Zn in maintaining DNA integrity and overall prostate health.
Zn and the mammary gland
The mammary gland is another secretory tissue that requires Zn for specific biological processes that are critical for proper tissue function. The role of Zn in the mammary gland is multifaceted, because the mammary gland is a dynamic tissue that undergoes dramatic morphological and functional changes. It requires a coordinated effort to provide sufficient Zn for tissue expansion during lactation, which is efficiently ameliorated following weaning. Interestingly, disruption of these processes is associated with breast disease. Understanding the Zn dependent mechanisms that regulate these processes is critical to understand mammary gland physiology. Herein, we discuss specific roles for Zn in mammary gland expansion, remodeling, and lactation, and in breast disease (Fig. 3).
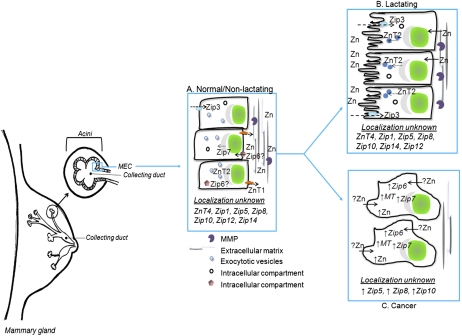
Figure 3.
Zn and Zn transporters involved in mammary gland function. Mammary gland is comprised of a network of branched ductal structures terminating at acinar units, also known as lobules. These structures are lined with MEC responsible for the production and secretion of milk into the collecting ducts for the developing neonate. (A) Although only localization of Zip3 and ZnT2 has been clearly established in normal/nonlactating tissue, ZnT4 is also known to be expressed. Zip6 and Zip7 are expressed in breast cells; however, their role in normal mammary gland is unknown. (B) During lactation, ZnT2 plays a major role in the secretion of Zn into milk, while Zip3 plays a role in the reuptake of Zn from the lumen. The localization and contribution of other Zn transporters during MEC differentiation and lactation remains to be determined and is an important question. (C) Hyper-accumulation of Zn has been associated with breast cancer. A limited number of Zn transporters (Zip5, Zip6, Zip7, Zip8, and Zip10) and MT have been shown to be overexpressed in this disease. However, their specific contribution to this phenotype and in MEC is remains to be delineated.
Architecturally, the mammary gland is comprised of a network of branched ductal structures terminating at acinar units. Each acini is lined with mammary epithelial cells (MEC) responsible for transferring nutrients into milk during lactation. From this perspective, MEC are the headquarters of milk production; thus, the regulation of MEC proliferation, differentiation, and function is paramount. Zn modulates cell proliferation, specifically through its role in regulating gene expression (73). For example, transcription factor IIIA contains 9 consecutive Zn finger motifs and is responsible for all cellular RNA transcription (74). Thus, ineffective modulation of transcription factor IIIA binding could lead to hyper- or hypoproliferation of MEC. There is a paucity of information on specific mechanisms through which Zn regulates mammary gland development; however, emerging data indicate that specific Zn binding transcription factors play important roles. Zfp289 has been localized in the cytoplasm, is expressed in the mammary gland, and expression is limited to developmental stages of active MEC proliferation and lobulo-alveolar (acini) development (75). This implicates Zfp289 as an important regulator of MEC proliferation by regulating downstream factors that are fundamental to cell growth and survival (75). Additionally, the ability to differentiate into a secretory tissue and regulate milk secretion is a vital function of the lactating mammary gland. LIM-ONLY (LMO1–4) is another family of Zn finger proteins implicated in MEC proliferation (76, 77). Lmo4 is expressed throughout mammary gland development, peaking during late pregnancy and diminishing throughout lactation (78). Lmo4−/− mice have condensed acini units and reduced MEC proliferation and milk production compared with Lmo4+/+ mice (78), illustrating the key role of this transcription factor in mammary gland morphogenesis. GATA proteins are another family of Zn binding transcription factors that play a role in breast development as an inducer of cell differentiation (79, 80). Recent reports identify GATA3 as a critical regulator of mammary gland morphogenesis (81) and luminal cell differentiation (82). Zn clearly plays an extensive role in mammary gland development and remodeling; however, it is not understood if and how Zn deficiency affects these processes. Further studies are needed to identify the role of Zn in modulating mammary gland phenotype.
Another Zn dependent function in the mammary gland is tissue expansion. The mammary gland dramatically expands during reproduction. Matrix metalloproteinases (MMP) are Zn dependent enzymes that degrade extracellular matrix and facilitate critical remodeling of this tissue. The mammary gland expresses MMP2, -3, -7, -9, -11, and -14 (83, 84). Until pregnancy, the mammary gland is maintained in a quiescent state. It is well accepted that MMP are regulated via transcription by various factors such as IL-1 (85) and TNFα (86). During MEC expansion (i.e. pregnancy and lactation), MMP3 (87) and MMP7 (88) expression levels are increased. However, at the cessation of lactation, expression of MMP2 (89), MMP3 (90), and MMP11 (91) are temporally upregulated, suggesting they play an important role in mammary gland involution and reorganization. It is interesting to note that the balance of enzymatic activity is dependent on the availability of cellular Zn, thus a decrease in activity could result in impaired remodeling and subsequent breast disease. Current information clearly suggests that Zn-regulated mechanisms play a central role in this process.
