Abstract
Background
Although schizophrenia patients are at high risk for tobacco use, the neurobiological basis of this comorbid association is not clear. White matter abnormalities have been described independently in schizophrenia and smoking cohorts. We sought to determine whether smoking and schizophrenia are associated with similar white matter abnormalities that could be biomarkers for the high risk of smoking in schizophrenia.
Methods
Whole brain white matter integrity (fractional anisotropy [FA]) was measured in 46 schizophrenia patients (32 smokers and 14 nonsmokers) and 69 healthy age matched controls (48 smokers and 21 nonsmokers).
Results
Schizophrenia and smoking status were independently and additively associated with reduced FA in left anterior thalamic radiation/anterior limb of the internal capsule, while significant FA decreases were identified in the bilateral uncinate fasciculus/inferior fronto-occipital fasciculus in schizophrenia and the left prefrontal area in smoking status separately.
Conclusions
Common and distinct patterns of impaired white matter are associated with schizophrenia and smoking. Particularly, the anatomical congruence of an additive white matter abnormality in the anterior thalamic radiation/anterior limb of the internal capsule suggests that this abnormal fiber connectivity between frontal cortex and striatum/thalamus may be a biomarker for the increased comorbid smoking in schizophrenia patients.
Keywords: nicotine, addiction, schizophrenia, DTI, white matter, FA
Introduction
The smoking rate in schizophrenia is disproportionately high (60–80%), about 3-fold higher than that in the general population (~20%) (1). However, the neurobiological basis of this high comorbidity remains unclear. Abnormalities of white matter integrity in schizophrenia, measured by diffusion tensor imaging (DTI), have been extensively reported. Notably, abnormalities in the uncinate fasciculus and the anterior limb of the internal capsule, including the anterior thalamic radiation, have been detected with regional (2,3), whole brain (4,5), and tract-based (6) analyses. Smoking is also associated with alterations in periventricular white matter intensity (7) and white matter integrity of the corpus callosum (8). The observed white matter abnormalities, thus far described separately in schizophrenia and smoking (2–8), and nicotine effects on brain functional connectivity in schizophrenia (9), raise the intriguing possibility of a shared mechanism between smoking and schizophrenia on abnormal fiber connectivity that may serve as a biomarker for this highly comorbid condition.
Our goal is to understand whether one or more of the impaired fiber bundles related to schizophrenia pathology are also associated with smoking status. An overlapped and additive (i.e., in the same direction) fiber abnormality could potentially increase the vulnerability of smoking in schizophrenia patients if the fiber change is causally related to smoking, or could be marking a concomitant secondary effect from smoking and schizophrenia. In either case, such finding would yield a circuit-based biomarker for the comorbid condition. Alternative hypotheses were that schizophrenia and smoking are associated with abnormalities in distinct fiber bundles; or smoking may even ‘reverse’ fiber abnormalities associated with schizophrenia (i.e., an interaction effect), which might be consistent with the self-medication theory where smoking is thought to reverse some dysfunctions or antipsychotic medication side effects associated with schizophrenia (10).
Methods and Materials
Participants
Data were collected from 46 schizophrenia patients (32 smokers and 14 non-smokers) and 69 matched controls (48 smokers and 21 non-smokers) after signing IRB approved consent forms (Table 1). The Structured Clinical Interview for DSM-IV diagnoses was used to make Axis I diagnosis in all participants. Major medical illnesses, neurological illnesses, or gross MRI structural abnormalities, substance dependence for 1 year or more or current substance abuse other than nicotine were exclusionary. A positive urine illicit drug test (TRIAGE®) was also exclusionary. Healthy control participants were without Axis I or II diagnoses. All but two patients were treated with antipsychotic medications: 42 on second-generation antipsychotics (29 smokers) and 2 on first-generation antipsychotics (1 smoker). There were no significant differences in the type and dosage of the antipsychotics between smokers and nonsmokers (Supplement: Table S1). The Brief Psychiatric Rating Scale (BPRS) was collected in all but two patients. Smokers smoked ≥10 cigarettes/day for more than one year. Non-smokers were never daily smokers (<100 cigarettes in their lifetime). Fagerström Test for Nicotine Dependence (FTND) and pack-year were collected in all but one smoker.
Table 1.
