Abstract
Stem cell proliferation, neuronal differentiation, cell survival, and migration in the central nervous system are all important steps in the normal process of neurogenesis. These mechanisms are highly active during gestational and early neonatal brain development. Additionally, in select regions of the brain, stem cells give rise to new neurons throughout the human lifespan. Recent work has revealed key roles for the essential trace element zinc in the control of both developmental and adult neurogenesis. Given the prevalence of zinc deficiency, these findings have implications for brain development, cognition, and the regulation of mood.
Introduction
Clearly, neurogenesis is an essential component of central nervous system (CNS)5 development. Not only do stem cells in the developing embryo undergo asymmetric division to form the embryonic notochord, neural tube, and neural crest, but these cells must travel via radial and tangential migration to their ultimate destination. Once there, the process of developmental neurogenesis continues with differentiation of stem cells into mature postmitotic neurons, aggregation, synaptogenesis, and synaptic pruning (1). Evidence also suggests that apoptotic and autophagic cell death are important parts of the processes leading to normal brain growth and plasticity during development (2, 3).
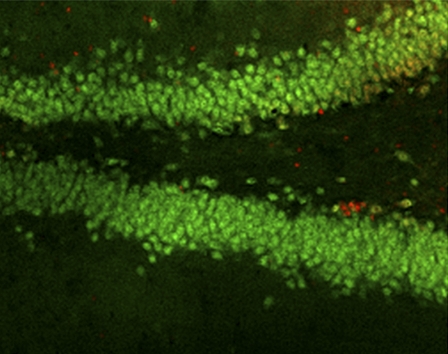
In addition to the importance of these processes in neurodevelopment, the last decade has seen an explosion in interest in the mechanisms that contribute to and govern adult neurogenesis. Although there has been evidence for adult neurogenesis in rodents for almost 50 y, it was the finding of stem cells in the adult human brain (4) that ignited new efforts to understand adult neuronal stem cell regulation. Adult neuronal stem cells, like their developmental counterparts, are capable not only of proliferation but also of differentiation into mature neuronal phenotypes. However, stem cells in the adult brain appear to be severely limited in number and largely isolated to specific brain regions such as the subventricular zone (SVZ) that surrounds the rostral end of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus. For example, Figure 1 shows the dense cell layer of the adult rat dentate gyrus labeled with the nuclear neuronal marker NeuN. Proliferating cells can be identified using immunohistochemistry to track uptake of the thymidine analogue bromodeoxyuridine (BrdU) or the expression of Ki67, a protein expressed in cells that are in all active phases of the cell cycle but not expressed in cells in G0.
Figure 1.
Stem cell proliferation in the adult rat hippocampus. NeuN labeled nuclei (green) define the mature neurons in the granular cell layer of the dentate gyrus. Proliferating cells, identified by the cell cycle marker Ki67 (red), are seen in the SGZ and hilus of the dentate. Adapted with permission from (20).
These 2 brain regions with significant neurogenesis potential are important for normal CNS function. The SVZ supplies neuroblasts that migrate along the rostral migratory stream toward the human and rat olfactory bulb, where they differentiate into interneurons (5) and participate in olfaction. Stem cells originally proliferating in the SGZ of the dentate migrate into the granular cell layer and differentiate into neurons that are integrated into the hippocampal circuitry (5, 6), a region of the brain known to participate in learning and memory. Additionally, as parts of the limbic system, both the olfactory bulb and the hippocampus regulate emotion.
A variety of factors, including age, stress, physical activity, antidepressant drugs, brain injury, stroke, seizure, and energy intake, have been shown to regulate adult neuronal stem cell proliferation, survival, and differentiation. This review will focus on our current understanding of the role of the essential trace metal zinc in the processes that lead to neurogenesis.
