Abstract
This study reports the administration of the Cambridge Neuropsychological Test Automated Battery system’s delayed matching to sample (DMTS) task to juvenile baboons. Nine subjects (female=5, male=4) were trained with delay intervals ranging from 0–80 seconds. Trial unique stimuli were utilized in combination with matching to sample, in contrast to non-matching to sample, to more accurately assess components of medial temporal lobe (hippocampal formation) mediated working memory. These parameters force subjects to rely on recognition for matching stimuli and overcome their innate tendency to choose novel stimuli (non-matching), thus increasing task difficulty. Testing with delays intervals of 0, 1, 2, 4, 8, and 16 seconds revealed decreased percent correct responding as delay intervals increased. An effect of 1 versus 3 distractor stimuli on accuracy was also noted. Increasing the number of distractors resulted in decreased observing response latencies. The increase in choice response latency seen with increasing delay interval was independent of number of distractor stimuli presented. There were no sex differences in task performance. Our laboratory is focused on understanding the functional consequences of suboptimal conditions during pregnancy and early postnatal life in offspring. The ability of juvenile baboons to perform the DMTS task demonstrates the utility of this non-human primate model in examining pre- and postnatal conditions that impact the development of working memory. Evaluation of causes and consequences of impaired working memory in a variety of human diseases will be assisted by the use of this task in nonhuman primate models of human health and disease.
Keywords: delayed response task, operant conditioning, baboon, working memory, hippocampus, prefrontal cortex, developmental programming
1. INTRODUCTION
Nonhuman primate (NHP) models are commonly used to determine the neurobiological underpinnings of human learning and memory processes with delayed response tasks, such as delayed matching to sample (DMTS) (Zola-Morgan and Squire, 1990;Paule et al., 1998;Castner et al., 2004;Murray et al., 2007;Buccafusco, 2008). Higher order brain functions associated with performance of delayed response tasks in NHP's can be assessed with a variety of approaches including combining operant conditioning with pharmacological challenges (Paule, 2005;Buccafusco, 2008), neuroanatomical lesions (Zola-Morgan and Squire, 1986;Funahashi et al., 1993;Alvarez et al., 1994), electrophysiological recordings (Maunsell et al., 1991;Wang et al., 2004) and neuroimaging techniques (Ranganath, 2006;Rypma, 2006) to name a few. Those studies have validated the importance of the prefrontal cortex (Robbins and Arnsten, 2009), medial temporal lobe and the hippocampus in performing delayed response tasks (Zola and Squire, 2001). However the contribution of the medial temporal lobe is still debated (Budson, 2009).
Working memory (categorized as short-term memory) is required for remembering information that varies unpredictably in time and/or in content such as when different stimuli govern the criterion response on different trials, so the cue that must be remembered varies from trial to trial (Rodriguez and Paule, 2009). It is this type of memory that is greatly impaired in many human diseases such as attention deficit/hyperactivity disorder (Rhodes et al., 2004), schizophrenia (Barnett et al., 2010), Parkinson’s (Kehagia et al., 2010), and Alzheimer’s disease (Germano and Kinsella, 2005). Indeed epidemiological studies show that suboptimal prenatal and early postnatal environments negatively influence normal development, and in particular brain development, which result in abnormal cognition and behavioral disorders in offspring (Galler et al., 1983a;Galler et al., 1983b;Galler et al., 1984a;Galler et al., 1984b;Galler et al., 1985a;Galler et al., 1985b;Galler et al., 1987a;Galler et al., 1987b;Galler et al., 1987c;Galler and Ramsey, 1989;Galler et al., 1990;Wadhwa, 2005). Development of NHP behavioral models that closely emulate human learning and memory will facilitate greater understanding of these disease processes. Delayed response tasks are an invaluable tool in deciphering the processes of working memory. Accordingly, investigations of working memory in the NHP offspring will facilitate the elucidation of the brain areas impacted by suboptimal developmental environments and greatly facilitate translation to the human.