Participant demographics and clinical variables
| Normal Controls | Schizophrenia Patients | Statistical test | ||||
|---|---|---|---|---|---|---|
| Non-smokers | Smokers | Non-smokers | Smokers | F or t | p | |
| Sample size | 21 | 48 | 14 | 32 | - | - |
| Age (mean±SD) | 36.0±8.7 | 35.5±9.6 | 36.8±12.9 | 35.2±10.5 | 0.09 | ns |
| Gender (m:f) | 15:6 | 45:3 | 10:4 | 30:2 | - | - |
| BPRS | ||||||
| Total (mean±SD) | - | - | 27.0±7.2 | 34.1±10.6 | 2.39 | 0.02 |
| Thought (mean±SD) | - | - | 4.1±2.8 | 7.0±4.0 | 2.47 | ns |
| Withdrawal (mean±SD) | - | - | 3.2±0.6 | 4.7±2.5 | 3.31 | 0.02 |
| Anxiety (mean±SD) | - | - | 5.8±2.4 | 7.3±3.6 | 1.50 | ns |
| Hostile (mean±SD) | - | - | 4.8±2.3 | 5.0±2.0 | 0.31 | ns |
| Activate (mean±SD) | - | - | 4.5±1.5 | 4.4±1.7 | 0.02 | ns |
| Psychosis (mean±SD) | - | - | 6.1±4.1 | 9.7±5.3 | 2.37 | ns |
| Smoking level | ||||||
| Pack-years (mean±SD) | - | 18.2±12.4 | - | 19.2±19.4 | 0.28 | ns |
| FTND (mean±SD) | - | 5.0±2.1 | - | 4.7±2.0 | 0.75 | ns |
| Age of smoking initiation (mean±SD) | - | 15.8±5.2 | - | 17.1±3.7 | 1.29 | ns |
Data acquisition and analysis
Whole-brain DTI was acquired on a Siemens 3T Allegra MRI scanner using the following parameters: TR/TE=5000/87ms, BW=1700Hz/Pixel, FOV=220×220mm, matrix size=128×128, 35 slices, thickness=4mm, a b=0 s/mm2 image and 12 1000s/mm2 images with diffusion gradients applied in 12 noncollinear directions. We employed a DTI analysis method (see Supplement for imaging and analysis details) using tract-based spatial statistics and an observer-independent method for aligning fractional anisotropy (FA) images across subjects.
A 2×2 ANOVA with disease and smoking factors was employed for whole brain FA comparisons. FA clusters were considered significant at p_FWE_corrected<0.05 (p_uncorrected=0.001 and minimal volume=49mm3). The averaged FA value was extracted from each participant from each significant cluster. Post-hoc analyses identified whether the schizophrenia-smoking effects in a significant cluster followed an “additive” pattern as in our primary hypothesis, or followed patterns more consistent with the alternative hypotheses. Correlation analyses with BPRS scores, pack-years, and FTND scores with each identified cluster were carried out, applying Bonferroni adjustment for multiple comparisons.
Result
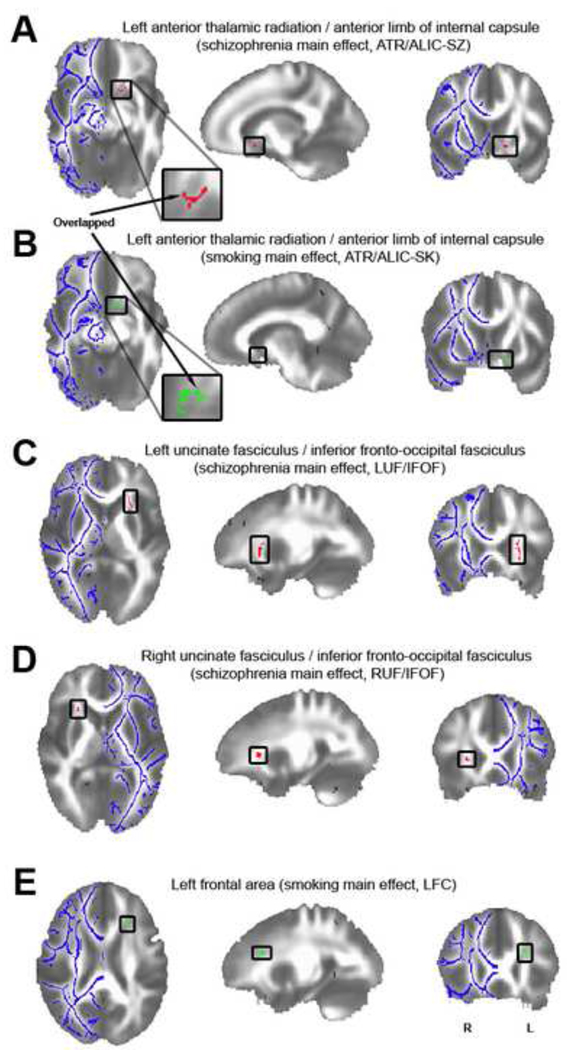
The whole brain 2×2 ANOVA identified one brain area, the left anterior thalamic radiation/anterior limb of internal capsule (ATR/ALIC) that showed significant main effects of both schizophrenia and smoking (Figure 1A and B). Post-hoc tests revealed that this effect was in the same direction and additive, such that FA in nonsmoking controls was significantly higher than that in smoking controls (t=4.590, p<0.001, suggesting a smoking effect independent of schizophrenia) and nonsmoking schizophrenia patients (t=6.125, p<0.001, suggesting a schizophrenia effect independent of smoking); while FA in smoking patients was the lowest and was significantly lower than that in smoking controls (t=3.621, p=0.001) (more details in Supplement).
