Abstract
Cannabis use and depressive disorders are thought to impair cognitive performance and psychosocial functioning. Both disorders co-occurring may compound the negative effects of these diagnoses. In this study we used the California Computerized Assessment Package (CalCAP) as the cognitive performance measure and the Addiction Severity Index (ASI) as the psychosocial functioning measure to compare depressed and non-depressed cannabis dependent individuals (n=108, 54 cannabis dependent only, 54 cannabis dependent and depressed/dysthymic). As predicted, cannabis dependent subjects with comorbid depression showed more psychosocial impairment than subjects with cannabis dependence alone. However contrary to our hypothesis, cannabis dependent subjects with comorbid depression showed less cognitive impairment in some CalCAP modules than subjects with cannabis dependence alone. Based on our results, we conclude that the additive effects of cannabis dependency and depression may only be limited to psychosocial domains, and may not extend to cognitive functioning.
Introduction
Cannabis is the most frequently used illicit drug in the United States. According to the 2006 National Household Survey on Drug Use,1 9% of people surveyed had used cannabis in the previous month, and 16% said they had used it in the previous year. Of all illicit substance use disorders in the United States, cannabis use disorders are the most common, with 1.5% of the population diagnosed as cannabis dependent or abusing.2 While cannabis use in the U.S. has remained steady through the late 1990s and into the early 2000s, incidence of DSM-IV cannabis abuse and dependence has risen significantly over the same time period.2
Although cannabis use is common, the full scope of its negative effects on psychosocial and cognitive areas is not completely understood. The decline in cognitive abilities specific to executive function such as in attention and memory, may be associated with long-term cannabis use.3, 4 Although rarely irreversible, it is unclear the duration of cognitive impairment in heavy cannabis users. Some residual effects appear to persist at least 7 days after last use, but by 28 days, cognitive deficits become immeasurable.5, 6 Visual processing speed, and immediate and delayed memory abilities showed significant deterioration in regular heavy cannabis users.7 Heavy cannabis users also appear to have impairments in mental flexibility and abstraction, along with other learning difficulties.8 Messinis et al. (2006) 9 found deficits in verbal memory, verbal fluency, attention, and psychomotor speed in long-term and short-term cannabis users. Impaired psychosocial functioning is also commonly linked to long-term cannabis use. Employment, social and family relations, physical and mental health, and global functioning can be substantially disrupted by frequent and chronic cannabis use. 10-13
The association between cannabis use and psychiatric diagnoses have been increasingly noted in recent literature. There is growing evidence confirming the long held suspicion that cannabis abuse may increase the risk of developing psychotic disorders.14, 15 Potential links between cannabis abuse and psychoses inherently garner a lot of attention due to the profoundly disabling qualities of psychotic disorders.16 Cannabis use has also been linked to other comorbid psychiatric diagnoses such as personality disorders, affective disorders, and anxiety disorders.17, 18 There is no consensus on whether cannabis use elicits the propensity of individuals to later develop a comorbid psychiatric disorder, or that pre-existing mental health conditions, or environmental conditions, ultimately lead to cannabis use. However, there is a general agreement that cannabis use and psychiatric diagnoses, when existing simultaneously, are inextricably connected.18 The occurrence of dysthymia and major depression in cannabis abusers is a problem that deserves increased attention. Some studies have shown a causal link between the two, with heavy cannabis use directly causing depression,19, 20 while others show individuals using cannabis to self-medicate existing depression.21-23
Depression alone can be extremely debilitating, both cognitively and psychosocially. Attentional and executive functioning limitations are prevalent in individuals with major depressive disorder (MDD) as are limitations in other neuropsychological domains. Memory, selective and sustained attention, and verbal and nonverbal learning performance levels are affected by MDD, indicative of a pervasive depressive symptomatology.24 A depressed individuals responsiveness to positive environmental cues is less than a non-depressed individual, and similarly, their processing of negative environmental feedback is extremely sensitive.25 Landro et al. (2001) 26 and Porter et al. (2003) 27 found neuropsychological deficits in depressed individuals, specifically in selective attention and working memory. Several groups have even found other more specific types of memory impairment in depressed patients.28-30 Depression has equally detrimental effects on social and occupational functioning, which are also found to persist after depressive episodes have subsided.31 Its impact on global and psychosocial functioning can often outweigh the cognitive impairment that has been more commonly associated with mood disorders.
Available evidence indicates that depression and cannabis dependence are commonly comorbid in the general population and in clinical samples; Nunes and Levin (2005) 32 note this frequent comorbidity in a meta-analysis of relevant pharmacotherapy clinical trials. The acute effects of both depression and cannabis use are well documented, and one could theoretically conclude that co-occuring symptomatology would have an additive effect on an individuals cognitive and psychosocial functioning. The cumulative influence of these diagnoses may increase negative outcomes in both areas.
In this study we examine the effects of depression on cognitive performance and psychosocial functioning in cannabis dependent individuals. Our primary and secondary hypotheses are, respectively: 1) The group with both depression and cannabis dependence will have slower reaction times and perform less accurately on cognitive measures than individuals who are only cannabis dependent, 2) The depressed cannabis dependent group will have greater impairment in psychosocial functioning as compared to the non-depressed cannabis dependent group.
Methods
Participants and Study Design
Fifty-four cannabis dependent (CD) and fifty-four diagnosed depressed/dysthmic cannabis dependent individuals (CD/MD), between the ages of 18 and 60, voluntarily chose to be evaluated at the Substance Treatment and Research Service. All participants were seeking treatment for cannabis use and/or depression. All participants were recruited from the New York City area through printed advertisements (newspapers, flyers, and advertisements on the subway) as well as radio and internet advertisements.