We have previously utilized the Cambridge Neuropsychological Test Automated Battery (CANTAB) system to develop training procedures and testing protocols in juvenile baboon subjects that allow the assessment of function through a series of cognitive challenges including simple discrimination, simple discrimination reversal, progressive ratio and intra- and extra-dimensional attention set shift tasks. These procedures assess associative learning, acquisition of task rule change, motivation, selective attention and attentional set-shifting respectively (Zurcher et al., 2010). Here we describe the training and responses of the same animal model to tasks that extend the battery of operant testing to include evaluation of working memory through the administration of trial-unique delayed matching to sample task using the CANTAB system. We report responses of test subjects with varying delay intervals and multiple visual distractors and provide brief interpretation of these responses in the contexts of attention, encoding, retention, discrimination, selective attention and retrieval. Since the baboon is phylogenetically very similar to the human, we believe this adaptation of the DMTS task using the CANTAB system will prove of translational value in assessing human behavior in health and disease states.
2. METHODS
2.1 Subjects
All procedures were approved by the Southwest Foundation for Biomedical Research (SFBR) and University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committees. Subjects were 9 adolescent baboons (Papio sp.) 3.9 ± 0.2 years of age at study onset and weighed within the normal range at this age (Coelho, Jr. et al., 1984). All baboons were born, nursed and mother-reared in SFBR group housing until transferred to the University of Texas Health Science Center’s Laboratory Animal Resources facility and habituated to single-cage housing for 1 month prior to operant conditioning.
Operant assessments were conducted between 9 a.m. and 4 p.m., Monday through Friday. Following behavioral assessment, subjects were fed with nonhuman primate chow (2050 Teklad Global 20% protein, 2.7 kcal/g metabolizable energy, Harlan Laboratories, USA). Daily chow rations were calculated prior to training by administering food ad libitum over 2 weeks and measuring average daily consumption, each subject was then fed this amount for the remainder of the study. No refusal to eat was observed. Water was available ad libitum. For enrichment and dietary supplementation, subjects received fruits in the late afternoon after behavioral testing three times weekly and were periodically given vitamins in treats. Lighting went on at 07.00 and off at 19.00h.
2.2. CANTAB apparatus
The Monkey CANTAB software and apparatus (models 80650/80652*C, Lafayette, IN, USA) were used to conduct behavioral testing. The testing station was constructed of an aluminum chassis incorporating a computer monitor with an infrared touch screen, a pellet dispenser (model 80209, Campden Instruments Ltd., Lafayette, IN, USA) and pellet trough situated onto a moveable trolley. This trolley was attached to the home cage. During training and testing the home cage permitted access to manipulate the CANTAB touch screen monitor situated 14 cm away. The controlling computer (IBM compatible Pentium IV) and LCD monitor were connected to the testing station by a 10-m cable and located behind a partition curtain in the testing laboratory removed from test subjects’ eyesight. This computer controlled the CANTAB software that generated the visual stimuli, task parameter/contingencies, and recorded the number of trials and errors made. The computer monitor ran in parallel with the touch screen monitor to display the location of the subject’s touches in real-time to the experimenter. A remote camera (Astak CM-842G wireless camera) was placed on top of the testing cage so the experimenter had a good view of the subject and CANTAB apparatus. Subjects were familiarized with the testing cage, CANTAB set-up, and camera before operant assessment.
2.3 Delayed Matching To Sample Task
The subjects had previously been trained and tested in simple discrimination, simple discrimination reversal, progressive ratio and intra- and extra-dimensional attention set shift tasks as described (Zurcher et al., 2010). The DMTS task parameters were adapted from rhesus monkey protocols (Rodriguez et al., 2010). Subjects were assessed in 30 minute sessions with a maximum of 120 trials. Two banana flavored food pellets were the positive reinforcement following correct stimulus choice selection. A ‘correction procedure’ (i.e. to eliminate side preference) was utilized during delay set 1 when a subject failed to meet criterion after five sessions; 3 out of 9 subjects required correction. The stimuli were taken from the Cambridge Cognition Pal 0 set, that generates 199,283 trial-unique stimuli by enabling the ‘vary colors’ selection which multiples the number of stimuli (83) by 2401 (see Table 1 for representative stimuli).
Table 1.
Sample of the shapes used during dmts task. Used with permission (Cardinal and Aitken, 2010;Cardinal, 2010).
 |
Initially, a sample stimulus was presented on the touch screen monitor for 30 seconds during which the subject made an observing response which was not reinforced. Observing response latency was the time elapsed between presentation of the sample stimulus and a press on the touch screen monitor. Failure to make an observing response ended the trial followed with a 5 second inter-trial interval. An observing response blanked the touch screen contemporaneous with a varying delay interval followed by presentation of choice stimuli for which a choice response was required. The correct stimulus and 1 or 3 distractor stimuli were simultaneously presented randomly at the 4 corners of the touch screen monitor during choice stimulus presentation. Thus, if the sample stimulus for a given trial was a triangle, the subject had to choose the triangle from the presented choice stimuli, only one of which would be the triangle. Subjects were allowed 30 seconds for choice stimulus responding (choice response latency). Five second inter-trial intervals followed both correct and incorrect choice stimuli selection.
