Abstract
RLIP76 is a stress-responsive membrane protein implicated in the regulation of multiple cellular signaling pathways. It represents the predominant glutathione-conjugate (GS-E) transporter in cells. We have shown that RLIP76 plays a crucial role in defending cancer cells from radiation and chemotherapeutic toxin-mediated apoptosis, and that its inhibition by antibodies or depletion by siRNA or antisense causes apoptosis in a number of cancer cell types. Recently, we have demonstrated for the first time that the striking anti-neoplastic effects with no evident toxicity in terms of either weight loss or metabolic effects are also demonstrable for the antibody, antisense and siRNA in a renal cell xenografts model of Caki-2 cells (Singhal et al., Cancer Res., 2009, 69: 4244). Present studies were performed to determine if RLIP76 targeting is more broadly applicable in other kidney cancer cell lines, to compare the signaling effects of RLIP76 antisense with kinase inhibitors used in treatment of renal cell carcinoma, and to determine whether kinase inhibitors were substrates for transport by RLIP76. Results of these studies show that sorafenib as well as sunitinib are substrates for transport by RLIP76 thus are competitive inhibitors of GS-E transport. Furthermore, kinase inhibition in the ERK as well as PI3K pathways by RLIP76 depletion is more profound and consistent and is more widely apparent in a number of renal carcinoma cell lines. These studies offer strong support for our overall hypothesis that RLIP76 is an overarching anti-apoptosis mechanism that, if inhibited, can be more broadly effective in the treatment of renal cell carcinoma.
Keywords: RLIP76, Kidney cancer, Drug-resistance, Radiation-resistance, Glutathione-conjugate transport
Introduction
Renal cell Carcinoma (RCC) affects ~ 55,000 Americans each year, resulting in ~ 15,000 deaths in the United States and ~120,000 deaths worldwide annually, making RCC one of the most lethal urologic cancers. RCC accounts for 2% of all cancers. The incidence of RCC has been steadily rising over the past 30 years. RCC have been classified histologically as clear cell, papillary, chromophobe, and collecting duct and medullary. The majority (~ 75%) of cases are clear-cell RCC. These are characteristically associated with loss of function of the von Hippel-Lindau (VHL) gene (e.g., chromosome 3p depletion, suppressed expression, or loss-of-function base substitutions). VHL mutations occur in ~ 60 % of RCCs.1 RCC is a highly treatment-resistant tumor type; however, advances in elucidating the molecular pathophysiology underlying RCC have led to the identification of promising targets for therapeutic intervention. In clear-cell RCC, mutations to the VHL gene involved with cellular responses to hypoxia, results in the up-regulation of many proteins necessary for tumor growth and survival, such as vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR), and platelet-derived growth factor (PDGF), which are involved in tumor-initiated angiogenesis. 2
Temsirolimus (Torisel, CCI-779), an mTOR kinase inhibitor, is an effective therapy in patients with poor-risk metastatic RCC. 3 The mTOR pathway seems to be important primary or alternative pathway in RCC, disrupting phosphoinositide 3-kinase (PI3K)/AKT signaling. Temsirolimus forms a complex with FK-506-binding protein-12, and this complex inhibits mTOR kinase activity and decreases HIFα level. 4 Sunitinib (Sutent, SU011248), an orally administered tyrosine kinase inhibitor that blocks the activity of VEGF and PDGFR, is the new reference standard for the first-line treatment of metastatic RCC. 5 Sorafinib (Nexavar, BAY 43-9006), one of the most promising agents of the class of Raf kinase inhibitors, has moved into phase II and III clinical trials. Sorafinib is an orally available, novel bi-aryl urea compound that prevents tumor growth by combining two anticancer properties, 1) inhibition of proliferation by targeting the ERK pathway, and 2) inhibition of angiogenesis by targeting the receptor tyrosine kinases VEGFR and PDGFR. 6
RLIP76, a ral-binding effector protein, belongs to the Ras family. Ras modulates many cellular processes involved in normal cell growth and malignant cell transformation and the activation of Ras is an important step in the pathogenesis of human cancers. Ras is located downstream of several receptor tyrosine kinases, including those for EGFR, VEGFR and PDGFR. 7 RLIP76 appears to exert multiple functions in cells, the relative importance of each of which is not known. We have demonstrated that the primary function of RLIP76 is the ATP-dependent trans-membrane transport of anionic metabolites to drive the process of endocytosis; this function of RLIP76 simultaneously protects cell from apoptosis, and functions to determine the rate at which receptor-ligand signaling (i.e. insulin, EGF, TGFβ) is terminated. Several lines of experimental evidence obtained from RLIP76-knockout animals as well as cancer cells indicate that the GS-E transport function of RLIP76 is of key importance in providing protection to cells from apoptosis inducing agents. 8-12
RLIP76 over-expression is frequently associated with neoplasia and normal cells are much less sensitive to apoptosis caused by reducing RLIP76 function by either depleting RLIP76 (by antisense or siRNA) or inhibiting its transport activity at the cell surface using specific antibodies. 8-13 Syngeneic mouse B16 melanomas 8 as well as xenografts of human lung and colon cancer 13 undergo durable and complete remissions with RLIP76 targeted therapy using antibodies, siRNA or antisense. Kidney cells characteristically express high levels of transporter proteins in their membranes that can contribute to drug-resistance, but targeting the ABC-transporter family protein has not been effective in improving the efficacy of chemotherapy in kidney cancer. Recently, we have shown marked success of this targeting approach in kidney cancer xenograft which revealed a broad spectrum and fundamental anti-neoplastic nature of this targeting approach. 14 Results of present studies establish that sunitinib and sorafenib are substrates for efflux from cells by RLIP76, demonstrate that targeting RLIP76 affects the ERK and PI3K pathway more consistently and profoundly than either sunitinib, sorafenib, or temsirolimus, and suggest that a strategy of targeting RLIP76 can be broadly applicable for renal cell carcinoma.
Materials and Methods
Reagents
Sunitinib (sutent), sorafenib (nexavar) and temsirolimus (torisel) were purchased from Toronto Research Chemicals Inc., Ontario, Canada. 3H-sunitinib (specific activity 15 Ci/mmol) and 3H-sorafenib (specific activity 25.2 Ci/mmol) were purchased from ViTrax Company, Placentia, CA. DOX was obtained from Adria Laboratories (Columbus, OH). 14C-DOX (specific activity 44.8 Ci/mmol) was purchased from NEN Life Sciences (Boston, MA). Source of anti-RLIP76 antibodies and DNP-SG were the same as previously described. 15 Anti-MRP1 and anti-Pgp antibodies were procured from Santa-Cruz Biotechnology (Santa Cruz, CA). FACE ERK1/2 and FACE PI3K kits were purchased from Active Motif (Carlsbad, CA). Ninety-six-well nitrocellulose membrane plates (pore size 0.45 μm) used in transport studies were purchased from the Millipore Co. (Bedford, MA).
Cell Lines and Cultures
Human kidney cancer (Caki-2, 786-O and A-498) cell lines were purchased from American Type Culture Collection, Manassas, VA. Human kidney normal (mesangial) cells were kindly provided by Dr. Rong Ma, University of North Texas Health Science Center, Fort Worth, TX. All cells were cultured at 37 °C in a humidified atmosphere of 5 % CO2 in RPMI-1640 medium supplemented with 10 % (v/v) heat-inactivated FBS and 1% (v/v) P/S solution.