Normal mammary gland Zn biology and lactation
In normal/nonlactating tissue, the regulation of Zn metabolism in MEC requires the integrated function of numerous Zn transporters to maintain mammary gland Zn homeostasis for proper cellular function. Several Zn importers (Zip1, -3, -5, -6, -7, -8, -10, -12, and -14) and Zn exporters (ZnT1,-2, and -4) have been detected in the breast cells [reviewed in (92)]. Zip1, -3, and -7 are ubiquitously expressed (93–95). As mentioned above, Zip3 localization suggests it imports Zn from prostate fluid (54). Conversely, Zip5 localization to the basolateral membrane of intestinal cells (96) suggests it imports Zn from systemic circulation. Interestingly, Zip6 is dually localized to the cell membrane and within an intracellular compartment in malignant breast tissue and malignant breast tumor (T47D) cells (97–99). Zip proteins may also export Zn from a subcellular compartment into the cytoplasm. For example, Zip7 exports Zn from the Golgi apparatus in lung fibroblasts (WI-38), prostate epithelial cells (RWPE1), erythroleukemia cells (K-562), and MEC (MCF-7) (95), and plays a key role in intracellular signaling through the epidermal growth factor receptor (100). Thus far, expression of only ZnT1, -2, and -4 has been documented in the mammary gland. ZnT1 exports Zn across the cell membrane (101, 102), whereas ZnT2 and ZnT4 imports Zn into intracellular compartments (102–104). Although numerous Zn transporters are expressed in breast tissue, we still lack specific information on their functional relevance and regulatory factors in the mammary gland.
Following cellular differentiation during lactation, MEC are responsible for the transfer of nutrients into milk to nourish the developing newborn. The mammary gland must transfer ∼1–3 μg Zn/d during lactation to meet the needs of the developing infant. When milk Zn levels are compromised, Zn deficiency results in failure to thrive, diarrhea, irritability, and dermatitis in the infant (105). Data on the mechanisms through which Zn is transferred into milk is limited. In 1997, a mutation in the ZNT4 gene was identified and proposed as a candidate gene for Zn transfer into milk, because a truncation mutation in the ZNT4 gene is associated with reduced milk Zn levels in mice (“lethal milk mouse”) (106). Attempts to identify analogous ZNT4 mutations in humans have been unsuccessful (107, 108). Although ZnT4 clearly plays a role in Zn transfer into milk, our current understanding of its contribution to Zn metabolism is limited. In contrast, our knowledge of the role of ZnT2 in milk Zn secretion has expanded in recent years. A single amino acid substitution in human ZnT2 reduces milk Zn concentration by ∼75% and results in severe dermatitis and alopecia in the nursing infant (108). Our group has demonstrated that prolactin directly regulates mammary gland Zn metabolism through transcriptional regulation of ZnT2 expression (64) and post-translational relocalization to the cell membrane for enhanced Zn secretion (104). More recently, we identified 2 distinct single nucleotide polymorphisms in ZnT2 that alter ZnT2 localization and impair Zn metabolism in cultured MEC (109). It is not currently understood if these single nucleotide polymorphisms are associated with low milk Zn levels in women. However, this highlights the critical role ZnT2 plays in the secretion of Zn into milk and begins to shed light on the importance of subcellular localization of Zn transporters and their unique role in mammary gland Zn metabolism.
Breast disease
Benign, noncancerous breast disease (BBD) accounts for ∼80% of all breast lesions diagnosed in women (110). Similar to breast cancer, BBD is a heterogeneous disease about which there is a paucity of information regarding etiology (111). BBD are typically associated with inflammation such as mastitis and benign tumor masses such as fibrocystic breast disease (FBD). Interestingly, higher Zn levels in breast tissue from women diagnosed with BBD has been demonstrated (112). This implicates a role for Zn dysregulation in breast diseases and underscores the importance in delineating the mechanisms attributing to Zn dysregulation to these pathologies. Mastitis is the most common inflammatory disorder of the breast arising from a bacterial infection or blocked milk ducts (113). Mastitis reduces milk yield (114) and negatively alters milk secretion (114). Evidence of mastitis-mediated changes in milk composition in cows indicates that protein and fat content are higher, whereas lactose and the abundance of specific milk proteins such as caseins are reduced (114). Mechanistically, the decline in milk production has been attributed to damage to MEC as a consequence of immunological defense mechanisms (115–117). Mastitis induces apoptosis, reduces MEC proliferation, and enhances MMP9 activity (117), suggesting an overall sloughing off or removal of MEC. Given the role of Zn in maintaining epithelial integrity (118), one could reason that Zn may act as a key protective mechanism against mastitis in the mammary gland. FBD is associated with irregular lumps or cysts and extensive breast discomfort (111). There is limited data on the direct role of Zn in FBD; however, women with low Zn intake have a higher incidence of FBD (119). Interestingly, aspirated fluid (from breast microcysts, breast secretions, and breast cyst fluid) and serum from FBD patients has higher levels of Zn α-2 glycoprotein (ZnGP) (120). In addition, women with advanced breast cancer have higher ZnGP levels in serum than patients with early breast cancer (121). The intracellular localization of ZnGP within the acini of human breast tissue suggests that MEC actively secrete ZnGP (122), although the function of this 43-kDa glycoprotein in the breast is currently unknown (123).