The DMTS task consisted of 6 delay intervals chosen randomly and interposed after initial (observing) responses were made to the sample stimuli: 20 trials per delay interval for a max of 120 trials. At the outset delay intervals were all set to 0 seconds; delay set 1. When overall choice accuracy (i.e., average accuracy of the 6 delay intervals) was between 60–70% for three consecutive sessions, or 71–84% for two consecutive session or 1 session equal to 85% or greater, the subsequent delay set was used: (0, 0, 0, 1, 1, and 1 = three values were set to 0 seconds and three to 1 second). In this manner, the following delay sets were incorporated into the DMTS task as subjects demonstrated criterion performance: 0, 0, 1, 1, 2, and 2 seconds (sec); 0, 1, 1, 2, 2, and 4 sec; 0, 1, 1, 2, 4, and 8 sec; 0, 1, 2, 4, 8, and 16 sec; 0, 2, 4, 8, 16, and 32 sec; 0, 4, 8, 16, 32, and 48 sec; 0, 8, 16, 32, 48, and 64 sec; 0, 16, 32, 48, 64, and 80 sec. These ten delay sets were administered with one distractor, however not all subjects attained performed criterion to delay set 10. Delay set 6 was assigned as the minimum set for which performance criterion was required to proceed to testing since all subjects attained this stage and thus is the only set presented comparing number of distractors. During testing, subjects were administered the following in consecutive sessions: 6th delay set with two distractors, 6th delay set with three distractors, 7th delay set with three distractors, the 8th delay set with three distractors and the 7th and 8th delay sets with one distractor for subjects which lacked those observations (4 of the 9). Performance criterion was not required during testing sessions.
2.4 Data Analysis
Data are summarized with the mean ± standard error. A sex comparison was performed with percent correct responding (accuracy) as the dependent variable and independent variables of delay length, number of distractors, observing response latency and choice response latency. A repeated measures linear model with an autoregressive order 1 autocorrelation matrix was utilized for comparisons using SAS Version 9.2 (SAS institute, Cary NC); statistical significance was set at p ≤ 0.05.
3. RESULTS
Subjects on average required 13 sessions (± 1.09) to train to delay set 6 with the faster learners acquiring criterion by the 10th session and the slower learners by the 18th session. Table 2 depicts each subject’s sex, age and delay set attained during training. No sex differences were determined for delay intervals under the 1 distractor (p=0.25) condition, so sexes were pooled for analyses. Comparisons for effects of delay interval revealed differences. Figure 1A shows percent correct responding per delay interval under the 1 distractor condition with accuracy following 0 or 1 sec delays higher than after 4, 8 and 16 sec delays (*, p<0.05) and accuracy following 2 or 4 sec delays higher than after 8 and 16 sec delays (+, p<0.05). No sex differences were determined for delay intervals under the 3 distractor (p=0.31) condition, so sexes were pooled for analyses. Figure 1B shows percent correct responding per delay interval under the 3 distractor condition with accuracy following 0 sec delays higher than after 8 and 16 sec delays (*, p<0.05) and accuracy following 1, 2, 4 and 8 sec delays higher than after 16 sec delays (+, p<0.05). Figure 1C shows comparisons of number of distractors per delay interval. An increase in number of distractors significantly reduced percent correct responding at 0, 1, 2, and 16 second delay intervals (*, p<0.05).
Table 2.
Male and female subjects of similar age were trained between 16–18 sessions to progress through the 10 delay sets under the 1 distractor condition. Since all subjects reached mastery of delay set 6, this set was used for the 3 distractor test.
| Subject | Sex | Age (yrs) | Delay set |
|---|---|---|---|
| 27237 | F | 4.6 | 7 |
| 27827 | F | 4.1 | 8 |
| 28368 | F | 3.7 | 8 |
| 28385 | F | 3.5 | 6 |
| 28497 | F | 3.5 | 10 |
| 26322 | M | 5.0 | 10 |
| 28473 | M | 3.6 | 6 |
| 28428 | M | 3.5 | 6 |
| 28476 | M | 3.5 | 10 |
Figure 1.