Participants underwent an extensive physical and psychiatric evaluation by a physician. The physical evaluation involved an assessment of prior medical conditions as well as a physical exam, electrocardiogram, and blood test. The psychiatric evaluation included the Structured Clinical Interview (SCID) 33 for Diagnostic and Statistical Manual of Mental Disorders (4th ed. [DSM-IV]); Axis I disorders.34 Participants with unstable medical conditions or Axis I disorders other than major depression or dysthymia were excluded from the study. Additionally, participants physiologically dependent on drugs other than cannabis and nicotine were excluded.
Participants in the CD only group were required to meet DSM-IV criteria for cannabis dependence. The CD group was enrolled in a clinical trial of Dronabinol for the treatment of cannabis dependence. Participants had not yet been administered Dronabinol at the time of assessment of cognitive and psychosocial functioning.
Participants in the depressed cannabis dependent group were required to meet DSM-IV criteria for cannabis dependence and also for major depressive disorder or dysthymia. The rationale for using both sub-types of depression in the CD/MD group is the relative similarity between both depression diagnoses. Not only are the treatment modalities for dysthymia and depression virtually congruent, but Klein et al. (1996) 35 notes little substantiation for qualitative differences in symptomatolgy between the two. 36-38 There is also a high level of comorbidity between dysthymia and substance abuse disorders, similar to that of depression. 20, 39 Inclusion in the CD/MD group required a score ≥ 12 on the Hamilton Depression Rating Scale (Ham-D).40-42 The Ham-D is a 21-question clinician administered multiple choice questionnaire that rates the severity of participants depressive symptomatology based on the participants subjective experience and the clinicians observations during the interview. The questionnaire rates the severity and pervasiveness of specific symptoms often observed in depression such as low mood, weight fluctuations, insomnia, agitation, and anxiety. Some items are scored on a 5-point scale, ranging from 0 = not present to 4= severe, and some are scored from 0-2 in subjective severity. The highest achievable score would be 67. Scores of 0-7 are considered normal, 8-13 are associated with mild depression, 14-18 moderate, 19-22 severe, and 23 or greater very severe depression. Furthermore, patients in the CD/MD group could not be taking any psychotropic medication (except specifically prescribed for treating insomnia). Individuals in the CD/MD group were enrolled in a clinical trial of Venlafaxine for the treatment of cannabis dependence and comorbid depressive or dysthymic disorders. Participants were not yet administered Venlafaxine at the time of assessment.
Setting
Participants were screened and tested at the Substance Treatment and Research Service (STARS) at the New York State Psychiatric Institute. STARS has two sites, one in mid-town Manhattan, and another located at Columbia-Presbyterian Medical Center in upper Manhattan.
Assessments
Psychosocial Functioning
Psychosocial information was collected using the Addiction Severity Index (ASI).43-45 The ASI is a widely used clinical assessment of severity of psychosocial problems stemming from substance addiction. The ASI provides severity scores for seven dimensions of psychosocial functioning: medical, employment, alcohol use, drug use, mental status, legal, and family/social relations. Composite scores are calculated to indicate severity of impairment in each psychosocial dimension. Scores range from .00 to 1.00 with higher scores representing greater need for treatment in that particular life area. A computerized version of the ASI, the ASI-Multimedia Version (ASI-MV®), was used for this study. The ASI-MV® is an interactive program that allows for client self-administration. Question and answer options are presented verbally as well as written on the screen. In clinical studies, the ASI-MV® has proven to have excellent reliability and validity.46 The average duration for one session of the ASI is 30-45 minutes
Cognitive Functioning
To assess cognitive functioning, participants were administered the California Computerized Assessment Package (CalCAP).47 The CalCAP is a continuous performance task designed to provide a standardized assessment of cognitive functions requiring focused and sustained attention. The CalCAP measures participants responses to 10 psychomotor reaction tasks relevant to several dimensions of cognitive functioning. Each assessment requires the participant to respond when presented with visual stimuli. These 10 psychomotor reaction tasks consisted of four simple reaction tests (e.g. participant responds every time a visual stimulus appears on the screen using their dominant hand and non-dominant hand) and six choice reaction tests that built on the simple reaction tasks by adding more complex areas of memory, language skills, and selective attention. The six choice reaction tasks were comprised of: 1) Choice Reaction (CR) time for single digits where participants were instructed to press a key when they saw a specific number (simple memory) 2) Sequential Reaction (SR) where participants responded when they saw two of the same number in succession (complex memory) 3) Lexical Discrimination (LD) where participants responded only when they saw a word that fit a specific category (task sensitive to disruptions in language skills) 4) Degraded Words (DW) with distraction where participants responded only if they saw the word “seven” with other words flashing on the periphery (visual selective attention) 5) Response Reversal (RR) and rapid visual scanning where participants ignored the central word and responded only to the words on the periphery (this task tested the participants ability to change cognitive set from the previous task and also required a more rapid visual scanning process across the entire display screen) 6) Form discrimination (FD) where participants were asked to respond only when two of three geometric figures were identical (tasks required participant to quickly compare non-nameable forms).48 All patients were administered the CalCAP in a quiet room to reduce distractions. The duration for one session of the CalCAP is approximately 20 minutes.
Drug use
The Recent Drug Use Questionnaire was used to assess lifetime history of drug use as well as use during the 30 days preceding the initial evaluation. By convention, all methods of cannabis administration were converted to joints by a system adopted at STARS: 3 bowls are equivalent to 1 joint, 1 blunt is equivalent to 3 joints. All participants reported smoking cannabis, no participants reported orally ingesting cannabis.
Dependent Measures
Cognitive performance was assessed with the CalCAP using two dependent variables: 1) Mean Reaction times (millisecond) on each of the 10 tasks were calculated for each patient, 2) Mean Accuracy of response was calculated for the 6 complex reaction tasks and was based on the proportion of true positive responses minus the proportion of false positive responses.