Finally, there is compelling evidence implicating dysregulated Zn homeostasis in the physiology of breast cancer [reviewed in (92,94)]. Expression of numerous Zn importers is markedly upregulated in some breast cancers (94). Current data suggest that select Zn transporters, including MT, Zip5, Zip6, Zip7, Zip8 and Zip10, are aberrantly expressed in breast cancer cells (92, 94, 99); however, the functional relevance of Zn hyper-accumulation in breast cancer is still unknown. Interestingly, the Zn hyper-accumulating phenotype associated with breast cancer is curiously reminiscent of Zn accumulation in the lactating mammary gland destined for secretion. This suggests that perhaps Zn hyper-accumulation in breast tumors reflects the activation of Zn accumulating mechanisms concurrent with inactivated secretory machinery. What remains to be understood is how the breast cell manages excess Zn accumulation and if Zn accumulation predisposes the mammary cell to malignancy or results from malignant transformation. Moreover, an interesting question reflects the role of genetic variation and mutations in Zn transporters in the initiation or progression of breast disease. Taken together, the extensive role for Zn in the breast and dysregulation of critical Zn modulators in breast disease suggests that Zn metabolism may provide interesting avenues for novel therapeutic interventions.
Conclusions
The pancreas, prostate, and mammary gland have unique requirements for Zn, providing Zn for key cellular processes as well as abundant Zn for secretion into pancreatic fluid, prostate fluid, and milk, respectively. Thus, it is not surprising that dysregulation of Zn metabolism in these tissues is closely associated with impaired cellular function, disrupted Zn secretion, and disease. The current understanding of the mechanisms responsible for managing Zn metabolism in these unique tissues remains immature. However, our current understanding does suggest that Zn-based intervention strategies may prove to be an invaluable addition to our arsenal of therapeutic approaches improving diagnostic and therapeutic capabilities. Key avenues to explore in the future clearly involve understanding the role of genetic variation and mutations in Zn management proteins in pancreas, prostate, and mammary gland function. Improving our understanding of how these unique tissues regulate Zn transport and metabolism and elucidating the effects of nutrition, environmental factors, and aging on Zn homeostasis are critical to improving human health and disease.
Acknowledgments
All authors wrote the paper. S.L.K. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by interdepartmental funds and the NIH (HD058614 to S.L.K.).
Authors disclosures: S. L. Kelleher, N. H. McCormick, V. Velasquez, and V. Lopez, no conflicts of interest.
Abbreviations used: BBD, benign breast disease; DM, diabetes mellitus; ER, endoplasmic reticulum; FBD, fibrocystic breast disease; MEC, mammary epithelial cell.
Literature Cited
- 1.Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763:711–22 [DOI] [PubMed] [Google Scholar]
- 2.Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. : Hediger MA, editor The ABC of solute carriers. New York: Springer-Verlag; 2003 [DOI] [PubMed] [Google Scholar]
- 3.Kambe T, Narita H, Yamaguchi-Iwai Y, Hirose J, Amano T, Sugiura N, Sasaki R, Mori K, Iwanaga T, et al. Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J Biol Chem. 2002;277:19049–55 [DOI] [PubMed] [Google Scholar]
- 4.Dufner-Beattie J, Langmade SJ, Wang F, Eide D, Andrews GK. Structure, function, and regulation of a subfamily of mouse zinc transporter genes. J Biol Chem. 2003;278:50142–50 [DOI] [PubMed] [Google Scholar]
- 5.Huber AM, Gershoff SN. Effect of zinc deficiency in rats on insulin release from the pancreas. J Nutr. 1973;103:1739–44 [DOI] [PubMed] [Google Scholar]
- 6.Jou MY, Philipps AF, Lonnerdal B. Maternal zinc deficiency in rats affects growth and glucose metabolism in the offspring by inducing insulin resistance postnatally. J Nutr. 2010;140:1621–7 [DOI] [PubMed] [Google Scholar]
- 7.Hall AG, Kelleher SL, Lonnerdal B, Philipps AF. A graded model of dietary zinc deficiency: effects on growth, insulin-like growth factor-I, and the glucose/insulin axis in weanling rats. J Pediatr Gastroenterol Nutr. 2005;41:72–80 [DOI] [PubMed] [Google Scholar]
- 8.Kinlaw WB, Levine AS, Morley JE, Silvis SE, McClain CJ. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med. 1983;75:273–7 [DOI] [PubMed] [Google Scholar]