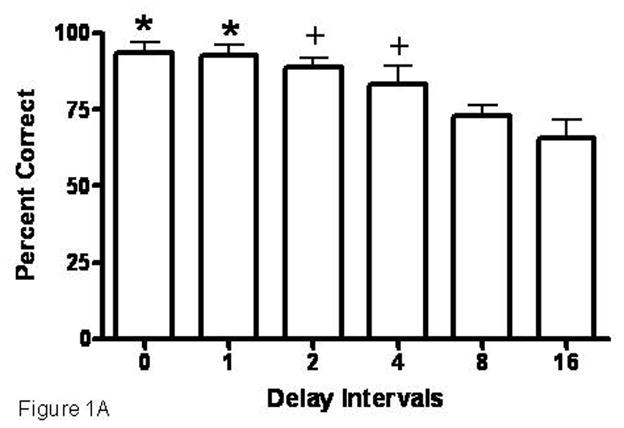


Figure 1A) Accuracy comparisons between delay intervals under 1 distractor condition. Accuracy following 0 or 1sec delay intervals is higher than after 4, 8 and 16 sec delays (*, p<0.05); accuracy following 2 or 4 sec delays is higher than after 8 and 16 sec delays (+, p<0.05). Mean ± SEM.
Figure 1B) Accuracy comparisons between delay intervals under 3 distractor condition. Accuracy following 0 sec delay intervals is higher than after 8 and 16 sec delays (*, p<0.05); accuracy following 1, 2, 4 and 8 sec delays is higher than after 16 sec delays (+, p<0.05). Mean ± SEM.
Figure 1C) Accuracy comparisons between 1 (open bar) and 3 (closed bar) distractor conditions. An effect of number of distractors was determined at 0, 1, 2, and 16 sec delays (*, p<0.05). Mean ± SEM.
No sex differences were determined for observing response latencies under the 1 distractor (p=0.19) or 3 distractor (p=0.26) conditions, so sexes were pooled for analyses. Comparing overall observing response latency under the 1 or 3 distractor conditions revealed a decrease in latency as number of distractors increased but the difference did not reach significance (Figure 2A). Observing response latency is independent of the delay interval and is therefore averaged across delays. No sex differences were determined for choice response latencies under the 1 distractor (p=0.45) or 3 distractor (p=0.88) conditions, so sexes were pooled for analyses. Comparing choice response latencies under the 1 or 3 distractor conditions revealed an increase in latency as the delay interval increased with no effect of distractor number. Choice response latencies increased for responses following 1, 2, 4, and 16 sec delays compared to no delay with 1 distractor (Figure 2B). Choice response latencies with 3 distractors increased for responses following delay intervals of 4 and 16 sec compared to 0 and 2 sec delays, and only after 16 sec delays as compared to 1 sec intervals (Figure 2C).
Figure 2.



Figure 2A) Observing response latency for all delay intervals under each distractor condition. Increasing number of distractors reduced observing response latency but did not reach significance p=0.3.
Figure 2B) Choice response latencies per delay intervals under 1 distractor condition. Latency following 0 sec delay intervals is shorter than after 1, 2, 4, and 16 sec delays (*, p<0.05). Mean ± SEM.
Figure 2C) Choice response latencies per delay intervals under 3 distractor condition. Latency following 0 and 2 sec delay intervals is shorter than after 4 and 16 sec delays (*, p<0.05); latency following 1 sec delays is shorter than after 16 sec delays (+, p<0.05). Mean ± SEM.
4. DISCUSSION
We have demonstrated that the CANTAB system can be used to successfully administer the DMTS task to juvenile (3.9 years old) baboons. The subjects were not naïve to CANTAB testing having participated in testing as described before (Zurcher et al., 2010). Even so, training for the present task took between 10–18 sessions (Table 2). We have shown that percent correct responding decreased with increasing delay intervals. Also, increasing the number of distractors presented from 1 to 3 distractors decreased response accuracy that, in turn, was associated with decrease in observing response latency. Choice response latency increased as delay intervals increased regardless of number of distractors. Finally, no sex differences were determined for behavioral endpoints.