Psychosocial functioning was assessed by comparing the composite scores on the seven ASI subscales: medical, employment, alcohol and drug use, family/social relationships, mental status and legal.
Data Analyses
All demographic and substance use data was analyzed using independent sample t-tests and Chi squares, using a significance level of 0.05. T-tests were used to compare the two groups on all psychosocial and cognitive modules. As a secondary analysis, multiple main effect linear regression models were used to assess the correlation between scores on the Hamilton Depression Rating Scale and the main outcome measures, ASI and CalCAP, after controlling for age and joints per week. All analyses comparing the groups on primary outcome measures were conducted using a Holm-Bonferroni adjusted significance level to account for multiple testing.
Results
Demographics
The sample (n=108) consisted of 86 males (79.6%), and was 27.8% Hispanic, 43.5% Caucasian, 23.1% Black, and 5.6% other. The average age was 37.5 (±10.4) and the average years of education were 14.0 (±2.6). 62.9% were employed, 33.3% were unemployed, and the remaining participants were students. The average household income for all participants was $55,500 (±59,566). As for marital status, 53.7% were single, 33.3% were married, and 13.0% were separated or divorced. The average age of first cannabis use episode was at 15.6 (±3.9) years old, and first regular use was at 18.8 (±5.1) years old. The average number of days per week of cannabis use was 6.5 (±1.1), and the average number of joints per day 6.3 (±8.1). There were no significant differences in baseline demographic and clinical characteristics between the CD group and the CD/MD group, except in average income [$74,895 (±73,606) vs. $36,104 (±31,480); t=-3.21; p=.002], with the CD group earning significantly more annually. As for alcohol, cocaine, and nicotine use, there were no significant differences between the groups, except for number of days per week nicotine was used, with the CD/MD group smoking cigarettes on average 2.85 days (±3.4) vs.1.65 (±2.9) for the CD group (t=1.99; p=.05) Within the CD/MD group, 43 (80%) were diagnosed with major depressive disorder, and 11 (20%) were diagnosed with dysthymic disorder, with no significant difference between the two diagnoses in Hamilton Depression ratings. There was a significant difference between the CD and CD/MD groups in their Hamilton Depression ratings, with the CD scoring a mean of 4.9 (±3.3), and the CD/MD scoring a mean of 18.4 (±4.1) (t=18.46; p<.0001).
CalCAP: Reaction time
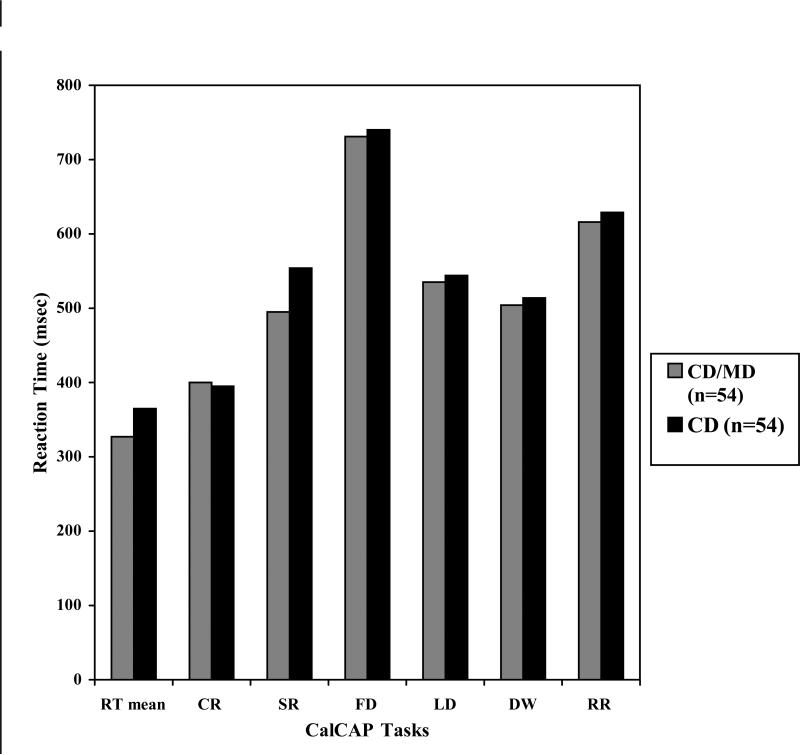
The simple reaction tasks were assessed at four separate time points during the administration of the CalCAP program, once at the beginning, twice in the middle and once at the end. This structure was embedded in the format of the CalCAP and remained the same for all participants. The three dominant hand simple reaction tasks were averaged for each participant and the non-dominant hand reaction time was omitted in the analyses. A Holm-Bonferroni correction was used to account for multiple testing and the results of the analyses showed a statistical trend with the CD/MD group recording a faster average reaction time as compared to the CD group (.327 seconds ±.060 vs. .365 ±.086; t=-2.403; df=95; p=.02). In terms of choice reaction time, the only significant difference between the two groups was in the sequential reaction task, with the CD/MD group similarly recording faster reaction times than the CD group (.495 seconds ±.074 vs. .555 ±.119; t=-2.89; df=95; p=.01).
CalCAP: Accuracy of Response
To calculate accuracy, relative proportion of true positive responses was subtracted from relative proportion of false positive responses. For sequential reaction accuracy, the CD/MD group scored significantly better than the CD group after a Holm-Bonferroni correction, with average means of 91% (±10%) and 79% (±27%), respectively (t=2.94; df=95; p=.004). There was also a similar statistical trend that occurred in the lexical discrimination module, with the CD/MD group, 69% (±15%), also achieving a higher mean accuracy percentage as compared with CD group, 65% (±20%) (t=-1.73; df=101; p=.09). All other choice reaction measures did not differ in overall mean accuracy.