- 9.Canfield WK, Hambidge KM, Johnson LK. Zinc nutriture in type I diabetes mellitus: relationship to growth measures and metabolic control. J Pediatr Gastroenterol Nutr. 1984;3:577–84 [DOI] [PubMed] [Google Scholar]
- 10.Jayaraman AK, Jayaraman S. Increased level of exogenous zinc induces cytotoxicity and up-regulates the expression of the ZnT-1 zinc transporter gene in pancreatic cancer cells. J Nutr Biochem. 2010;22:79–88 [DOI] [PubMed] [Google Scholar]
- 11.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci USA. 2007;104:18636–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Zhang Y, Bharadwaj U, Zhai QJ, Ahern CH, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, et al. Down-regulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin Cancer Res. 2009;15:5993–6001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girish BN, Rajesh G, Vaidyanathan K, Balakrishnan V. Zinc status in chronic pancreatitis and its relationship with exocrine and endocrine insufficiency. JOP. 2009;10:651–6 [PubMed] [Google Scholar]
- 14.Milnerowicz H, Jablonowska M, Bizon A. Change of zinc, copper, and metallothionein concentrations and the copper-zinc superoxide dismutase activity in patients with pancreatitis. Pancreas. 2009;38:681–8 [DOI] [PubMed] [Google Scholar]
- 15.Yavuz N, Unal E, Dogan M, Kiziler AR, Aydemir B, Titiz I. Serum free prostate-specific antigen and zinc levels in experimental acute pancreatitis. Biol Trace Elem Res. 2005;106:205–9 [DOI] [PubMed] [Google Scholar]
- 16.Bojarski K, Dabrowski A, Wallner G, Maciejewski R. Shift of zinc in serum and tissues in course of experimental acute pancreatitis. Ann Univ Mariae Curie Sklodowska Med. 2002;57:74–8 [PubMed] [Google Scholar]
- 17.Cameron AR, Anil S, Sutherland E, Harthill J, Rena G. Zinc-dependent effects of small molecules on the insulin-sensitive transcription factor FOXO1a and gluconeogenic genes. Metallomics. 2010;2:195–203 [DOI] [PubMed] [Google Scholar]
- 18.Ramracheya R, Ward C, Shigeto M, Walker JN, Amisten S, Zhang Q, Johnson PR, Rorsman P, Braun M. Membrane potential-dependent inactivation of voltage-gated ion channels in alpha-cells inhibits glucagon secretion from human islets. Diabetes. 2010;59:2198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slucca M, Harmon JS, Oseid EA, Bryan J, Robertson RP. ATP-sensitive K+ channel mediates the zinc switch-off signal for glucagon response during glucose deprivation. Diabetes. 2010;59:128–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gyulkhandanyan AV, Lu H, Lee SC, Bhattacharjee A, Wijesekara N, Fox JE, MacDonald PE, Chimienti F, Dai FF, et al. Investigation of transport mechanisms and regulation of intracellular Zn2+ in pancreatic alpha-cells. J Biol Chem. 2008;283:10184–97 [DOI] [PubMed] [Google Scholar]
- 21.Kim BJ, Kim YH, Kim S, Kim JW, Koh JY, Oh SH, Lee MK, Kim KW, Lee MS. Zinc as a paracrine effector in pancreatic islet cell death. Diabetes. 2000;49:367–72 [DOI] [PubMed] [Google Scholar]
- 22.Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J Clin Invest. 1998;101:2377–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller SR. Role of the insulin-regulated aminopeptidase IRAP in insulin action and diabetes. Biol Pharm Bull. 2004;27:761–4 [DOI] [PubMed] [Google Scholar]
- 24.Keller SR, Scott HM, Mastick CC, Aebersold R, Lienhard GE. Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from Glut4 vesicles. J Biol Chem. 1995;270:23612–8 [DOI] [PubMed] [Google Scholar]
- 25.Tang X, Shay NF. Zinc has an insulin-like effect on glucose transport mediated by phosphoinositol-3-kinase and Akt in 3T3–L1 fibroblasts and adipocytes. J Nutr. 2001;131:1414–20 [DOI] [PubMed] [Google Scholar]
- 26.Dufner-Beattie J, Kuo YM, Gitschier J, Andrews GK. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J Biol Chem. 2004;279:49082–90 [DOI] [PubMed] [Google Scholar]
- 27.Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53:2330–7 [DOI] [PubMed] [Google Scholar]
- 28.Coffman FD, Dunn MF. Insulin-metal ion interactions: the binding of divalent cations to insulin hexamers and tetramers and the assembly of insulin hexamers. Biochemistry. 1988;27:6179–87 [DOI] [PubMed] [Google Scholar]
- 29.Albiston AL, Yeatman HR, Pham V, Fuller SJ, Diwakarla S, Fernando RN, Chai SY. Distinct distribution of GLUT4 and insulin regulated aminopeptidase in the mouse kidney. Regul Pept. 2011;166:83–9 [DOI] [PubMed] [Google Scholar]