RLIP76 siRNA
RLIP76 siRNA was purchased from Dharmacon Research (Lafayette, CO), as described previously. 8
RLIP76 phosphorothioate anti-sense DNA
RLIP76-antisense was purchased from Biosynthesis, Inc., (Lewisville, TX), as described previously. 8
Preparations of total crude membrane fractions for Western blot analyses
Crude membrane fractions were prepared from the normal and cancer cells using established procedures as described previously. 8 Levels of RLIP76 protein in normal and cancer cells were measured by ELISA. Purified rec-RLIP76 with purity assessed by amino acid composition was used to generate calibration curves.
Purification of RLIP76
DNP-SG affinity purification of RLIP76 from human kidney cells was carried out in a manner identical to that described previously for human lung cancer cell lines. SDS-PAGE and Western blot analyses were performed by the method as described previously. 16
Preparation of RLIP76-liposomes
rec-RLIP76 was purified by DNP-SG affinity as previously described from transformed E.coli BL21 expressing the full-length (1968 bp) RLIP76 cDNA in PET30a (+) prokaryotic expression plasmid and purity was confirmed by SDS-PAGE, Western-blot, amino-acid composition and MALDI-MS. To prepare proteoliposomes, purified RLIP76 was dialyzed against reconstitution buffer (10 mM Tris-HCl, pH 7.4, 4 mM MgCl2, 1 mM EGTA, 100 mM KCl, 40 mM sucrose, 2.8 mM BME, 0.05 mM BHT, and 0.025% polidocanol). An aqueous emulsion of soybean asolectin (40 mg/ml) and cholesterol (10 mg/ml) was prepared in the reconstitution buffer by sonication, from which a 100 μl aliquot was added to 0.9 ml of dialyzed purified RLIP76 protein. After sonication of the resulting mixture for 30 s at 50 W, 200 mg of SM-2 Bio-beads pre-equilibrated with reconstitution buffer (without polidocanol) were added to initiate vesiculation, and after 4 h incubation at 4 °C, SM-2 beads were removed by centrifugation at 3000 × g and the vesicles (RLIP76-liposomes) were collected. Control-liposomes were prepared using an equal amount of crude protein from E. coli not expressing RLIP76. 15 Functional reconstitution of purified kidney cancer cell RLIP76 in artificial liposomes was performed similarly.
Transport studies in artificial liposomes
Transport studies in proteoliposomes were done by the same method as described previously. No-protein liposomes were used as negative controls. 15
Transport studies in IOVs
Inside-out vesicles (IOVs) were prepared from the human kidney cell lines according to the method as described by us for the K562 cells. 15 Transport studies of 14C-DOX, 3H-DNP-SG, 3H-sunitinib and 3H-sorafenib in IOVs were performed by the method as described previously. 15 ATP-dependent uptake of 14C-DOX was determined by subtracting the radio-activity (cpm) of the control without ATP from that of the experimental containing ATP and the transport of DOX was calculated in terms of pmol/min/mg IOV protein. In one of the controls, IOV was excluded while the other control was incubated with an equal amount of heat-inactivated IOV. Each determination was performed in triplicate. The transport of 3H-DNPSG, 3H-sunitinib and 3H-sorafenib were measured in a similar manner.
Transport studies in vesicles coated with antibodies
ATP-dependent transport of 14C-DOX, 3H-DNP-SG, 3H-sunitinib and 3H-sorafenib in IOVs or purified reconstituted liposomes, coated with different antibodies was measured as described previously. 17 Briefly, either IOV or purified reconstituted liposomes (20 μg or 0.25 μg protein/30 μl reaction mixture, respectively) were incubated separately with I μg of each anti-RLIP76, anti-MRP1, and anti-Pgp antibodies for 30 min at room temperature. In one of the controls, IgG was excluded while the other control was treated with an equal amount of pre-immune IgG. After incubation, the ATP-dependent transport of 14C-DOX, 3H-DNP-SG, 3H-sunitinib and 3H-sorafenib were measured.
Drug-sensitivity assay
Cell density measurements were performed using a hemocytometer to count reproductive cells resistant to staining with trypan blue. Approximately 20,000 cells were seeded into each well of 96-well plates containing 160 μl medium. Post 24 h incubation, 40 μl aliquots of drug concentrations ranging from 0.1 μM to 100 μM was then added to eight replicate wells to assess the IC50 of drug. After 96 h incubation, 20 μl of 5 mg/ml MTT was introduced to each well and incubated for 2 h. The plates were centrifuged and cells were subsequently dissolved in 100 μl DMSO with gentle shaking for 2 h at room temperature, followed by measurement of OD at 570 nm. Inhibition of RLIP76 expression in cells by RLIP76 antibodies were measured by incubating the cells with RLIP76 antibodies (40 μg/ml final conc.) for 24 h prior to MTT assay. Depletion of RLIP76 expression in cells by RLIP76 siRNA and RLIP76 antisense were measured as follows: cells were incubated for 3 h with either RLIP76 siRNA (20 μg/ml final conc.) or RLIP76 anti-sense (10 μg/ml final conc.) in Transmessenger Transfection Reagent (Qiagen) or Maxfect transfection reagent (MoleculA), respectively, according to the manufacturer provided protocol. Cells were then washed with PBS, followed by 48 h incubation at 37 °C in medium before MTT assay. 8, 13, 14
Colorimetric assay for the detection of total ERK1/2, phospho-ERK1/2, total PI3K and phospho-PI3K
Kidney cancer cells were seeded in 96 well plate (~50, 000 cells per well) and treated with 20 μM sunitinib, sorafenib and temsirolimus, and RLIP76 antisense (10 μg/ml final conc.) for 24h and were fixed with 4% formaldehyde in PBS for 20 minutes at room temperature followed by washing with wash buffer. The vendor-provided method was used to determine total and phosphorylated proteins using phospho-specific antibodies (Active Motif).
Colony forming assay
Cells (0.1 × 106 cells / 500 μl) were irradiated at 100, 200, 500 and 1000 cGY (6 × 106 volt-photon/min) for 1.25 min at the Texas Cancer Center, Arlington, TX, using Varian Clinac 21EX Linear accelerator, and 50 and 100 μl aliquots were placed in 60 mm size petri dishes, separately, in a total volume of 4 ml of medium. After 7 days, un-irradiated and irradiated cells were stained with methylene blue, and colonies were counted using Innotech Alpha Imager HP. 12
Statistical Analyses
All data were evaluated with a two-tailed unpaired student’s t test or compared by one-way ANOVA and are expressed as the mean ± SD. A value of P < 0.05 was considered statistically significant.
Results
RLIP76 is over-expressed in human kidney cancer cells
Western-blot analyses of crude membrane protein extracts revealed that the Caki-2, 786-O and A-498 renal carcinoma cell lines over-express RLIP76 protein as compared with mesangial cells (Fig. 1).
Figure 1. Comparison of RLIP76 levels and transport activity in kidney malignant versus non-malignant cells.