Overall, the DMTS task is a valuable tool for measuring working memory. However, there are some limitations which should be considered. [1] Ceiling and floor effects. The DMTS task can suffer from ceiling effects if task parameters are not sufficiently challenging. Response accuracies even at long delay intervals can be very high which makes detection of experimentally induced alterations in memory difficult to assess (Rodriguez and Paule, 2009). Lengthening delay intervals and/or increasing complexity of stimuli or number of distractors so that discrimination between sample and choice stimuli is more demanding can usually resolve this problem (Rodriguez and Paule, 2009). As evident by the findings reported here, increasing the number of distractors during the test phase can decrease accuracy following no delay conditions which demonstrates effects on encoding. Conversely, if the task is too difficult a floor effect can result in which case delay intervals can be shortened or the difficulty of the discrimination can be made less challenging (Rodriguez and Paule, 2009). Delay intervals in DMTS task administered to human or other nonhuman primates range from 2–80 seconds with 12 seconds intervals most common in human testing (Coghill et al., 2007;Rodriguez et al., 2010). The delay intervals administered in this study are well within the assessment range of human capabilities and established tasks protocols.
[2] Side preference. Another potential pitfall of delayed response tasks is side preference. In tasks administering a limited number of response locations, subjects frequently develop a position preference that results in a majority of their choice responses being restricted to one side, i.e. either the right or left response position in a two choice paradigm (Rodriguez and Paule, 2009). As a consequence on approximately 50% of all trials the subject’s response to one location can result in a correct response and reinforcement (Angeli et al., 1993). Even when such biases are not observed under control conditions they can be induced through experimental design (such as with drug administration) complicating response interpretation (Baron and Wenger, 2001). However, the protocol described here for juvenile baboon avoided this potential problem since choice stimuli were presented randomly at the corners of the touch screen. In addition 1 or 3 distractors ensured a high degree of sample and distractor stimuli placement uncertainty.
The DMTS task allows for the assessment of mnemonic processes (i.e. discrimination, encoding and retention) which are relevant to human cognition and executive function, such as attention, strategy formation, reaction time in complex situations, and memory for recent events. With this approach we have demonstrated that which a number of studies have shown; percent accuracy of the matching response is proportional to the length of the delay (Paule et al., 1998). Also, the effect of distractors on accuracy we report are in agreement with previous studies (Rodriguez and Paule, 2009). Results from response latencies deserve further consideration. Decreases in observing response latency under the 3 distractor situation, although not significantly different, implies subjects were attentive to sample stimulus by responding quickly, but in doing so decreased the amount of time needed to encode into short-term memory. This decrease in encoding led to decreased retention or maintenance of sample stimulus during delay intervals. Lastly, choice response latencies increased as the delay interval increased which implies impaired discrimination and retrieval resulting in increased error during choice stimulus selection. The increase in choice response latencies was independent of distractor number and is similar to behavioral profiles reported in children (Chelonis et al., 2000). The use of trial unique stimuli recruited the utilization of medial temporal lobe components in addition to the prefrontal cortical areas typically relied upon task performance (Stern et al., 2001;Hunkin et al., 2002). No sex differences were determined which is consistent with findings of children administered DMTS (Chelonis et al., 2000). Under experimental conditions, these measures can be assessed to differentiate effects on working memory function.
In summary, the establishment of a DMTS paradigm using the CANTAB in juvenile baboons provides a method for detecting and elucidating the biological underpinnings of working memory under controlled experimental conditions that are extremely difficult to achieve in humans. Although various laboratories have administered delayed non-matching to sample (DNMS) (Weed et al., 1999;Taffe et al., 1999;Golub, 2002;Weed et al., 2004), DMTS (Katner et al., 2004;Buccafusco, 2008;Rodriguez et al., 2010), or delayed/concurrent delayed match to position (DMP/CDMP) tasks (Spinelli et al., 2004;Spinelli et al., 2006) to rhesus monkeys and marmosets, we are the first to establish the feasibility of utilizing the CANTAB system to administer the DMTS task to baboons and incorporating trial-unique stimuli with the same temporal parameters used in the clinical setting. The performance accuracies we report are comparable to the behavioral profiles shown in studies which administered DNMS or CDMP tasks with similar delay and distractor conditions (Weed et al., 1999;Spinelli et al., 2004). However, it should be noted that DMTS tasks are more challenging than DNMS tasks because NHP’s have a tendency to response to novel stimuli, a confounding characteristic that is disallowed by the DMTS design. In agreement with our findings, Katner et al., (2004) has reported approximately 75% correct responding following 2 sec delays in rhesus monkeys administered the CANTAB DMTS task with 3 distractors, which is identical to what we demonstrate in this study.