- 30.Hambidge KM, Miller LV, Westcott JE, Sheng X, Krebs NF. Zinc bioavailability and homeostasis. Am J Clin Nutr. 2010;91:S1478–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vehik K, Dabelea D. The changing epidemiology of type 1 diabetes: why is it going through the roof? Diabetes Metab Res Rev. 2011;27:3–13 [DOI] [PubMed] [Google Scholar]
- 32.Guo L, Lichten LA, Ryu MS, Liuzzi JP, Wang F, Cousins RJ. STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells. Proc Natl Acad Sci USA. 2010;107:2818–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harding MM, Hodgkin DC, Kennedy AF, O'Conor A, Weitzmann PD. The crystal structure of insulin. II. An investigation of rhombohedral zinc insulin crystals and a report of other crystalline forms. J Mol Biol. 1966;16:212–26 [DOI] [PubMed] [Google Scholar]
- 34.Ohly P, Dohle C, Abel J, Seissler J, Gleichmann H. Zinc sulphate induces metallothionein in pancreatic islets of mice and protects against diabetes induced by multiple low doses of streptozotocin. Diabetologia. 2000;43:1020–30 [DOI] [PubMed] [Google Scholar]
- 35.Failla ML, Kiser RA. Altered tissue content and cytosol distribution of trace metals in experimental diabetes. J Nutr. 1981;111:1900–9 [DOI] [PubMed] [Google Scholar]
- 36.Adachi Y, Yoshida J, Kodera Y, Kiss T, Jakusch T, Enyedy EA, Yoshikawa Y, Sakurai H. Oral administration of a zinc complex improves type 2 diabetes and metabolic syndromes. Biochem Biophys Res Commun. 2006;351:165–70 [DOI] [PubMed] [Google Scholar]
- 37.Lemaire K, Ravier MA, Schraenen A, Creemers JW, Van de Plas R, Granvik M, Van Lommel L, Waelkens E, Chimienti F, et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA. 2009;106:14872–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104:17040–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chimienti F, Favier A, Seve M. ZnT-8, a pancreatic beta-cell-specific zinc transporter. Biometals. 2005;18:313–7 [DOI] [PubMed] [Google Scholar]
- 40.Cauchi S, Del Guerra S, Choquet H, D'Aleo V, Groves CJ, Lupi R, McCarthy MI, Froguel P, Marchetti P. Meta-analysis and functional effects of the SLC30A8 rs13266634 polymorphism on isolated human pancreatic islets. Mol Genet Metab. 2010;100:77–82 [DOI] [PubMed] [Google Scholar]
- 41.Ellison LF. Measuring the effect of including multiple cancers in survival analyses using data from the Canadian Cancer Registry. Cancer Epidemiol. 2010;34:550–5 [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Bharadwaj U, Logsdon CD, Chen C, Yao Q, Li M. ZIP4 regulates pancreatic cancer cell growth by activating IL-6/STAT3 pathway through zinc finger transcription factor CREB. Clin Cancer Res. 2010;16:1423–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Chen C, Yao Q, Li M. ZIP4 upregulates the expression of neuropilin-1, vascular endothelial growth factor, and matrix metalloproteases in pancreatic cancer cell lines and xenografts. Cancer Biol Ther. 2010;9:236–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner B. Acute pancreatitis: symptoms, diagnosis and management. Nurs Times. 2003;99:38–40 [PubMed] [Google Scholar]
- 45.Mawson CA, Fischer MI. The occurrence of zinc in the human prostate gland. Can J Med Sci. 1952;30:336–9 [DOI] [PubMed] [Google Scholar]
- 46.Sorensen MB, Stoltenberg M, Danscher G, Ernst E. Chelation of intracellular zinc ions affects human sperm cell motility. Mol Hum Reprod. 1999;5:338–41 [DOI] [PubMed] [Google Scholar]
- 47.Yoshida K, Kawano N, Yoshiike M, Yoshida M, Iwamoto T, Morisawa M. Physiological roles of semenogelin I and zinc in sperm motility and semen coagulation on ejaculation in humans. Mol Hum Reprod. 2008;14:151–6 [DOI] [PubMed] [Google Scholar]
- 48.Costello LC, Franklin RB. Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate. 1998;35:285–96 [DOI] [PubMed] [Google Scholar]
- 49.Fair WR, Couch J, Wehner N. Prostatic antibacterial factor. Identity and significance. Urology. 1976;7:169–77 [DOI] [PubMed] [Google Scholar]
- 50.Cho YH, Lee SJ, Lee JY, Kim SW, Lee CB, Lee WY, Yoon MS. Antibacterial effect of intraprostatic zinc injection in a rat model of chronic bacterial prostatitis. Int J Antimicrob Agents. 2002;19:576–82 [DOI] [PubMed] [Google Scholar]
- 51.Costello LC, Franklin RB, Feng P. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion. 2005;5:143–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello LC. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer. 2005;4:32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iguchi K, Otsuka T, Usui S, Sugimura Y, Hirano K. Correlation between ZIP2 messenger RNA expression and zinc level in rat lateral prostate. Biol Trace Elem Res. 2006;112:159–67 [DOI] [PubMed] [Google Scholar]