Figure 1.
A: A significant schizophrenia (SZ) main effect in the left anterior thalamic radiation/anterior limb of the internal capsule (ATR/ALIC) following whole brain analysis (ATR/ALIC-SZ, MNI: 14.4 -11.9 -9.3, p_uncorrected=0.001, volume=52mm3).
B: A significant smoking (SK) main effect in the left ATR/ALIC (ATR/ALIC-SK, MNI: 10.1 - 8.7 -10.2, p_uncorrected=0.001, volume=59mm3). The arrows point out the overlapped fibers (volume=14mm3) of A and B.
C: Illustration of the significant schizophrenia main effect in the left uncinate fasciculus/inferior fronto-occipital fasciculus (UF/IFOF) (LUF/IFOF, MNI: 24.7 -18.4 4.9, p_uncorrected=0.001, volume=370mm3).
D: Illustration of the significant schizophrenia main effect in the right UF/IFOF (RUF/IFOF, MNI: -25.2 -20.3 5.1, p_uncorrected=0.001, volume=57mm3).
E: Localization of the significant smoking main effect in the left frontal area (LFC, MNI: 24.0 -22.9 21.2, p_uncorrected=0.001, volume=64mm3).
The FA clusters are projected onto a white matter skeleton (shown in blue on the contralateral hemisphere). Red clusters show significant schizophrenia main effects while green clusters show significant smoking main effects.
Additionally, two clusters in the left and right uncinate fasciculus/inferior fronto-occipital fasciculus (UF/IFOF) (Figure 1C and D) showed main effects of schizophrenia only. Finally, a cluster in the left frontal cortex area (Figure 1E) showed a main effect of smoking only. No significant interactions were found in the whole brain analysis. The averaged FA data of each group from each cluster are given in Supplement: Figure S1 and Table S2. See Supplement for details of post-hoc results.
After Bonferroni correction for multiple comparisons, there was no significant correlation between FA in any of these clusters and BPRS (total or any sub-scores), FTND or pack-years in any group. See Supplement for exploratory results.
Discussion
Using whole-brain voxel-wised DTI analysis methods, we found significantly reduced white matter integrity in the left anterior thalamic radiation/anterior limb of internal capsule (ATR/ALIC) that was independently and additively associated with schizophrenia patients and smokers. White matter alterations in ATR/ALIC have previously been associated with schizophrenia (3,5,6). This fiber tract provides connections between the thalamus/striatum and frontal cortical regions (11,12), and comprises a system that plays a role in reward and emotional processes (11,12), both of which are compromised in schizophrenia patients (13) and smokers (14). The main new finding from our study is that the smoking effect on FA was both independent of schizophrenia and was apparently additive with the disease effect, with schizophrenia smokers demonstrating the most impaired white matter integrity in this region (Figure 1A and 1B and Supplement: Figure S1). Although we cannot completely rule out a secondary effect of medication in patients, similar effects seen in controls argue against medication as a driving factor.