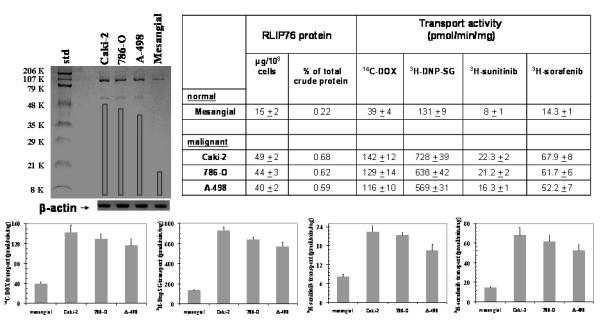
Aliquots of crude membrane fractions of malignant cells (Caki-2, 786-O and A-498) and non-malignant cells (mesangial), containing 200 μg protein were used for SDS-PAGE and Western-blotting against anti-RLIP76 IgG. Results were quantified by scanning densitometry. β-actin was used as an internal control. Cell lines were cultured in respective medium and homogenate was prepared from 100 × 106 cells. RLIP76 was purified from total crude membrane fraction using DNP-SG affinity, and quantified by ELISA. For transport studies, in-side-out vesicles (IOVs) were prepared from 20 × 106 cells.15 Transport-activity was calculated from measurements of uptake of 14C-DOX (sp. activity, 8.7 × 104 cpm/nmol), 3H-DNP-SG (sp. activity, 3.6 × 103 cpm/nmol), 3H-sunitinib (sp. activity, 4.5 × 104 cpm/nmol) and 3H-sorafenib (sp. activity, 3.9 × 104 cpm/nmol), into the IOVs (20 μg/ 30 μl reaction mixture) in the absence or presence of 4 mM ATP. Each transport experiment was done in triplicates in three separate experiments (n = 9). Please note the differences in scales between the panels.
RLIP76 accounts for the majority of the GS-E and DOX-transport in mouse tissues
In order to establish the role of RLIP76 in the transport of GS-E and DOX in mouse kidney, we utilized our previously generated RLIP76 knock-out mice colonies, based on genotyping. The results of Western-blot analysis of mouse kidney and brain tissues using anti-RLIP76 IgG showed as expected, the presence of RLIP76 in the wild-type (RLIP76+/+) and its absence in knockout (RLIP76−/−) mice kidney and brain tissues (see supplementary data). Consistent with the transport function of RLIP76 in IOVs prepared from kidney and brain tissues for GS-E and DOX, the ATP-dependent transport of both 3H-DNPSG and 14C-DOX in IOVs prepared from RLIP76−/− mice tissues, was only about 20% of that observed in RLIP76+/+ mouse. That this 80% loss in transport activity in RLIP76−/− mice was in fact due to RLIP76 deletion and was confirmed by the results of transport studies with IOVs prepared from tissues of RLIP76−/− mice supplemented with purified RLIP76 (see supplementary data). We have previously shown that RLIP76 can be delivered to tissues in-vivo by injecting RLIP76 encapsulated in proteoliposomes. 9 RLIP76−/− mice were injected with 200 μg/0.2 ml purified RLIP76 liposomes, i.p. and the presence of RLIP76 was examined by Western-blot and immuno-histochemistry. RLIP76 was clearly detected at levels comparable to the RLIP76+/+ mice in mouse tissues 24 h after a single RLIP76-liposomes dose. However, there was no detectable RLIP76 in RLIP76−/− animals given control-liposomes (see supplementary data). These results are consistent with our previous studies showing successful delivery of RLIP76 to mice tissues in-vivo. The transport activity for 14C-DOX as well as 3H-DNP-SG in the IOVs prepared from kidney and brain tissues of RLIP76 supplemented mice was comparable to that of RLIP76+/+ mice confirming that the loss of transport-activity in RLIP76−/− was in fact due to RLIP76 deletion, and consistent with the observed transport function of RLIP76 for GS-E and DOX in cell culture studies. Furthermore, the transport-activity for 14C-DOX and 3H-DNPSG in the IOVs prepared from kidney and brain tissues of RLIP76-antisense treated mice was comparable to that of RLIP76−/− mice or ~ 20% of that observed in RLIP76+/+ mouse (see supplementary data) and also in Western-blot analyses against anti-RLIP76 IgG, RLIP76 expression was undetectable in the tissues of RLIP76 antisense treated mice. 14
The specificity of RLIP76 antibody has been demonstrated previously by showing that the major bands recognized by this antibody are internal fragments, alternative splice variants, and aggregates of internal fragments of RLIP76. 15 RLIP76 was purified from all three malignant cells as well as the cultured mesangial cells. RLIP76 protein represented only 0.22% of total crude membrane protein in mesangial cells, consistent with the relative abundance in other normal cell lines such as lung-endothelial, lung-epithelial, aorta-vascular smooth muscle, and umbilical vein endothelial cells (0.14 - 0.19%). 8 In contrast, in all renal cell carcinoma, RLIP76 represented a higher fraction of total membrane protein (0.59 - 0.68 %) (Fig 1).
Because cytotoxic drugs are generally ineffective in kidney cancers, and used only infrequently now, as compared with the ‘non-cytotoxic’ targeted drugs such as sunitinib, sorafenib, and temsirolimus, it is most reasonable to consider the question of whether the efflux of these agents could be affected by RLIP76. The transport-activity of the crude membrane vesicles was determined using radio-labeled substrates including 14C-DOX, 3H-DNP-SG, 3H-sunitinib and 3H-sorafenib. The maximal velocity for transport of all four substrates in the crude membrane vesicles from cancer cells was 2.5 to 5.5 fold greater than that from mesangial cells. These studies for the first time directly demonstrated that both sorafenib and sunitinib are subject to efflux catalyzed by RLIP76. Indeed, we found that sunitinib, a generally more effective kinase inhibitor and more active clinically, was a poorer substrate for transport by RLIP76 as compared with sorafenib. Taken together, these findings indicate that RLIP76 expression is generally increased in renal cell carcinoma, and this increase in expression is comparable to that seen in lung, colon, and melanoma cells, 8, 13 and that the differences in transport activities between normal and malignant cells as well as between different kinase inhibitors could have direct implications in the cancer specificity as well as efficacy of these two approved agents for human renal cell carcinoma.
Functional reconstitution of RLIP76 purified from kidney cancer cells
RLIP76 from detergent extracted membrane fraction of human kidney cancer (Caki-2) cells was purified by DNPSG affinity chromatography as described previously to purify RLIP76 from lung cancer cells (16). In SDS-PAGE, purified RLIP76 from Caki-2 cells showed two bands corresponding to 95 and 67 kDa, and both bands were recognized by anti-RLIP76 IgG in Western blot analyses (Fig. 2A). The transport activity of RLIP76 was established initially using purified protein from lung and breast cancer cells and subsequently by reconstituting it into artificial liposomes (18). Caki-2 cells purified RLIP76 was reconstituted into artificial proteoliposomes prepared from soybean asolectin: cholesterol (4:1) with a protein: lipid ratio of 50 μg protein/5 mg lipid/ml reconstitution buffer. This method yields liposomes which have been extensively characterized previously, showing an intra-vesicular volume of 18 ± 2 μl/ml extra-vesicular volume (by inulin-exclusion study) and median diameter of 0.5 μm by transmission electron microscopy and are suitable for transport studies (19). Western blot analysis of the pellet and supernatant fractions of 104,000 × g centrifugation of RLIP76-proteoliposomes revealed that >95% of total RLIP76 was found within the pellet fraction indicating excellent incorporation of the protein within vesicles.
Figure 2. Transport of 14C-DOX, 3H-DNPSG, 3H-sunitinib and 3H-sorafenib by reconstituted RLIP76 purified from Caki-2 cells.