- 54.Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol Cancer. 2007;6:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kirschke CP, Huang L. Expression of the ZNT (SLC30) family members in the epithelium of the mouse prostate during sexual maturation. J Mol Histol. 2008;39:359–70 [DOI] [PubMed] [Google Scholar]
- 56.Fujimoto N, Akimoto Y, Suzuki T, Kitamura S, Ohta S. Identification of prostatic-secreted proteins in mice by mass spectrometric analysis and evaluation of lobe-specific and androgen-dependent mRNA expression. J Endocrinol. 2006;190:793–803 [DOI] [PubMed] [Google Scholar]
- 57.Ishihara K, Yamazaki T, Ishida Y, Suzuki T, Oda K, Nagao M, Yamaguchi-Iwai Y, Kambe T. Zinc transport complexes contribute to the homeostatic maintenance of secretory pathway function in vertebrate cells. J Biol Chem. 2006;281:17743–50 [DOI] [PubMed] [Google Scholar]
- 58.Ellis CD, Macdiarmid CW, Eide DJ. Heteromeric protein complexes mediate zinc transport into the secretory pathway of eukaryotic cells. J Biol Chem. 2005;280:28811–8 [DOI] [PubMed] [Google Scholar]
- 59.Fukunaka A, Suzuki T, Kurokawa Y, Yamazaki T, Fujiwara N, Ishihara K, Migaki H, Okumura K, Masuda S, et al. Demonstration and characterization of the heterodimerization of ZnT5 and ZnT6 in the early secretory pathway. J Biol Chem. 2009;284:30798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCormick N, Velasquez V, Finney L, Vogt S, Kelleher SL. X-ray fluorescence microscopy reveals accumulation and secretion of discrete intracellular zinc pools in the lactating mouse mammary gland. PLoS ONE. 2010;5:e11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, Franklin RB, Costello LC. Prolactin and testosterone regulation of mitochondrial zinc in prostate epithelial cells. Prostate. 1997;30:26–32 [DOI] [PubMed] [Google Scholar]
- 62.Elzanaty S. Association between age and epididymal and accessory sex gland function and their relation to sperm motility. Arch Androl. 2007;53:149–56 [DOI] [PubMed] [Google Scholar]
- 63.Iguchi K, Morihara N, Usui S, Hayama M, Sugimura Y, Hirano K. Castration- and aging-induced changes in the expression of zinc transporter and metallothionein in rat prostate. J Androl. Epub 2010 Aug 26 [DOI] [PubMed] [Google Scholar]
- 64.Qian L, Lopez V, Seo YA, Kelleher SL. Prolactin regulates ZNT2 expression through the JAK2/STAT5 signaling pathway in mammary cells. Am J Physiol Cell Physiol. 2009;297:C369–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaichick VYe, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol. 1997;29:565–74 [DOI] [PubMed] [Google Scholar]
- 66.Zaichick VY, Sviridova TV, Zaichick SV. Zinc concentration in human prostatic fluid: normal, chronic prostatitis, adenoma and cancer. Int Urol Nephrol. 1996;28:687–94 [DOI] [PubMed] [Google Scholar]
- 67.Costello LC, Liu Y, Franklin RB, Kennedy MC. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J Biol Chem. 1997;272:28875–81 [DOI] [PubMed] [Google Scholar]
- 68.Feng P, Li T, Guan Z, Franklin RB, Costello LC. The involvement of Bax in zinc-induced mitochondrial apoptogenesis in malignant prostate cells. Mol Cancer. 2008;7:25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463:211–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bianchi-Frias D, Vakar-Lopez F, Coleman IM, Plymate SR, Reed MJ, Nelson PS. The effects of aging on the molecular and cellular composition of the prostate microenvironment. PLoS ONE. 2010;5:p.i.12501–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song Y, Elias V, Loban A, Scrimgeour AG, Ho E. Marginal zinc deficiency increases oxidative DNA damage in the prostate after chronic exercise. Free Radic Biol Med. 2010;48:82–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan M, Song Y, Wong CP, Hardin K, Ho E. Zinc deficiency alters DNA damage response genes in normal human prostate epithelial cells. J Nutr. 2008;138:667–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bohnsack BL, Hirschi KK. Nutrient regulation of cell cycle progression. Annu Rev Nutr. 2004;24:433–53 [DOI] [PubMed] [Google Scholar]
- 74.Miller J, Fairall L, Rhodes D. A novel method for the purification of the Xenopus transcription factor IIIA. Nucleic Acids Res. 1989;17:9185–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh J, Itahana Y, Parrinello S, Murata K, Desprez PY. Molecular cloning and characterization of a zinc finger protein involved in Id-1-stimulated mammary epithelial cell growth. J Biol Chem. 2001;276:11852–8 [DOI] [PubMed] [Google Scholar]