Current status of knowledge
Brain zinc functions
The roles of zinc in the developing and adult brain (and other organ systems) are in part due to the fact that zinc is an essential catalytic component of ∼80 different mammalian enzymes. Many of these enzymes, such as DNA and RNA polymerases, histone deacetylases (7), and DNA ligases (8), are clearly needed for normal DNA replication and cellular proliferation. Other zinc dependent enzymes, including metalloproteinases and many dehydrogenases in intermediary metabolism (9), also play important roles in normal CNS function. Additionally, zinc plays an essential structural role in a family of DNA binding transcription factors known as zinc-finger proteins (10, 11). Nuclear receptors, such as those that mediate the transcriptional roles of retinoic acid, vitamin D, thyroid hormone, glucocorticoids, and estrogen in the brain, are all zinc-finger proteins (12). All of these receptors are known to regulate key genes involved in cellular proliferation, brain development, and neurogenesis.
In addition to the zinc that is bound to enzymes, transcription factors, and other proteins, ∼20% of CNS zinc is in the free form and is associated with presynaptic vesicles of glutamatergic neurons (13). Although neurons containing free zinc are found in many regions of the brain, including the cortex, amygdala, and olfactory bulb, the neurons of the hippocampus appear to have the highest concentrations of free zinc. The zinc in these vesicles is released into the synaptic cleft, where it modulates the activity of a variety of postsynaptic receptors, including N-methyl-D-aspartate receptors, γ-aminobutyric acid receptors, and voltage-gated calcium channels (14, 15). N-methyl-D-aspartate receptor subunit expression has also been shown to be regulated by zinc (16).
Zinc and developmental neurogenesis
Stem cell proliferation.
Zinc supplementation provided in the drinking water (4 mmol/L) to pregnant mice resulted in an apparent increase in the number of proliferating cells that took up BrdU in the SVZ of the newborn pups (17). Although this was a very preliminary report that lacked quantification of the numbers of cells in the SVZ or SGZ, the work did suggest a link between zinc availability and neuronal stem cell proliferation in the developing brain. Other work found that zinc supplementation (100 mg/kg) increased expression of the stem cell marker nestin in the developing cerebellum and cortex compared with pups born to dams fed the zinc adequate (30 mg/kg) diet (18).
Zinc deficiency appears to reduce stem cell proliferation during brain development. When female mice were fed a severely deficient (1 mg Zn/kg) or marginally deficient (5 mg Zn/kg) zinc diet from the first day of pregnancy, significant reductions in nestin were measured in the pups beginning on embryonic d 10.5 through postnatal d 10 (18). Future work will be needed to determine whether deficits associated with developmental zinc deficiency, such as impaired long-term potentiation and learning and memory, are the result of alterations in neuronal stem cell proliferation. This is an important area of investigation, because it appears that these deficits cannot be reversed by subsequent addition of zinc to the diet after weaning (18).
Several investigators have contributed to our understanding of the mechanisms responsible for the role of zinc in stem cell proliferation during development. For example, when dams were fed either severely or marginally zinc deficient diets from the first day of gestation, binding of the transcription factor activator protein 1 to DNA was elevated in pup brain at gestational d 19 compared with controls. Given the large number of activator protein 1 responsive genes, these data suggest that even marginal zinc deficiency can result in significant changes in gene expression during development. DNA binding of NF-κB and nuclear factor of activated T-cell was also decreased by both severe and marginal deficiency (19). These findings are significant, because both of these transcription factors are known to participate in regulation of the cell cycle. The connection between zinc and the regulation of neuronal precursor proliferation and neurogenesis is further supported by the finding that other genes associated with the cell cycle, specifically those regulating the transition from G1 to S phase, are differentially regulated by zinc deficiency (20, 21). Another mechanism that may be responsible for reduced stem cell proliferation is the fact that zinc deficiency can induce elevations in glucocorticoid hormones (22). This mechanism represents a significant problem for the developing nervous system, because high glucocorticoid levels during gestation have been shown to reduce stem cell proliferation in the dentate gyrus of the hippocampus of newborn monkeys (23).
Neuronal differentiation.
Not only does zinc deficiency impair developmental neurogenesis via reductions in neuronal precursor proliferation, there is also early evidence suggesting that zinc deficiency may impair neuronal differentiation during this period as well. Zinc deficiency during lactation impaired the dendritic differentiation of cerebellar stellate, basket, and Purkinjie cells of 21-d-old rats compared with pair-fed controls (24, 25). Although the impact of zinc deficiency on neuronal migration has not been directly studied, it should be noted that elevated levels of glucocorticoids delay the radial migration of neurons during development of the cerebral cortex (26). Thus, it is likely that zinc mediated elevations in glucocorticoids would impair migration of cells undergoing differentiation during development.