ASI Composite Scores
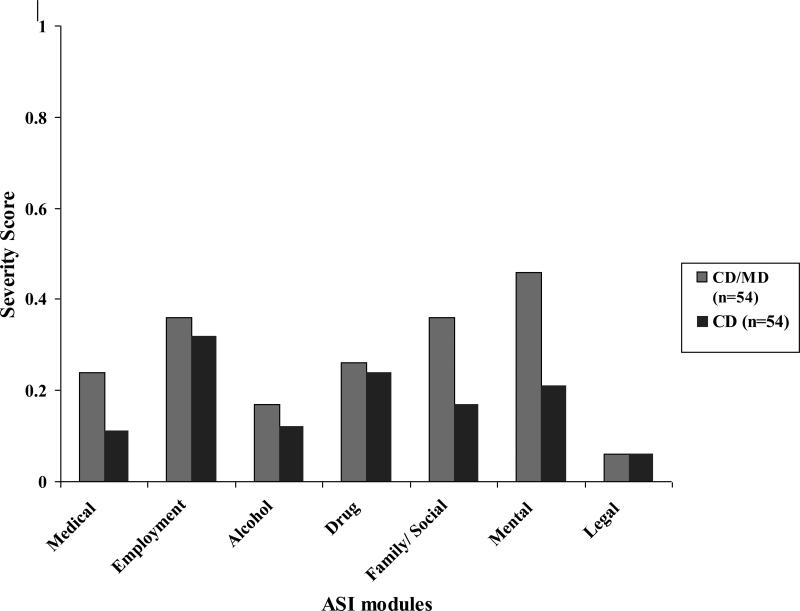
There are seven assessed psychosocial domains that receive composite scores based on the participants perceived severity of dysfunction in that area. For the medical domain, the mean score for the CD group was .11 (±.21) vs. .24 (±.27) for the CD/MD group, with the CD/MD group showing a significantly greater impairment compared with CD group (t=2.65; p=.01). For family/social relationships, the CD group mean score was .17 (±.17), and the CD/MD group mean score was significantly greater in severity at .36 (±.24) (t=4.52; p<.0001). The last domain assessed in the ASI was mental status, which showed a less marked impairment for the CD group versus the CD/MD group, .21 (±.19) vs. .46 (±.15); t=7.38; p<.0001. No significant difference in severity scoring was detected for employment, alcohol use, drug use, or legal domains.
Severity of Depression and Cognitive and Psychosocial measures
The results of the linear regression analysis of Ham-D scores showed very similar predictive outcomes on the ASI and CalCAP. The analysis was done so that age and number of joints per week were controlled for. When looking at composite ASI scores for both groups, the linear regression model showed that Ham-D was significantly correlated with the medical (t= 2.81, p= .01), drug use (t= 2.58, p= .01), mental status (t= 8.60, p=.0001 ), and family/social relations (t=6.66, p=.0001 ) modules. Per unit increase on the Ham-D, there was on average a .009 point increase in the medical score on the ASI, .004 point increase on the drug use score, .018 point increase on the mental status score, and .017 point increase on the family/social relations score. All other ASI modules showed no significant linear correlation.
The CalCAP regression analysis showed statistical trends for linear negative correlations between the Ham-D and the dominant hand simple reaction time mean and sequential reaction time, and a positive correlation with the Ham-D and sequential accuracy, when age and joints per week were controlled for. After a Holm-Bonferroni correction was applied to account for multiple testing, the dominant hand reaction time means revealed a trend in the data, showing a negative correlation with Ham-D scores such that per unit increase on Ham-D, there was an average of .002 second decrease on the participants reaction time (t=-1.90; p= .06). Similarly, for every unit increase on the Ham-D, there was an average of .004 second decrease on sequential reaction time measure (t= -2.65; p=.01). In terms of accuracy, the sequential reaction task linear regression analysis showed a similar trend with a .7 percent increase in accuracy for every unit increase of Ham-D (t=2.50; p=.01). There was no detectable significant correlation on any CalCAP module after accounting for multiple testing.
Discussion
The results of the analyses may suggest an increased need for psychosocial intervention for cannabis dependent and depressed treatment seeking patients. However, the assumption that this population is more cognitively impaired than the solely cannabis dependent population may in fact be false, evident in that the depressed group had the same, if not better, reaction time and precision on several cognitive tasks. Although the data is consistent with our hypothesis that psychosocial areas are more severely impacted in a depressed cannabis dependent population than one without depression, our hypothesis that cognitive impairment is also more severely impacted was not supported. The conventional understanding of depression is that it affects individuals on multiple levels. The accepted clinical paradigm regarding depression is that it distresses normal psychosocial functioning and impedes cognitive functioning.49-51, 31 By a different rationale, a similar assertion is made in the field regarding the impact of chronic cannabis use, with both areas being impaired.5, 12, 4, 13 Our results did not replicate what is the standard agreed notion of the effect of depression on cognitive ability, but the data did show that psychosocial functioning would be negatively affected.