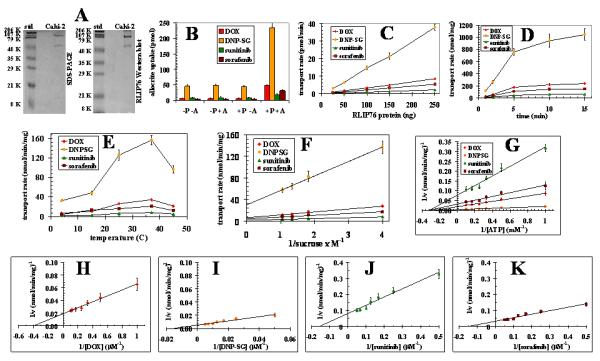
DNP-SG affinity purified RLIP76 from Caki-2 kidney cancer cells was applied to SDS-PAGE and subjected to Western blot analyses against anti-RLIP76 IgG as a primary antibody. SDS-PAGE was stained with Coomassie Brilliant Blue-R250 and Western blots were developed using horseradish peroxidase-conjugated goat-anti-rabbit-IgG as secondary antibody (panel A). Purified RLIP76 was reconstituted into artificial liposomes and uptake of radio-labeled 14C-DOX, 3H-DNPSG, 3H-sunitinib and 3H-sorafenib by control- or RLIP76-proteoliposomes were measured in the presence or absence of ATP. Vesicles equivalent to 250 ng of purified RLIP76 were used per 30 μl filtered reaction mixture in all panels except C. Total radioactivity retained by 0.45 μm filters in 96-well plates was determined after solubilizing filters in scintillation fluid, converted to pmol using the specific activity of 14C-DOX (87.4 cpm/pmol), 3H-DNPSG (3.7 cpm/pmol), 3H-sunitinib (43.5 cpm/pmol) and 3H-sorafenib (39.2 cpm / pmol), Each point represents an average and SD calculated from 12 measurements [control (−P) or RLIP76-proteoliposomes (+P), without (−A) or with ATP (+), in triplicates]. Uptake in RLIP76-proteoliposomes with ATP is significantly greater than other groups (p<0.005) (panel B). All transport studies were carried out using 250 ng protein/assay except when protein was varied (panel C). Incubation time was 5 min except for time dependence studies (panel D). Temperature was 37 °C except for temperature dependence studies (panel E). External sucrose concentration was 40 mM, except for studies of osmolar dependence (panel F). ATP was 4 mM except in ATP-dependence studies (panel G). DOX was 3.6 μM except in DOX-dependence studies (panel H). DNP-SG was 100 μM except in DNPSG-dependence studies (panel I). Sunitinib was 10 μM except in sunitinib-dependence studies (panel J). Sorafenib was 10 μM except in sorafenib-dependence studies (panel K). The proteoliposomes prepared from RLIP76 purified from Caki-2 cells. Mean values ± SD for three experiments in triplicates are shown.
We performed the kinetics of transport of DOX, DNPSG, sunitinib and sorafenib by RLIP76 purified from Caki-2 cells (Fig. 2). For transport studies, uptake of 14C-DOX, 3H-DnpSG, 3H-sunitinib and 3H-sorafenib, with or without ATP, was measured in proteoliposomes reconstituted with purified RLIP76. Control liposomes were reconstituted in the presence of an equal amount of crude protein. Thus, 14C-DOX, 3H-DNPSG, 3H-sunitinib and 3H-sorafenib transport was measured by uptake in the absence of ATP from that observed in its presence. The presence of ATP caused an increase in uptake of 14C-DOX, 3H-DNPSG, 3H-sunitinib and 3H-sorafenib only in proteoliposomes reconstituted with RLIP76 and not in control liposomes (Fig. 2B). ATP-dependent uptake of 14C-DOX, 3H-DNPSG, 3H-sunitinib and 3H-sorafenib were linear with respect to the amount of RLIP76 used for reconstitution of vesicles (Fig. 2C). The uptake was time dependent in a manner consistent with uptake by a single compartment (Fig. 2D). Optimal temperature for transport was found to be 37 °C, with progressive inactivation above 45 °C (Fig. 2E), and sensitive to osmolarity of extra-vesicular medium (Fig. 2F). Saturable kinetics for the transport by these proteoliposomes was observed for ATP (Fig. 2G) and DOX, DNPSG, sunitinib and sorafenib (Fig. 2H-K). All transport studies with Caki-2 RLIP76 proteoliposomes indicated that 14C-DOX, 3H-DNPSG, 3H-sunitinib and 3H-sorafenib uptake were identical in control-liposomes in the absence or presence of ATP, and equal to the uptake observed in RLIP76-proteoliposomes in the absence of ATP (Fig. 2B). Because background binding of DOX, DNPSG, sunitinib and sorafenib to the filtration membranes was unaffected by the presence of liposomes or nucleotides, its exclusion had no effect on calculations of ATP-dependent uptake rates. Please note the differences in scales between the figure panels. Collectively, increased 14C-DOX, 3H-DNPSG, 3H-sunitinib and 3H-sorafenib uptake was demonstrable only in the presence of both RLIP76-proteoliposomes and ATP and not in control-liposomes without or with ATP, or in RLIP76-proteoliposomes without ATP (Fig. 2B). Kinetic properties of 14C-DOX, 3H-DNPSG, 3H-sunitinib and 3H-sorafenib transport by Caki-2 RLIP76 showed interesting similarities and differences (Table I). The observed Km for DOX, DNPSG, sunitinib and sorafenib was 3.2 μM, 68 μM, 7.2 μM, 10.4 μM, respectively, and for ATP was 3.5 ± 3 mM. The calculated Vmax 48, 168, 12.5, and 28 nmol/min/mg for DOX, DNPSG, sunitinib and sorafenib, respectively (Table I).
Table I.
Comparison of kinetics parameters of 14C-DOX, 3H-DNPSG, 3H-sunitinib and 3H-sorafenib transport by RLIP76 purified from human kidney cancer Caki-2 cells
| Parameters | |
|---|---|
| 14C-DOX transport | |
| Km for ATP (mM) | 3.5 ± 0.27 |
| Km for DOX (μM) | 3.2 ± 0.22 |
| Vmax of DOX transport (nmol/min/mg) | 48 ± 3.2 |
| 3H-DNPSG transport | |
| Km for ATP (mM) | 3.8 ± 0.21 |
| Km for DNPSG (μM) | 6 8± 8.3 |
| Vmax of DNPSG transport (nmol/min/mg) | 168 ± 12 |
| 3H-sunitinib transport | |
| Km for ATP (mM) | 3.3 ± 0.34 |
| Km for sunitinib (μM) | 7.2 ± 0.54 |
| Vmax of sunitinib transport (nmol/min/mg) | 12.5 ± 0.8 |
| 3H-sorafenib transport | |
| Km for ATP (mM) | 3.1 ± 0.17 |
| Km for sorafenib (μM) | 10.4 ± 1.1 |
| Vmax of sorafenib transport (nmol/min/mg) | 28 ± 1.6 |
To ensure that the entire transport observed was due to the RLIP76, and not an artifact, immuno-titration studies were performed in which RLIP76 proteoliposomes were incubated with pre-immune, anti-RLIP76, anti-MRP1 and anti-Pgp IgG. Results of these studies clearly demonstrated that no detectable immunological cross-reactivity between them, and complete abrogation of RLIP76 transport activity by anti-RLIP76 IgG. In contrast, anti-MRP1 and anti-Pgp antibodies had no significant effect on the transport activity in RLIP76 proteoliposomes. Together these results confirmed that these transporters (RLIP76, MRP1 and Pgp) were immunologically distinct and the antibodies used in these experiments were specific (Fig. 3).