Zinc and adult neurogenesis
Recent work has established that zinc regulates all 3 stages of adult neurogenesis: cell proliferation, stem cell survival, and neuronal differentiation.
Stem cell proliferation.
The first report of a role for zinc in the regulation of adult neuronal stem cell proliferation was published in 2008 and showed that 3 wk of a zinc deficient diet (1 mg/kg) resulted in a 50% reduction in the number of cells that were positive for the proliferation marker Ki67 in the adult (2 mo old) rat dentate SGZ (20). This initial publication also used zinc deficient and zinc adequate human neuronal precursor cells (NT-2) to implicate p53 mediated alterations in genes associated with zinc regulation of the G1 and S phases of the cell cycle. Subsequent work showed that 5 wk of a zinc deficient diet (0.85 mg/kg) fed to weanling mice produced similar decreases in the number of BrdU labeled cells in the dentate (27) and that 6 wk of a more moderately deficient diet (2.7 mg/kg) resulted in significant decreases in BrdU labeled cells as well as reductions in the cell cycle marker Ki67 (28). Decreases in BrdU positive cells in the dentate following 4 wk of zinc deficiency were reversed by 2 wk of feeding with a zinc adequate diet (28).
In vitro work using a variety of neuronal precursor cells that proliferate in culture has confirmed a role for zinc in CNS cell proliferation and helped to elucidate the mechanisms responsible for the action of zinc. For example, Corniola et al. (20) not only reported that zinc deficiency significantly reduced BrdU labeling of human NT-2 cells but also showed a role for the tumor suppressor p53. Zinc deficiency resulted in p53 nuclear translocation with subsequent regulation of down-stream genes such as reprimo, which induces cell cycle arrest (20). Similarly, zinc deficiency also resulted in increased p53 in IMR-32 neuroblastoma cells. This was accompanied by a reduction in cyclin D1 and E protein levels as well as phosphorylation of the mitogen-activated kinase extracellular-signal-regulated kinase, leading to cell cycle arrest at G0/G1 (21).
Neuronal precursor survival.
Reductions in the number of neuronal precursors during zinc deficiency are not only the result of reduced cell proliferation but also occur because of reduced cell survival. The first report of increased apoptosis in the SGZ of the dentate showed that when adult rats were subjected to a zinc deficient diet for 3 wk, there was an increase in terminal deoxynucleotidyl transferase dUTP nick end labeling labeled (TUNEL) cells compared with pair-fed controls (20). This finding was confirmed in mice fed a diet low in zinc for 5 wk, where increased TUNEL labeling in this region of the hippocampus was accompanied by activation of Fas, FasL, apoptosis-inducing factor, and caspase-3 (27).
In vitro analysis of zinc deficient NT-2 and IMR-32 cells shows a cascade of events triggered by p53. Down-stream p53 targets, including tissue inhibitor of metalloproteinase-3, TGFβ, and retinoblastoma-1, appear to play a p53 dependent role in inducing apoptosis in neuronal precursor cells (20). There is also clear evidence for the role of mitochondria in apoptosis associated with zinc deficiency. Zinc deficiency induces mitochondrial generation of reactive oxygen species, translocation of the apoptotic factor Bcl-2-associated death promoter (BAD) protein to the mitochondria, release of cytochrome c into the cytosol, activation of caspase-3, and poly (ADP-ribose) polymerase (PARP) cleavage (20, 21). Together, these mechanisms appear to play a significant role in reducing the numbers of neuronal precursor cells and inhibiting neurogenesis.
Neuronal differentiation.