- 76.Dawid IB, Breen JJ, Toyama R. LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet. 1998;14:156–62 [DOI] [PubMed] [Google Scholar]
- 77.Sum EY, O'Reilly LA, Jonas N, Lindeman GJ, Visvader JE. The LIM domain protein Lmo4 is highly expressed in proliferating mouse epithelial tissues. J Histochem Cytochem. 2005;53:475–86 [DOI] [PubMed] [Google Scholar]
- 78.Sum EY, Shackleton M, Hahm K, Thomas RM, O'Reilly LA, Wagner KU, Lindeman GJ, Visvader JE. Loss of the LIM domain protein Lmo4 in the mammary gland during pregnancy impedes lobuloalveolar development. Oncogene. 2005;24:4820–8 [DOI] [PubMed] [Google Scholar]
- 79.Bresnick EH, Lee HY, Fujiwara T, Johnson KD, Keles S. GATA switches as developmental drivers. J Biol Chem. 2010;285:31087–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naylor MJ, Ormandy CJ. Gata-3 and mammary cell fate. Breast Cancer Res. 2007;9:302–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–9 [DOI] [PubMed] [Google Scholar]
- 82.Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rudolph-Owen LA, Matrisian LM. Matrix metalloproteinases in remodeling of the normal and neoplastic mammary gland. J Mammary Gland Biol Neoplasia. 1998;3:177–89 [DOI] [PubMed] [Google Scholar]
- 84.Benaud C, Dickson RB, Thompson EW. Roles of the matrix metalloproteinases in mammary gland development and cancer. Breast Cancer Res Treat. 1998;50:97–116 [DOI] [PubMed] [Google Scholar]
- 85.Matrisian LM, Hogan BL. Growth factor-regulated proteases and extracellular matrix remodeling during mammalian development. Curr Top Dev Biol. 1990;24:219–59 [DOI] [PubMed] [Google Scholar]
- 86.Brenner DA, O'Hara M, Angel P, Chojkier M, Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989;337:661–3 [DOI] [PubMed] [Google Scholar]
- 87.Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162:1123–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rudolph-Owen LA, Cannon P, Matrisian LM. Overexpression of the matrix metalloproteinase matrilysin results in premature mammary gland differentiation and male infertility. Mol Biol Cell. 1998;9:421–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 regulates adipogenesis during mammary gland involution. J Cell Biol. 2001;152:693–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rio MC, Lefebvre O, Santavicca M, Noel A, Chenard MP, Anglard P, Byrne JA, Okada A, Regnier CH, et al. Stromelysin-3 in the biology of the normal and neoplastic mammary gland. J Mammary Gland Biol Neoplasia. 1996;1:231–40 [DOI] [PubMed] [Google Scholar]
- 92.Kelleher SL, Seo YA, Lopez V. Mammary gland zinc metabolism: regulation and dysregulation. Genes Nutr. 2009;4:83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dressman MA, Walz TM, Lavedan C, Barnes L, Buchholtz S, Kwon I, Ellis MJ, Polymeropoulos MH. Genes that co-cluster with estrogen receptor alpha in microarray analysis of breast biopsies. Pharmacogenomics J. 2001;1:135–41 [DOI] [PubMed] [Google Scholar]
- 94.Taylor KM, Morgan HE, Smart K, Zahari NM, Pumford S, Ellis IO, Robertson JF, Nicholson RI. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol Med. 2007;13:396–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang L, Kirschke CP, Zhang Y, Yu YY. The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J Biol Chem. 2005;280:15456–63 [DOI] [PubMed] [Google Scholar]
- 96.Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). Biol Chem. 2007;388:1301–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kasper G, Weiser AA, Rump A, Sparbier K, Dahl E, Hartmann A, Wild P, Schwidetzky U, Castanos-Velez E, et al. Expression levels of the putative zinc transporter LIV-1 are associated with a better outcome of breast cancer patients. Int J Cancer. 2005;117:961–73 [DOI] [PubMed] [Google Scholar]
- 98.Taylor KM, Morgan HE, Johnson A, Hadley LJ, Nicholson RI. Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem J. 2003;375:51–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lopez V, Kelleher SL. Zip6-attenuation promotes epithelial-to-mesenchymal transition in ductal breast tumor (T47D) cells. Exp Cell Res. 2010;316:366–75 [DOI] [PubMed] [Google Scholar]
- 100.Taylor KM, Vichova P, Jordan N, Hiscox S, Hendley R, Nicholson RI. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer Cells. Endocrinology. 2008;149:4912–20 [DOI] [PubMed] [Google Scholar]