Our model did not test subjects in asymptotic performance nor adjust delay intervals to individual subject performance as others have shown (Buccafusco, 2008). To emulate the clinical assessment of untrained human subjects administered an acute task challenge; we administered a test session with an increase in distractor number at a specific delay set. The clinical relevance of this type of task is further supported by the observation that DMTS performance in humans correlates significantly with IQ (Paule et al., 1999). By inference, use of these tasks in animal studies should provide direct access to important aspects of animal intelligence. Availability of this task in the baboon using the CANTAB system will allow direct comparison of age, drug and developmentally related differences in both normative development and important challenges to mental health using this well documented nonhuman primate species. In particular, our future goals include the application of CANTAB testing in developing understanding of the consequences of suboptimal nutrition during pregnancy on offspring neurodevelopment and it’s mental health consequences.
Acknowledgments
HD57480 (TQB, MJN), HD21350-17 (PWN, MJN) and an NIH Postdoctoral Supplement (JSR HD21350-17).
We sincerely thank Dr. Joel E. Michalek and Yumin Chen from the Department of Epidemiology and Biostatistics for the statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alvarez P, Zola-Morgan S, Squire LR. The animal model of human amnesia: long-term memory impaired and short-term memory intact. Proc Natl Acad Sci U S A. 1994;91:5637–5641. doi: 10.1073/pnas.91.12.5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli SJ, Murray EA, Mishkin M. Hippocampectomized monkeys can remember one place but not two. Neuropsychologia. 1993;31:1021–1030. doi: 10.1016/0028-3932(93)90030-4. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Robbins TW, Leeson VC, Sahakian BJ, Joyce EM, Blackwell AD. Assessing cognitive function in clinical trials of schizophrenia. Neurosci Biobehav Rev. 2010;34:1161–1177. doi: 10.1016/j.neubiorev.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Baron SP, Wenger GR. Effects of drugs of abuse on response accuracy and bias under a delayed matching-to-sample procedure in squirrel monkeys. Behav Pharmacol. 2001;12:247–256. doi: 10.1097/00008877-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ. Estimation of working memory in macaques for studying drugs for the treatment of cognitive disorders. J Alzheimers Dis. 2008;15:709–720. doi: 10.3233/jad-2008-15414. [DOI] [PubMed] [Google Scholar]
- Budson AE. Understanding memory dysfunction. Neurologist. 2009;15:71–79. doi: 10.1097/NRL.0b013e318188040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN. MonkeyCantab, version 7.6, computer software. 2010. Ref Type: Internet Communication. [Google Scholar]
- Cardinal RN, Aitken MR. Whisker: A client-server high-performance multimedia research control system. Behav Res Methods. 2010;42:1059–1071. doi: 10.3758/BRM.42.4.1059. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS, Williams GV. Animal models of working memory: insights for targeting cognitive dysfunction in schizophrenia. Psychopharmacology (Berl) 2004;174:111–125. doi: 10.1007/s00213-003-1710-9. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, niels-Shaw JL, Blake DJ, Paule MG. Developmental aspects of delayed matching-to-sample task performance in children. Neurotoxicol Teratol. 2000;22:683–694. doi: 10.1016/s0892-0362(00)00090-8. [DOI] [PubMed] [Google Scholar]
- Coelho AM, Jr, Glassman DM, Bramblett CA. The relation of adiposity and body size to chronological age in olive baboons. Growth. 1984;48:445–454. [PubMed] [Google Scholar]
- Coghill DR, Rhodes SM, Matthews K. The neuropsychological effects of chronic methylphenidate on drug-naive boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:954–962. doi: 10.1016/j.biopsych.2006.12.030. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas”. J Neurosci. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler JR, Ramsey F. A follow-up study of the influence of early malnutrition on development: behavior at home and at school. J Am Acad Child Adolesc Psychiatry. 1989;28:254–261. doi: 10.1097/00004583-198903000-00018. [DOI] [PubMed] [Google Scholar]