Proliferating cells of the SGZ differentiate into neurons with processes that extend into the granular cell layer. One marker of neuronal differentiation is the expression of doublecortin (DCX). Zinc deficiency has been shown to reduce hippocampal DCX levels (27, 28). Immunohistochemistry revealed a reduction in the number of cells double labeled with BrdU and DCX (27). It should be noted that the reduction in DCX both in Western analysis and immunohistochemistry could be explained simply by the reduction in the number of proliferating cells, without an independent effect of zinc on newly differentiating neurons. However, in zinc deficient mice, DCX+ cells also had shortened processes and reduced neuronal branching (27), suggesting that zinc deficiency does indeed impair neurogenesis by reducing neuronal differentiation.
Conclusions
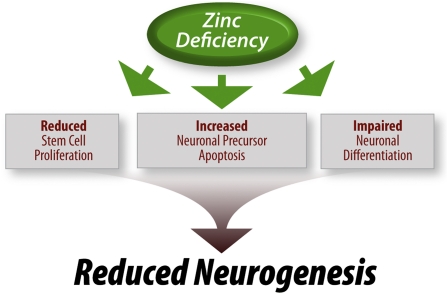
The available data suggest that zinc deficiency, both during development and adulthood, reduces neurogenesis by limiting the number of proliferating neuronal precursor cells in the CNS. As illustrated in Figure 2, this is the result of both a reduction in cell proliferation and an increase in apoptotic cell death. It also appears that neurogenesis is impaired by a reduction in the differentiation of stem cells into mature, functional neurons. It is not yet known what role zinc nutriture may play in the migration of developing neurons or adult neuronal precursors.
Figure 2.
Zinc regulation of neurogenesis. Current data suggest that the trace element zinc regulates multiple stages of neurogenesis, including cell proliferation, survival, and differentiation.
The question that now must be answered is: What are the implications of zinc deficiency induced reductions in neurogenesis for humans? In the developmental period, the need for normal neurogenesis in cognitive and motor development is clear. However, it is not yet known what role zinc regulation of stem cell proliferation, survival, differentiation, and migration may play in the development of human cognitive or behavioral functions. Regardless of mechanism, recent reports of widespread world-wide zinc deficiency during pregnancy (29, 30) highlight the need for future work on the role of zinc in developmental neurogenesis. This is particularly true given that early zinc deprivation may have long-term cognitive implications that cannot be corrected by later zinc supplementation (18).
The implications of reduced stem cell proliferation and neurogenesis in the adult are more complex. In animal models, antidepressant drugs and other treatments for depression such as electroconvulsive shock therapy and exercise increase cell proliferation in the SGZ. In humans, this relationship is likely to be more complicated. However, it does appear that normalization of neurogenesis may be at least in part responsible for the efficacy of antidepressant drugs (31). These considerations are important, because a number of groups have shown increased depression-like and anxiety-like behaviors in zinc deficient rodents (32–34). Furthermore, zinc appears to enhance the efficacy of antidepressant therapies. A double-blind, placebo controlled study showed that 25 mg/d of supplemental zinc improved the ability of several antidepressant drugs to reduce depression scores in patients with major unipolar depression (35). It is not known to what degree the zinc and antidepressant drugs may be acting synergistically to increase stem cell proliferation and neurogenesis in these patients. However, given the possible association between zinc, neurogenesis, and mood disorders, health care professionals who treat patients with mood disorders should recommend a zinc adequate diet or, where appropriate, zinc supplementation, for patients experiencing depression (36).
Acknowledgments
Both authors wrote the paper. C.W.L. had primary responsibility for final content. Both authors read and approved the final manuscript.
Footnotes
Supported by NIH grant GM081382 and the U.S. Army Medical Research and Materiel Command (MRMC).
Author disclosures: C. W. Levenson and D. Morris, no conflicts of interest.
Abbreviations used: BrdU, bromodeoxyuridine; CNS, central nervous system; DCX, doublecortin; SGZ, subgranular zone; SVZ, subventricular zone.