As demonstrated by this study, the impact of co-occurring cannabis dependence and depression is not clear. If we assumed that cognitive and psychosocial dysfunction would be compounded when both CD and MD are present, than the CD only group should have had greater cognitive and psychosocial functioning than those with CD/MD, however this is not entirely the case. In terms of the cognitive functioning, the CD/MD group had a similar, if not slightly faster, mean reaction time on the simple reaction tasks, and in the sequential reaction task (complex memory) they had better reactions and accuracy. For all other cognitive modules, there were no statistically significant differences between the two groups, however several trends in the data were noted. In terms of psychosocial functioning, the conventional thinking surrounding depression was supported in that the CD/MD group had more severe problems in the medical, family/social, and mental status domains.31
The trends in the linear regression analysis reaffirmed that an increase in depressive symptoms (Ham-D score) may be accompanied by a slight increase in accuracy and decrease in reaction time on a few CalCAP modules, and significantly higher overall ASI composite scores. Although elevated Ham-D scores are not always directly linked to a depression diagnosis, it is a standard indicator of depressive symptomology. The hypothesis that psychosocial areas will be more severely impacted for cannabis dependent people with depression was supported by the regression analysis, with increasing Ham-D scores corresponding to increasing levels of difficultly functioning in many critical areas. Although it could not be definitively said that increasing Ham-D scores also corresponded to better performance on the CalCAP's cognitive assessment, the trends in the data support the possibility of this occurrence. The observed statistical trend in the Ham-D's correlation to participants CalCAP performance makes it that much more salient that exacerbated cognitive deficiencies do not arise from a concurrent cannabis dependence and depression symptomology.
In an effort to unmask the unique differences between these two groups and aid in tailor-making treatment for these populations with the potential for greater success, a discrepancy was uncovered between what is known and the outcome of this research. It appears that addressing, or even just being aware of intensified cognitive differences of cannabis treatment seekers with depression is not warranted. In fact the opposite may be true, with this population showing slightly more competency on some cognitive performance tasks than the non-depressed group. It might be that this group was more diligent about attempting to score their absolute best on the cognitive tasks in an effort to garner greater approval. It could also be that the non-depressed group simply did not try as hard because the outcome was not tied to any issues of self-worth and there was little motivation to do well. When compared with a normative control population, both the CD group and the CD/MD group were well within normal limits for all the areas assessed in the CalCAP.47 Despite an enormous body of research looking into the effects of depression on cognitive functioning and the specific cognitive abilities that are impacted such as memory, verbal and nonverbal learning, selective and sustained attention, alertness, cognitive flexibility, problem-solving, and planning, according to the data it seems that concurrent cannabis dependence and depression does not lead to greater than expected cognitive deficiencies.49, 52
As for psychosocial domains, the need for intervention in these areas is supported by the data. The non-depressed group was not entirely unaffected in relevant areas, but there is undoubtedly a need for addressing domains that are negatively impacted by a comorbid diagnosis. Individuals with depression often report physical symptomology associated with their depression that may inhibit emotional recovery, and develop detrimental social relationships linked to their dysphoric mood and blunted affect, both supported by our findings.53 One of the most important caveats of these data that certainly warrants further examination is that depressed CD individuals are similar to depressed non-users, and that self-medication of cannabis does not seem to dull the participants perceived difficulty within certain areas of their life. Depressed CD individuals are likely hindered in recovery from both substance abuse and mood disorder diagnoses because of their pervasive global functioning impairment.
There are limitations of the study, which should be considered when interpreting the results. (i) The time of day at which subjects were tested was not consistent. Although subjects were all tested between 9am and 5pm, the time testing occurred could have affected CalCAP scores. (ii) This investigation only used one cognitive test battery, and a more robust cognitive measure may elucidate additional strengths and weaknesses of both groups. A more comprehensive neuropsychological battery may expose more typical results. (iii) While all volunteers underwent thorough medical screening, and exclusion criteria for the depressed group included currently being prescribed psychotropic medication by another physician (except for the treatment of acute insomnia), this study did not account for other prescribed or over-the-counter medications participants may have been taking at the time of testing, or for psychotropic medications being taken by the non-depressed group. (iv) Furthermore, although subjects were regularly screened for drugs of abuse, they were not necessarily screened immediately before taking the CalCAP. There is the possibility that some subjects may have been under the influence of non-prescribed or illicit drugs, including cannabis, which may have inhibited peak performance. And while subjects did not appear to be under the influence of cannabis, residual effects from their last use could have subtly impaired their performance. When looking at last reported alcohol and cannabis use, 19% of CD participants used alcohol the night before their test, drinking an average of 2.7 drinks (±1.2), while 25% of CD/MD participants used alcohol the day prior to their test, drinking an average of 1.9 drinks (±1.4). As for cannabis, 90% of the CD group used cannabis the day before the test, while 84% of the CD/MD group used the day prior to test day. There was no difference between the two groups in terms of alcohol or cannabis use the day before the CalCAP. (v) In terms of psychosocial outcomes, it is possible that the significant discrepancy in annual income influenced participants subjective opinion of certain areas of functioning. (vi) A possible reason for the disparity in several cognitive measure outcomes could be that cognitively adept cannabis dependent individuals with a negative view of their status as a substance user may be at a higher risk for depression.
Due to the counterintuitive nature of this study's finding that the depressed subgroup showed the same, if not less, cognitive impairment as compared with non-depressed sub-group, we hope that future studies will attempt to verify this result. Furthermore, although the CalCAP is effective in assessing reaction times, speed of information processing, rapid visual scanning, form discrimination, brief memory, and divided attention, other measures may provide a broader picture of cognitive functioning and other relevant cognitive comparisons between depressed and non-depressed cannabis dependent patients. Ideally, future studies will utilize a broader variety of cognitive tests in order to attain convergent validity of their findings. Nonetheless, the outcome of this study showed that cognitive functioning may be the least affected area from this comorbidity, somewhat counter to our accepted understanding of depressions impact on cognition. A greater understanding of the complexity of this population will ultimately prove helpful in creating a flexible treatment model that can be effective for various cannabis dependent subgroups.
Figure 1.
Mean CalCAP reaction times
Figure 2.
Mean ASI composite scores
Table 1.