Figure 3. Dose dependent inhibition of 14C-DOX, 3H-DnpSG, 3H-sunitinib and 3H-sorafenib transport in purified RLIP76 reconstituted vesicles from Caki-2 cells by anti-RLIP76, anti-MRP and anti-Pgp IgG.
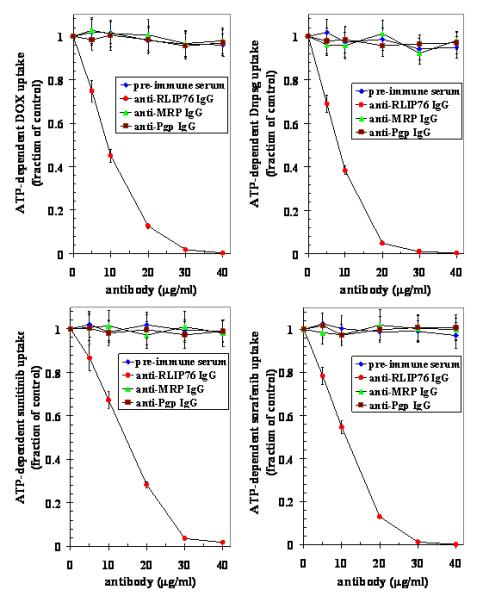
Proteoliposomes were incubated in the presence of varying concentration of antibodies (5-40 μg/ml transport reaction) for 30 min followed by determination of 14C-DOX, 3H-DnpSG, 3H-sunitinib and 3H-sorafenib uptake by proteoliposomes in the absence or presence of ATP as described in Methods section. Results presented are mean and SD from three experiments in triplicates. (◆), pre-immune serum; (●), anti-RLIP76 IgG; (▲), anti-MRP1 IgG and (■), anti-Pgp IgG.
Relative contribution of RLIP76 to the total transport of 14C-DOX, 3H-DNPSG, 3H-sunitinib and 3H-sorafenib in kidney cancer cells
RLIP76 plays a significant role in DOX and DNP-SG transport and resistance in kidney cancer, and its role in the transport of drugs in cancer cells has also been suggested. 14 DOX is a common allocrite transported by RLIP76, 15 MRP1 and MDR1.20, 21 Since our previous studies indicate that RLIP76 is the predominant transporter of DOX as well as GS-E in lung and prostate cancer cells, we quantitated the relative contributions of RLIP76 in the transport of 14C-DOX, 3H-DNPSG (a model GS-E allocrite), 3H-sunitinib and 3H-sorafenib in kidney cancer cells using an immunological approach. Specific inhibition of the transport mediated by RLIP76, MRP and Pgp by their respective antibodies has been used by us to successfully quantitate RLIP76-, MRP- and Pgp-mediated transport in cells. 17 Antibodies specificities were confirmed by Ouchterlony double immuno-diffusion experiments which showed that anti-RLIP76 IgG did not recognize MRP or Pgp and vice versa. 16 We therefore, designed experiments in which IOVs prepared from kidney cancer cells, and separately coated with anti-RLIP76 IgG, anti-MRP IgG, or anti-Pgp IgG were used to measure the ATP-dependent uptake of 14C-DOX, 3H-DNPSG, 3H-sunitinib and 3H-sorafenib. Optimal concentration of antibodies to be used for specific inhibition of transport was determined in titration studies where varying concentration of each antibody was used to coat the membrane-vesicles. Anti-RLIP76 IgG, which recognized only RLIP76 in crude extracts of kidney cancer cells, inhibited 55±14 % of total 14C-DOX, 3H-DNPSG, 3H-sunitinib and 3H-sorafenib transport in IOVs. Anti-MRP IgG also inhibited transport in a saturable manner, but maximal inhibition was less (29±9 %) as compared to that observed with anti-RLIP76 IgG. Anti-Pgp IgG had a small but detectable inhibitory effect on transport (11±5 %). These results also established that <40 μg/ml of each of the antibody quantitatively inhibited transport-activity of their respective antigen present in the amount of vesicles used in these experiments. In the vesicle coated with the mixture of the all three antibodies almost complete abrogation (91±6 %) of transport was observed. In control vesicles coated with pre-immune IgG, the transport-activity remained unaffected. These results demonstrated that RLIP76, MRP, and Pgp together constitute nearly all ATP-dependent transport-activity in these membranes. Furthermore, these results established that RLIP76 accounted for a major portion (about two-thirds) of the ATP-dependent transport of 14C-DOX, 3H-DNPSG, 3H-sunitinib and 3H-sorafenib in kidney cancer cells.
RLIP76 inhibition or depletion caused cytotoxicity preferentially in malignant cells
We have studied a number of siRNA and phosphorothioate antisense DNA molecules targeted to different regions of RLIP76, and concluded that the most specific and effective depletion occurs when targeting the nucleotide region 510-555 (encoding aa171-185). The methods used to select and design the particular siRNA and antisense DNA constructs are published and the resulting targeting agents have been shown to be effective and selective in depleting RLIP76. 8 The cytotoxic effect of RLIP76 inhibition by RLIP76 antibodies or the depletion of RLIP76 by using either siRNA or antisense (R508) was assessed by an established MTT cytotoxicity method. 8 Whereas the mesangial cells were spared from the cytotoxic effects of the antibody, siRNA or antisense, all malignant cell lines were significantly inhibited by all three agents (Fig. 4). Sparing of mesangial cells in these studies is reminiscent of similar sparing of lung endothelial and epithelial, vascular smooth muscle endothelial cells. 8 Similarly, the consistent and broad-spectrum effect in different kidney cancer cells is also consistent with the same effect in NSCLC and SCLC as well as other cancer cell lines including melanoma, prostate cancer, ovarian cancer, and leukemia. 8
Figure 4. Selective toxicity of anti-RLIP76 IgG, RLIP76-siRNA and RLIP76-antisense towards kidney cancer cells.
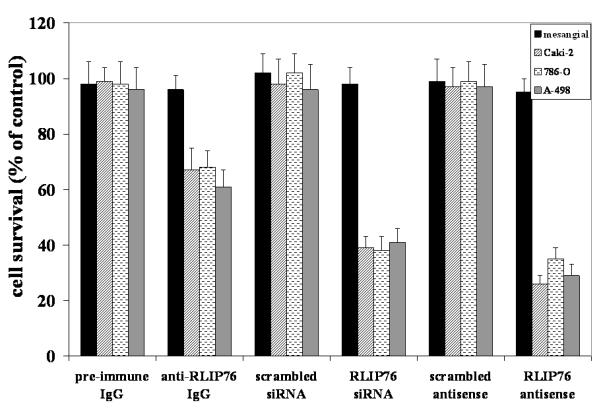
Effect of anti-RLIP76 IgG (40 μg/ml final concentration) on cell survival was determined by MTT assay. Depletion of RLIP76 expression by RLIP76-siRNA (20 μg/ml final conc.) and RLIP76-antisense (10 μg/ml final conc.) was done, using Transmessenger Transfection Reagent kit (Qiagen), and Maxfect Transfection Reagent (Molecula, Inc.), according to the manufacturer’s instructions, respectively. Cell-survival was measured by MTT cytotoxicity assay 24 h after treatment. Values are presented as mean ± SD from two separate determinations with eight replicates each (n = 16).