Literature Cited
- 1.Hollyday M. Neurogenesis in the vertebrate neural tube. Int J Dev Neurosci. 2001;19:161–73 [DOI] [PubMed] [Google Scholar]
- 2.Cecconi F, Di Bartolomeo S, Nardacci R, Fuoco C, Corazzari M, Giunta L, Romagnoli A, Stoykova A, Chowdhury K, et al. A novel role for autophagy in neurodevelopment. Autophagy. 2007;3:506–8 [DOI] [PubMed] [Google Scholar]
- 3.Johnston MV. Clinical disorders of brain plasticity. Brain Dev. 2004;26:73–80 [DOI] [PubMed] [Google Scholar]
- 4.Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7 [DOI] [PubMed] [Google Scholar]
- 5.Whitman MC, Greer CA. Adult neurogenesis and the olfactory system. Prog Neurobiol. 2009;89:162–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim DA, Huang YC, Alvarez-Buylla A. The adult neural stem cell niche: lessons for future neural cell replacement strategies. Neurosurg Clin N Am. 2007;18:81–92 [DOI] [PubMed] [Google Scholar]
- 7.Marks PA. Histone deacetylase inhibitors: a chemical genetics approach to understanding cellular functions. Biochim Biophys Acta. 2010;1799:717–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotner-Gohara E, Kim IK, Hammel M, Tainer JA, Tomkinson AE, Ellenberger T. Human DNA ligase III recognizes DNA ends by dynamic switching between two DNA-bound states. Biochemistry. 2010;49:6165–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tapiero H, Tew KD. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother. 2003;57:399–411 [DOI] [PubMed] [Google Scholar]
- 10.O’Halloran TV. Transition metals in control of gene expression. Science. 1993;261:715–25 [DOI] [PubMed] [Google Scholar]
- 11.Klug A, Schwabe JW. Protein motifs 5. Zinc fingers. FASEB J. 1995;9:597–604 [PubMed] [Google Scholar]
- 12.Freedman LP, Luisi BF. On the mechanism of DNA binding by nuclear hormone receptors: a structural and functional perspective. J Cell Biochem. 1993;51:140–50 [DOI] [PubMed] [Google Scholar]
- 13.Takeda A, Tamano H. Insight into zinc signaling from dietary zinc deficiency. Brain Res Rev. 2009;62:33–44 [DOI] [PubMed] [Google Scholar]
- 14.Stoll L, Hall J, Van Buren N, Hall A, Knight L, Morgan A, Zuger S, Van Deusen H, Gentile L. Differential regulation of ionotropic glutamate receptors. Biophys J. 2007;92:1343–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matias CM, Matos NC, Arif M, Dionisio JC, Quinta-Ferreira ME. Effect of the zinc chelator N,N,N’,N’-tetrakis (2-pyridylmethyl)ethylenediamine (TPEN) on hippocampal mossy fiber calcium signals and on synaptic transmission. Biol Res. 2006;39:521–30 [DOI] [PubMed] [Google Scholar]
- 16.Chowanadisai W, Kelleher SL, Lönnerdal B. Maternal zinc deficiency reduces NMDA receptor expression in neonatal rat brain, which persists into early adulthood. J Neurochem. 2005;94:510–9 [DOI] [PubMed] [Google Scholar]
- 17.Azman MS, Wan Suadi WS, Ilhami M, Mutalib MS, Rahman MT. Zinc intake during pregnancy increases the proliferation at ventricular zone of the newborn brain. Nutr Neurosci. 2009;12:9–12 [DOI] [PubMed] [Google Scholar]
- 18.Wang FD, Wei B, Ling WK, Fa JZ, Jun SG, Nai HJ. Maternal zinc deficiency impairs brain nestin expression in prenatal and postnatal mice. Cell Res. 2001;11:135–41 [DOI] [PubMed] [Google Scholar]
- 19.Aimo L, Mackenzie GG, Keenan AH, Oteiza PI. Gestational zinc deficiency affects the regulation of transcription factors AP-1, NF-κB and NFAT in fetal brain. J Nutr Biochem. 2010;21:1069–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corniola RS, Tassabehji NM, Hare J, Sharma G, Levenson CW. Zinc deficiency impairs neuronal precursor cell proliferation and induces apoptosis via p53-mediated mechanisms. Brain Res. 2008;1237:52–61 [DOI] [PubMed] [Google Scholar]