- Galler JR, Ramsey F, Solimano G. The influence of early malnutrition on subsequent behavioral development III. Learning disabilities as a sequel to malnutrition. Pediatr Res. 1984a;18:309–313. doi: 10.1203/00006450-198404000-00001. [DOI] [PubMed] [Google Scholar]
- Galler JR, Ramsey F, Solimano G. A follow-up study of the effects of early malnutrition on subsequent development. II. Fine motor skills in adolescence. Pediatr Res. 1985a;19:524–527. doi: 10.1203/00006450-198506000-00004. [DOI] [PubMed] [Google Scholar]
- Galler JR, Ramsey F, Solimano G. Influence of early malnutrition on subsequent behavioral development. V. Child’s behavior at home. J Am Acad Child Psychiatry. 1985b;24:58–64. doi: 10.1016/s0002-7138(09)60410-6. [DOI] [PubMed] [Google Scholar]
- Galler JR, Ramsey F, Solimano G, Kucharski LT, Harrison R. The influence of early malnutrition on subsequent behavioral development. IV. Soft neurologic signs. Pediatr Res. 1984b;18:826–832. doi: 10.1203/00006450-198409000-00004. [DOI] [PubMed] [Google Scholar]
- Galler JR, Ramsey F, Solimano G, Lowell WE. The influence of early malnutrition on subsequent behavioral development. II. Classroom behavior. J Am Acad Child Psychiatry. 1983a;22:16–22. doi: 10.1097/00004583-198301000-00003. [DOI] [PubMed] [Google Scholar]
- Galler JR, Ramsey F, Solimano G, Lowell WE, Mason E. The influence of early malnutrition on subsequent behavioral development. I. Degree of impairment in intellectual performance. J Am Acad Child Psychiatry. 1983b;22:8–15. doi: 10.1097/00004583-198301000-00002. [DOI] [PubMed] [Google Scholar]
- Galler JR, Ramsey FC, Forde V, Salt P, Archer E. Long-term effects of early kwashiorkor compared with marasmus. II. Intellectual performance. J Pediatr Gastroenterol Nutr. 1987a;6:847–854. doi: 10.1097/00005176-198711000-00005. [DOI] [PubMed] [Google Scholar]
- Galler JR, Ramsey FC, Forde V, Salt P, Archer E. Long-term effects of early kwashiorkor compared with marasmus. II. Intellectual performance. J Pediatr Gastroenterol Nutr. 1987b;6:847–854. doi: 10.1097/00005176-198711000-00005. [DOI] [PubMed] [Google Scholar]
- Galler JR, Ramsey FC, Morley DS, Archer E, Salt P. The long-term effects of early kwashiorkor compared with marasmus. IV. Performance on the national high school entrance examination. Pediatr Res. 1990;28:235–239. doi: 10.1203/00006450-199009000-00018. [DOI] [PubMed] [Google Scholar]
- Galler JR, Ramsey FC, Salt P, Archer E. Long-term effects of early kwashiorkor compared with marasmus. III. Fine motor skills. J Pediatr Gastroenterol Nutr. 1987c;6:855–859. doi: 10.1097/00005176-198711000-00006. [DOI] [PubMed] [Google Scholar]
- Germano C, Kinsella GJ. Working memory and learning in early Alzheimer’s disease. Neuropsychol Rev. 2005;15:1–10. doi: 10.1007/s11065-005-3583-7. [DOI] [PubMed] [Google Scholar]
- Golub MS. Cognitive testing (delayed non-match to sample) during oral treatment of female adolescent monkeys with the estrogenic pesticide methoxychlor. Neurotoxicol Teratol. 2002;24:87–92. doi: 10.1016/s0892-0362(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Hunkin NM, Mayes AR, Gregory LJ, Nicholas AK, Nunn JA, Brammer MJ, Bullmore ET, Williams SC. Novelty-related activation within the medial temporal lobes. Neuropsychologia. 2002;40:1456–1464. doi: 10.1016/s0028-3932(01)00200-7. [DOI] [PubMed] [Google Scholar]
- Katner SN, Davis SA, Kirsten AJ, Taffe MA. Effects of nicotine and mecamylamine on cognition in rhesus monkeys. Psychopharmacology (Berl) 2004;175:225–240. doi: 10.1007/s00213-004-1804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;20:199–204. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Sclar G, Nealey TA, DePriest DD. Extraretinal representations in area V4 in the macaque monkey. Vis Neurosci. 1991;7:561–573. doi: 10.1017/s095252380001035x. [DOI] [PubMed] [Google Scholar]
- Murray EA, O’Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J Neurosci. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule MG. Chronic drug exposures during development in nonhuman primates: models of brain dysfunction in humans. Front Biosci. 2005;10:2240–2249. doi: 10.2741/1693. [DOI] [PubMed] [Google Scholar]