Membrane localization of RLIP76
Present studies of flow-cytometry of intact kidney cancer cells show clear cell-surface presence of RLIP76 which is greater in malignant as compared with mesangial cells (Fig. 5A). Immuno-histochemistry studies using anti-RLIP76 antibodies were performed with live, unfixed Caki-2 cells and examined by confocal laser microscopy, which revealed a staining pattern consistent with cell surface recognition. Under these conditions, no cell surface signal was detected when the pre-immune serum IgG was used as the primary antibody (Fig. 5B).
Figure 5. Cell surface antigen analysis.
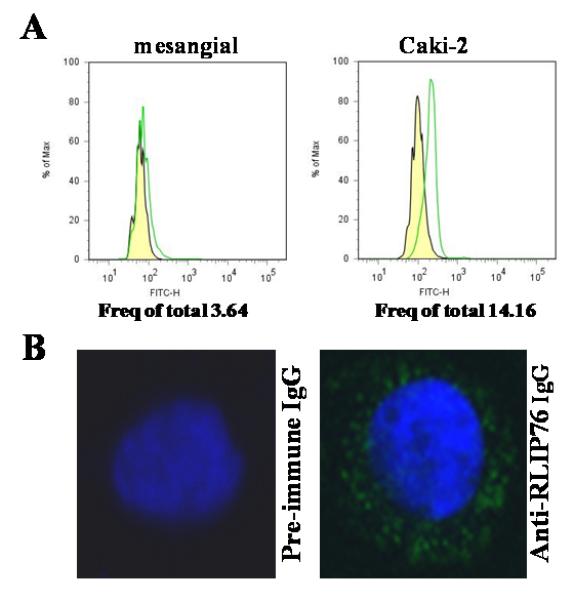
Flow-cytometric analyses were carried out on cell suspensions (106 cells/ml) obtained by incubating monolayer cell cultures with Trypsin-EDTA. For the determination of cell-surface RLIP76, the mesangial (normal) as well as Caki-2 kidney cancer cells were incubated for 20 min at 4 °C with anti-RLIP76 IgG as a primary antibody. After washing with phosphate buffered saline containing NaN3, and 1% FBS, cells were incubated for 20 min at 4 °C with a FITC-conjugated goat-anti-rabbit as secondary antibody, followed by washing and than cells were immediately analyzed using BD LSR11 flow-cytometer systems using Flow Jo 7.2.2 analysis software. Yellow: pre-immune IgG + secondary antibody; Green: anti-RLIP76 IgG + secondary antibody (panel A). For cell-surface localization of RLIP76 in Caki-2 cells, cells were grown on glass cover-slips and live, unfixed cells were subjected to immuno-histochemistry using pre-immune and anti-RLIP76 IgG as the primary antibody and FITC-conjugated goat anti-rabbit IgG as the secondary antibody. DAPI was used as a nuclear counter-stain (blue-fluorescence). Slides were analyzed using a Lecia TCS SP5 confocal microscope (panel B).
RLIP76 confers drug resistance in kidney cancer cells
RLIP76 is a very broad-specificity transporter of chemotherapeutic drugs, in addition to GS-E. Therefore, the sensitivity of kidney cancer cells towards DOX, sorafenib, sunitinib and temsirolimus were compared using an MTT assay (Fig. 6). The IC50 for all four drugs were measured with 96 h of exposure time. Abrogation of this resistance by specific anti-RLIP76 IgG further indicates that this effect is specifically mediated by RLIP76 (data not shown).
Figure 6. DOX, Sorafenib, Sunitinib and Temsirolimus IC50 values in renal carcinoma.
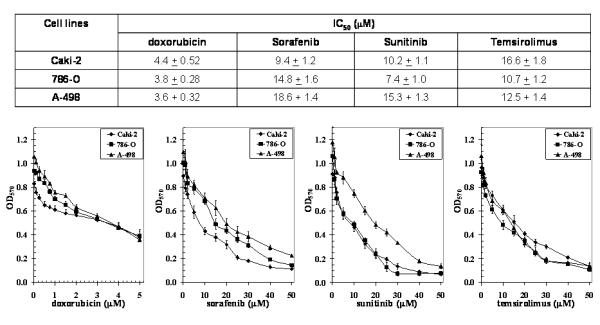
Drug-sensitivity assays were performed using MTT to determine IC50 values. The values are presented as mean ± SD from three separate determinations with eight replicates each (n = 24). Please note the differences in scales between the figures.
Change in phosphorylated ERK and PI3K caused by sorafenib, sunitinib, temsirolimus and RLIP76 antisense
In present studies, we also investigated the mechanisms of anti-neoplastic effects in kidney cancer cell lines by comparing the ratio of phosphorylated / total ERK and PI3K in cells treated with either RLIP76-antisense, sunitinib, sorafenib or temsirolimus. Results of these studies showed that RLIP76-depletion resulted in reduction of P-ERK and P-PI3K consistently in all malignant renal cell carcinoma, and to a significantly greater extent by RLIP76-antisense compared to that of any of the three clinically available renal cell carcinoma drugs known to target these pathways. As expected, sorafenib and sunitinib affected the ERK pathway more than the PI3K pathway which was preferentially affected by temsirolimus; however, these effects were in-consistent between different cell lines as compared with the effect of RLIP76-antisense (Fig. 7).
Figure 7. Percent change in phosphorylated ERK and PI3K caused by sorafenib, sunitinib, temsirolimus and RLIP76-antisense.
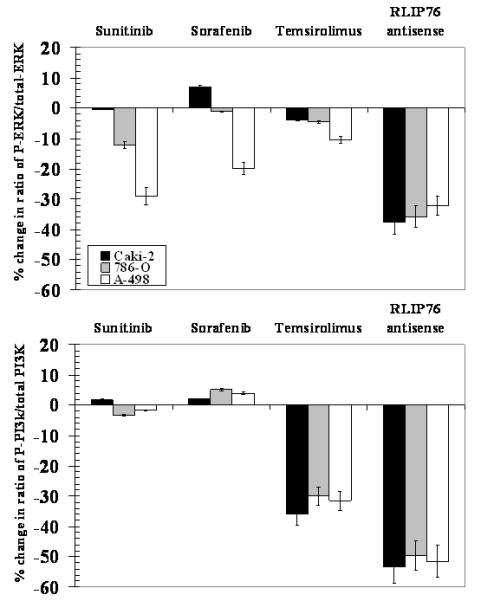
Kidney cancer cells were seeded in 96 well plate (~50, 000 cells per well) and treated with 20 μM sunitinib, sorafenib and temsirolimus, and RLIP76-antisense (10 μg/ml) for 24 h and were fixed with 4% formaldehyde in PBS for 20 minutes at room temperature followed by washing with wash buffer. The vendor-provided method was used to determine total and phosphorylated proteins using phospho-specific antibodies (Active Motif). The results presented represent the percent change in phosphorylated protein compared with corresponding untreated cells.