- 21.Adamo AM, Zago MP, Mackenzie GG, Aimo L, Keen CL, Keenan A, Oteiza PI. The role of zinc in the modulation of neuronal proliferation and apoptosis. Neurotox Res. 2010;17:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamano H, Kan F, Oku N, Takeda A. Ameliorative effect of Yokukansan on social isolation-induced aggressive behavior of zinc-deficient young mice. Brain Res Bull. 2010;83:351–5 [DOI] [PubMed] [Google Scholar]
- 23.Tauber SC, Schlumbohm C, Schilg L, Fuchs E, Nau R, Gerber J. Intrauterine exposure to dexamethasone impairs proliferation but not neuronal differentiation in the dentate gyrus of newborn common marmoset monkeys. Brain Pathol. 2006;16:209–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dvergsten CL, Johnson LA, Sandstead HH. Alterations in the postnatal development of the cerebellar cortex due to zinc deficiency. III. Impaired dendritic differentiation of basket and stellate cells. Brain Res. 1984;318:21–6 [DOI] [PubMed] [Google Scholar]
- 25.Dvergsten CL, Fosmire GJ, Ollerich DA, Sandstead HH. Alterations in the postnatal development of the cerebellar cortex due to zinc deficiency. II. Impaired maturation of Purkinje cells. Brain Res. 1984;318:11–20 [DOI] [PubMed] [Google Scholar]
- 26.Fukumoto K, Morita T, Mayanagi T, Tanokashira D, Yoshida T, Sakai A, Sobue K. Detrimental effects of glucocorticoids on neuronal migration during brain development. Mol Psychiatry. 2009;14:1119–31 [DOI] [PubMed] [Google Scholar]
- 27.Gao HL, Zheng W, Xin N, Chi ZH, Wang ZY, Chen J, Wang ZY. Zinc deficiency reduces neurogenesis accompanied by neuronal apoptosis through caspase-dependent and -independent signaling pathways. Neurotox Res. 2009;16:416–25 [DOI] [PubMed] [Google Scholar]
- 28.Suh SW, Won SJ, Hamby AM, Yoo BH, Fan Y, Sheline CT, Tamano H, Takeda A, Liu J. Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J Cereb Blood Flow Metab. 2009;29:1579–88 [DOI] [PubMed] [Google Scholar]
- 29.Pathak P, Kapil U, Dwivedi SN, Singh R. Serum zinc levels amongst pregnant women in a rural block of Haryana state, India. Asia Pac J Clin Nutr. 2008;17:276–9 [PubMed] [Google Scholar]
- 30.Kamau-Mbuthia E, Elmadfa I. Diet quality of pregnant women attending an antenatal clinic in Nakuru, Kenya. Ann Nutr Metab. 2007;51:324–30 [DOI] [PubMed] [Google Scholar]
- 31.Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, Oomen CA, Czéh B. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17 [DOI] [PubMed] [Google Scholar]
- 32.Takeda A, Tamano H, Kan F, Itoh H, Oku N. Anxiety-like behavior of young rats after 2-week zinc deprivation. Behav Brain Res. 2007;177:1–6 [DOI] [PubMed] [Google Scholar]
- 33.Tassabehji NM, Corniola RS, Alshingiti A, Levenson CW. Zinc deficiency induces depression-like symptoms in adult rats. Physiol Behav. 2008;95:365–9 [DOI] [PubMed] [Google Scholar]
- 34.Whittle N, Lubec G, Singewald N. Zinc deficiency induces enhanced depression-like behaviour and altered limbic activation reversed by antidepressant treatment in mice. Amino Acids. 2009;36:147–58 [DOI] [PubMed] [Google Scholar]
- 35.Siwek M, Dudek D, Paul IA, Sowa-Kućma M, Zieba A, Popik P, Pilc A, Nowak G. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: a double blind, placebo-controlled study. J Affect Disord. 2009;118:187–95 [DOI] [PubMed] [Google Scholar]
- 36.Cope EC, Levenson CW. Role of zinc in the development and treatment of mood disorders. Curr Opin Clin Nutr Metab Care. 2010;13:685–9 [DOI] [PubMed] [Google Scholar]