- 101.Liuzzi JP, Bobo JA, Cui L, McMahon RJ, Cousins RJ. Zinc transporters 1, 2 and 4 are differentially expressed and localized in rats during pregnancy and lactation. J Nutr. 2003;133:342–51 [DOI] [PubMed] [Google Scholar]
- 102.Kelleher SL, Lonnerdal B. Zn transporter levels and localization change throughout lactation in rat mammary gland and are regulated by Zn in mammary cells. J Nutr. 2003;133:3378–85 [DOI] [PubMed] [Google Scholar]
- 103.Michalczyk A, Varigos G, Catto-Smith A, Blomeley RC, Ackland ML. Analysis of zinc transporter, hZnT4 (Slc30A4), gene expression in a mammary gland disorder leading to reduced zinc secretion into milk. Hum Genet. 2003;113:202–10 [DOI] [PubMed] [Google Scholar]
- 104.Lopez V, Kelleher SL. Zinc transporter-2 (ZnT2) variants are localized to distinct subcellular compartments and functionally transport zinc. Biochem J. 2009;422:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ackland ML, Michalczyk A. Zinc deficiency and its inherited disorders: a review. Genes Nutr. 2006;1:41–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang L, Gitschier J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat Genet. 1997;17:292–7 [DOI] [PubMed] [Google Scholar]
- 107.Michalczyk AA, Allen J, Blomeley RC, Ackland ML. Constitutive expression of hZnT4 zinc transporter in human breast epithelial cells. Biochem J. 2002;364:105–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chowanadisai W, Lonnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J Biol Chem. 2006;281:39699–707 [DOI] [PubMed] [Google Scholar]
- 109.Seo YA, Kelleher SL. Functional analysis of two single nucleotide polymorphisms in SLC30A2 (ZnT2): implications for mammary gland function and breast disease in women. Physiol Genomics. 2010;42A:219–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353:275–85 [DOI] [PubMed] [Google Scholar]
- 111.Guray M, Sahin AA. Benign breast diseases: classification, diagnosis, and management. Oncologist. 2006;11:435–49 [DOI] [PubMed] [Google Scholar]
- 112.Cui Y, Vogt S, Olson N, Glass AG, Rohan TE. Levels of zinc, selenium, calcium, and iron in benign breast tissue and risk of subsequent breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1682–5 [DOI] [PubMed] [Google Scholar]
- 113.Amir LH, Forster DA, Lumley J, McLachlan H. A descriptive study of mastitis in Australian breastfeeding women: incidence and determinants. BMC Public Health. 2007;7:62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shuster DE, Harmon RJ, Jackson JA, Hemken RW. Suppression of milk production during endotoxin-induced mastitis. J Dairy Sci. 1991;74:3763–74 [DOI] [PubMed] [Google Scholar]
- 115.Harmon RJ, Heald CW. Migration of polymorphonuclear leukocytes into the bovine mammary gland during experimentally induced Staphylococcus aureus mastitis. Am J Vet Res. 1982;43:992–8 [PubMed] [Google Scholar]
- 116.Capuco AV, Paape MJ, Nickerson SC. In vitro study of polymorphonuclear leukocyte damage to mammary tissues of lactating cows. Am J Vet Res. 1986;47:663–8 [PubMed] [Google Scholar]
- 117.Long E, Capuco AV, Wood DL, Sonstegard T, Tomita G, Paape MJ, Zhao X. Escherichia coli induces apoptosis and proliferation of mammary cells. Cell Death Differ. 2001;8:808–16 [DOI] [PubMed] [Google Scholar]
- 118.Sordillo LM, Shafer-Weaver K, DeRosa D. Immunobiology of the mammary gland. J Dairy Sci. 1997;80:1851–65 [DOI] [PubMed] [Google Scholar]
- 119.Vobecky J, Simard A, Vobecky JS, Ghadirian P, Lamothe-Guay M, Falardeau M. Nutritional profile of women with fibrocystic breast disease. Int J Epidemiol. 1993;22:989–99 [DOI] [PubMed] [Google Scholar]
- 120.Bundred NJ, Miller WR, Walker RA. An immunohistochemical study of the tissue distribution of the breast cyst fluid protein, zinc alpha 2 glycoprotein. Histopathology. 1987;11:603–10 [DOI] [PubMed] [Google Scholar]
- 121.Bundred NJ, Scott WN, Davies SJ, Miller WR, Mansel RE. Zinc alpha-2 glycoprotein levels in serum and breast fluids: a potential marker of apocrine activity. Eur J Cancer. 1991;27:549–52 [DOI] [PubMed] [Google Scholar]
- 122.Tada T, Ohkubo I, Niwa M, Sasaki M, Tateyama H, Eimoto T. Immunohistochemical localization of Zn-alpha 2-glycoprotein in normal human tissues. J Histochem Cytochem. 1991;39:1221–6 [DOI] [PubMed] [Google Scholar]
- 123.Hassan MI, Waheed A, Yadav S, Singh TP, Ahmad F. Zinc alpha 2-glycoprotein: a multidisciplinary protein. Mol Cancer Res. 2008;6:892–906 [DOI] [PubMed] [Google Scholar]