Demographic Characteristics
| CD/MD (n=54) | CD (n=54) | P value | |
|---|---|---|---|
| Gender | |||
| Male | 42 (77.7%) | 44 (81.4%) | df= 1; χ2 = .23; p= .63 |
| Female | 12 (22.3%) | 10 (19.6%) | |
| Race | |||
| Hispanic | 12 (22.3%) | 18 (33.3%) | df= 3; χ2 = 2.25; p= .52 |
| Black | 14 (25.9%) | 11 (20.4%) | |
| Caucasian | 24 (44.4%) | 23 (42.6%) | |
| Other Race | 4 (7.4%) | 2 (3.7%) | |
| Age | 37.65 ±11.15 | 37.35 ±9.65 | t106= .15; p=.88 |
| Education (years) | 13.76 ±2.82 | 14.34 ±2.44 | t104= -1.14; p=.26 |
| Marital Status | |||
| Single | 29 (53.7%) | 29 (53.7%) | df= 3; χ2 = 6.44; p=.09 |
| Married w/spouse | 14 (25.9%) | 22 (40.7%) | |
| Separated | 6 (11.1%) | 2 (3.7%) | |
| Divorced | 5 (9.3%) | 1 (1.9%) | |
| Current Employment | |||
| Employed | 29 (53.7%) | 39 (72.2%) | df= 2; χ2 = 4.25; p= .12 |
| Unemployed | 23 (42.6%) | 13 (24.1%) | |
| Student | 2 (3.7%) | 2 (3.7%) | |
| Income | 36,104.55±31,480.32 | 74,895.59±73,606.07 | t86= -3.21; p=.002 |
Table 2.
Patterns of cannabis and other drug use
| CD/MD | CD | p value | |
|---|---|---|---|
| Cannabis use | |||
| Age of first use (years) | 15.92 ±4.56 | 15.26 ±3.15 | t104= .868; p=.39 |
| Age of regular use | 19.33 ±5.86 | 18.25 ±4.10 | t103= 1.098; p=.28 |
| Number days per week used | 6.44 ±1.65 | 6.58 ±.84 | t105= -.551; p=.58 |
| Amt of cannabis used per using day in prior 7 days (joints) | 6.57 ±6.94 | 5.96 ±9.16 | t104= .389; p= .70 |
| Alcohol use | |||
| Number days per week used | 1.83 ±2.37 | 1.69 ±2.21 | t105= .328; p=.74 |
| Amt of alcohol used per using day in prior 7 days (drinks) | 2.63 ±3.36 | 2.24 ±2.89 | t106=.645; p=.52 |
| Cocaine/Crack* use | |||
| Number of days per week used | .09 ±.44 .13 ±.95* |
.19 ±.55 .04 ±.19* |
t106=-.959; p=.34 t106=.700; p=.49* |
| Amt of cocaine/crack* used per using day in prior 7 days (dollar amount) | 4.07 ±20.69 2.78 ±20.41* |
3.74 ±14.04 1.94 ±13.61* |
t106=.098; p=.92 t106=.250; p=.80* |
Table 3.
CalCAP Accuracy of Response
| CD/MD (%) | CD (%) | p value | |
|---|---|---|---|
| Complex Reaction Tasks | |||
| CR | 98 ±4 | 95 ±13 | t95= .952; p= .34 |
| SR | 91 ±10 | 79 ±27 | t95= 2.937; p= .004 |
| FD | 65 ±20 | 69 ±15 | t92=-1.166; p= .27 |
| LD | 78 ±33 | 87 ±20 | t101=-1.731; p= .09 |
| DW | 92 ±9 | 91 ±10 | t94= .444; p= .66 |
| RR | 87 ±11 | 84 ±13 | t92= 1.282; p= .20 |
Table 4.
Regression Analysis- Severity of Depression and Cognitive/Psychosocial
| Variable | B (Standard error) | tdf | P value |
|---|---|---|---|
| ASI | |||
| Medical | |||
| Constant | -.052 (.103) | t3= -.508 | p= .61 |
| HAM-D | .009 (.003) | t3= 2.81 | p= .01* |
| Age | .003 (.002) | t3= 1.27 | p= .21 |
| Joints per day | .002 (.003) | t3= .50 | p= .62 |
| Mental Status | |||
| Constant | .176 (.069) | t3= 2.55 | p= .01 |
| HAM-D | .018 (.002) | t3= 8.60 | p <.0001* |
| Age | -.001 (.002) | t3= -.90 | p= .37 |
| Joints per day | .000 (.002) | t3= -.38 | p= .70 |
| Family/social | |||
| Constant | .066 (.083) | t3= .80 | p= .43 |
| HAM-D | .017 (.003) | t3= 6.66 | p <.0001* |
| Age | .000 (.003) | t3= .12 | p= .91 |
| Joints per day | -.001 (.002) | t3= -.42 | p= .68 |
| Drug | |||
| Constant | .220 (.046) | t3= 4.74 | p<.0001 |
| HAM-D | .004 (.001) | t3= 2.58 | p= .012* |
| Age | .000 (.001) | t3= -.35 | p= .72 |
| Joints per day | -2.60E-5 (.001) | t3= -.02 | p= .99 |
| CALCAP | |||
| Simple reaction time mean | |||
| Constant | 303.609 (33.016) | t3= 9.20 | p<.0001 |
| HAM-D | -1.945 (1.023) | t3= -1.90 | p= .06* |
| Age | 1.863 (.774) | t3=2.41 | p= .02 |
| Joints per day | -.631 (.967) | t3= -.65 | p= .52 |
| SR reaction time | |||
| Constant | 574.737 (45.499) | t3=12.63 | p<.0001 |
| HAM-D | -3.730 (1.410) | t3= -2.65 | p= .01* |
| Age | -.049 (1.067) | t3= -.05 | p= .96 |
| Joints per day | -.279 (1.333) | t3= -.21 | p= .84 |
| SR Accuracy | |||
| Constant | .761 (.095) | t3=7.97 | p<.0001 |
| HAM-D | .007 (.003) | t3= 2.50 | p= .01* |
| Age | .000 (.002) | t3= .13 | p= .90 |
| Joints per day | -.001 (.003) | t3= -.48 | p= .63 |
Acknowledgements
This research was supported by NIDA grants P50DA09236, R01DA15451, K23021209, and K00465. We would also recognize the extraordinary help and support from all the staff at the Substance Treatment and Research Service (STARS) for their clinical research and administrative support.