- Paule MG, Bushnell PJ, Maurissen JP, Wenger GR, Buccafusco JJ, Chelonis JJ, Elliott R. Symposium overview: the use of delayed matching-to-sample procedures in studies of short-term memory in animals and humans. Neurotoxicol Teratol. 1998;20:493–502. doi: 10.1016/s0892-0362(98)00013-0. [DOI] [PubMed] [Google Scholar]
- Paule MG, Chelonis JJ, Buffalo EA, Blake DJ, Casey PH. Operant test battery performance in children: correlation with IQ. Neurotoxicol Teratol. 1999;21:223–230. doi: 10.1016/s0892-0362(98)00045-2. [DOI] [PubMed] [Google Scholar]
- Ranganath C. Working memory for visual objects: complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139:277–289. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- Rhodes SM, Coghill DR, Matthews K. Methylphenidate restores visual memory, but not working memory function in attention deficit-hyperkinetic disorder. Psychopharmacology (Berl) 2004;175:319–330. doi: 10.1007/s00213-004-1833-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto- executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JS, Morris SM, Hotchkiss CE, Doerge DR, Allen RR, Mattison DR, Paule MG. The effects of chronic methylphenidate administration on operant test battery performance in juvenile rhesus monkeys. Neurotoxicol Teratol. 2010;32:142–151. doi: 10.1016/j.ntt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JS, Paule MG. Working memory: delayed response tasks in monkeys. In: Buccafusco JJ, editor. Methods of behavior analysis in neuroscience. Boca Raton: Taylor & Francis Group, LLC, CRC Press; 2009. [PubMed] [Google Scholar]
- Rypma B. Factors controlling neural activity during delayed-response task performance: testing a memory organization hypothesis of prefrontal function. Neuroscience. 2006;139:223–235. doi: 10.1016/j.neuroscience.2005.07.062. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Ballard T, Feldon J, Higgins GA, Pryce CR. Enhancing effects of nicotine and impairing effects of scopolamine on distinct aspects of performance in computerized attention and working memory tasks in marmoset monkeys. Neuropharmacology. 2006;51:238–250. doi: 10.1016/j.neuropharm.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Pennanen L, Dettling AC, Feldon J, Higgins GA, Pryce CR. Performance of the marmoset monkey on computerized tasks of attention and working memory. Brain Res Cogn Brain Res. 2004;19:123–137. doi: 10.1016/j.cogbrainres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME. Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus. 2001;11:337–346. doi: 10.1002/hipo.1048. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gold LH. Scopolamine alters rhesus monkey performance on a novel neuropsychological test battery. Brain Res Cogn Brain Res. 1999;8:203–212. doi: 10.1016/s0926-6410(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Weed MR, Gold LH, Polis I, Koob GF, Fox HS, Taffe MA. Impaired performance on a rhesus monkey neuropsychological testing battery following simian immunodeficiency virus infection. AIDS Res Hum Retroviruses. 2004;20:77–89. doi: 10.1089/088922204322749521. [DOI] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR. Relationship between magnitude of damage to the hippocampus and impaired recognition memory in monkeys. Hippocampus. 2001;11:92–98. doi: 10.1002/hipo.1027. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Memory impairment in monkeys following lesions limited to the hippocampus. Behav Neurosci. 1986;100:155–160. doi: 10.1037//0735-7044.100.2.155. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. The neuropsychology of memory. Parallel findings in humans and nonhuman primates. Ann N Y Acad Sci. 1990;608:434–450. doi: 10.1111/j.1749-6632.1990.tb48905.x. [DOI] [PubMed] [Google Scholar]
- Zurcher NR, Rodriguez JS, Jenkins SL, Keenan K, Bartlett TQ, McDonald TJ, Nathanielsz PW, Nijland MJ. Performance of juvenile baboons on neuropsychological tests assessing associative learning, motivation and attention. J Neurosci Methods. 2010;188:219–225. doi: 10.1016/j.jneumeth.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