Radiation sensitivity
We have shown that RLIP76 knockout mice are extremely sensitive to irradiation, and in recent studies, we have also demonstrated that the overall effect of RLIP76 depletion is almost an order of magnitude greater than the effects exerted by important signaling proteins including Akt, JNK and MEK. 10 Based on these previous studies in knockout animals and other histologies of cancer cells, we reasoned that RLIP76 modulation would directly affect radiation-sensitivity and resistance of kidney cancer cells. To test this postulate, we determined X-irradiation sensitivity of the Caki-2 kidney cancer cells in dose-response studies utilizing 100-1000 cGY single dose X-irradiation, followed by colony-forming assays. Cells pretreated with RLIP76-liposomes were least sensitive to radiation. Delivery of rec-RLIP76 to cells via a liposomal-delivery system completely reversed radiation-sensitivity. At each radiation dose, survival was significantly more when the cells were pretreated with RLIP76-liposomes before radiation-exposure (Fig. 8). The physiological significance of RLIP76-mediated transport of endogenously generated GS-E (e.g., conjugate of 4HNE) is further indicated by results of our studies showing that RLIP76-enriched cells are resistant to radiation-toxicity. In these studies, Caki-2 cells were loaded with RLIP76 by incubating them with RLIP76 encapsulated in artificial-liposomes. As shown in figure 8, cells enriched with RLIP76 were resistant to radiation as compared to controls. These results suggest that the electrophilic products of lipid-peroxide caused by reactive-oxygen-species (ROS) generated during radiation may account for the cytotoxicity caused by radiation and that RLIP76-mediated transport of GSH-conjugates of these electrophiles provides protection from radiation. These findings confirmed prior findings that RLIP76-liposomes are excellent radio-protective agents. 10
Figure 8. Radiation protections by RLIP76-liposomal delivery.
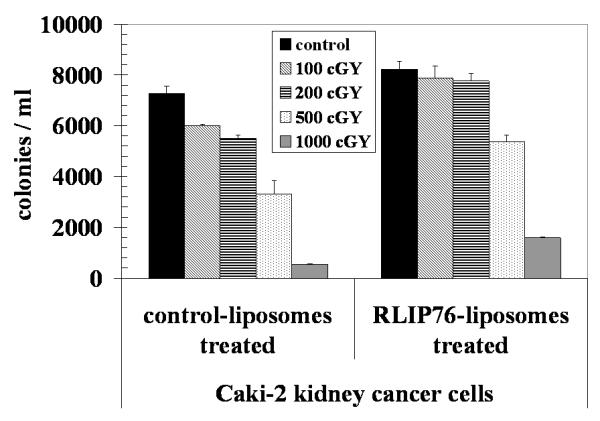
2.5 × 103 Caki-2 cells were treated with control and RLIP76-liposomes (50 μg/ml final conc.) for 24 h prior to radiation at 100, 200, 500 and 1000 cGY (6 MeV photons, 1.25 min) at the Texas Cancer Center, Arlington, TX. After 7 days, cells were stained with methylene blue and the colonies were counted using an Innotech Alpha Imager HP.12 The results presented are the mean and s.d. from three separate experiments.
The dramatic effect of RLIP76-liposomes in providing complete protection from radiation-toxicity has direct implications for treatment of radiation-toxicity. We believe that RLIP76-liposomes are excellent candidates for development as a radio-protective agent which may have broad applicability, particularly given that these liposomes are capable of delivering sustained levels of RLIP76 in all tissue, including brain. 9 These findings also indicate that these liposomes may be useful as vehicles for delivery of drugs, antisense-therapies and other therapies to the brain. These striking results not only confirm the validity of RLIP76 as a target but also strongly support our model for RLIP76 in which it functions to protect cells from stress through its transport-activity.
Discussion
The cancer specific apoptosis caused by RLIP76 depletion or inhibition has been shown in lung and colon cancer xenografts as well as a syngeneic mouse melanoma model. 8, 13 Recently, we have demonstrated that i.p. administration of RLIP76 antisense, siRNA or anti-RLIP76 IgG to nude mice leads to nearly complete and sustained regression of kidney cancer xenografts tumors. 14 It should be noted that xenograft studies of sorafenib, sunitinib, temsirolimus, or other agents in development have not demonstrated this dramatic effect. These findings indicate that RLIP76 is a key survival protein for kidney cancer cell, and its depletion/inhibition results in regression of human kidney cancer xenografts without any apparent toxicity to animals. Since both anti-RLIP76 antibodies and antisense produce similar effects, our findings support the assertion that anti-apoptotic and stress-protective effects of RLIP76 are primarily related to its transport-activity.
The function of RLIP76 as a multi-specific stress-protective GS-E / drug-transporter is necessary for the characteristic apoptosis-resistant phenotype of kidney cancer. Kidney cancer cells frequently have mutations in the VHL tumor-suppressor gene which result in inappropriate activation of Hif-1α, thereby driving the transcription and secretion of growth factors including VEGF, PDGF, TGFα, and EGF (type 1 ligand), and erythropoietin. 22-24 These in turn bind their cognate receptors and activate signaling in the Ras and PI3K pathways. 23 Ras pathway is activated in over half of all kidney cancers. 25 Interruption of this signaling is known to induce apoptosis in kidney cancer cells, and is the basis for sorafenib, sunitinib, and other receptor tyrosine-kinase inhibitors which can terminate this signaling. Our present studies show that phosphorylation of ERK and PI3K is dramatically and consistently decreased in all human kidney cancer cell lines, to a greater extent by RLIP76-antisense than that observed with sunitinib, sorafenib, or temsirolimus. Furthermore, our studies using radio-labeled 3H-sorafenib and 3H-sunitinib demonstrate for the first time that these two drugs are substrates for transport by RLIP76, thus by definition competitive inhibitors of GS-HNE transport. Present studies also demonstrate a role of RLIP76 in mediating radiation-resistance in kidney cancer cells. Because a primary mechanism of killing after X-irradiation is through the genotoxic effects of lipid-hydroperoxides produced as a consequence of OH. radical, produced as a result of radiation, the findings offer additional evidence for the overall model in which RLIP76 protects cells by removing toxins generated during oxidative-stress.
Because kidney cancers have significantly increased content of RLIP76 as compared to normal tissues, we reasoned that the cyto-protective function of RLIP76 could be playing an important role in kidney cancer. This reasoning was bolstered by recent observations that targeting the mTOR pathway provides a valuable and effective therapy for kidney cancer; recent studies by others have shown that RLIP76 functions through an mTOR independent protective mechanism. 24, 26 Thus, we consider these findings highly significant and in support of the hypothesis that 1) RLIP76 is necessary for kidney cancer cells to resist cell death, and 2) differential toxic effects towards cancer and normal cells may be sufficient for further development of this therapeutic agent in human clinical studies.
In general, most chemotherapy agents have failed to be clinically very useful in the treatment of kidney cancers, though some responses to chemotherapy drugs are seen in selected patients. Cytotoxic chemotherapy drugs that have been used in the treatment of kidney cancers include platinum compounds (cisplatin, carboplatin, and oxaliplatin), anti-metabolites (5-fluorouracil, gemicitabine, and methotrexate), taxane compounds (paclitaxel, docetaxel), anthracycline (DOX), and vinca-alkaloids (vinblastine, vincristine, and vinorelbine). Single agent response rates with these drugs do not exceed 10%. Of these agents, the platinum compounds are known to form GSH-conjugates which are subject to transport by RLIP76. The anthracyclines and vinca-alkaloids are direct substrates for transport by RLIP76. 8-15 In contrast, taxanes (taxol and paclitaxel) are not substrates for transport by RLIP76. On this basis, we can predict that RLIP76 depletion or inhibition will preferentially enhance the toxicity of those drugs which are its transport substrates (allocrites), and will have no or less-significant effect on the toxicity of drugs which are not its allocrites. Furthermore, present studies are likely to make major impact on our efforts for developing effective regimens for treatment of kidney cancer.