References
- 1.Substance Abuse and Mental Health Services Administration (SAMHSA) Office of Applied Studies (2007), Results from the 2006 National Survey on Drug Use and Health: National Findings. 2007 September;:07–4293. [Google Scholar]
- 2.Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991-1992 and 2001-2002. JAMA. 2004;291(17):2114–21. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- 3.Pope HG, Jr., Gruber AJ, Yurgelun-Todd D. Residual nueropsychologic effects of cannabis. Curr Psychiatry Rep. 2001;3(6):507–12. doi: 10.1007/s11920-001-0045-7. [DOI] [PubMed] [Google Scholar]
- 4.Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287(9):1123–31. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- 5.Pope HG, Jr., Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58(10):909–15. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- 6.Pope HG, Jr., Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive measures in long-term cannabis users. J. Clin. Pharmacol. 2002;42:41S–47S. doi: 10.1002/j.1552-4604.2002.tb06002.x. [DOI] [PubMed] [Google Scholar]
- 7.Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana—a comparison with pre-drug performance. Neurotoxicology and Teratology. 2005;27(2):231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Pope HG, Jr., Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275(5):521–7. [PubMed] [Google Scholar]
- 9.Messinis L, Kyprianidou A, Malefaki S, Papathanasopoulos P. Neuropsychological deficits in long-term frequent cannabis users. Neurology. 2006;65(5):737–9. doi: 10.1212/01.wnl.0000201279.83203.c6. [DOI] [PubMed] [Google Scholar]
- 10.Lehman WE, Simpson DD. Employee substance use and on-the-job behaviors. J Appl Psychol. 1992;77(3):309–21. doi: 10.1037/0021-9010.77.3.309. [DOI] [PubMed] [Google Scholar]
- 11.Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000;95(11):1621–30. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- 12.Eisen SA, Chantarujikapong S, Xian H, Lyons MJ, Toomey R, True WR, Scherrer JF, Goldberg J, Tsuang MT. Does marijuana use have residual adverse effects on self-reported health measures, socio-demographics and quality of life? A monozygotic co-twin control study in men. Addiction. 2002;97(9):1137–44. doi: 10.1046/j.1360-0443.2002.00120.x. [DOI] [PubMed] [Google Scholar]
- 13.Gruber AJ, Pope HG, Hudson JI, Yurgelun-Todd D. Attributes of long-term heavy cannabis users: a case-control study. Psychol Med. 2003;33(8):1415–22. doi: 10.1017/s0033291703008560. [DOI] [PubMed] [Google Scholar]
- 14.Hall W, Degenhardt L. Cannabis use and psychosis: a review of clinical and epidemiological evidence. Australian and New Zealand Journal of Psychiatry. 2000;34(1):26–34. doi: 10.1046/j.1440-1614.2000.00685.x. [DOI] [PubMed] [Google Scholar]
- 15.Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. The Lancet. 2007;370(9584):319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 16.Hall W. Cannabis use and psychosis. Drug Alcohol Rev. 1998;17(4):433–44. doi: 10.1080/09595239800187271. [DOI] [PubMed] [Google Scholar]
- 17.Agosti V, Levin FR. Predictors of Cannabis Dependence Recovery Among Epidemiological Survey Respondents in the United States. The American Journal of Drug and Alcohol Abuse. 2007;33(1):81–88. doi: 10.1080/00952990601087364. [DOI] [PubMed] [Google Scholar]
- 18.Arendt M, Munk-Jorgensen P. Heavy cannabis users seeking treatment- prevalence of psychiatric disorders. Soc Psychiatry Psychiatr Epidemiol. 2004;39(2):97–105. doi: 10.1007/s00127-004-0719-7. [DOI] [PubMed] [Google Scholar]
- 19.Bovasso GB. Cannabis abuse as a risk factor for depressive symptoms. Am J Psychiatry. 2001;158(12):2033–7. doi: 10.1176/appi.ajp.158.12.2033. [DOI] [PubMed] [Google Scholar]
- 20.Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression.”. Addiction. 2003;98(11):1493–504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 21.Gruber AJ, Pope HG, Jr., Brown ME. Do patients use marijuana as an antidepressant? Depression. 1996;4(2):77–80. doi: 10.1002/(SICI)1522-7162(1996)4:2<77::AID-DEPR7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 22.Harder VS, Morral AR, Arkes J. Marijuana use and depression among adults: Testing for causal associations. Addiction. 2006;101(10):1463–72. doi: 10.1111/j.1360-0443.2006.01545.x. [DOI] [PubMed] [Google Scholar]
- 23.Schofield D, Tennant C, Nash L, Degenhardt L, Cornish A, Hobbs C, Brennan G. Reasons for cannabis use in psychosis. Aust N Z J Psychiatry. 2006;40(6-7):570–4. doi: 10.1080/j.1440-1614.2006.01840.x. [DOI] [PubMed] [Google Scholar]
- 24.Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. J Affect Disord. 2005;89(1-3):125–35. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. Am J Psychiatry. 2007;164(4):608–16. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- 26.Landro NI, Stiles TC, Sletvold H. Neuropsychological function in nonpsychotic unipolar major depression. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14(4):233–40. [PubMed] [Google Scholar]
- 27.Porter RJ, Gallagher P, Thompson JM, Young AH. Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry. 2003;182:214–220. doi: 10.1192/bjp.182.3.214. [DOI] [PubMed] [Google Scholar]