The characteristics of the ATR/ALIC FA abnormality are consistent with our primary hypothesis and appear to represent a novel white matter biomarker associated with the high incidence of smoking in schizophrenia. Although the current finding does not provide direct evidence on whether the ATR/ALIC abnormality is a cause of increased smoking in schizophrenia or a consequence of smoking and schizophrenia, this result suggests the possibility that the schizophrenia pathology impacts some of the same neural paths that influence smoking. The independent effect rules out the possibility that the FA reduction is secondary to either smoking or schizophrenia-related factors alone, although it does not rule out that the two conditions could have independently exerted a secondary effect on the same fiber region. Additional studies are required to determine whether it is a cause (predisposition) rather than a consequence (secondary effect) of the comorbidity.
There were no significant associations between FA and specific smoking-related measures (i.e. FTND, pack-years), suggesting a general smoking phenotype relationship, rather than one related to either degree of addiction or total cigarette exposure. Nevertheless, we recently reported that the resting state functional connectivity strength between striatum and anterior cingulate cortex (15), areas connected by fibers within the currently identified region (11,12), is significantly associated with nicotine addiction severity. This anatomical coherence raises interesting questions for further studies to address the potential causal relationship between fibers and functional connectivity abnormalities in smokers.
Our findings from the other significant clusters appear more specific to separate schizophrenia or smoking effects. The FA decrease in UF/IFOF in schizophrenia patients has been previously reported (2,4,6). The uncinate fasciculus originates in the temporal lobe and terminates in the frontal cortex and is believed to play a key role linking cognition and emotion (16). Further, impaired inferior fronto-occipital fasciculus, a long associative fiber tract (11,12), is associated with memory and language decline (17). Because these cognitive processes are altered in schizophrenia (18), such structural abnormalities may be associated with these dysfunctions.
In contrast, a left frontal cluster showed a significant main effect of smoking that had no significant schizophrenia main effect (but see Supplement for further discussion). Impairments in attention and working memory accuracy have been associated with chronic smoking (19) and these cognitive functions are known to involve the prefrontal lobe (20). Therefore, the FA abnormality in this area could be consistent with smoking-related attention and working memory alterations.
In conclusion, a common effect of disease and smoking on fibers within the anterior thalamic radiation/anterior limb of the internal capsule is a novel finding and supports a hypothesis that this specific white matter change may index an underlying neurobiological link between smoking and schizophrenia, and raise the interesting possibility that the increased smoking vulnerability in schizophrenia may be associated with this “incidental” overlap of schizophrenia pathology onto a smoking-related phenotype.
Supplementary Material
Acknowledgment
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institute on Health grants MH70644 and N01-DA-5-9909, and the Maryland Cigarette Restitution Fund Program – Other Tobacco-Related Diseases Research Grant. We thank Brittany Buchholz, Kimberly Modo, and Loretta Spurgeon for their assistance in the conduct of the study.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- 2.Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, Haznedar MM, et al. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophr Res. 2007;92:211–224. doi: 10.1016/j.schres.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 4.Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, Lawrie SM. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- 5.Sussmann JE, Lymer GK, McKirdy J, Moorhead TW, Maniega SM, Job D, Hall J, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009;11:11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 6.McIntosh AM, Maniega SM, Lymer GK, McKirdy J, Hall J, Sussmann JE, Bastin ME, et al. White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda H, Kitani M. Cigarette smoking is correlated with the periventricular hyperintensity grade of brain magnetic resonance imaging. Stroke. 1996;27:645–649. doi: 10.1161/01.str.27.4.645. [DOI] [PubMed] [Google Scholar]
- 8.Paul RH, Grieve SM, Niaura R, David SP, Laidlaw DH, Cohen R, Sweet L, et al. Chronic cigarette smoking and the microstructural integrity of white matter in healthy adults: a diffusion tensor imaging study. Nicotine Tob Res. 2008;10:137–147. doi: 10.1080/14622200701767829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobsen LK, D'Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15:429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- 11.Mori S, Wakana S, Nagae-poetscher L, van Zijl P. MRI Atlas of Human White Matter. San Diego, CA: Elsevier; 2005. [Google Scholar]
- 12.Schmahmann J, Pandya D. Fiber Pathways of the Brain. Oxford University Press; 2006. [Google Scholar]
- 13.Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol Psychiatry. 2008;64:62–69. doi: 10.1016/j.biopsych.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, Horner WE, et al. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78:813–824. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 15.Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, et al. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 17.McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Dale AM, et al. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buss AH, Lang PJ. Psychological deficit in schizophrenia: I. affect, reinforcement, and concept attainment. J Abnorm Psychol. 1965;70:2–24. doi: 10.1037/h0021685. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.