Supplementary Material
Acknowledgments
This study was supported in part by NIH Grants CA 77495 and CA 104661, Cancer Research Foundation of North Texas and Institute for Cancer Research & the Joe & Jessie Crump Fund for Medical Education. We thank Abhijit Bugde and Dr. Kate Luby-Phelps from The Live Cell Imaging Core Facility at The University of Texas Southwestern Medical Center at Dallas for help in imaging using the Lecia TCS SP5 confocal microscope. The authors also thank Xiangle Sun, Core Facility at the University of North Texas Health Science Center, Fort Worth, TX, for helping in Flow Cytometry and Laser Capture Micro-dissection, supported by NIH grant (ISIORR018999-01A1).
The abbreviations used are
- RLIP76
Ral-interacting protein
- GSH
glutathione
- GS-E
glutathione-conjugates
- DNP-SG
dinitrophenyl-S-glutathione
- DOX
doxorubicin
- MDR
multi-drug resistance
- Pgp
P-glycoprotein
- MRP1
multi-drug resistance protein
- R508
RLIP76 anti-sense phosphorothioate deoxyoligonucleotide.
References
- 1.Yao M, Yoshida M, Kishida T, Nakaigawa N, Baba M, Kobayashi K, Miura T, Moriyama M, Nagashima Y, Nakatani Y, Kubota Y, Kondo K. VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst. 2002;94:1569–75. doi: 10.1093/jnci/94.20.1569. [DOI] [PubMed] [Google Scholar]
- 2.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–7. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 3.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 4.Thomas GV, Tran C, Mellinghoff IK, Welsbie DS, Chan E, Fueger B, Czernin J, Sawyers CL. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–7. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 5.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37. [PubMed] [Google Scholar]
- 6.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 7.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 8.Singhal SS, Awasthi YC, Awasthi S. Regression of melanoma in a murine model by RLIP76 depletion. Cancer Res. 2006;66:2354–60. doi: 10.1158/0008-5472.CAN-05-3534. [DOI] [PubMed] [Google Scholar]
- 9.Awasthi S, Singhal SS, Yadav S, Singhal J, Drake K, Nadkar A, Zajac E, Wickramarachchi D, Rowe N, Yacoub A, Boor P, Dwivedi S, et al. RLIP76 is a major determinant of radiation sensitivity. Cancer Res. 2005;65:6022–8. doi: 10.1158/0008-5472.CAN-05-0968. [DOI] [PubMed] [Google Scholar]
- 10.Singhal J, Singhal SS, Yadav S, Warnke M, Yacoub A, Dent P, Sharma R, Awasthi YC, Armstrong D, Awasthi S. RLIP76 in defense of radiation poisoning. Int J Rad Oncol Biol Phys. 2008;72:553–61. doi: 10.1016/j.ijrobp.2008.06.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuckler D, Singhal J, Singhal SS, Yadav S, Awasthi YC, Awasthi S. RLIP76 transports vinorelbine and mediates drug resistance in non-small cell lung cancer. Cancer Res. 2005;65:991–8. [PubMed] [Google Scholar]
- 12.Singhal SS, Yadav S, Drake K, Singhal J, Awasthi S. Hsf-1 and POB1 induce drug-sensitivity and apoptosis by inhibiting Ralbp1. J Biol Chem. 2008;283:19714–29. doi: 10.1074/jbc.M708703200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singhal SS, Singhal J, Yadav S, Dwivedi S, Boor P, Awasthi YC, Awasthi S. Regression of lung and colon cancer xenografts by depleting or inhibiting RLIP76. Cancer Res. 2007;67:4382–9. doi: 10.1158/0008-5472.CAN-06-4124. [DOI] [PubMed] [Google Scholar]
- 14.Singhal SS, Singhal J, Yadav S, Sahu M, Awasthi YC, Awasthi S. RLIP76: A target for kidney cancer therapy. Cancer Res. 2009;69:4244–51. doi: 10.1158/0008-5472.CAN-08-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awasthi S, Cheng J, Singhal SS, Saini MK, Pandya U, Pikula S, Pikula J, Singh SV, Zimniak P, Awasthi YC. Novel function of human RLIP76: ATP-dependent transport of glutathione conjugates and doxorubicin. Biochemistry. 2000;39:9327–34. doi: 10.1021/bi992964c. [DOI] [PubMed] [Google Scholar]
- 16.Singhal SS, Singhal J, Sharma R, Singh SV, Zimniak P, Awasthi YC, Awasthi S. Role of RLIP76 in lung cancer doxorubicin resistance: I. The ATPase activity of RLIP76 correlates with doxorubicin and 4HNE resistance in lung cancer cells. Int J Oncol. 2003;22:365–75. [PubMed] [Google Scholar]
- 17.Awasthi S, Singhal SS, Singhal J, Cheng J, Zimniak P, Awasthi YC. Role of RLIP76 in lung cancer doxorubicin resistance II. Doxorubicin transport in lung cancer by RLIP76. Int J Oncol. 2003;22:713–20. [PubMed] [Google Scholar]
- 18.Singhal SS, Singhal J, Nair MP, Lacko AG, Awasthi YC, Awasthi S. Doxorubicin transport by RALBP1 and ABCG2 in lung and breast cancer. Int J Oncol. 2007;30:717–25. [PubMed] [Google Scholar]
- 19.Awasthi S, Singhal SS, Pikula S, Piper JT, Srivastava SK, Torman RT, Pikula J, Lin JT, Singh SV, Zimniak P, Awasthi YC. ATP-dependent human erythrocyte glutathione-conjugate transporter. Functional reconstitution of transport activity. Biochemistry. 1998;37:5239–48. doi: 10.1021/bi972131r. [DOI] [PubMed] [Google Scholar]
- 20.Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Over-expression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–4. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 21.Gottesman MM, Pastan I. Biochemistry of multidrug-resistance mediated by the multidrug-transporter. Annu Rev Biochem. 1993;62:385–27. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 22.Bankhead C. Three new drugs available to fight kidney cancer. J Natl Cancer Inst. 2006;98:1181–2. doi: 10.1093/jnci/djj384. [DOI] [PubMed] [Google Scholar]
- 23.Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597–12. doi: 10.1016/S0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- 24.Ma WW, Jimeno A. Temsirolimus. Drugs Today. 2007;43:659–69. doi: 10.1358/dot.2007.43.10.1148059. [DOI] [PubMed] [Google Scholar]
- 25.Sridhar SS, Hedley D, Siu LL. Raf kinase as a target for anticancer therapeutics. Mol Cancer Ther. 2005;4:677–85. doi: 10.1158/1535-7163.MCT-04-0297. [DOI] [PubMed] [Google Scholar]
- 26.Panner A, Nakamura JL, Parsa AT, Rodriguez-Viciana P, Berger MS, Stokoe D, Pieper RO. mTOR-independent translational control of the extrinsic cell death pathway by RalA. Mol Cell Biol. 2006;26:7345–57. doi: 10.1128/MCB.00126-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