- 28.Barch DM, Sheline YI, Csernansky, Synder AZ. Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol Psychiatry. 2003;53(5):376–84. doi: 10.1016/s0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- 29.Egeland J, Sundet K, Rund BR, Asbjornsen A, Hugdahl K, Landro NI, Lund A, Roness A, Stordal KI. Sensitivity and specificity of memory dysfunction in schizophrenia: a comparison with major depression. J Clin Exp Nueropsychol. 2003;25(1):79–93. doi: 10.1076/jcen.25.1.79.13630. [DOI] [PubMed] [Google Scholar]
- 30.Ellwart T, Rinck M, Becker ES. Selective memory and memory deficits in depressed inpatients. Depression Anxiety. 2003;17(4):197–206. doi: 10.1002/da.10102. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy N, Foy K, Sherazi R, McDonough M, McKeon P. Long-term social functioning after depression treated by psychiatrists: a review. Bipolar Disord. 2007;9(1-2):25–37. doi: 10.1111/j.1399-5618.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 32.Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291(15):1887–96. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, William JBW. Biometrics Research Department. New York State Psychiatric Institute; 1995. Structured Clinical Interview for DSM-IV Axis I Disorders- Patient Edition (SCID-I/P, Version 2.0) [Google Scholar]
- 34.American Psychiatric Association (APA) 4th Edition American Psychiatric Press; Washington, DC: 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 35.Klein DN, Kocsis JH, McCullough JP, Holzer CE, 3rd, Hirschfeld RM, Keller MB. Symptomatology in dysthymic and major depressive disorder. Psychiatr Clin North Am. 1996;19(1):41–53. doi: 10.1016/s0193-953x(05)70272-0. [DOI] [PubMed] [Google Scholar]
- 36.Westermeyer J, Eames SL, Nugent S. Comorbid dysthymia and substance disorder: Treatment history and cost. Am J Psychiatry. 1998;155(11):1556–60. doi: 10.1176/ajp.155.11.1556. [DOI] [PubMed] [Google Scholar]
- 37.Cuijpers P, van Straten A, Schuurmans J, van Oppen P, Hollon SD, Andersson G. Psychotherapy for chronic major depression and dysthymia: A meta-analysis. Clin Psychol Rev. 2009 Sep 12; doi: 10.1016/j.cpr.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Silva de Lima M, Hotopf M. A comparison of active drugs for treatment of dysthymia. Cochrane Database Syst Rev. 2003;(3):CD004047. doi: 10.1002/14651858.CD004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eames SL, Westermeyer J, Crosby RD. Substance use and abuse among patients with comorbid dysthymia and substance disorder. Am J Drug Alcohol Abuse. 1998;24(4):541–50. doi: 10.3109/00952999809019606. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knesevich JW, Biggs JT, Clayton PJ, Ziegler VE. Validity of the Hamilton Rating Scale for depression. Br J Psychiatry. 1977;131:49–52. doi: 10.1192/bjp.131.1.49. [DOI] [PubMed] [Google Scholar]
- 42.O'Hara MW, Rehm LP. Hamilton Rating Scale for Depression: reliability and validity of judgments of novice raters. J Consult Clin Psychol. 1983;51(2):318–9. doi: 10.1037//0022-006x.51.2.318. [DOI] [PubMed] [Google Scholar]
- 43.Hodgins DC, el-Guebaly N. More data on the Addiction Severity Index. Reliability and validity with the mentally ill substance abuser. J Nerv Ment Dis. 1992;180(3):197–201. doi: 10.1097/00005053-199203000-00009. [DOI] [PubMed] [Google Scholar]
- 44.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 45.Bovasso GB, Alterman AI, Cacciola JS, Cook TG. Predictive validity of the Addiction Severity Index's composite scores in the assessment of 2-year outcomes in a methadone maintenance population. Psychol Addict Behav. 2001;15(3):171–6. [PubMed] [Google Scholar]
- 46.Butler SF, Budman SH, Goldman RJ, Newman FL, Beckley KE, Trottier D, Cacciola JS. Initial validation of a computer-administered Addiction Severity Index: the ASI-MV. Psychol Addict Behav. 2001;15(1):4–12. doi: 10.1037/0893-164x.15.1.4. [DOI] [PubMed] [Google Scholar]
- 47.Miller EN, Satz P, Visscher B. Computerized and conventional neuropsychological assessment of HIV-1-infected homosexual men. Neurology. 1991;41(10):1608–16. doi: 10.1212/wnl.41.10.1608. [DOI] [PubMed] [Google Scholar]
- 48.Brooks DJ, Vosburg SK, Evans SM, Levin FR. Assessment of cognitive functioning of methadone-maintenance patients: impact of adult ADHD and current cocaine dependence. J Addict Dis. 2006;25(4):15–25. doi: 10.1300/J069v25n04_02. [DOI] [PubMed] [Google Scholar]
- 49.Austin MP, Ross M, Murray C, O'Carroll RE, Ebmeier KP, Goodwin GM. Cognitive function in major depression. J Affect Disord. 1992;25(1):21–29. doi: 10.1016/0165-0327(92)90089-o. [DOI] [PubMed] [Google Scholar]
- 50.Ottowitz WE, Dougherty DD, Savage CR. The neural network basis for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harv Rev Psychiatry. 2002;10(2):86–99. doi: 10.1080/10673220216210. [DOI] [PubMed] [Google Scholar]
- 51.Solomon DA, Leon AC, Endicott J, Mueller TI, Coryell W, Shea MT, Keller MB. Psychosocial impairment and recurrence of major depression. Compr Psychiatry. 2004;45(6):423–30. doi: 10.1016/j.comppsych.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11(3):111–9. [PubMed] [Google Scholar]
- 53.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]